Abstract
The progenitors of the gametes, the primordial germ cells (PGCs) are typically specified early in the development in positions, which are distinct from the gonad. These cells then migrate toward the gonad where they differentiate into sperms and eggs. Here, we study the role of the germ cells in somatic development and particularly the role of the germ line in the sex differentiation in zebrafish. To this end, we ablated the germ cells using two independent methods and followed the development of the experimental fish. First, PGCs were ablated by knocking down the function of dead end, a gene important for the survival of this lineage. Second, a method to eliminate the PGCs using the toxin–antitoxin components of the parD bacterial genetic system was used. Specifically, we expressed a bacterial toxin Kid preferentially in the PGCs and at the same time protected somatic cells by uniformly expressing the specific antidote Kis. Our results demonstrate an unexpected role for the germ line in promoting female development because PGC-ablated fish invariably developed as males.
Keywords: cell ablation, parD, sex determination, primordial germ cell, dead end
In most organisms, sex is determined during early stages of development. In Drosophila melanogaster and Caenorhabditis elegans, the primary signal for sex determination is the ratio of X chromosomes to the autosomes. In these organisms, XX animals become hermaphrodites (in worms) or female (in flies), whereas XY and XO animals develop as males (1). Similarly, sex determination in mammals and birds depends on the chromosomal constitution of the organism. In mammals, it is controlled by the Y-linked SRY gene, which initiates a cascade of genetic and cellular events leading to testicular differentiation (2, 3). In birds, the females are ZW (the heterogametic sex), and the males are ZZ (the homogametic sex), but master male- or female-promoting genes have not yet been identified, and the precise mechanism of sex determination remains unclear (4). In contrast to the dominant role of the genetic composition of the individual, in crocodilians, many turtles, and some lizards, environmental conditions play a major role in sex determination (5). In these species, heteromorphic sex chromosomes have not been identified, and sex is controlled by egg-incubation temperature.
In fish, environmental as well as genetic (chromosome-based) mechanisms have been implicated in sex determination. For example, in medaka and some poeciliid fishes, sex chromosomes can be distinguished from the autosomes (6, 7). Interestingly however, even fish with established sex chromosomes show strong dependence on environmental cues, the most prominent of which is temperature, but other factors such as pH, pollutants, and social effects have been shown to influence sex determination as well (8, 9).
In other fish such as zebrafish and the European eel, morphological differences in the chromosomes of the two sexes have not been identified by classical karyotyping. Furthermore, in zebrafish, a chromosomal locus controlling sex determination has not been found, implicating polygenetic or environmental signals in sex determination (10).
Sexual development culminates in formation of functional gametes. Sperms and eggs are derived from a specific cell type, namely, primordial germ cells (PGCs), which are set aside early during development to differentiate independently from the soma (11–13). The decision of PGCs to develop into sperm or eggs varies among the species with examples of mechanisms involving both cell-autonomous control and inductive cues. In mammals, the decision to differentiate into male gametes depends on signals from somatic cells (14), whereas in Drosophila, this process is mediated by cell-autonomous as well as inductive signals (15).
In fish, the mechanisms governing the sexual fate of the PGCs are not clear. In some species, such as the channel catfish and medaka, an undifferentiated gonad develops, which then gives rise to ovaries in females and testes in males (16, 17). In other fish species, including zebrafish, an ovary-like structure is initially formed in all embryos. This structure subsequently develops into ovaries in females, or after the death of the oocytes, into testis in males (18, 19).
Despite the extensive signaling between the germ cells and the somatic cells in the gonads of mouse or Drosophila, the presence of PGCs does not seem to be important for somatic sexual differentiation in these species because both sexual types are generated by animals bearing mutations causing germ cell depletion (20, 21). These data imply a unidirectional sex-determining signaling from the soma to the germ cells.
In this study, we have examined the role of the germ line in somatic development of zebrafish. For that purpose, we have developed a method for targeted cell ablation in zebrafish that was applied to the PGCs. This method is based on the bicistronic protein killer bacterial system parD. Preferential expression of the toxin kid in the PGCs and concomitant expression of the natural antidote kis allowed specific ablation of the PGCs. We show that embryos depleted of the germ cells develop into sterile male fish thus, uncovering an unexpected role of the PGCs in fish sexual development.
Materials and Methods
Fish Strains. All experiments were performed by using WT fish of the AB and Tl genetic background.
