Abstract
Rhythmicity is critical for the generation of rhythmic behaviors and higher brain functions. This review discusses common mechanisms of rhythm generation, including the role of synaptic inhibition and excitation, with a focus on the mammalian respiratory network. This network generates three phases of breathing and is highly integrated with brain regions associated with numerous non-ventilatory behaviors. We hypothesize that during evolution multiple rhythmogenic microcircuits were recruited to accommodate the generation of each breathing phase. While these microcircuits relied primarily on excitatory mechanisms, synaptic inhibition became increasingly important to coordinate the different microcircuits and to integrate breathing into a rich behavioral repertoire that links breathing to sensory processing, arousal, and emotions as well as learning and memory.
Introduction
Rhythmicity is involved in almost all behaviors and brain functions [1••,2]. This includes the generation of rhythmic behaviors, communication, encoding, attention, learning and memory [3,4]. Thus, understanding rhythmogenesis is a core issue in neuroscience. Rhythmogenesis can be studied in microcircuits isolated from invertebrates [5,6], mammalian [7•,8•,9,10,11•,12], and non-mammalian vertebrates [13,14••,15•,16] as well as fully integrated behavioral systems [17••,18–20,21•,22,23]. Yet, the quest to unravel the mechanisms underlying rhythmogenesis has been a difficult journey. Concepts developed using intact systems have often conflicted with those obtained from isolated networks. In particular in mammalian studies, some discrepancies have been attributed to developmental differences, since many in vivo studies were conducted in adults, while in vitro experiments have often been limited to neonates [24•], a difficulty that can be overcome by studying non-mammalian model systems [25•].
Mechanisms commonly found in rhythm generating networks include reciprocal inhibition [26•,27,28], rhythmic pacemaker properties [11•,29–33] and recurrent excitatory network mechanisms [10,34•,35,36•]. However, their roles within a given network vary and are often different than originally hypothesized. We learned that the relative contribution of neuronal mechanisms is not fixed, but dynamically regulated, resulting in state-dependent re-configuration of neuronal networks [37–40]. Mechanisms that are essential in one condition can become non-essential contributors in another state even within the same network [41].
This review will discuss the mechanisms underlying rhythm generation in a variety of brain networks with a focus on the respiratory rhythm-generating network. This network has well-defined physiological roles and it is amenable to a rigorous cellular analysis [17••,42–44]. Moreover, the respiratory rhythm is integrated with the activity of numerous networks distributed throughout the brainstem and forebrain [45•,46]. A comparative approach among vertebrates may provide important insights into how multiple rhythmic circuits become functionally intertwined to produce and coordinate ventilatory and non-ventilatory behaviors. By unraveling the complexities of the breathing rhythm, we may also gain insights into rhythmicity involved in other CNS functions ranging from locomotion to memory and emotion.
Rhythm generating networks and the role of synaptic inhibition
Most rhythm generating networks are heterogeneous, consisting of silent, tonic and rhythmically bursting neurons (Figure 1a) [2,47]. Neurons endowed with these discharge patterns form the building blocks of neuronal networks and are often incorporated into computational models. One of those models, the so-called half-center oscillator (HCO) was one of the first models to mechanistically explain rhythmogenesis [48–50] and has been particularly influential. In this model, two non-rhythmic cells or groups of cells are coupled by mutual inhibitory connections that give rise to antiphasic rhythmicity in the presence of an excitatory drive [51].
Figure 1.
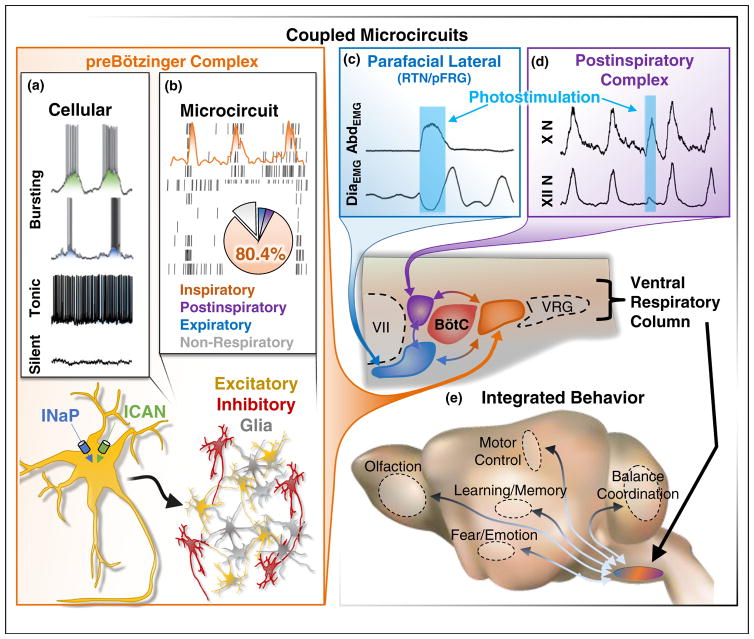
Microcircuits in mammalian respiratory rhythm generation. (a) Isolated preBötC neurons have various activity patterns including bursting ‘pacemaker’, tonic firing, and silent that are largely determined by conductance characteristics including persistent sodium (INaP) and non-specific cation (ICAN) currents (Adapted with permission from [96]). (b) The microcircuit constituting the preBötC consists of glia and both excitatory and inhibitory neurons that primarily fire (>80%) in phase with the inspiratory phase of breathing (Adapted with permission from [71]). (c and d) In vivo optogenetic manipulations of respiratory microcircuits coupled to the preBötC. (c) The parafacial lateral region (pFL; blue) is a conditional oscillator that generates active expiration visualized in abdominal (Abd) activity; excitation of this microcircuit elicits an AbdEMG burst and inhibits diaphragm activity (DiaEMG) (Adapted with permission from [100]). (d) The post-inspiratory complex (PiCo; purple) generates postinspiration visualized in the vagus nerve (X N); stimulation of this microcircuit elicits a vagal nerve burst and delays the onset of inspiratory activity observed in hypoglossal motor output (XII N). BötC, Bötzinger complex; VRG, ventral respiratory group. (e) The contribution of each microcircuit to the generation of the respiratory rhythm is dynamically regulated and integrated with brain regions controlling distinct respiratory-related and non-respiratory behaviors.
Mutually inhibitory connections are ubiquitous in rhythm generating networks [44,52,53], and variations of the HCO are found in some models of respiration [54] and locomotion [55]. However, to what extent reciprocal inhibition operates as a rhythmogenic mechanism is still an open question. In lamprey swimming, reciprocal inhibition regulates left and right coordination, yet each hemisegment can generate rhythmicity even without inhibition [56]. Similarly, in the respiratory network rhythmicity persists after blockade of synaptic inhibition in lamprey and frogs [25•,57], in isolated mammalian respiratory microcircuits [44] and in intact mammals [19,58•].
