Abstract
Host immune responses are crucial for combating enteropathogenic infections including Campylobacter jejuni. Within 1 week following peroral C. jejuni infection, secondary abiotic IL-10–/– mice develop severe immunopathological sequelae affecting the colon (ulcerative enterocolitis). In the present study, we addressed whether pathogen-induced pro-inflammatory immune responses could also be observed in the small intestines dependent on the innate receptor nucleotide-oligomerization-domain-protein 2 (Nod2). Within 7 days following peroral infection, C. jejuni stably colonized the gastrointestinal tract of both IL-10–/– mice lacking Nod2 (Nod2–/– IL-10–/–) and IL-10–/– controls displaying bloody diarrhea with similar frequencies. Numbers of apoptotic and regenerating epithelial cells increased in the small intestines of C. jejuni-infected mice of either genotype that were accompanied by elevated ileal T and B lymphocyte counts. Notably, ileal T cell numbers were higher in C. jejuni-infected Nod2–/– IL-10–/– as compared to IL-10–/– counterparts. Furthermore, multifold increased concentrations of pro-inflammatory cytokines including IFN-γ, TNF, and MCP-1 could be measured in small intestinal ex vivo biopsies derived from C. jejuni-infected mice of either genotype. In conclusion, C. jejuni-induced pro-inflammatory immune responses affected the small intestines of both Nod2–/– IL-10–/– and IL-10–/– mice, whereas ileal T lymphocyte numbers were even higher in the former.
Keywords: small intestine, Campylobacter jejuni, nucleotide-oligomerization-domain-2, murine IL-10–/– infection model, pro-inflammatory immune responses
Introduction
Host immune responses are pivotal for combating enteropathogenic infections. The nucleotide-binding oligomerization domain (Nod)-like receptors constitute an important family of intracellular pattern recognition receptors that regulate host immunity by sensing microbial products and damage-associated factors [1]. Among these, Nod2 is expressed by innate (including monocytes, macrophages, and dendritic cells) and adaptive (such as T lymphocytes) immune cell populations [2, 3] and is activated by muramyl dipeptide (MDP), a constituent of bacterial peptidoglycans [4]. Activation of the Nod2 signaling pathway confers resistance against a broad variety of bacterial species including enteropathogens [1, 5–7]. Among these, Campylobacter jejuni are colonizing domestic and wild animals as commensals. Humans, however, become infected via the food chain by consumption of contaminated livestock animals or surface water [8, 9]. Depending on the virulence of the acquired strain and the host immune status, infected individuals present with symptoms of varying degree. Whereas most patients display rather mild malaise and watery diarrhea, others suffer from severe ulcerative enterocolitis with fever, abdominal cramps, and inflammatory, bloody diarrhea [10, 11]. Despite the progressively increasing prevalence of human campylobacteriosis cases, molecular and cellular events involved in disease development are not fully understood. For a long time, in vivo studies have been hampered by a lack of suitable animal models. We have recently shown that secondary abiotic mice generated by broad-spectrum antibiotic treatment (virtually lacking an intestinal microbiota) developed wasting non-selflimiting ulcerative enterocolitis within 1 week following peroral C. jejuni infection mimicking key features of campylobacteriosis in severely immunocompromized patients [12–16]. Importantly, the large intestinal immunopathology was mediated by distinct innate immune receptors such as Toll-like receptor (TLR) -2 and -4 [12, 17]. However, pathogen-induced inflammatory responses in the small intestines have not been reported so far.
Given the colon as predilection site of C. jejuni infection, we addressed in the present study 1) whether C. jejuni-induced inflammatory sequelae could also be observed in the small intestines of secondary abiotic IL-10–/– and, if so, 2) whether Nod2 was involved in mediating small intestinal immunopathology.