DNA Constructs and RNA Synthesis. Diphtheria toxin (DT) A chain was amplified by PCR from the plasmid pCGmIL3 (22) by using the primers 807 (GGCATGGGCGCTGATGATGTTGTTG) and 808 (TTATCGCCTGACACGATTTCCTGCA) and cloned into an RNA expression vector upstream of the nanos1 (nos1) 3′-UTR (23).
Kid and Kis coding sequences were amplified from plasmids pcIneoKid and p424Met25K (24).
For kid, A023 (CGGGATCCACCATGGAAAGAGGGGAAATCT) and A024 (CCCTCGAGTCAAGTCAGAATAGTGGACAGG) were used, and A025 (CCCTCGAGCCATGCATACCACCCGACTGAA) and A026 (TAAAGCGGCCGCTCAGATTTCCTCCTGACC) were used to amplify the kis ORF. The kid ORF was cloned into an RNA expression vector upstream of the nos1 3′-UTR, and the kis ORF was cloned in an RNA expression vector in between the Xenopus 3′- and 5′-UTR.
Caped sense mRNA was synthesized in vitro by using the Message Machine kit (Ambion, Austin, TX) and injected in the quantities indicated in the text into embryos of the AB genetic background.
Whole-Mount in Situ Hybridization. In situ hybridization was performed as described in ref. 25, with modification according to Hauptmann and Gerster (26).
Time-Lapse Analysis of PGC Migration. For the time-lapse analysis, control and experimental embryos whose PGCs were labeled with GFP by injection of GFP-nos1 3′-UTR (23) were oriented in agarose ramps and overlaid with 0.3× Danieau's solution (27). Time-lapse movies were generated by using metamorph software (Universal Imaging, Downingtown, PA) controlling an Axioplan2 microscope (Zeiss).
Histology. We fixed 25-, 35-, and 90-day-old WT and dead end (dnd) morpholino oligonucleotide (MO)-injected embryos in 4% paraformaldehyde for 48 h. After dehydration in ethanol and clearing in toluene, the specimens were infiltrated with paraffin, embedded, and sectioned. The sections (8 μm thick) were stained with hematoxylin–eosin and visualized by using an Axioplan2 microscope.
Hormone Treatments. WT AB/Tl embryos injected with dnd antisense MOs were treated with the hormone [10 μl/liter of 1.5 ng/μl estrogen (17α-ethynylestradiol; Sigma) in 70% ethanol] or as a control, with the solvent alone (10 μl/liter of 70% ethanol) as described in ref. 28. The fish were treated between the ages 20 and 110 days with 30% daily water exchange. After the treatment, the fish were examined morphologically, their behavior was assayed by crossing them to WT females and the presence of gonadal structures was determined by dissection.
Results
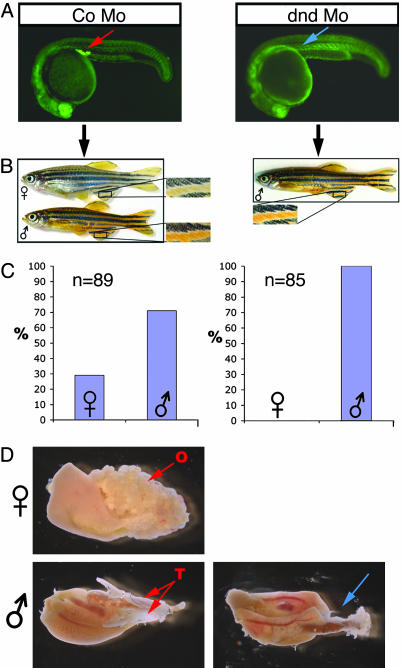
Embryos Lacking Germ Cells Develop into Sterile Adult Males. To determine the role of the germ line in somatic development of zebrafish, we ablated the PGCs by using dnd antisense MOs. As described (29, 30), dnd is essential for normal migration and survival of PGCs, and therefore, embryos devoid of this protein develop to become sterile adults. Interestingly, in contrast to embryos injected with control mopholino that developed into fish of both sexes, all embryos injected with dnd mopholino developed into fish that appeared phenotypically males as determined by their body shape and color (Fig. 1 A–C). Moreover, when these males were mated with WT females, the fish displayed normal male sexual behavior, as judged by their ability to induce females to lay eggs. Nevertheless, as expected from the early loss of PGCs, the males were sterile and did not fertilize the eggs. To characterize the dnd MO phenotype further, we examined the adult fish. This analysis revealed the lack of any gonadal structures in these fish (Fig. 1D). These results clearly show that Dead End function is essential for female development as well as for proper development of the gonad. However, this requirement could represent either a specific and direct function of dnd in sex determination or an indirect consequence of the loss of PGCs or the gonad. To distinguish between these two possibilities, we set out to ablate the PGCs by using an independent method and to determine the effect of the treatment on sex determination in zebrafish.