Yet, inhibitory mechanisms have important functions within the respiratory network [24•,58•]. Concrete insights were gained from studying the preBötzinger complex (preBötC; Figure 1), the first rhythmogenic microcircuit identified within the mammalian respiratory network [12,59]. This network, located in the ventrolateral medulla, is both necessary and sufficient for generating respiratory rhythm [9,12,60]. A large proportion of preBötC neurons are inhibitory (Figure 1b) [61], and optogenetic manipulations have revealed that these neurons provide important temporal cues by specifically mediating afferent input in the intact network [19]. Another medullary region, the Bötzinger complex (BötC; Figures 1 and 3c), contains primarily inhibitory neurons that are thought to play a critical role in forming the different phases of breathing [62]. However, BötC neurons are not well defined and optogenetic approaches probing the functional roles of these neurons are still missing.
Figure 3.
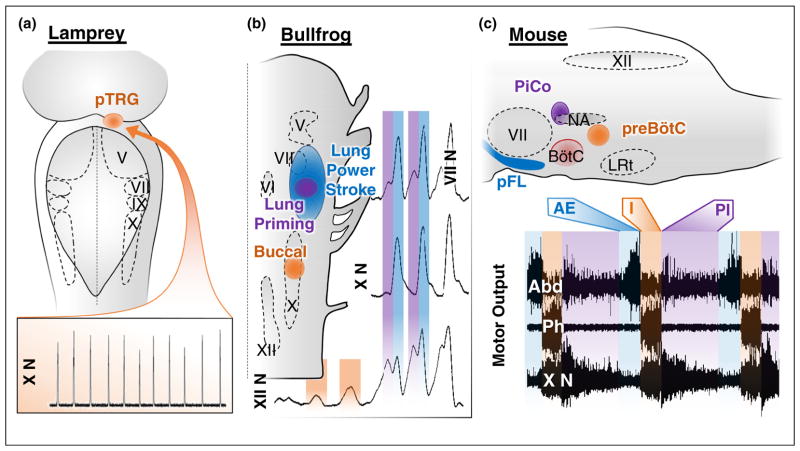
Vertebrate rhythmogenic respiratory microcircuits. (a) Dorsal representation of respiratory rhythm generation in lamprey, which seems to be localized to a single microcircuit located in the pons — the para trigeminal respiratory group (pTRG). The pTRG utilizes excitatory mechanisms for rhythm generation and may be homologous to the mammalian preBötC. Respiratory activity in this primitive vertebrate generates gill movements, which can be observed in vagus nerve (X N) motor output (bottom trace) (Adapted with permission from [25•]). (b) Dorsal representation of respiratory microcircuits located near cranial nerve nuclei in bullfrogs. It is thought that three distinct respiratory oscillators generate the buccal (orange), lung priming (purple), and lung powerstroke (blue) rhythms. These respiratory activities can be differentially observed in cranial nerve activity, as shown here in facial (VII N), vagus (X N) and hypoglossal (XII N) nerve motor output (Adapted with permission from [14••]). (c) Sagittal representation of the three identified excitatory rhythmogenic respiratory microcircuits in the mouse. The ‘triple-oscillator’ hypothesis: three anatomically distinct coupled excitatory microcircuits generate the three phases of the mammalian breathing rhythm — the preBötC, PiCo, and pFL, generate inspiration (I), postinspiration (PI), and active expiration (AE), respectively. These breathing phases are observed in motor output from respiratory-related nerves (Abd, abdominal; Ph, phrenic; cVN, cervical vagus nerve), which is precisely coordinated to produce a breath (Adapted with permission from [101]). BötC, Bötzinger complex, NA, nucleus ambiguus; LRt, lateral reticular nucleus.
Inhibitory mechanisms have also been studied in non-mammalian vertebrates. For the buccal rhythm generating network in bullfrogs, a sensitivity to changes in chloride-dependent conductances has been demonstrated [14••], but the exact role of inhibition remains unclear. In lamprey, respiratory frequency is controlled by inhibition, but like mammalian respiratory microcircuits, inhibition is not necessary for rhythmogenesis [16].
The role of excitatory mechanisms in rhythm generation
The critical role of synaptic excitation within the respiratory network has never been questioned. Two principle rhythmogenic mechanisms have been proposed for excitatory networks in general: (1) Interconnected endogenous bursting, pacemaker neurons: In its extreme, the temporal characteristics of amplitude and period are defined by the intrinsic membrane properties of pacemakers [63,64]. (2) Excitatory interactions between non-pacemakers: In this configuration temporal and amplitude characteristics are largely defined by synaptic dynamics [27]. Yet, neither of these ‘extreme’ network configurations is likely realized in actual networks, since synaptic and intrinsic mechanisms are intimately interwoven (Figure 2) [44].
Figure 2.
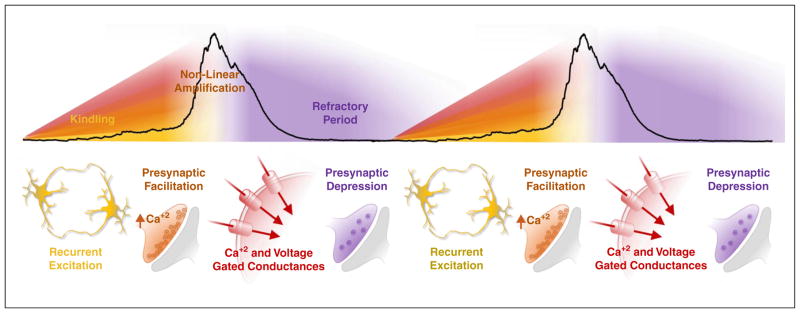
The anatomy of an inspiratory population burst. Recurrent synaptic excitation among sparsely connected neurons begins to increase action potential rates and build network excitability. As presynaptic Ca+2 summates, vesicle release probability increases, strengthening synaptic transmission. Burst generating Ca+2 and voltage gated conductances become increasingly active as neurons in the network depolarize, leading to non-linear amplification of action potential rates and network synchrony. The high rate of action potentials generated during the population burst depletes the ready-releasable pool of synaptic vesicles, which reduces synaptic transmission, leading to the loss of synchronization, termination of the burst, and a refractory period for inspiratory activity.
