Abstract
The central administration of the fatty acid synthase (FAS) inhibitor, C75, rapidly suppresses the expression of orexigenic neuropeptides [neuropeptide Y (NPY) and agouti-related protein (AgRP)] and activates expression of anorexigenic neuropeptides [proopiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART)] in the hypothalamus. The combined actions of these changes inhibit food intake and decrease body weight. Intracerebroventricular injection of C75 appears to rapidly inhibit the secretion of ghrelin by hypothalamic explants ex vivo and by the stomach in vivo. Ghrelin administered intracerebroventricularly reverses the anorexic effect of C75, suggesting that C75 acts upstream of ghrelin. Because ghrelin-producing neurons are known to form synapses onto NPY/AgRP neurons, we suggest that the reversal of C75-induced anorexia by ghrelin may be mediated by NPY/AgRP neurons. This hypothesis is supported by the finding that ghrelin reverses the C75-induced inactivation (assessed by c-Fos expression) of neurons in the arcuate nucleus that express NPY (assessed by immunohistochemical costaining). These effects closely correlate with appropriate changes downstream in the expression of the hypothalamic neuropeptides that regulate feeding behavior, i.e., down-regulation of the expression of NPY and AgRP and up-regulation of the expression of proopiomelanocortin/α-melanocyte-stimulating hormone, provoked by C75 and reversed by ghrelin. We propose a model in which ghrelin secretion plays an intermediary role between malonyl-CoA, the substrate of fatty acid synthase, and the neural circuitry regulating energy homeostasis.
Keywords: c-Fos, hypothalamus, malonyl-CoA, neuropeptide Y
Ghrelin is a small peptide that binds to the growth hormone (GH) secretagogue receptor (GHS-R) in the pituitary and stimulates GH secretion (1). In addition to its role as a secretagogue, ghrelin when administered peripherally or centrally stimulates food intake and increases body weight gain (2–5). The orexigenic action of ghrelin is independent of its role in promoting the secretion of GH (2, 6, 7). Ghrelin contains 28 aa and undergoes a covalent modification by which an octanoyl group becomes covalently attached to Ser-3 (8). This modification is essential both for its orexigenic activity and for GH release.
Ghrelin is expressed and secreted into the circulatory system by a subset of endocrine cells in the oxyntic glands of the stomach where it increases gastric motility and acid secretion (9). Secretion of ghrelin as well as other peptide hormones produced by the stomach and small intestine [e.g., cholecystokinin (CCK) and peptide YY (PYY)] respond to mechanical or nutrient stimuli (10). However, CCK and PYY are anorexic, their secretion being increased by such stimuli, whereas ghrelin is orexigenic, its secretion being suppressed by such stimuli (11). These hormones seem to bind to their cognate receptors on afferent nerves of the vagal system within the gastrointestinal tract. In addition, however, PYY and ghrelin can reach the hypothalamus via the circulatory system. Receptors for both of these peptides are expressed by neurons of the vagal system and the hypothalamus (10, 12).
Ghrelin is also expressed in the hypothalamus (3), which is the major site of its orexigenic action (13, 14). Ghrelin-producing neurons in the hypothalamus are located within and adjacent to the arcuate nucleus (Arc) and interact with neuropeptide Y (NPY)/agouti-related protein (AgRP) neurons through GH secretagogue receptors (GHS-Rs) (13, 15). The fact that the orexigenic action of ghrelin is mediated by NPY and/or AgRP is supported by the finding that intracerebroventricularly (i.c.v.) administered ghrelin rapidly increases the expression of NPY and AgRP mRNA in the Arc (16). Although disruption of either the NPY or AgRP gene produces only a modest effect, disruption of both genes abolishes the orexigenic effect of ghrelin (4, 5). Thus, it appears that ghrelin action depends on functional NPY/AgRP neurons (17). Because NPY/AgRP neurons impinge on proopiomelanocortin (POMC)/α-melanocyte-stimulating hormone (αMSH)-producing neurons (5, 18), the melanocortin pathway also appears to be affected by ghrelin, albeit indirectly (17).