Fig. 1.
dead end MO-injected embryos develop into sterile adult male fish. (A) Fluorescent images of 24-hpf embryos injected with 200 pg of control antisense MO or dnd MO and GFP-nos1 3′-UTR to visualize the PGCs. The red arrow indicates the germ cells in control embryos, and the blue arrow indicates the region where the PGC are normally found. (B) Adult AB fish (6 months old) developed from control (Left) or dnd MO-injected (Right) embryos. Insets are magnifications of the male and female fins showing the typical sexually dimorphic pigmentation. (C) A quantitative analysis of the female/male ratio. (D) Dissected gonads from 180-day-old control and dnd MO-treated fish. Red arrows indicate ovary (O) or testis (T) in the control fish. The blue arrow indicates the region of the gonad in a phenotypically appearing male fish developed from a dnd MO-injected embryo.
Germ Cell Ablation Using DT. To ablate zebrafish PGCs by an independent method, we decided to express bacterial toxins in these cells. One-cell-stage embryos were injected with mRNA encoding for the DT catalytic A chain (31) fused to the 3′-UTR of the zebrafish nos1 gene, which directs the expression of the DT protein preferentially to the PGCs (23). Embryos injected with ≥0.05 pg of the Diphtheria fusion mRNA died within the first hours of their development with severe malformations (data not shown). Reduction of the injected amounts of RNA led to a decrease in the observed malformations, which presumably resulted from residual toxin expression in the somatic cells (Fig. 6 A and C, which is published as supporting information on the PNAS web site). Our attempts to define injection conditions in which the injected embryos would lose all of their PGCs yet develop to reach adulthood failed (Fig. 6 B and D). This result probably reflects a high sensitivity to DT of some somatic cell lineages that are essential for viability. Thus, under conditions in which all of the PGCs were ablated, a low level of DT in somatic cells led to embryonic lethality.
Prokaryotic Toxin Kid and Antitoxin Kis Are Functional in Zebrafish. To overcome the problem of toxicity to somatic cells, we chose to use a system that allows ablating the PGCs while protecting somatic cells from the toxin. The prokaryotic parD system consists of a toxin, kid, and an antidote, kis. In cells expressing the Kid protein, cell growth is inhibited, whereas coexpression of Kis inactivates the toxin and neutralizes this effect (32). This system has been shown to inhibit cell proliferation in yeast, frog embryos and in mammalian cell lines, where it also induces cell death (24), but ablation of a specific cell type in a developing organism by using this system has not been demonstrated.
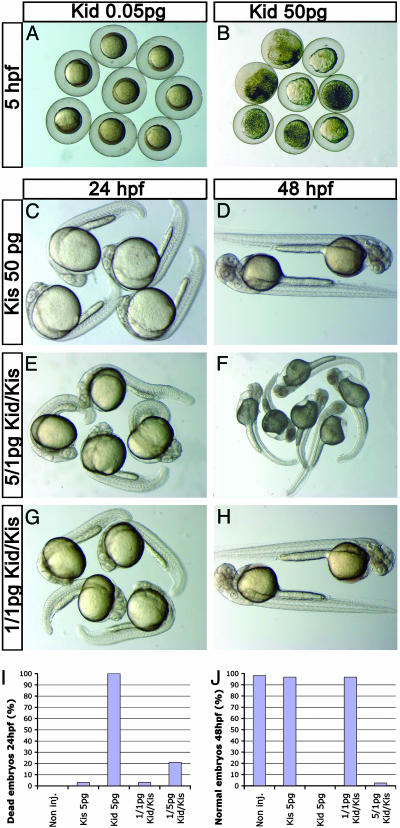
To determine whether the toxin antitoxin components of the parD system could function in zebrafish, we expressed the toxin preferentially in the PGCs as described above [injection of mRNA, which included the kid ORF fused to 3′-UTR of the nos1 gene]. Indeed, this treatment effectively eliminated the PGCs, demonstrating that Kid is functional in zebrafish cells (see below). Nevertheless, similar to our findings using DT, injections of kid-nos1 3′-UTR resulted in somatic defects and embryonic death. The extent of the Kid-induced embryonic death was concentration-dependent, such that embryos injected with 50 pg of the toxin fusion mRNA died at the very early developmental stages, whereas amounts of <0.05 pg had no effect on the embryos (Fig. 2 A and B). Uniform somatic expression of the antidote upon injecting 50 pg of kis-globin (glo) 3′-UTR mRNA by itself did not lead to any visible affect on embryos at 24 h after fertilization (hpf), 48 hpf (Fig. 2 C and D), and adults. Importantly, coinjection of the antidote mRNA effectively neutralized the deleterious effects of Kid on somatic development. Specifically, embryos coinjected with 5 pg of kid-nos1 3′-UTR and 1 pg of kis-glo UTR mRNA appeared morphologically normal at 24 hpf, thus sharply contrasting embryos that did not receive the antidote (Fig. 2 E, I, and J). Nevertheless, these embryos did exhibit somatic defects at 48 hpf (Fig. 2F). Embryos injected with a ratio of 1:1 pg from the toxin mRNA/antidote mRNA showed PGC loss (see below) but appeared normal at 24 and 48 hpf (Fig. 2 G and H) and could be raised to adulthood.