Methods
Generation of secondary abiotic mice and C. jejuni infection
Female IL-10–/– mice and IL-10–/– mice lacking Nod2 (Nod2–/– IL-10–/–) mice (all in C57BL/6j background) were reared and kept within the same specific pathogen-free (SPF) unit of the Forschungseinrichtungen für Experimentelle Medizin (FEM, Charité – University Medicine Berlin). To counteract physiological colonization resistance and assure stable intestinal colonization of the pathogen [18], secondary abiotic (i.e., gnotobiotic) mice virtually lacking an intestinal microbiota were generated by broad-spectrum antibiotic treatment for 8 weeks as described previously [18, 19]. Three days prior infection, the antibiotic cocktail was withdrawn and replaced by autoclaved tap water. Mice were then perorally infected with 109 colony forming units (CFU) of viable C. jejuni strain 81-176 in a volume of 0.3 ml phosphate buffered saline (PBS; Gibco, Life Technologies, UK) on two consecutive days (days 0 and 1) by gavage as described earlier [18]. To prevent mice from contaminations, animals were continuously maintained in a sterile environment (autoclaved food and drinking water or sterile antibiotic cocktail) and handled under strict aseptic conditions.
Clinical conditions
To assess clinical signs of C. jejuni-induced infection on a daily basis, a standardized cumulative clinical score (maximum 12 points), addressing the occurrence of blood in feces (0: no blood; 2: microscopic detection of blood by the Guajac method using Haemoccult, Beckman Coulter/PCD, Germany; 4: macroscopic blood visible), diarrhea (0: formed feces; 2: pasty feces; 4: liquid feces), and the clinical aspect (0: normal; 2: ruffled fur, less locomotion; 4: isolation, severely compromized locomotion, pre-final aspect) was used as described earlier [12, 20, 21].
Sampling procedures and immunohistochemistry
Mice were sacrificed at day 7 p.i. by isofluran treatment (Abbott, Germany). Ileal ex vivo biopsies were asserved under sterile conditions and collected from each mouse in parallel for microbiological, histopathological, immunohistopathological, and immunological analyses.
In situ immunohistochemical analysis of ileal paraffin sections was performed as described elsewhere [14, 20–22]. Primary antibodies against cleaved caspase-3 (Asp175, Cell Signaling, USA, 1:200), Ki67 (TEC3; Dako, Denmark; 1:100), CD3 (#N1580; Dako; 1:10), FOXP3 (FJK-16s; eBioscience, Germany; 1:100), and B220 (eBioscience; 1:200) were used. For each animal, the average number of positively stained cells within at least six high power fields (HPF, 0.287 mm2, 400× magnification) were determined microscopically by a blinded independent investigator.
Quantitative analysis of bacterial colonization
Viable C. jejuni were quantitatively assessed in feces over time p.i. or at time of necropsy (i.e., day 7 p.i.) in luminal samples taken from the gastrointestinal tract (i.e., stomach, duodenum, ileum, and colon), dissolved in sterile PBS and serial dilutions streaked onto Karmali- and Columbia-Agar supplemented with 5% sheep blood (Oxoid, Germany) for 2 days at 37 °C under microaerobic conditions using CampyGen gas packs (Oxoid). The respective weights of fecal or gastrointestinal luminal samples were determined by the difference of the sample weights before and after asservation. The detection limit of viable pathogens was ≈100 CFU per g.
Cytokine detection in supernatants of small intestinal ex vivo biopsies
Ileal ex vivo biopsies were cut longitudinally and washed in PBS. Strips of approximately 1 cm2 of small intestinal tissues were placed into 24-flat-bottom well culture plates (Nunc, Germany) containing 500 μl serum-free RPMI 1640 medium (Gibco, Life Technologies, UK) supplemented with penicillin (100 U/ml) and streptomycin (100 μg/ml; PAA Laboratories, Germany). After 18 h at 37 °C, culture supernatants were tested for IFN-γ, TNF, MCP-1, and IL-6 by the Mouse Inflammation Cytometric Bead Assay (CBA; BD Biosciences, Germany) on a BD FACS-Canto II flow cytometer (BD Biosciences). Nitric oxide (NO) was measured by the Griess reaction as described earlier [19].
Statistical analysis
Medians and levels of significance were determined using Mann–Whitney test (GraphPad Prism v5, La Jolla, CA, USA) as indicated. Two-sided probability (p) values ≤ 0.05 were considered significant. Experiments were repeated at least twice.
