Abstract
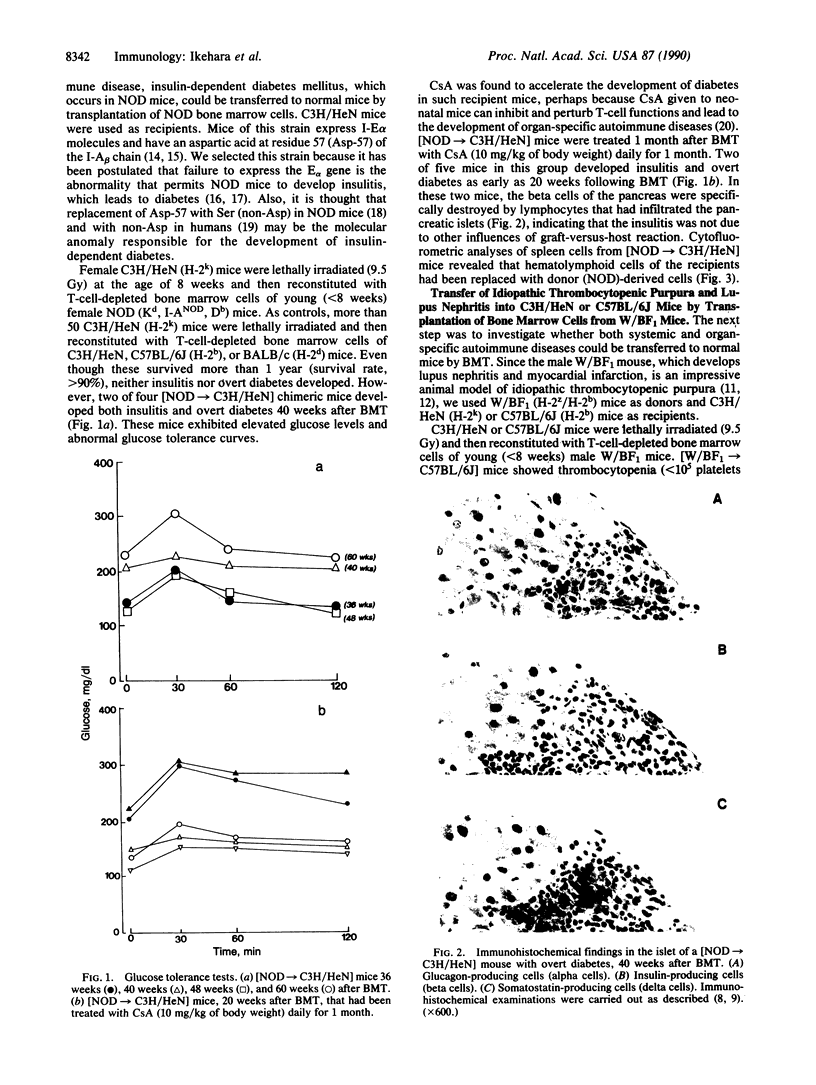
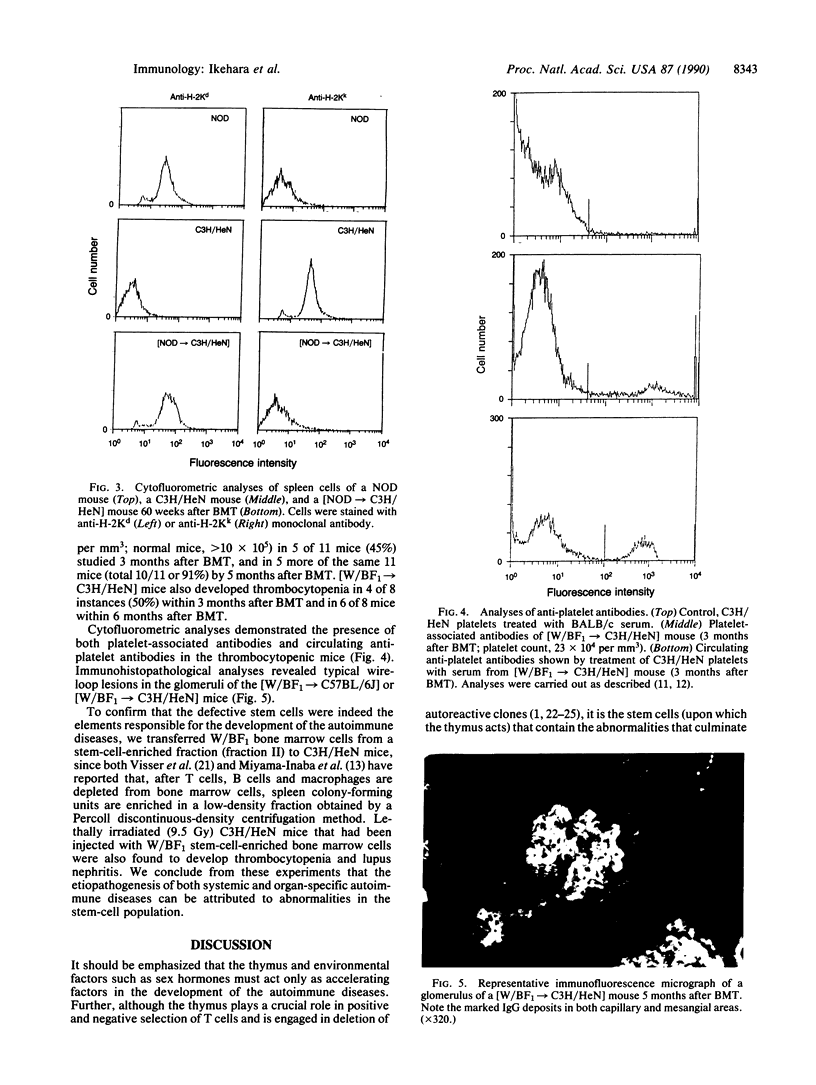
Transplantation of bone marrow cells from nonobese diabetic (NOD) mice, a model for type 1 diabetes mellitus, to C3H/HeN mice, which express I-E alpha molecules and have aspartic acid at residue 57 of the I-A beta chain, induced insulitis followed by overt diabetes in the recipient C3H/HeN mice more than 40 weeks after bone marrow transplantation. When cyclosporin A, which perturbs T-cell functions, was injected intraperitoneally into [NOD----C3H/HeN] chimeric mice daily for 1 month, the chimeric mice developed insulitis and overt diabetes within 20 weeks following bone marrow transplantation. Transplantation of bone marrow cells from (NZW x BXSB)F1 mice, which develop lupus nephritis, myocardial infarction, and idiopathic thrombocytopenic purpura, into C3H/HeN or C57BL/6J mice induced in the recipient strains both lupus nephritis and idiopathic thrombocytopenic purpura more than 3 months after transplantation. Transplantation of a stem-cell-enriched population from (NZW x BXSB)F1 mice into normal mice also induced autoimmune disease in the recipients. These results indicate that both systemic autoimmune disease and organ-specific autoimmune disease originate from defects that reside within the stem cells; the thymus and environmental factors such as sex hormones appear to act only as accelerating factors.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acha-Orbea H., McDevitt H. O. The first external domain of the nonobese diabetic mouse class II I-A beta chain is unique. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2435–2439. doi: 10.1073/pnas.84.8.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akizuki M., Reeves J. P., Steinberg A. D. Expression of autoimmunity by NZB/NZW marrow. Clin Immunol Immunopathol. 1978 Jul;10(3):247–250. doi: 10.1016/0090-1229(78)90177-0. [DOI] [PubMed] [Google Scholar]
- Estess P., Begovich A. B., Koo M., Jones P. P., McDevitt H. O. Sequence analysis and structure-function correlations of murine q, k, u, s, and f haplotype I-A beta cDNA clones. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3594–3598. doi: 10.1073/pnas.83.11.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara S., Good R. A., Nakamura T., Sekita K., Inoue S., Oo M. M., Muso E., Ogawa K., Hamashima Y. Rationale for bone marrow transplantation in the treatment of autoimmune diseases. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2483–2487. doi: 10.1073/pnas.82.8.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara S., Ohtsuki H., Good R. A., Asamoto H., Nakamura T., Sekita K., Muso E., Tochino Y., Ida T., Kuzuya H. Prevention of type I diabetes in nonobese diabetic mice by allogenic bone marrow transplantation. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7743–7747. doi: 10.1073/pnas.82.22.7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara S., Yasumizu R., Inaba M., Izui S., Hayakawa K., Sekita K., Toki J., Sugiura K., Iwai H., Nakamura T. Long-term observations of autoimmune-prone mice treated for autoimmune disease by allogeneic bone marrow transplantation. Proc Natl Acad Sci U S A. 1989 May;86(9):3306–3310. doi: 10.1073/pnas.86.9.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyonouchi H., Kincade P. W., Good R. A., Fernandes G. Reciprocal transfer of abnormalities in clonable B lymphocytes and myeloid progenitors between NZB and DBA/2 mice. J Immunol. 1981 Sep;127(3):1232–1235. [PubMed] [Google Scholar]
- Kappler J. W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Koide Y., Yoshida T. O. The unique nucleotide sequence of the A beta gene in the NOD mouse is shared with its nondiabetic sister strains, the ILI and the CTS mouse. Int Immunol. 1990;2(2):189–192. doi: 10.1093/intimm/2.2.189. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Schneider R., Lees R. K., Howe R. C., Acha-Orbea H., Festenstein H., Zinkernagel R. M., Hengartner H. T-cell receptor V beta use predicts reactivity and tolerance to Mlsa-encoded antigens. Nature. 1988 Mar 3;332(6159):40–45. doi: 10.1038/332040a0. [DOI] [PubMed] [Google Scholar]
- Miyama-Inaba M., Ogata H., Toki J., Kuma S., Sugiura K., Yasumizu R., Ikehara S. Isolation of murine pluripotent hemopoietic stem cells in the Go phase. Biochem Biophys Res Commun. 1987 Sep 15;147(2):687–694. doi: 10.1016/0006-291x(87)90985-5. [DOI] [PubMed] [Google Scholar]
- Mizutani H., Furubayashi T., Kuriu A., Take H., Tomiyama Y., Yoshida H., Nakamura Y., Inaba M., Kurata Y., Yonezawa T. Analyses of thrombocytopenia in idiopathic thrombocytopenic purpura-prone mice by platelet transfer experiments between (NZW x BXSB)F1 and normal mice. Blood. 1990 May 1;75(9):1809–1812. [PubMed] [Google Scholar]
- Morton J. I., Siegel B. V. Transplantation of autoimmune potential. I. Development of antinuclear antibodies in H-2 histocompatible recipients of bone marrow from New Zealand Black mice. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2162–2165. doi: 10.1073/pnas.71.6.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Ikehara S., Good R. A., Inoe S., Sekita K., Furukawa F., Tanaka H., Oo M. M., Hamashima Y. Abnormal stem cells in autoimmune-prone mice are responsible for premature thymic involution. Thymus. 1985;7(3):151–160. [PubMed] [Google Scholar]