Fig. 2.
The prokaryotic toxin Kid and antitoxin Kis are functional in zebrafish. (A and B) High concentrations of Kid result in rapid embryonic lethality in embryos injected with kid-nos1 3′-UTR RNA. (C and D) Embryonic development is not affected by high levels of somatically expressed antidote kis-glo UTR RNA. The deleterious effect of 5 pg of Kid on somatic cell development is counteracted efficiently by 1 pg of kis-glo UTR for up to 24 hpf (E), but not at 48 hpf (F). (G and H) A ratio of 1:1 pg of kid-nos1 3′UTR/kis-glo UTR mRNAs allows proper somatic development during the first 2 days of development. (I and J) Quantitative representation of Kid-induced somatic phenotypes during the first 2 days of development.
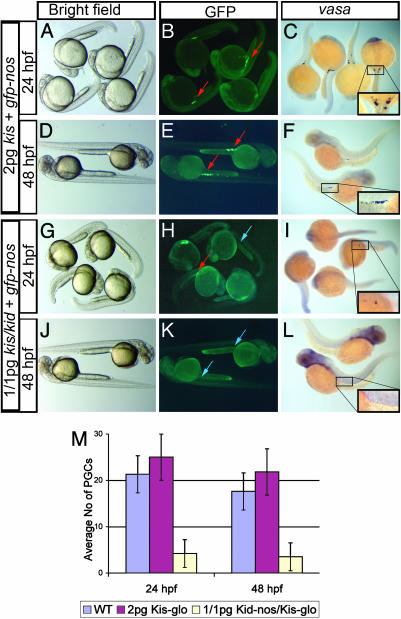
Embryos Injected with kid/kis Constructs Lack PGCs and Develop as Sterile Male Adult Fish. To examine further the effect of PGCs on sex determination, we compared control embryos injected with 2 pg of kis-glo UTR (Fig. 3 A–F) with those injected with 1 pg of each kid and kis RNA constructs. These embryos consequently exhibited dramatically reduced number of fluorescent PGCs or lacked them at 24 hpf (Fig. 3H). By 48 hpf, most toxin-injected embryos had no fluorescent PGCs (Fig. 3K), an observation confirmed by in situ hybridization using the germ-cell-specific marker vasa (Fig. 3 F and L). Significantly, these embryos showed no somatic defects during their development (Fig. 3 G and J) and could be raised to adulthood.
Fig. 3.
kid and kis allow effective ablation of the germ line without affecting embryonic morphology. (A and D) Control embryos injected with 2 pg of the antidote RNA developed without apparent somatic defects and are morphologically similar to embryos injected with 1 pg of kid-nos1 3′UTR plus 1 pg of kis-glo (G and J). Reduction in the number and lack of GFP-labeled (B, E, H, and K) or vasa RNA-labeled (C, F, I, and L) germ cells at 24 and 48 hpf. Red arrows indicate the germ cells, and blue arrows indicate the regions where PGCs are normally found in the fluorescent images (B, E, H, and K). The same regions are boxed and magnified in C, F, I, and L.(M) A quantitative representation of the number of germ cells after different treatment at 24 and 48 hpf, as determined by in situ hybridization using vasa antisense RNA probe.
Analysis of kid/kis-treated fish revealed high efficiency of the germ cell ablation manifested in frequent generation of sterile adult fish (54 of 71). We attribute the presence of the 17 fertile fish (4 females and 13 males) to a small number of germ cells that survived the treatment and succeeded in populating the gonad. Importantly, all sterile fish appeared to be phenotypically males and were capable of inducing females to lay eggs. In contrast with the striking male bias in the experimental fish, only 44 of 87 control fish developed as males (Fig. 3M). Dissection of the sterile experimental fish revealed that, similar to dnd MO-injected fish, these males also lacked gonadal structures (data not shown).