Ethics statement
All animal experiments were conducted according to the European Guidelines for animal welfare (2010/63/EU) with approval of the commission for animal experiments headed by the “Landesamt für Gesundheit und Soziales” (LaGeSo, Berlin, registration number G0135/10). Animal welfare was monitored twice daily by assessment of clinical conditions.
Results
Gastrointestinal colonization densities of C. jejuni following peroral infection of secondary abiotic IL-10–/– mice lacking Nod2
In order to overcome physiological colonization resistance provided by the complex conventional gastrointestinal microbiota, secondary abiotic IL-10–/– mice lacking Nod2 and IL-10–/– controls were generated by quintuple antimicrobial treatment. Following cessation of the antibiotic cocktail and a 3-day washout phase, mice were perorally infected with 109 CFU C. jejuni strain 81-176 by gavage. A kinetic survey of C. jejuni counts in fecal samples revealed that mice of either genotype could be stably colonized by the pathogen with median loads of approximately 109 CFU per gram (n.s., Fig. 1). At day of necropsy (i.e., day 7 p.i.), Nod2–/– IL-10–/– and IL-10–/– mice harbored C. jejuni alongside the gastrointestinal tract with highest loads in the colonic lumen (i.e., approximately 109 CFU per gram), whereas 107 CFU per gram could be cultured from the ileal lumen of mice of either genotype (n.s.; Fig. 2).
Fig. 1.
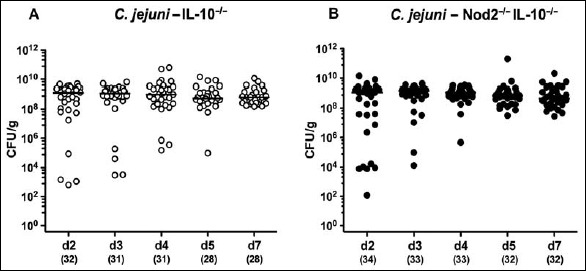
Intestinal C. jejuni strain 81-176 loads over time in perorally infected secondary abiotic IL-10–/– mice lacking Nod2. Secondary abiotic (A) IL-10–/– (white circles) and (B) IL-10–/– mice lacking Nod2 (Nod2–/– IL-10–/–; black circles) were generated by broad-spectrum antibiotic treatment and perorally infected with C. jejuni strain 81-176 by gavage at day (d) 0 and d1. Pathogenic loads were determined in fecal samples (colony forming units per gram, CFU/g) by culture over time postinfection as indicated. Medians (black bars) and numbers of analyzed mice are given in parentheses. Data were pooled from four independent experiments
Fig. 2.
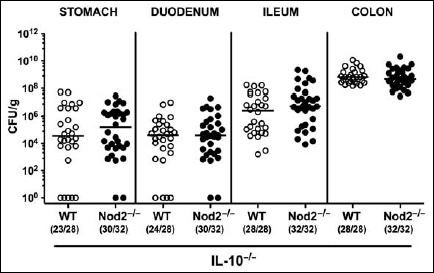
Gastrointestinal colonization properties of C. jejuni strain 81-176 following peroral infection of secondary abiotic IL-10–/– mice lacking Nod2. Secondary abiotic IL-10–/– (WT IL-10–/–; white circles) and IL-10–/– mice lacking Nod2 (Nod2–/– IL-10–/–; black circles) were generated by broad-spectrum antibiotic treatment and perorally infected with C. jejuni strain 81-176 by gavage at day (d) 0 and d1. Pathogenic loads (colony forming units per gram, CFU/g) were assessed in distinct parts of the gastrointestinal tract at d7 p.i. by culture. Medians (black bars) and numbers of mice harboring C. jejuni strain 81-176 out of the total number of analyzed animals are given in parentheses. Data were pooled from four independent experiments
Clinical sequelae upon C. jejuni infection of secondary abiotic IL-10–/– mice lacking Nod2
As early as 4 days following C. jejuni infection, approximately 50% of mice of either genotype exhibited bloody diarrhea, whereas at day 7 p.i., fecal blood-positivity rates were 81.3% and 92.9% in Nod2–/– IL-10–/– and IL-10–/– mice, respectively (Fig. 3).