Glutamatergic, non-NMDA-dependent, synaptic transmission is the essential rhythmogenic mechanism within the isolated preBötC, which is similar to other respiratory microcircuits in mammals [65••,66] and non-mammalian vertebrates [25•]. Synchronization is established by recurrent excitation [67], which leads to pre-synaptic facilitation [27] and the activation of burst generating conductances (Figure 2) [68,69]. Two conductances that promote the non-linear amplification of excitatory synaptic interactions are the persistent sodium (INaP) and calcium activated non-specific cation (ICAN) currents (Figure 1a) [70]. Conductances promoting bursting have also been implicated in respiratory rhythm generation in turtles [32].
Multiarray recordings reveal that the majority of preBötC neurons are weakly spiking or tonically spiking, while silent and intrinsic bursting neurons constitute a minority [71,72]. This distribution of spiking profile is likely the result of a gradient distribution of ionic conductances [30]. Indeed, a fundamental question is whether different ionic conductance ratios are randomly distributed among respiratory neurons, or whether this distribution follows specific rules. One possibility is that conductance ratio is regulated to shift the network towards intrinsically spiking modes based on energy consumption principles. For example, bursting activity patterns require a dynamic overlap between inward and outward currents [63,73], which is not energy efficient [74]. This could explain why bursting neurons are relatively rare within the preBötC. By contrast, a small overlap between Na+ and K+ currents gives the most efficient energy consumption; most likely such dynamics promote tonic spiking activity [74], which is also the activity most frequently observed in the pre-BötC [71,72].
However, preBötC neurons cannot simply be classified according to their discharge pattern in isolation since the strength of discharge can also vary with respect to a given respiratory phase [71]. Neurons within the preBötC exhibit a high degree of cycle-to-cycle variability with regard to which neurons lead each successive population burst, as well as the timing jitter of spike patterns of individual rhythmic neurons [72]. Based on this finding it can be concluded that neurons are stochastically activated during synchronized population bursts [72,75]. This suggests that there is not a particular cell type that plays a distinct functional role in kindling or terminating the rhythm, although neurons with increased excitability (which includes bursting neurons) tend to discharge earlier. The stochastic phase distribution of preBötC neurons seems to be a result of sparse connectivity within the network, which is consistent with cross-correlation analysis of 10,778 cells recorded in multicellular experiments, indicating a connectivity probability of only 1% [72].
The critical rhythmogenic preBötC neurons are derived from progenitors expressing the transcription factor Dbx1 during development (henceforth referred to as ‘Dbx1 neurons’). Identification of these and other respiratory neurons has greatly facilitated our understanding of synaptic excitation in rhythmogenesis [76••,77]. The synaptic interactions between glutamatergic Dbx1-derived neurons dynamically regulate both burst frequency and burst termination [76••]. The volley of action potentials generated during synchronization of Dbx1 neurons depletes the ready-releasable pool of synaptic vesicles (i.e., pre-synaptic depression) resulting in a ‘refractory period’ for activating the subsequent Dbx1 burst (Figure 2) [34•]. Thus, the degree of network synchronization likely influences the magnitude of pre-synaptic depression. Specifically, one might expect that a high degree of network synchrony may lead to a respiratory burst with larger amplitude, resulting in greater pre-synaptic depression and a longer refractory period. Interestingly, this refractory period lasts on average approximately 2 seconds in vitro, which is incompatible with breathing frequencies typically observed in vivo. This raises an important question: To what extent do synaptic dynamics support the broad range of breathing frequencies observed in vivo? The answer is likely complex, since aside from local excitatory and inhibitory mechanisms, the rhythmogenic properties of Dbx1 neurons are influenced by the activity of other microcircuits and a rich cellular milieu of neuromodulatory and glial interactions.
The role of glia in the generation of rhythmic activity
Neuron–glia interactions are increasingly being considered important for generating physiological and pathophysiological rhythmicity in the brain [78]. Although glia do not synchronize with each other through mechanisms of glutamatergic synaptic transmission, they are capable of synchronous activity via other mechanisms such as gap junctions [79]. The resulting Ca+2 oscillations or ‘waves’ between interconnected glia have important network functions [80•], and models of astrocyte–neuron interactions suggest that cytosolic calcium dynamics within astrocytes can induce or modulate network synchronization by up- or down-regulating synaptic transmission [81,82].
In the mammalian respiratory network, there is ample evidence that aside from their well-known role in maintaining network homeostasis [83], glia are critical for enhancing the system’s responsiveness to pH, PCO2 and PO2 [84••,85•,86]. There is increasing awareness that central neuronal networks are hypoxia sensitive [43], which may critically depend on glial cells [85•,86]. Interestingly, the processes involved in the hypoxic response share common pathways with the inflammatory response [87,88]. In this context, not only astrocytes, but also microglia may play important roles, as demonstrated for the release of pro-inflammatory cytokines within the preBötC [87,89]. An important, yet unresolved question is to what extent glial oscillations influence neuronal synchrony during the ongoing respiratory rhythm. Also unresolved is to what extent the role of glial cells is conserved throughout evolution.
Coupled oscillators in respiratory rhythmogenesis and their evolution
The preBötC likely originated from similar rhythm generating structures present in early vertebrates [15•]. In lamprey, the respiratory rhythm generator is located in the pons, in the so-called para-trigeminal respiratory group (pTRG) (Figure 3a). This differs from the respiratory rhythm-generating network of dogfish, carp and tench that seems to span the length of the brainstem [14••] including medullary regions. Evidence for a localized microcircuit comes from bullfrogs: the gill/buccal rhythmogenic network is located in rhombomere 7/8, similar to the preBötC [14••]. However, a fundamental problem in assessing homology is that some markers that define the preBötC, such as somatostatin, are not conserved among different vertebrate species [90]. The use of transcription factors expressed during development may provide more detailed insights into the evolution of neuronal networks as exemplified by comparative studies exploring the evolution of neurons expressing the transcription factor Phox2b [91•] or Atoh1 [77]. But to the best of our knowledge, this comparative information is currently missing for Dbx1 neurons.
In mammals, the preBötC has been considered the noeud vital, because of its important role in breathing [92]. Yet, this network is primarily responsible for inspiration, and multi-array recordings indicate that roughly 80% of preBötC neurons are active during the inspiratory phase (Figure 1b) [71,72]. There is increasing evidence that anatomically distinct microcircuits are responsible for generating the other phases of breathing.