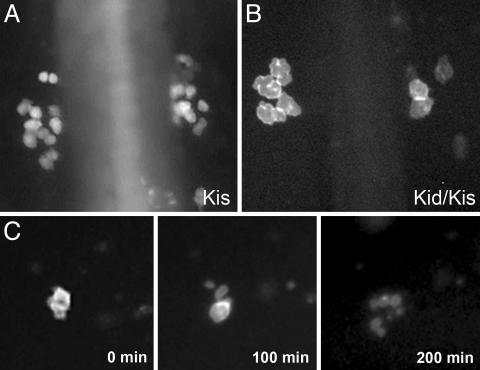
By time-lapse movie, we recorded fluorescently labeled germ cells in kid/kis-treated embryos. This analysis demonstrated that PGCs appear abnormally large relative to the WT germ cells (Fig. 4 A and B and Movies 1 and 2, which are published as supporting information on the PNAS web site), presumably signifying an effect on cell proliferation by Kid (24). These large germ cells subsequently die while exhibiting morphology typical of apoptotic cells (Fig. 4C).
Fig. 4.
PGCs in kid-nos1 3′UTR treated embryos exhibit abnormal cell morphology culminating in cell death. Fluorescently labeled germ cells in kis-glo UTR (A) and kid-nos1 3′UTR/kis-glo UTR-injected (B) embryos at 12 hpf are shown. More PGCs are found in the control embryos and these appear smaller in size relative to kid-treated PGCs. (C) Frames from a time-lapse movie of a kid-nos1 3′-UTR-treated embryo showing a PGC undergoing cell death (see Movie 2).
These results demonstrate that the germ cells are essential for female development and that the phenotype observed in dnd MO-treated fish results from germ cells loss rather than reflecting a specific function of the dnd gene in this process.
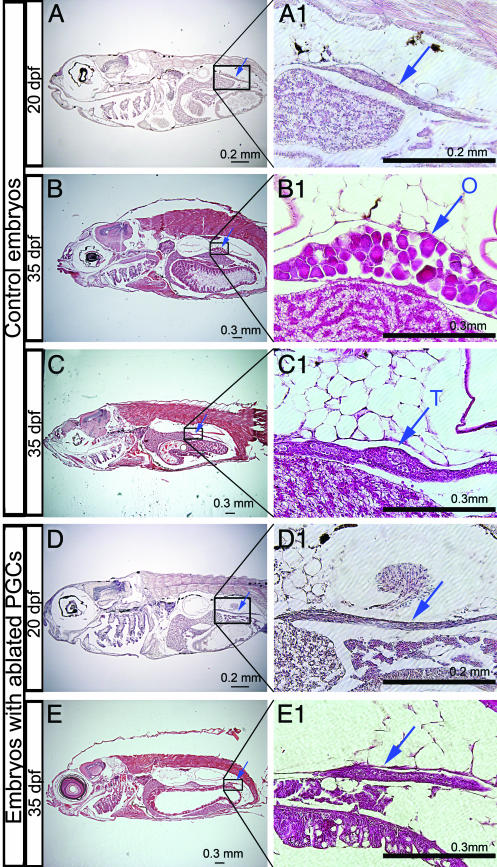
Germ Cells Are Important for Gonad Survival. To investigate the role of germ cells in gonad development, we sectioned fish derived from embryos in which the germ line had been ablated by using dnd MO. It has been shown that initially [25–35 days after fertilization (dpf)] the zebrafish gonads do not undergo morphological sex-specific differentiation and appear similar to an ovary (19). Sex differentiation in the male gonad is first manifested by massive early-oocyte death. Before this transition, we find that the gonadal tissue shows similar morphology in both control and dnd MO-treated fish (e.g., at 20 dpf; Fig. 5 A and D). However, shortly upon gonad differentiation, approximately half of the control embryos developed ovaries that were full with oocytes (35 dpf; Fig. 5B), whereas the rest developed testis (Fig. 5C). In contrast, in the experimental fish the gonadal structure appeared smaller in size and histologically uniform. Moreover, at 90 dpf, no gonad-like structures were observed in dnd MO-injected fish, suggesting that the gonads degenerated in the absence of PGCs. Hence, our results imply that in zebrafish germ cells are not required for the formation of the gonad but, rather, are essential for the differentiation and survival of this organ.
Fig. 5.