- Nishimoto H., Kikutani H., Yamamura K., Kishimoto T. Prevention of autoimmune insulitis by expression of I-E molecules in NOD mice. 1987 Jul 30-Aug 5Nature. 328(6129):432–434. doi: 10.1038/328432a0. [DOI] [PubMed] [Google Scholar]
- Oyaizu N., Yasumizu R., Miyama-Inaba M., Nomura S., Yoshida H., Miyawaki S., Shibata Y., Mitsuoka S., Yasunaga K., Morii S. (NZW x BXSB)F1 mouse. A new animal model of idiopathic thrombocytopenic purpura. J Exp Med. 1988 Jun 1;167(6):2017–2022. doi: 10.1084/jem.167.6.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich E. P., Sherwin R. S., Kanagawa O., Janeway C. A., Jr An explanation for the protective effect of the MHC class II I-E molecule in murine diabetes. Nature. 1989 Sep 28;341(6240):326–328. doi: 10.1038/341326a0. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S., Sakaguchi N. Organ-specific autoimmune disease induced in mice by elimination of T cell subsets. V. Neonatal administration of cyclosporin A causes autoimmune disease. J Immunol. 1989 Jan 15;142(2):471–480. [PubMed] [Google Scholar]
- Schneider R., Lees R. K., Pedrazzini T., Zinkernagel R. M., Hengartner H., MacDonald H. R. Postnatal disappearance of self-reactive (V beta 6+) cells from the thymus of Mlsa mice. Implications for T cell development and autoimmunity. J Exp Med. 1989 Jun 1;169(6):2149–2158. doi: 10.1084/jem.169.6.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer P. A., Balderas R. S., McEvilly R. J., Bobardt M., Theofilopoulos A. N. Tolerance-related V beta clonal deletions in normal CD4-8-, TCR-alpha/beta + and abnormal lpr and gld cell populations. J Exp Med. 1989 Dec 1;170(6):1869–1877. doi: 10.1084/jem.170.6.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J., Lo D., Gao E. K., Ron Y. T cell selection in the thymus. Immunol Rev. 1988 Jan;101:173–190. doi: 10.1111/j.1600-065x.1988.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Dixon F. J. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- Todd J. A., Bell J. I., McDevitt H. O. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987 Oct 15;329(6140):599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- Visser J. W., Bauman J. G., Mulder A. H., Eliason J. F., de Leeuw A. M. Isolation of murine pluripotent hemopoietic stem cells. J Exp Med. 1984 Jun 1;159(6):1576–1590. doi: 10.1084/jem.159.6.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumizu R., Sugiura K., Iwai H., Inaba M., Makino S., Ida T., Imura H., Hamashima Y., Good R. A., Ikehara S. Treatment of type 1 diabetes mellitus in non-obese diabetic mice by transplantation of allogeneic bone marrow and pancreatic tissue. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6555–6557. doi: 10.1073/pnas.84.18.6555. [DOI] [PMC free article] [PubMed] [Google Scholar]