A microcircuit in the parafacial respiratory group (pFRG) of the medulla, termed the pFL, generates active expiration (Figures 1c and 3c) [21•]. This respiratory phase is conditionally recruited during high metabolic demand (e.g., exercise). Like the preBötC, the pFL is an autonomous rhythm generator that depends on excitatory mechanisms, and is modulated by inhibition [93]. The pFL is temporally coupled with the preBötC [66], perhaps by a specific neuronal population that expresses the transcription factor Atoh1 [77]. A theoretical model incorporating these inspiratory and expiratory microcircuits hypothesizes a ‘hand-shake’ mechanism where the inspiratory CPG inhibits the expiratory CPG, which in turn excites the inspiratory network via post-inhibitory rebound [62]. However, to mimic the experimental behavior, the frequency of the expiratory CPG must be faster than the inspiratory network, and this has not yet been confirmed experimentally [62]. The pFL is located in rhombomere 4/5 and it has been hypothesized that the pFL is a homologue of the lung burst generator in frogs (Figure 3b) [13], but further research is necessary to better define this potential homology.
An additional mammalian microcircuit, the postinspiratory complex (PiCo), was recently discovered rostral to the preBötC. Optogenetic manipulations suggest that PiCo is both necessary and sufficient for generating postinspiration (Figure 1d) [65••], the dominant expiratory phase of breathing under control conditions. PiCo is an autonomous excitatory rhythm generator that is temporally coordinated with the preBötC through GABAergic inhibition [65••]. The discovery of this additional oscillator suggests that each of the three phases of breathing present in mammals is generated by a distinct excitatory CPG — the so-called ‘triple oscillator hypothesis’ (Figure 3c) [65••]. Although the presence of three rhythmogenic networks has also been predicted in frogs (Figure 3b) [13], it is too early to know whether PiCo is homologous to one of the networks identified in non-mammalian vertebrates.
From an evolutionary perspective, it has been hypothesized that, as breathing evolved from buccal/brachial ventilation to aspiration-driven ventilation, additional oscillatory networks located more caudally and closer to the cranial nerves became involved in rhythm generation (Figure 3) [14••,25•]. In mammals, we hypothesize that the three phases of breathing evolved through the recruitment of separate excitatory rhythmogenic micro-circuits in the medulla. Mechanisms of inhibition have likely evolved in parallel to integrate and coordinate these microcircuits. Indeed, modeling, recording and lesioning experiments implicate extensive inhibitory interactions between distinct compartments of the wider mammalian respiratory network [24•]. Yet, since each microcircuit evolved with its own rhythmogenic mechanisms, rhythmicity can still persist even if stripped from inhibitory control.
In addition to coordinating the numerous pump and upper airway muscles involved in the three phases of breathing, respiratory microcircuits had to become integrated with circuits underlying a multitude of non-ventilatory behaviors (Figure 1e). In mammals, each phase of breathing is associated with distinct behaviors and conditions. Inspiration is expressed during eupnea, gasping and sighing, and is associated with olfaction, whisking [94], arousal [95], and specific emotional conditions [96], while postinspiration is associated with swallowing, vocalization, breath-holding and coughing [97•]. Thus, it is likely that each respiratory microcircuit is specifically connected to the brain regions that control these behaviors. For example, sighs can be generated by distinct connectivity within [96,98], and outside the preBötC [17••]. Similarly, sensory behaviors such as whisking and sniffing [94,99] have distinct connectivity with the preBötC. Continuing to unravel how respiratory rhythm generating microcircuits interact with networks associated with other behaviors will be an important issue for future studies.
The emerging concept is that complex behaviors can be generated through interactions between multiple rhythmogenic microcircuits; each with their own functional properties that allow them to be differentially reconfigured under various modulatory conditions in the intact network. As organisms evolved and the behavioral repertoire increased, these microcircuits required increased coordination. This seems to be established by inhibitory interactions. By comparing rhythmogenic mechanisms and their integration across vertebrates, we may gain critical insights not only into the generation of breathing, but also into the evolution of rhythmicity that is so critical for higher order brain functions.
Acknowledgments
Supported by grants from the National Institute of Health (HL090554 and HL126523-01.
Footnotes
Conflicts of interest statement
The authors do not have any conflict of interest.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1••.Watson BO, Buzsaki G. Sleep, memory & brain rhythms. Daedalus. 2015;144:67–82. doi: 10.1162/DAED_a_00318. A very interesting review on the role of rhythmic activity in the generation of higher brain functions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramirez JM, Tryba AK, Pena F. Pacemaker neurons and neuronal networks: an integrative view. Curr Opin Neurobiol. 2004;14:665–674. doi: 10.1016/j.conb.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Wilson MA, Varela C, Remondes M. Phase organization of network computations. Curr Opin Neurobiol. 2015;31:250–253. doi: 10.1016/j.conb.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler JL, Paulsen O. Hippocampal network oscillations –recent insights from in vitro experiments. Curr Opin Neurobiol. 2015;31:40–44. doi: 10.1016/j.conb.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 5.Wagenaar DA. A classic model animal in the 21st century: recent lessons from the leech nervous system. J Exp Biol. 2015;218:3353–3359. doi: 10.1242/jeb.113860. [DOI] [PubMed] [Google Scholar]
- 6.Marder E, Gutierrez GJ, Nusbaum MP. Complicating connectomes: electrical coupling creates parallel pathways and degenerate circuit mechanisms. Dev Neurobiol. 2016 doi: 10.1002/dneu.22410. http://dx.doi.org/10.1002/dneu.22410. [DOI] [PMC free article] [PubMed]