The formation of the zebrafish gonads does not depend on colonization by PGCs. (A–C) Hematoxylin–eosin-stained paraffin sections of 20- and 35-dpf fish derived from embryos injected with control MO. (A1–C1) Magnifications of the boxed regions of the gonad in A–C. Blue arrows indicate the gonads labeled as testis (T), ovary (O), or undifferentiated (left blank). (D and E) Sections of 20- and 35-dpf fish injected with 200 pg of dnd MO. Arrows indicate gonadal tissue, which is detected at the correct position (D1 and E1).
The Role of the Gonad in Sex Hormone Regulation. The analysis of fish lacking the germ line demonstrated a critical role for this lineage in female sexual differentiation. A compelling hypothesis would be that the function of the germ line in this context is to support the survival of the gonad that, in turn, is important for controlling the levels of the sex hormones testosterone and estrogen.
To examine this option, we have generated germ-line-depleted fish by knocking down the function of dnd and treated these fish with estrogen, thus supplementing the sex hormone that presumably is normally generated in the female gonad. Indeed, we could bypass the requirement for the gonad for female development by providing the germ-line-ablated fish with estrogen; 12 of 18 fish treated in this manner developed into adult fish that appear phenotypically females despite the lack of gonadal structures (the sex of the other six fish could not be determined). In addition to the female morphological characteristics, the experimental fish were unable to induce WT females to lay eggs. Specifically, none of the phenotypically female estrogen-treated fish were able to induce egg lay, as compared with 13 of 16 germ-line-ablated control (not treated with the hormone) fish.
Discussion
In this study, we examined the role of the germ line in the development of somatic tissues in zebrafish. We used two independent methods to ablate the germ cells, and both methods resulted in the generation of sterile adults. Remarkably, all of these sterile adults developed as males, as judged by morphological and behavioral criteria. Therefore, we conclude that the germ line is essential for the development of female zebrafish but is dispensable for the development of male somatic tissues with the exception of the gonad.
Targeted cell ablation is commonly used as a tool for studying the role of a particular cell line in the multicellular context of the entire organism. Genetic methods for expressing toxic molecules under the control of tissue specific promoters allow high specificity of cell targeting (33, 34). Nevertheless, in many cases, as shown in our experiments with DT, even minute amounts of the toxin presented outside of the targeted cells can compromise the viability of the organism. Obstacles of a similar nature often prevent the use of targeted cell ablation for medical purposes, such as in cancer treatment. Our results clearly show that toxin–antitoxin pairs such as the prokaryotic Kid and Kis proteins can be successfully used to refine the specificity of the toxic action to a well defined cell population, the PGCs in this case. These findings demonstrate that these proteins can be applied for highly specific ablation of targeted eukaryotic cells, and they support the general idea that they could become invaluable tools in developmental studies and anticancer therapies (24).
Sex determination in zebrafish is affected by environmental factors and exogenous hormonal treatments (8). Also, removal of the germ cells acts as a sexual modulator promoting male development. It could be hypothesized that environmental cues and lack of germ cells operate through a common mechanism namely, the endocrine system. Thus, the crosstalk among the germ line, somatic gonadal tissue, and additional somatic tissues that are relevant for sex differentiation could be influenced by environmental factors.
Molecules that are likely to participate in such signaling are sex-determining hormones, such as testosterone or estrogen (8). A key enzyme controlling the relative levels of sex hormones, cytochrome P450, catalyzes the transition of testosterone into estrogen (35). Inhibition of the biochemical activity of cytochrome P450 by using a nonsteroidal aromatase inhibitor, fadrozole, promotes male development, conceivably because of its effect on the hormonal balance of the organism (36–38). Consistent with the critical role of the ovary in zebrafish feminization, this enzyme is expressed at higher levels in the ovary than in the testes (38). Furthermore, the observed elevated expression in the follicles surrounding maturing oocytes (38) hints to a potential crosstalk between the germ cells, particularly the oocytes, and this key regulator of the hormonal balance in zebrafish. In our experiments, the absence of germ-line cells resulted in lack of gonadal tissue, presumably decreasing the conversion of testosterone into estrogen. Consistent with this suggestion, treating fish that lack the gonad with estrogen resulted in the production of adult fish exhibiting female characteristics. Thus, once female gonadal tissue is determined through primary signals (currently unknown), this tissue could be responsible for specifying and maintaining the feminized fate of somatic tissue by converting testosterone into estrogen. Whereas the germ line has an essential role in female somatic development, it is dispensable for the male development and behavior. Therefore, with respect to the role of the germ line, male development represents the “default state,” which is then modified in female fish. Apart from its role in female development, irrespective of the gender, zebrafish germ cells are crucial for the survival of the gonads, but not for their formation. Therefore, integrity of the somatic tissue in the gonad of the fish depends on the germ line. This result differs from findings in mammals where in the absence of germ cells the gonads are present albeit in a smaller size (21).