Fig. 3.
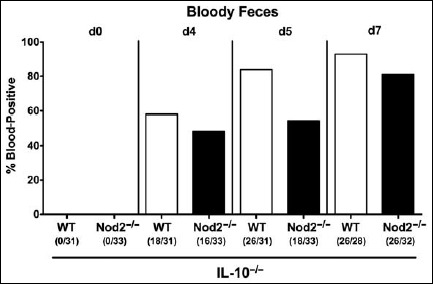
Kinetic survey of bloody feces in secondary abiotic IL-10–/– mice lacking Nod2 following C. jejuni strain 81-176 infection. Secondary abiotic (A) IL-10–/– (white bars) and (B) IL-10–/– mice lacking Nod2 (Nod2–/– IL-10–/–; black bars) were generated by broad-spectrum antibiotic treatment and perorally infected with C. jejuni strain 81-176 by gavage at day (d) 0 and d1. Microscopic or macroscopic ccurrence of blood in fecal samples before and after infection was assessed as described in methods. Bars indicate relative abundances of blood-positive fecal samples (in %). Absolute numbers of animals with blood-positive fecal samples out of the total number of analyzed mice are further given in parentheses. Data were pooled from four independent experiments
Small intestinal microscopic sequelae upon C. jejuni infection of secondary abiotic IL-10–/– mice lacking Nod2
Even though the large intestine is known to be the predilection site of C. jejuni-induced inflammation in secondary abiotic IL-10–/– mice (i.e., acute enterocolitis [18]), we next addressed whether C. jejuni infection was accompanied by distinct microscopic changes also within the small intestinal tract. Since apoptosis is regarded as diagnostic marker for histopathological grading of intestinal inflammation including campylobacteriosis [18], we stained ileal paraffin sections with caspase-3 antibodies. Remarkably, numbers of apoptotic epithelial cells more than doubled in ilea of both Nod2–/– IL-10–/– and IL-10–/– mice until day 7 p.i. (p < 0.001 and p < 0.01 versus naive controls, respectively; Fig. 4A). These C. jejuni-induced increases in apoptotic cells were accompanied by elevated numbers of Ki67+ cells in the ileal epithelium of mice of either genotype at day 7 p.i. (p < 0.05 versus naive; Fig. 4B), indicative for regenerative/proliferative responses counteracting small intestinal cell damage following enteropathogenic infection.
Fig. 4.
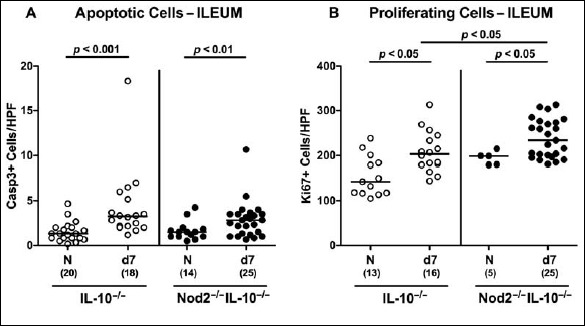
Microscopic inflammatory sequelae in small intestines of C. jejuni strain 81-176 infected secondary abiotic IL-10–/– mice lacking Nod2. Secondary abiotic IL-10–/– (white circles) and IL-10–/– mice lacking Nod2 (Nod2–/– IL-10–/–; black circles) were generated by broad-spectrum antibiotic treatment and perorally infected with C. jejuni strain 81-176 by gavage at day (d) 0 and d1. The average numbers of ileal epithelial (A) apoptotic cells (positive for caspase-3, Casp3) and (B) proliferating/regenerating cells (positive for Ki67) from six high power fields (HPF, 400× magnification) per animal were determined microscopically in immunohistochemically stained ileal paraffin sections at d7 following C. jejuni infection. Naive (N) mice served as uninfected controls. Medians (black bars), levels of significance (p values) determined by Mann–Whitney U test, and numbers of analyzed animals (in parentheses) are indicated. Data were pooled from three independent experiments
Small intestinal immune cell responses upon C. jejuni infection of secondary abiotic IL-10–/– mice lacking Nod2
Since recruitment of pro-inflammatory immune cell populations to the site of infection is one of the key features of campylobacteriosis [18], we next quantitatively assessed numbers of distinct immune cell subsets applying in situ immunohistochemical staining of ileal paraffin sections at day 7 p.i. Adaptive immune cells such as T and B lymphocytes had inceased in the small intestinal mucosa and lamina propria until day 7 following C. jejuni infection (p < 0.05–0.001; Fig. 5A,C), whereas elevated Treg numbers could be observed only in the small intestines of IL-10–/– mice lacking Nod2 at day 7 p.i. (p < 0.05; Fig. 5B). Remarkably, ileal T lymphocyte counts were higher in C. jejuni-infected Nod2–/– IL-10–/– as compared to IL-10–/– mice (p < 0.05; Fig. 5A). Notably, small intestinal innate immune cell populations such as macrophages and monocytes as well as neutrophils were virtually unaffected by C. jejuni infection of mice (not shown).