- 7•.Revill AL, Vann NC, Akins VT, Kottick A, Gray PA, Del Negro CA, Funk GD. Dbx1 precursor cells are a source of inspiratory XII premotoneurons. Elife. 2015;4 doi: 10.7554/eLife.12301. This study demonstrates that Dbx1 neurons are not only involved in respiratory rhythm generation, but also act a premotor neurons for the hypoglossal motor output. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8•.Salkoff DB, Zagha E, Yuzgec O, McCormick DA. Synaptic mechanisms of tight spike synchrony at gamma frequency in cerebral cortex. J Neurosci. 2015;35:10236–10251. doi: 10.1523/JNEUROSCI.0828-15.2015. An elegant study characterizing the role of excitatory and inhibitory synaptic mechanisms in the generation of the cortical gamma rhythm and spike synchrony. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lieske SP, Thoby-Brisson M, Telgkamp P, Ramirez JM. Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps. Nat Neurosci. 2000;3:600–607. doi: 10.1038/75776. [DOI] [PubMed] [Google Scholar]
- 10.Song H, Hayes JA, Vann NC, Drew LaMar M, Del Negro CA. Mechanisms Leading to rhythm cessation in the respiratory prebotzinger complex due to piecewise cumulative neuronal deletions (1,2,3) eNeuro. 2015:2. doi: 10.1523/ENEURO.0031-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11•.Phillips WS, Herly M, Del Negro CA, Rekling JC. Organotypic slice cultures containing the preBotzinger complex generate respiratory-like rhythms. J Neurophysiol. 2016;115:1063–1070. doi: 10.1152/jn.00904.2015. Description of a new in vitro approach for culturing the respiratory microcircuit located within the preBötzinger complex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baghdadwala MI, Duchcherer M, Paramonov J, Wilson RJ. Three brainstem areas involved in respiratory rhythm generation in bullfrogs. J Physiol. 2015;593:2941–2954. doi: 10.1113/JP270380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14••.Baghdadwala MI, Duchcherer M, Trask WM, Gray PA, Wilson RJ. Diving into the mammalian swamp of respiratory rhythm generation with the bullfrog. Respir Physiol Neurobiol. 2016;224:37–51. doi: 10.1016/j.resp.2015.09.005. A comparative review detailing the similarities between respiratory circuits in frogs and mammals, also providing clues as to the evolution of the mammalian respiratory system. [DOI] [PubMed] [Google Scholar]
- 15•.Hoffman M, Taylor BE, Harris MB. Evolution of lung breathing from a lungless primitive vertebrate. Respir Physiol Neurobiol. 2016;224:11–16. doi: 10.1016/j.resp.2015.09.016. An excellent review discussing the evolution of lung breathing in vertebrates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cinelli E, Mutolo D, Contini M, Pantaleo T, Bongianni F. Inhibitory control of ascending glutamatergic projections to the lamprey respiratory rhythm generator. Neuroscience. 2016;326:126–140. doi: 10.1016/j.neuroscience.2016.03.063. [DOI] [PubMed] [Google Scholar]
- 17••.Li P, Janczewski WA, Yackle K, Kam K, Pagliardini S, Krasnow MA, Feldman JL. The peptidergic control circuit for sighing. Nature. 2016;530:293–297. doi: 10.1038/nature16964. An elegant study dissecting the modulatory pathway that regulates the generation of the sigh. A good example for the use of modern genetic screening approaches to identify specific neuronal subpopulations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson J, Manseau F, Ducharme G, Amilhon B, Vigneault E, El Mestikawy S, Williams S. Optogenetic activation of septal glutamatergic neurons drive hippocampal theta rhythms. J Neurosci. 2016;36:3016–3023. doi: 10.1523/JNEUROSCI.2141-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janczewski WA, Tashima A, Hsu P, Cui Y, Feldman JL. Role of inhibition in respiratory pattern generation. J Neurosci. 2013;33:5454–5465. doi: 10.1523/JNEUROSCI.1595-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBotzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci. 2001;4:927–930. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21•.Huckstepp RT, Cardoza KP, Henderson LE, Feldman JL. Role of parafacial nuclei in control of breathing in adult rats. J Neurosci. 2015;35:1052–1067. doi: 10.1523/JNEUROSCI.2953-14.2015. A recent study characterizing the role of the parafacial nucleus, an area which contains the presumed microcircuit for the generation of active expiration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kato S, Kaplan HS, Schrodel T, Skora S, Lindsay TH, Yemini E, Lockery S, Zimmer M. Global brain dynamics embed the motor command sequence of Caenorhabditis elegans. Cell. 2015;163:656–669. doi: 10.1016/j.cell.2015.09.034. [DOI] [PubMed] [Google Scholar]
- 23.Roberts WM, Augustine SB, Lawton KJ, Lindsay TH, Thiele TR, Izquierdo EJ, Faumont S, Lindsay RA, Britton MC, Pokala N, Bargmann CI, et al. A stochastic neuronal model predicts random search behaviors at multiple spatial scales in C. elegans. Elife. 2016:5. doi: 10.7554/eLife.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24•.Richter DW, Smith JC. Respiratory rhythm generation in vivo. Physiology (Bethesda) 2014;29:58–71. doi: 10.1152/physiol.00035.2013. A comprehensive review detailing evidence for the role of inhibitory mechanisms in the generation of the three phase breathing rhythm within the intact respiratory network. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.Bongianni F, Mutolo D, Cinelli E, Pantaleo T. Neural mechanisms underlying respiratory rhythm generation in the lamprey. Respir Physiol Neurobiol. 2016;224:17–26. doi: 10.1016/j.resp.2014.09.003. This review focuses on the lamprey’s paratrigeminal respiratory group (pTRG), the characterization of the area, exitatory, inhibitory and peptide signaling that govern the pTRG. [DOI] [PubMed] [Google Scholar]
- 26•.Rosa E, Jr, Skilling QM, Stein W. Effects of reciprocal inhibitory coupling in model neurons. Biosystems. 2015;127:73–83. doi: 10.1016/j.biosystems.2014.11.002. An interesting study characterizing mechanisms of rhythm generation in the classical half center oscillator. [DOI] [PubMed] [Google Scholar]