Our findings are of practical importance for fish ecology. First, chemicals in the environment affecting germ cell development and migration in fish (e.g., ref. 39) constitute a severe threat for the fish populations. The alteration of the sex ratio amplified by the fact that sterile males are able to mate with WT females, thereby producing unfertilized eggs, would lead to a rapid decrease in population size. Second, when required, the kid/kis method for ablating the germ line could be used for generating genetically modified fish whose fertility could be controlled by induction of Kid toxicity. Such a system could be valuable in preventing the contamination of WT fish populations with genetically modified fish, which represents a major concern hampering the use of genetically modified fish in the aquaculture (40).
Supplementary Material
Acknowledgments
We thank members of the E.R. laboratory for discussions, Daniel Gillet for constructs, Bruce Draper and Cecilia Moens for communicating results before publication, and Michal Reichman-Fried and Karin Dumstrei for critical comments on the manuscript. This work was supported by grants from the Deutsche Forschungsgemeinschaft, the Volkswagen-Stiftung (to E.R.), and the Swiss National Science Foundation (to J.S.).
Author contributions: K.S., J.S., and E.R. designed research; K.S. and J.S. performed research; K.S. and E.R. analyzed data; G.d.l.C.-M. contributed new reagents/analytic tools; and K.S. and E.R. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: PGC, primordial germ cell; dnd, dead end; nos1, nanos1; glo, globin; MO, morpholino oligonucleotide; DT, Diphtheria toxin; hpf, hours after fertilization; dpf, days after fertilization.
References
- 1.Cline, T. W. & Meyer, B. J. (1996) Annu. Rev. Genet. 30, 637–702. [DOI] [PubMed] [Google Scholar]
- 2.Swain, A. & Lovell-Badge, R. (1999) Genes Dev. 13, 755–767. [DOI] [PubMed] [Google Scholar]
- 3.Capel, B. (2000) Mech. Dev. 92, 89–103. [DOI] [PubMed] [Google Scholar]
- 4.Smith, C. A. & Sinclair, A. H. (2004) BioEssays 26, 120–132. [DOI] [PubMed] [Google Scholar]
- 5.Western, P. S. & Sinclair, A. H. (2001) J. Exp. Zool. 290, 624–631. [DOI] [PubMed] [Google Scholar]
- 6.Matsuda, M., Nagahama, Y., Shinomiya, A., Sato, T., Matsuda, C., Kobayashi, T., Morrey, C. E., Shibata, N., Asakawa, S., Shimizu, N., et al. (2002) Nature 417, 559–563. [DOI] [PubMed] [Google Scholar]
- 7.Volff, J. N. & Schartl, M. (2001) Genetica 111, 101–110. [DOI] [PubMed] [Google Scholar]
- 8.Baroiller, J. F. & D'Cotta, H. (2001) Comp. Biochem. Physiol. C Toxicol. Pharmacol. 130, 399–409. [DOI] [PubMed] [Google Scholar]
- 9.Lee, Y. H., Du, J. L., Yueh, W. S., Lin, B. Y., Huang, J. D., Lee, C. Y., Lee, M. F., Lau, E. L., Lee, F. Y., Morrey, C., et al. (2001) J. Exp. Zool. 290, 715–726. [DOI] [PubMed] [Google Scholar]
- 10.Traut, W. & Winking, H. (2001) Chromosome Res. 9, 659–672. [DOI] [PubMed] [Google Scholar]
- 11.Seydoux, G. & Schedl, T. (2001) Int. Rev. Cytol. 203, 139–185. [DOI] [PubMed] [Google Scholar]
- 12.Williamson, A. & Lehmann, R. (1996) Annu. Rev. Cell Dev. Biol. 12, 365–391. [DOI] [PubMed] [Google Scholar]
- 13.Wylie, C. (1999) Cell 96, 165–174. [DOI] [PubMed] [Google Scholar]
- 14.McLaren, A. (1995) Philos. Trans. R. Soc. London B 350, 229–233. [DOI] [PubMed] [Google Scholar]
- 15.Schutt, C. & Nothiger, R. (2000) Development (Cambridge, U.K.) 127, 667–677. [DOI] [PubMed] [Google Scholar]