C75, a potent inhibitor of fatty acid synthase (FAS), has an anorexic effect when administered to mice peripherally or centrally (19–21). When administered by i.c.v. injection, C75 rapidly (in 2 h or less) represses expression of the mRNAs encoding the orexigenic neuropeptides NPY and AgRP and activates expression of the anorexic neuropeptide mRNAs that encode POMC/α-melanocyte-stimulating hormone (αMSH) and cocaine- and amphetamine-regulated transcript (CART). These effects of C75 are reversed by centrally administered NPY (19, 21), which indicates that NPY acts downstream of C75. Here we provide evidence that centrally administered C75 rapidly blocks the secretion of ghrelin from hypothalamic explants and from the stomach and that the effects of C75 on ghrelin secretion and food intake are mediated through NPY/AgRP neurons.
Materials and Methods
Handling of Mice, i.c.v. Injections, Immunohistochemical Staining, and Neuropeptide mRNA Analyses. Animal experiments were performed in accordance with guidelines of The Johns Hopkins University School of Medicine Institutional Animal Care and Use Committee. Male BALB/c mice (20–25 g) from Charles River Laboratories were acclimated for 1 week to a 12-h light (from 0130 to 1330 hours)/12-h dark (from 1330 to 0130 hours) cycle at 22°C and were fed standard laboratory chow ad libitum. The basic protocol for C75 and/or ghrelin treatment included a 23- or 24-h fast beginning at the start of the dark cycle, then i.c.v. injection of C75 [10 μg in RPMI 1640 medium/vehicle (Invitrogen, Carlsbad, CA)] or vehicle, followed by i.c.v. injection (where indicated) of ghrelin (0.2 μg) or vehicle. i.c.v. injections into the lateral ventricle of mice anesthetized with isoflurane were performed as previously described (21). After i.c.v. injection(s) either the mice were given free access to food (refed) or fasting was continued. Where indicated, food intake was then measured over the next 0.5–2 h. Hypothalami were removed for analysis within 30–60 sec of euthanasia. For analysis of total, active octanoyl-, and des-octanoyl-ghrelin levels, tissue or plasma was prepared as described (22) and subjected to RIAs according to the manufacturer's (Phoenix Pharmaceuticals, Belmont, CA) instructions. In some cases octanoyl- and des-octanoyl-ghrelin were separated by HPLC (22) and then subjected to SDS/PAGE and immunoblotting with specific antibody to total ghrelin (Phoenix Pharmaceuticals). Neuropeptide mRNA measurements were conducted by real-time PCR with appropriate neuropeptide primers as described (23). Immunohistochemical staining with antibodies specific for c-Fos (Oncogene, Cambridge, MA) and NPY (Immunostar, Hudson, WI) was performed on brain sections of hypothalami from anesthetized mice that had been subjected to transcardial perfusion as previously described (24).
Hypothalamic Explants. After removal of the brain, hypothalami (bordered by the optic chiasma, mammillary bodies, and amygdaloid sulcus) were quickly dissected and placed in cold (4°C) Krebs–Ringer bicarbonate buffer (140 mM NaCl, 5 mM KCl, 3.7 mM CaCl2·2H2O, 0.88 mM MgSO4·7H2O, 25 mM NaHCO3, and 5 mM glucose, pH 7.4) previously gassed with a 95% O2/5% CO2 mixture. Sets of two hypothalami were rinsed three times in 0.5 ml of cold (4°C) buffer and transferred to 0.5 ml of buffer prewarmed to 37°C in Krebs–Ringer bicarbonate buffer in 4-ml scintillation tubes for a preincubation period of 30 min. After removal of the preincubation medium, the explants were incubated for 30 min in 0.5 ml of fresh Krebs–Ringer bicarbonate buffer (37°C). Incubations were conducted in the Dubnoff metabolic shaker (50 cycles per min, under 95% O2/5% CO2). At the end of the experiment hypothalami were homogenized in cold Krebs–Ringer bicarbonate buffer, centrifuged at 16,000 × g for 5 min. The ghrelin content of the explants and ghrelin secreted into the medium were determined by RIA (Phoenix Pharmaceuticals and Linco Research, St. Charles, MO).