- 27.Kintos N, Nusbaum MP, Nadim F. Convergent neuromodulation onto a network neuron can have divergent effects at the network level. J Comput Neurosci. 2016;40:113–135. doi: 10.1007/s10827-015-0587-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlingloff D, Kali S, Freund TF, Hajos N, Gulyas AI. Mechanisms of sharp wave initiation and ripple generation. J Neurosci. 2014;34:11385–11398. doi: 10.1523/JNEUROSCI.0867-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Firl A, Ke JB, Zhang L, Fuerst PG, Singer JH, Feller MB. Elucidating the role of AII amacrine cells in glutamatergic retinal waves. J Neurosci. 2015;35:1675–1686. doi: 10.1523/JNEUROSCI.3291-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramirez JM, Koch H, Garcia AJ, 3rd, Doi A, Zanella S. The role of spiking and bursting pacemakers in the neuronal control of breathing. J Biol Phys. 2011;37:241–261. doi: 10.1007/s10867-011-9214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hooper RM, Tikidji-Hamburyan RA, Canavier CC, Prinz AA. Feedback control of variability in the cycle period of a central pattern generator. J Neurophysiol. 2015;114:2741–2752. doi: 10.1152/jn.00365.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson SM, Hedrick MS, Krause BM, Nilles JP, Chapman MA. Respiratory neuron characterization reveals intrinsic bursting properties in isolated adult turtle brainstems (Trachemys scripta) Respir Physiol Neurobiol. 2016;224:52–61. doi: 10.1016/j.resp.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Selverston AI. Invertebrate central pattern generator circuits. Philos Trans R Soc Lond B Biol Sci. 2010;365:2329–2345. doi: 10.1098/rstb.2009.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Kottick A, Del Negro CA. Synaptic depression influences inspiratory-expiratory phase transition in Dbx1 interneurons of the prebotzinger complex in neonatal mice. J Neurosci. 2015;35:11606–11611. doi: 10.1523/JNEUROSCI.0351-15.2015. An important experimental study demonstrating that presynaptic depression among Dbx1 neurons leads to burst termination and results in a refractory period for Dbx1 activity in preBötC brainstem slices. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Budd JM. Theta oscillations by synaptic excitation in a neocortical circuit model. Proc Biol Sci. 2005;272:101–109. doi: 10.1098/rspb.2004.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.Guerrier C, Hayes JA, Fortin G, Holcman D. Robust network oscillations during mammalian respiratory rhythm generation driven by synaptic dynamics. Proc Natl Acad Sci U S A. 2015;112:9728–9733. doi: 10.1073/pnas.1421997112. An important study using theoretical models to demonstrate that robust rhythms can be generated by recurrent excitation and synaptic facilitation and depression in a sparsely connected network. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nieto-Posadas A, Flores-Martinez E, Lorea-Hernandez JJ, Rivera-Angulo AJ, Perez-Ortega JE, Bargas J, Pena-Ortega F. Change in network connectivity during fictive-gasping generation in hypoxia. prevention by a metabolic intermediate. Front Physiol. 2014;5:p265. doi: 10.3389/fphys.2014.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sinha M, Narayanan R. HCN channels enhance spike phase coherence and regulate the phase of spikes and LFPs in the theta-frequency range. Proc Natl Acad Sci U S A. 2015;112:E2207–E2216. doi: 10.1073/pnas.1419017112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koch H, Garcia AJ, 3rd, Ramirez JM. Network reconfiguration and neuronal plasticity in rhythm-generating networks. Integr Comp Biol. 2011;51:856–868. doi: 10.1093/icb/icr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weimann JM, Meyrand P, Marder E. Neurons that form multiple pattern generators: identification and multiple activity patterns of gastric/pyloric neurons in the crab stomatogastric system. J Neurophysiol. 1991;65:111–122. doi: 10.1152/jn.1991.65.1.111. [DOI] [PubMed] [Google Scholar]
- 41.Pena F, Parkis MA, Tryba AK, Ramirez JM. Differential contribution of pacemaker properties to the generation of respiratory rhythms during normoxia and hypoxia. Neuron. 2004;43:105–117. doi: 10.1016/j.neuron.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 42.Picardo MC, Weragalaarachchi KT, Akins VT, Del Negro CA. Physiological and morphological properties of Dbx1-derived respiratory neurons in the pre-Botzinger complex of neonatal mice. J Physiol. 2013;591:2687–2703. doi: 10.1113/jphysiol.2012.250118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zanella S, Doi A, Garcia AJ, 3rd, Elsen F, Kirsch S, Wei AD, Ramirez JM. When norepinephrine becomes a driver of breathing irregularities: how intermittent hypoxia fundamentally alters the modulatory response of the respiratory network. J Neurosci. 2014;34:36–50. doi: 10.1523/JNEUROSCI.3644-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramirez JM, Doi A, Garcia AJ, 3rd, Elsen FP, Koch H, Wei AD. The cellular building blocks of breathing. Compr Physiol. 2012;2:2683–2731. doi: 10.1002/cphy.c110033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45•.Kleinfeld D, Deschenes M, Wang F, Moore JD. More than a rhythm of life: breathing as a binder of orofacial sensation. Nat Neurosci. 2014;17:647–651. doi: 10.1038/nn.3693. A review considering experimental evidence that the breathing rhythm serves as a common ‘clock’ that integrates sensory behaviors such as whisking and licking with respiratory motor output. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen Chi V, Muller C, Wolfenstetter T, Yanovsky Y, Draguhn A, Tort AB, Brankack J. Hippocampal respiration-driven rhythm distinct from theta oscillations in awake mice. J Neurosci. 2016;36:162–177. doi: 10.1523/JNEUROSCI.2848-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raghuraman S, Garcia AJ, Anderson TM, Twede VD, Curtice KJ, Chase K, Ramirez JM, Olivera BM, Teichert RW. Defining modulatory inputs into CNS neuronal subclasses by functional pharmacological profiling. Proc Natl Acad Sci U S A. 2014;111:6449–6454. doi: 10.1073/pnas.1404421111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stuart DG, Hultborn H. Thomas Graham Brown (1882–1965), Anders Lundberg (1920−), and the neural control of stepping. Brain Res Rev. 2008;59:74–95. doi: 10.1016/j.brainresrev.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 49.Sharp AA, Skinner FK, Marder E. Mechanisms of oscillation in dynamic clamp constructed two-cell half-center circuits. J Neurophysiol. 1996;76:867–883. doi: 10.1152/jn.1996.76.2.867. [DOI] [PubMed] [Google Scholar]
- 50.Brown GT. The intrinsic factors in the act of progression in the mammal. Proc R Soc Lond. 1911;84B:308–319. [Google Scholar]
- 51.Mouser C, Bose A, Nadim F. The role of electrical coupling in generating and modulating oscillations in a neuronal network. Math Biosci. 2016;278:11–21. doi: 10.1016/j.mbs.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weaver AL, Roffman RC, Norris BJ, Calabrese RL. A role for compromise: synaptic inhibition and electrical coupling interact to control phasing in the leech heartbeat CpG. Front Behav Neurosci. 2010:4. doi: 10.3389/fnbeh.2010.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roberts A, Li WC, Soffe SR. Roles for inhibition: studies on networks controlling swimming in young frog tadpoles. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2008;194:185–193. doi: 10.1007/s00359-007-0273-3. [DOI] [PubMed] [Google Scholar]