Fig. 5.
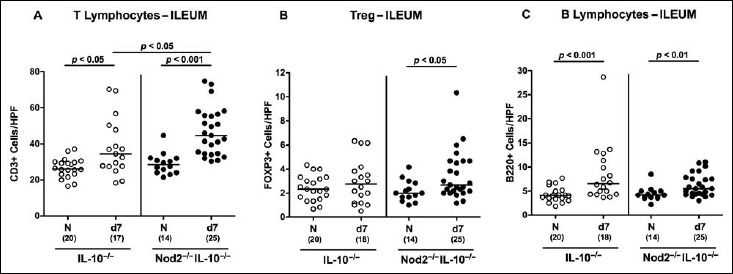
Small intestinal immune cell responses in C. jejuni strain 81-176 infected secondary abiotic IL-10–/– mice lacking Nod2. Secondary abiotic IL-10–/– (white circles) and IL-10–/– mice lacking Nod2 (Nod2–/– IL-10–/–; black circles) were generated by broad-spectrum antibiotic treatment and perorally infected with C. jejuni strain 81-176 by gavage at day (d) 0 and d1. The average numbers of ileal epithelial (A) T lymphocytes (positive for CD3), (B) regulatory T cells (Treg; positive for FOXP3), and (C) B lymphocytes (positive for B220) from six high power fields (HPF, 400× magnification) per animal were determined microscopically in immunohistochemically stained ileal paraffin sections at d7 following C. jejuni infection. Naive (N) mice served as uninfected controls. Medians (black bars), levels of significance (p values) determined by Mann–Whitney U test and numbers of analyzed animals (in parentheses) are indicated. Data were pooled from three independent experiments
Small intestinal cytokine responses upon C. jejuni infection of secondary abiotic IL-10–/– mice lacking Nod2
We next measured pro-inflammatory cytokine secretion in supernatants of ileal ex vivo biopsies at day 7 p.i. Ileal IFN-γ, TNF, and MCP-1 concentrations were elevated in small intestines of C. jejuni-infected mice of either genotype (p < 0.05–0.001; Fig. 6A–C). Ileal secretion of nitric oxide was more pronounced in C. jejuni-infected IL-10–/– mice as compared to naive controls (p < 0.05; Fig. 6D), but did not reach statistical significance in Nod2–/– IL-10–/– mice (n.s., Fig. 6D). In both Nod2–/– IL-10–/– and IL-10–/– mice, a trend towards higher small intestinal IL-6 concentrations could be observed at day 7 p.i. (n.s.; Fig. 6E).
Fig. 6.