- 16.Patino, R., Davis, K., Schoore, J. E., Uguz, C., Strussmann, C. A., Parker, N. C., Simco, B. A. & Gousie, C. A. (1996) J. Exp. Zool. 276, 209–218. [Google Scholar]
- 17.Tanaka, M., Kinoshita, M., Kobayashi, D. & Nagahama, Y. (2001) Proc. Natl. Acad. Sci. USA 98, 2544–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura, M. (1984) Aquaculture 43, 83–90. [Google Scholar]
- 19.Uchida, D., Yamashita, M., Kitano, T. & Iguchi, T. (2002) J. Exp. Biol. 205, 711–718. [DOI] [PubMed] [Google Scholar]
- 20.Jongens, T. A., Hay, B., Jan, L. Y. & Jan, Y. N. (1992) Cell 70, 569–584. [DOI] [PubMed] [Google Scholar]
- 21.Beck, A. R., Miller, I. J., Anderson, P. & Streuli, M. (1998) Proc. Natl. Acad. Sci. USA 95, 2331–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liger, D., vanderSpek, J. C., Gaillard, C., Cansier, C., Murphy, J. R., Leboulch, P. & Gillet, D. (1997) FEBS Lett. 406, 157–161. [DOI] [PubMed] [Google Scholar]
- 23.Küprunner, M., Thisse, C., Thisse, B. & Raz, E. (2001) Genes Dev. 15, 2877–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de la Cueva-Méndez, G., Mills, A. D., Clay-Farrace, L., Diaz-Orejas, R. & Laskey, R. A. (2003) EMBO J. 22, 246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jowett, T. & Lettice, L. (1994) Trends Genet. 10, 73–74. [DOI] [PubMed] [Google Scholar]
- 26.Hauptmann, G. & Gerster, T. (1994) Trends Genet. 10, 266. [DOI] [PubMed] [Google Scholar]
- 27.Westerfield, M. (1995) The Zebrafish Book (Univ. of Oregon Press, Eugene).
- 28.Andersen, L., Holbech, H., Gessbo, A., Norrgren, L. & Petersen, G. I. (2003) Comp. Biochem. Physiol. C Comp. Pharmacol. 134, 365–374. [DOI] [PubMed] [Google Scholar]
- 29.Weidinger, G., Stebler, J., Slanchev, K., Dumstrei, K., Wise, C., Lovell-Badge, R., Thisse, C., Thisse, B. & Raz, E. (2003) Curr. Biol. 13, 1429–1434. [DOI] [PubMed] [Google Scholar]
- 30.Ciruna, B., Weidinger, G., Knaut, H., Thisse, B., Thisse, C., Raz, E. & Schier, A. F. (2002) Proc. Natl. Acad. Sci. USA 99, 14919–14924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lord, J. M., Smith, D. C. & Roberts, L. M. (1999) Cell. Microbiol. 1, 85–91. [DOI] [PubMed] [Google Scholar]
- 32.Ruiz-Echevarria, M. J., Berzal-Herranz, A., Gerdes, K. & Diaz-Orejas, R. (1991) Mol. Microbiol. 5, 2685–2693. [DOI] [PubMed] [Google Scholar]
- 33.Arase, K., Saijo, K., Watanabe, H., Konno, A., Arase, H. & Saito, T. (1999) Proc. Natl. Acad. Sci. USA 96, 9264–9268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roman, G., Endo, K., Zong, L. & Davis, R. L. (2001) Proc. Natl. Acad. Sci. USA 98, 12602–12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Callard, G. V., Tchoudakova, A. V., Kishida, M. & Wood, E. (2001) J. Steroid Biochem. Mol. Biol. 79, 305–314. [DOI] [PubMed] [Google Scholar]
- 36.Uchida, D., Yamashita, M., Kitano, T. & Iguchi, T. (2004) Comp. Biochem. Physiol. A Mol. Integr. Physiol. 137, 11–20. [DOI] [PubMed] [Google Scholar]
- 37.Fenske, M. & Segner, H. (2004) Aquat. Toxicol. 67, 105–126. [DOI] [PubMed] [Google Scholar]
- 38.Chiang, E. F., Yan, Y. L., Guiguen, Y., Postlethwait, J. & Chung, B. (2001) Mol. Biol. Evol. 18, 542–550. [DOI] [PubMed] [Google Scholar]
- 39.Thorpe, J. L., Doitsidou, M., Ho, S.-Y., Raz, E. & Farber, S. A. (2004) Dev. Cell 6, 295–302. [DOI] [PubMed] [Google Scholar]
- 40.Stokstad, E. (2002) Science 297, 1797–1799. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.