- 54.Lindsey BG, Rybak IA, Smith JC. Computational models and emergent properties of respiratory neural networks. Compr Physiol. 2012;2:1619–1670. doi: 10.1002/cphy.c110016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rybak IA, Shevtsova NA, Lafreniere-Roula M, McCrea DA. Modelling spinal circuitry involved in locomotor pattern generation: insights from deletions during fictive locomotion. J Physiol. 2006;577:617–639. doi: 10.1113/jphysiol.2006.118703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cangiano L, Grillner S. Mechanisms of rhythm generation in a spinal locomotor network deprived of crossed connections: the lamprey hemicord. J Neurosci. 2005;25:923–935. doi: 10.1523/JNEUROSCI.2301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leclere R, Straus C, Similowski T, Bodineau L, Fiamma MN. Persistent lung oscillator response to CO2 after buccal oscillator inhibition in the adult frog. Respir Physiol Neurobiol. 2012;183:166–169. doi: 10.1016/j.resp.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 58•.Sherman D, Worrell JW, Cui Y, Feldman JL. Optogenetic perturbation of preBotzinger complex inhibitory neurons modulates respiratory pattern. Nat Neurosci. 2015;18:408–414. doi: 10.1038/nn.3938. The study utilizes powerful optogenetic techniques in vivo to examine how glycinergic preBötC neurons contribute to the respiratory rhythm. The authors demonstrate that inhibitory mechanisms are important for shaping the pattern and frequency of rhythmogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwarzacher SW, Rub U, Deller T. Neuroanatomical characteristics of the human pre-Botzinger complex and its involvement in neurodegenerative brainstem diseases. Brain. 2011;134:24–35. doi: 10.1093/brain/awq327. [DOI] [PubMed] [Google Scholar]
- 60.Gray PA, Hayes JA, Ling GY, Llona I, Tupal S, Picardo MC, Ross SE, Hirata T, Corbin JG, Eugenin J, Del Negro CA. Developmental origin of preBotzinger complex respiratory neurons. J Neurosci. 2010;30:14883–14895. doi: 10.1523/JNEUROSCI.4031-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morgado-Valle C, Baca SM, Feldman JL. Glycinergic pacemaker neurons in preBotzinger complex of neonatal mouse. J Neurosci. 2010;30:3634–3639. doi: 10.1523/JNEUROSCI.3040-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wittmeier S, Song G, Duffin J, Poon CS. Pacemakers handshake synchronization mechanism of mammalian respiratory rhythmogenesis. Proc Natl Acad Sci U S A. 2008;105:18000–18005. doi: 10.1073/pnas.0809377105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rybak IA, Molkov YI, Jasinski PE, Shevtsova NA, Smith JC. Rhythmic bursting in the pre-Botzinger complex. mechanisms and models. Prog Brain Res. 2014;209:1–23. doi: 10.1016/B978-0-444-63274-6.00001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dunmyre JR, Del Negro CA, Rubin JE. Interactions of persistent sodium and calcium-activated nonspecific cationic currents yield dynamically distinct bursting regimes in a model of respiratory neurons. J Comput Neurosci. 2011;31:305–328. doi: 10.1007/s10827-010-0311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65••.Anderson TM, Garcia AJ, iii, Baertsch NA, Pollak J, Bloom JC, Wei AD, Rai KG, Ramirez JM. A novel excitatory network for the control of breathing. Nature. 2016;536:76–80. doi: 10.1038/nature18944. This recent study identifies an additional excitatory respiratory micro-circuit in mammals that generates the postinspiratory phase of the breathing rhythm, termed the postinspiratory complex (PiCo). Optogenetic approaches in vitro and in vivo demonstrate that PiCo is necessary and sufficient for postinspiratory activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huckstepp RT, Henderson LE, Cardoza KP, Feldman JL. Interactions between respiratory oscillators in adult rats. Elife. 2016:5. doi: 10.7554/eLife.14203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kam K, Worrell JW, Janczewski WA, Cui Y, Feldman JL. Distinct inspiratory rhythm and pattern generating mechanisms in the preBotzinger complex. J Neurosci. 2013;33:9235–9245. doi: 10.1523/JNEUROSCI.4143-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Del Negro CA, Hayes JA, Pace RW, Brush BR, Teruyama R, Feldman JL. Synaptically activated burst-generating conductances may underlie a group-pacemaker mechanism for respiratory rhythm generation in mammals. Prog Brain Res. 2010;187:111–136. doi: 10.1016/B978-0-444-53613-6.00008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rubin JE, Hayes JA, Mendenhall JL, Del Negro CA. Calcium-activated nonspecific cation current and synaptic depression promote network-dependent burst oscillations. Proc Natl Acad Sci U S A. 2009;106:2939–2944. doi: 10.1073/pnas.0808776106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pace RW, Mackay DD, Feldman JL, Del Negro CA. Inspiratory bursts in the preBotzinger complex depend on a calcium-activated non-specific cation current linked to glutamate receptors in neonatal mice. J Physiol. 2007;582:113–125. doi: 10.1113/jphysiol.2007.133660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carroll MS, Viemari JC, Ramirez JM. Patterns of inspiratory phase-dependent activity in the in vitro respiratory network. J Neurophysiol. 2013;109:285–295. doi: 10.1152/jn.00619.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carroll MS, Ramirez JM. Cycle-by-cycle assembly of respiratory network activity is dynamic and stochastic. J Neurophysiol. 2013;109:296–305. doi: 10.1152/jn.00830.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hill AA, Lu J, Masino MA, Olsen OH, Calabrese RL. A model of a segmental oscillator in the leech heartbeat neuronal network. J Comput Neurosci. 2001;10:281–302. doi: 10.1023/a:1011216131638. [DOI] [PubMed] [Google Scholar]
- 74.Alle H, Roth A, Geiger JR. Energy-efficient action potentials in hippocampal mossy fibers. Science. 2009;325:1405–1408. doi: 10.1126/science.1174331. [DOI] [PubMed] [Google Scholar]
- 75.Oke Y, Boiroux D, Miwakeichi F, Oku Y. Stochastic activation among inspiratory cells in the pre-Botzinger complex of the rat medulla revealed by Ca(2+) imaging. Neurosci Lett. 2015;595:12–17. doi: 10.1016/j.neulet.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 76••.Wang X, Hayes JA, Revill AL, Song H, Kottick A, Vann NC, LaMar MD, Picardo MC, Akins VT, Funk GD, Del Negro CA. Laser ablation of Dbx1 neurons in the pre-Botzinger complex stops inspiratory rhythm and impairs output in neonatal mice. Elife. 2014;3:e03427. doi: 10.7554/eLife.03427. An important study using sophisticated techniques to demonstrate the necessary role of a specific subset of genetically defined neurons in generating the preBötC rhythm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tupal S, Huang WH, Picardo MC, Ling GY, Del Negro CA, Zoghbi HY, Gray PA. Atoh1-dependent rhombic lip neurons are required for temporal delay between independent respiratory oscillators in embryonic mice. Elife. 2014;3:pe02265. doi: 10.7554/eLife.02265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morquette P, Verdier D, Kadala A, Fethiere J, Philippe AG, Robitaille R, Kolta A. An astrocyte-dependent mechanism for neuronal rhythmogenesis. Nat Neurosci. 2015;18:844–854. doi: 10.1038/nn.4013. [DOI] [PubMed] [Google Scholar]