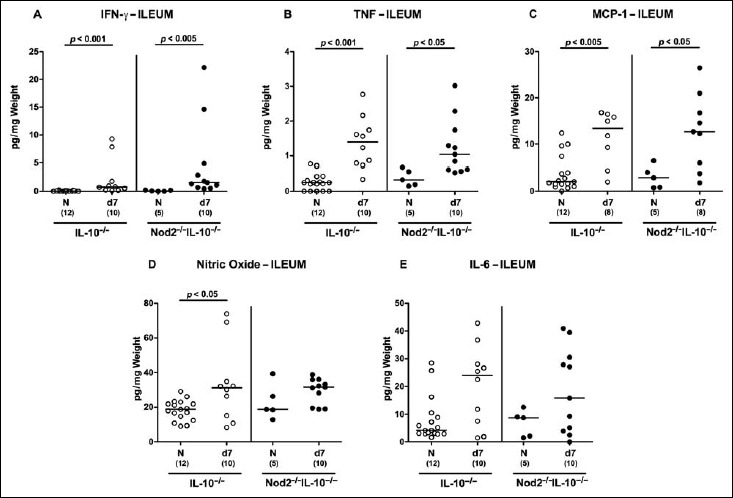
Ileal secretion of pro-inflammatory cytokines in C. jejuni strain 81-176 infected secondary abiotic IL-10–/– mice lacking Nod2. Secondary abiotic IL-10–/– (white circles) and IL-10–/– mice lacking Nod2 (Nod2–/– IL-10–/–; black circles) were generated by broad-spectrum antibiotic treatment and perorally infected with C. jejuni strain 81-176 by gavage at day (d) 0 and d1. (A) IFN-γ, (B) TNF, (C) MCP-1, (D) nitric oxide, and (E) IL-6 concentrations were determined in supernatants of ileal ex vivo biopsies at d7 postinfection. Naive (N) mice served as uninfected controls. Medians (black bars), level of significance (p value) determined by Mann–Whitney U test, and numbers of analyzed animals (in parentheses) are indicated. Data were pooled from two independent experiments
In summary, 1) C. jejuni is able to induce pro-inflammatory responses also in the small intestines of secondary abiotic IL-10–/– mice. 2) Nod2–/– IL-10–/– mice display higher ileal T cell numbers upon C. jejuni infection as compared to IL-10–/– controls.
Discussion
In the present study, we demonstrate for the first time that C. jejuni infection induces overt pro-inflammatory immune responses in the small intestines of IL-10–/– mice. So far, the colon has been known as predilection site of C. jejuni infection in the murine IL-10–/– infection model as shown previously by us and others. Within 2 to 35 days following C. jejuni infection, conventionally colonized IL-10–/– mice developed typhlocolitis affecting the colon and caecum including the ileocaecal junction [23–25]. Our group could further demonstrate that, even within less than 1 week following peroral C. jejuni infection, secondary abiotic IL-10–/– mice developed severe ulcerative enterocolitis with inflammatory, bloody diarrhea and succumbed to acute immunopathological sequelae [12–15]. As shown here, intestinal disease was not restricted to the colon, given that also the small intestines were affected upon C. jejuni infection as indicated by pronounced apoptosis in ileal epithelia and an enhanced influx of adaptive immune cells including T and B lymphocytes into the small intestinal mucosa and lamina propria that was accompanied by accelerated ileal secretion of pro-inflammatory cytokines such as IFN-γ, TNF, and MCP-1. The fact that immunopathological responses to C. jejuni infection of secondary abiotic IL-10–/– mice could additionally be observed in extra-intestinal organs including liver and kidney, but also in systemic compartments including spleen and serum [13], points towards a C. jejuni-induced systemic inflammatory response syndrome.
In the present study, we also addressed whether Nod2 was involved in mediating small intestinal pro-inflammatory immune responses upon C. jejuni infection. Whereas ileal apoptosis and pro-inflammatory cytokine secretion were Nod2-independent, higher numbers of T lymphocytes could be determined in the ileal mucosa and lamina propria of infected Nod2–/– IL-10–/– mice as compared to IL-10–/– controls. This is well in line with a C. jejuni-induced elevation of T cell counts in the large intestines of secondary abiotic IL-10–/– mice lacking Nod2 (submitted article) and Nod2–/– mice (article in revision). The same holds true for proliferating intestinal epithelial cells, given that higher Ki67+ cell numbers could be determined in small (present study) and large intestines (submitted article) of C. jejuni-infected secondary abiotic Nod2–/– IL-10–/– mice as well as in Nod2–/– mice (article in revision) as compared to respective infected controls. These results point towards more distinct regenerative properties of intestinal epithelia counteracting C. jejuni-induced cell damage in mice lacking Nod2.