- 79.Yin J, Wang SY, Song YF, Jing YH. Gap junction communication: alternative pattern of signal exchange in glia and neuron. Sheng Li Ke Xue Jin Zhan. 2012;43:183–187. [PubMed] [Google Scholar]
- 80•.Bazargani N, Attwell D. Astrocyte calcium signaling: the third wave. Nat Neurosci. 2016;19:182–189. doi: 10.1038/nn.4201. This review details the current understanding of neuron–glia interactions with a specific focus on the funcitonal implications of astrocyte intracellular calcium dynamics. [DOI] [PubMed] [Google Scholar]
- 81.Amiri M, Hosseinmardi N, Bahrami F, Janahmadi M. Astrocyte-neuron interaction as a mechanism responsible for generation of neural synchrony: a study based on modeling and experiments. J Comput Neurosci. 2013;34:489–504. doi: 10.1007/s10827-012-0432-6. [DOI] [PubMed] [Google Scholar]
- 82.Nadkarni S, Jung P, Levine L. Astrocytes optimize the synaptic transmission of information. PLoS Comput Biol. 2008:30. doi: 10.1371/journal.pcbi.1000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Olsen ML, Khakh BS, Skatchkov SN, Zhou M, Lee CJ, Rouach N. New insights on astrocyte ion channels: critical for homeostasis and neuronglia signaling. J Neurosci. 2015;35:13827–13835. doi: 10.1523/JNEUROSCI.2603-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84••.Funk GD, Rajani V, Alvares TS, Revill AL, Zhang Y, Chu NY, Biancardi V, Linhares-Taxini C, Katzell A, Reklow R. Neuroglia and their roles in central respiratory control; an overview. Comp Biochem Physiol A Mol Integr Physiol. 2015;186:83–95. doi: 10.1016/j.cbpa.2015.01.010. A comprehensive review of the different types of neuroglia and their contribution to chemosensing and homeostasis in respiratory control. [DOI] [PubMed] [Google Scholar]
- 85•.Angelova PR, Kasymov V, Christie I, Sheikhbahaei S, Turovsky E, Marina N, Korsak A, Zwicker J, Teschemacher AG, Ackland GL, Funk GD, et al. Functional oxygen sensitivity of astrocytes. J Neurosci. 2015;35:10460–10473. doi: 10.1523/JNEUROSCI.0045-15.2015. The authors use experimental techniques to define the cellular mechanisms of astrocyte oxygen sensing and its functional implications for the respiratory rhythm during local hypoxia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huxtable AG, Smith SM, Vinit S, Watters JJ, Mitchell GS. Systemic LPS induces spinal inflammatory gene expression and impairs phrenic long-term facilitation following acute intermittent hypoxia. J Appl Physiol (1985) 2013;114:879–887. doi: 10.1152/japplphysiol.01347.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Basaran KE, Villongco M, Ho B, Ellis E, Zarndt R, Antonova J, Hopkins SR, Powell FL. Ibuprofen blunts ventilatory acclimatization to sustained hypoxia in humans. PLoS ONE. 2016;11:pe0146087. doi: 10.1371/journal.pone.0146087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lorea-Hernandez JJ, Morales T, Rivera-Angulo AJ, Alcantara-Gonzalez D, Pena-Ortega F. Microglia modulate respiratory rhythm generation and autoresuscitation. Glia. 2016;64:603–619. doi: 10.1002/glia.22951. [DOI] [PubMed] [Google Scholar]
- 90.Tupal S, Rieger MA, Ling GY, Park TJ, Dougherty JD, Goodchild AK, Gray PA. Testing the role of preBotzinger complex somatostatin neurons in respiratory and vocal behaviors. Eur J Neurosci. 2014;40:3067–3077. doi: 10.1111/ejn.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91•.Albersheim-Carter J, Blubaum A, Ballagh IH, Missaghi K, Siuda ER, McMurray G, Bass AH, Dubuc R, Kelley DB, Schmidt MF, Wilson RJ, et al. Testing the evolutionary conservation of vocal motoneurons in vertebrates. Respir Physiol Neurobiol. 2016;224:2–10. doi: 10.1016/j.resp.2015.06.010. A set of comparative studies detailing how vocalization and the important neuronal complexes involved in the action, are conserved in different species. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ramirez JM. The human pre-Botzinger complex identified. Brain. 2011;134:8–10. doi: 10.1093/brain/awq357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Onimaru H, Ikeda K, Kawakami K. Phox2b, RTN/pFRG neurons and respiratory rhythmogenesis. Respir Physiol Neurobiol. 2009;168:13–18. doi: 10.1016/j.resp.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 94.Kleinfeld D, Deschenes M, Ulanovsky N. Whisking, sniffing, and the hippocampal theta-rhythm: a tale of two oscillators. PLOS Biol. 2016;14:e1002385. doi: 10.1371/journal.pbio.1002385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Burke PG, Kanbar R, Viar KE, Stornetta RL, Guyenet PG. Selective optogenetic stimulation of the retrotrapezoid nucleus in sleeping rats activates breathing without changing blood pressure or causing arousal or sighs. J Appl Physiol (1985) 2015;118:1491–1501. doi: 10.1152/japplphysiol.00164.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ramirez JM. The integrative role of the sigh in psychology, physiology, pathology, and neurobiology. Prog Brain Res. 2014;209:91–129. doi: 10.1016/B978-0-444-63274-6.00006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97•.Dutschmann M, Jones SE, Subramanian HH, Stanic D, Bautista TG. The physiological significance of postinspiration in respiratory control. Prog Brain Res. 2014;212:113–130. doi: 10.1016/B978-0-444-63488-7.00007-0. A comprehensive review of the postinspiratory phase of breathing, its associated behaviors, and its significance in health and disease. [DOI] [PubMed] [Google Scholar]
- 98.Lieske SP, Ramirez JM. Pattern-specific synaptic mechanisms in a multifunctional network. I. Effects of alterations in synapse strength. J Neurophysiol. 2006;95:1323–1333. doi: 10.1152/jn.00505.2004. [DOI] [PubMed] [Google Scholar]
- 99.Deschenes M, Takatoh J, Kurnikova A, Moore JD, Demers M, Elbaz M, Furuta T, Wang F, Kleinfeld D. Inhibition, not excitation, drives rhythmic whisking. Neuron. 2016;90:374–387. doi: 10.1016/j.neuron.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pagliardini S, Janczewski WA, Tan W, Dickson CT, Deisseroth K, Feldman JL. Active expiration induced by excitation of ventral medulla in adult anesthetized rats. J Neurosci. 2011;31:2895–2905. doi: 10.1523/JNEUROSCI.5338-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zoccal DB, Simms AE, Bonagamba LG, Braga VA, Pickering AE, Paton JF, Machado BH. Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J Physiol. 2008;586:3253–3265. doi: 10.1113/jphysiol.2008.154187. [DOI] [PMC free article] [PubMed] [Google Scholar]