Taken together, C. jejuni infection of secondary abiotic IL-10–/– mice resulted in pro-inflammatory responses in the small intestines, whereas increases in ileal T cell numbers were even more pronounced in IL-10–/– mice lacking Nod2.
In conclusion, not only large but also small intestinal changes need to be assessed during enteropathogenic infection studies employing IL-10–/– mice.
Acknowledgements
We thank Michaela Wattrodt, Ursula Rüschendorf, Silvia Schulze, Alexandra Bittroff-Leben, Ines Puschendorf, Gernot Reifenberger, Ulrike Hagen, Uwe Lohmann, and the staff of the animal research facility at Charité – University Medicine Berlin for excellent technical assistance and animal breeding.
Funding Statement
Funding sources: This work was supported by grants from the German Research Foun dation (DFG) to A.F. and S.B. (SFB633, TP A7), M.M.H. (SFB633, TP B6), M.E.A. and U.G. (SFB633, Immuco), and from the German Federal Ministery of Education and Research (BMBF) to S.B. (TP1.1).
The funders had no role in study design, data collection and analysis, and decision to publish or preparation of the article.
References
- 1.Shaw MH, Reimer T, Kim YG, Nunez G: NOD-like receptors (NLRs): bona fide intracellular microbial sensors. Curr Opin Immunol 20, 377–382 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G: Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem 276, 4812–4818 (2001) [DOI] [PubMed] [Google Scholar]
- 3.Tada H, Aiba S, Shibata K, Ohteki T, Takada H: Synergistic effect of Nod1 and Nod2 agonists with toll-like receptor agonists on human dendritic cells to generate interleukin-12 and T helper type 1 cells. Infect Immun 73, 7967–7976 (2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inohara N, Nunez G: NODs: intracellular proteins involved in inflammation and apoptosis. Nat Rev Immunol 3, 371–382 (2003) [DOI] [PubMed] [Google Scholar]
- 5.Girardin SE, Travassos LH, Herve M, Blanot D, Boneca IG, Philpott DJ, Sansonetti PJ, Mengin-Lecreulx D: Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J Biol Chem 278(43), 41702–41708 (2003) [DOI] [PubMed] [Google Scholar]
- 6.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ: Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem 278, 8869–8872 (2003) [DOI] [PubMed] [Google Scholar]
- 7.Grimes CL, Ariyananda Lde Z, Melnyk JE, O’Shea EK: The innate immune protein Nod2 binds directly to MDP, a bacterial cell wall fragment. J Am Chem Soc 134, 13535–13537 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guerry P, Szymanski CM: Campylobacter sugars sticking out. Trends Microbiol 16, 428–435 (2008) [DOI] [PubMed] [Google Scholar]
- 9.Lane JA, Mehra RK, Carrington SD, Hickey RM: The food glycome: a sou rce of protection against pathogen colonization in the gastrointestinal tract. Int J Food Microbiol 142, 1–13 (2010) [DOI] [PubMed] [Google Scholar]
- 10.Kist M, Bereswill S: Campylobacter jejuni. Contrib Microbiol 8, 150–165 (2001) [DOI] [PubMed] [Google Scholar]
- 11.Backert S, Tegtmeyer N, Crónín TÓ, Böhm M, Heimesaat MM. (2017): Human Campylobacteriosis. Campylobacter – Features, Detection, and Prevention of Foodborne Disease, ed. Klein G, Elsevier, London, pp. 1–16 [Google Scholar]
- 12.Haag LM, Fischer A, Otto B, Plickert R, Kühl AA, Göbel UB, Bereswill S, Heimesaat MM: Campylobacter jejuni induces acute enterocolitis in gnotobiotic IL-10–/– mice via Toll-like-receptor-2 and -4 signaling. PLoS One 7, e40761 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heimesaat MM, Alutis M, Grundmann U, Fischer A, Tegtmeyer N, Böhm M, Kühl AA, Göbel UB, Backert S, Bereswill S: The role of serine protease HtrA in acute ulcerative enterocolitis and extra-intestinal immune responses during Campylobacter jejuni infection of gnotobiotic IL-10 deficient mice. Front Cell Infect Microbiol 4, 77 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heimesaat MM, Lugert R, Fischer A, Alutis M, Kühl AA, Zautner AE, Tareen AM, Göbel UB, Bereswill S: Impact of Campylobacter jejuni cj0268c knockout mutation on intestinal colonization, translocation, and induction of immunopathology in gnotobiotic IL-10 deficient mice. PLoS One 9, e90148 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiebiger U, Bereswill S, Heimesaat MM: Dissecting the interplay between intestinal microbiota and host immunity in health and disease: lessons learned from germfree and gnotobiotic animal models. Eur J Microbiol Immunol (Bp) 6, 253–271 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golz G, Alter T, Bereswill S, Heimesaat MM: The immunopathogenic potential of Arcobacter butzleri – lessons from a meta-analysis of murine infection studies. PLoS One 11, e0159685 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Otto B, Haag LM, Fischer A, Plickert R, Kühl AA, Göbel UB, Heimesaat MM, Bereswill S: Campylobacter jejuni induces extra-intestinal immune responses via Toll-like-receptor-4 signaling in conventional IL-10 deficient mice with chronic colitis. Eur J Microbiol Immunol (Bp) 2, 210–219 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bereswill S, Fischer A, Plickert R, Haag LM, Otto B, Kühl AA, Dasti JI, Zautner AE, Muñoz M, Loddenkemper C, Gross U, Göbel UB, Heimesaat MM: Novel murine infection models provide deep insights into the “ménage à trois” of Campylobacter jejuni, microbiota and host innate immunity. PLoS One 6, e20953 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heimesaat MM, Bereswill S, Fischer A, Fuchs D, Struck D, Niebergall J, Jahn HK, Dunay IR, Moter A, Gescher DM, Schumann RR, Göbel UB, Liesenfeld O: Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J Immunol 177, 8785–8795 (2006) [DOI] [PubMed] [Google Scholar]
- 20.Alutis ME, Grundmann U, Fischer A, Hagen U, Kühl AA, Göbel UB, Bereswill S, Heimesaat MM: The role of gelatinases in Campylobacter jejuni infection of gnotobiotic mice. Eur J Microbiol Immunol (Bp) 5, 256–267 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alutis ME, Grundmann U, Hagen U, Fischer A, Kühl AA, Göbel UB, Bereswill S, Heimesaat MM: Matrix metalloproteinase-2 mediates intestinal immunopathogenesis in Campylobacter jejuni-infected infant mice. Eur J Microbiol Immunol (Bp) 5, 188–198 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heimesaat MM, Nogai A, Bereswill S, Plickert R, Fischer A, Loddenkemper C, Steinhoff U, Tchaptchet S, Thiel E, Freudenberg MA, Göbel UB, Uharek L: MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut 59, 1079–1087 (2010) [DOI] [PubMed] [Google Scholar]
- 23.Mansfield LS, Bell JA, Wilson DL, Murphy AJ, Elsheikha HM, Rathinam VA, Fierro BR, Linz JE, Young VB: C57BL/6 and congenic interleukin-10-deficient mice can serve as models of Campylobacter jejuni colonization and enteritis. Infect Immun 75, 1099–1115 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bell JA, St Charles JL, Murphy AJ, Rathinam VA, Plovanich-Jones AE, Stanley EL, Wolf JE, Gettings JR, Whittam TS, Mansfield LS: Multiple factors interact to produce responses resembling spectrum of human disease in Campylobacter jejuni infected C57BL/6 IL-10–/– mice. BMC Microbiol 9, 57 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bell JA, Jerome JP, Plovanich-Jones AE, Smith EJ, Gettings JR, Kim HY, Landgraf JR, Lefébure T, Kopper JJ, Rathinam VA, St Charles JL, Buffa BA, Brooks AP, Poe SA, Eaton KA, Stanhope MJ, Mansfield LS: Outcome of infection of C57BL/6 IL-10(–/–) mice with Campylobacter jejuni strains is correlated with genome content of open reading frames up- and down-regulated in vivo. Microb Pathog 54, 1–19 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]