Results
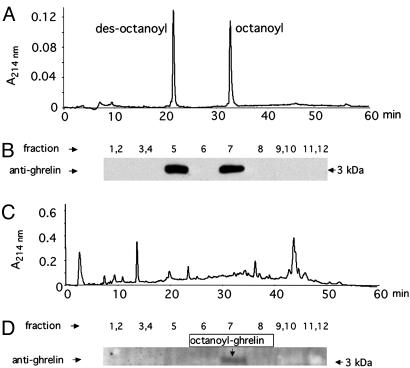
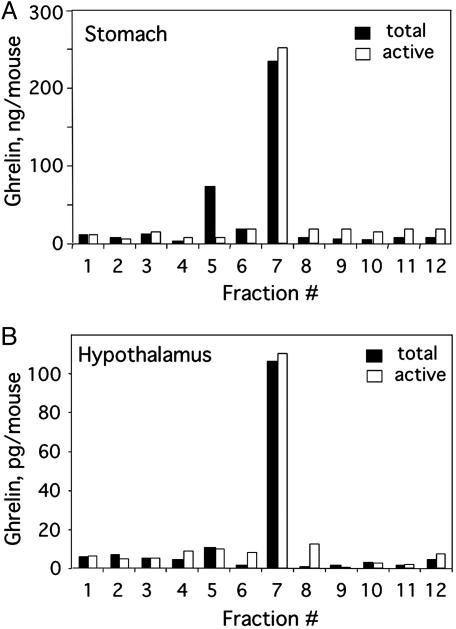
Analysis of Octanoyl- and Des-Octanoyl-Ghrelin in the Hypothalamus and Stomach. Two forms of ghrelin, octanoyl- and des-octanoylghrelin, are expressed both by the central nervous system (CNS) and the stomach of mice (3, 22). Octanoylation is essential for biological activity of ghrelin in the CNS (8, 25). Preliminary to quantifying tissue ghrelin, it was shown that the two forms can be resolved by HPLC (Fig. 1A), the separation being verified by SDS/PAGE and immunoblotting with anti-ghrelin antibody (Fig. 1B). Using this procedure, we determined that the predominant form in stomach tissue is the octanoylated form of ghrelin (Fig. 1 C and D). Because of the much lower level in hypothalamic tissue, ghrelin could not be detected by immunoblotting (data not shown). Therefore, a RIA was used to detect total and active octanoyl-ghrelin in conjunction with HPLC separation. As shown in Fig. 2, octanoyl-ghrelin immunoreactivity (IR) accounts for ≈90% of the ghrelin IR in hypothalamus tissue and 77% of total ghrelin IR in stomach tissue. This analysis showed that the ghrelin level in hypothalamic tissue is much lower than that in stomach tissue. Despite this low level, recent evidence indicates that hypothalamic ghrelin is a principal contributor to the regulation of feeding behavior (4, 5).
Fig. 1.
Separation and analysis of octanoyl- and des-octanoyl-ghrelin. (A) Pure octanoyl- and des-octanoyl-ghrelin were separated by HPLC on a C18 reverse-phase column by using a linear gradient. (B) Pooled or individual fractions from the HPLC run were subjected to SDS/PAGE (15% acrylamide), after which the gels were immunoblotted with antibody (Phoenix) that recognized both forms of ghrelin. (C) Washed stomach tissue from mice was extracted and separated by HPLC. (D) Pooled or individual fractions were subjected to SDS/PAGE and then immunoblotted with antibody as in B.
Fig. 2.
Distribution of octanoyl- and des-octanoyl-ghrelin in the stomach and hypothalamus. Stomach (A) and hypothalamic (B) tissue was extracted and subjected to HPLC to separate octanoyl- and des-octanoyl-ghrelin. HPLC fractions were assayed for total and active octanoyl-ghrelin by RIA.
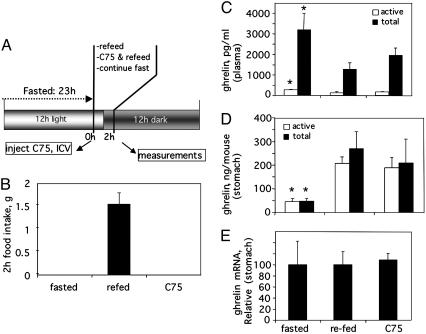
Central Administration of C75 Decreases Blood Ghrelin While Increasing Stomach Ghrelin. To assess the short-term effect of centrally administered C75 on blood and stomach ghrelin levels, C75 was administered by i.c.v. injection to mice that had been fasted for 23 h to ensure a robust appetite. The mice were then refed ad libitum or fasting was continued. The treatment protocol is summarized in Fig. 3A. Over the next 2 h food intake of the refed animals was substantial, whereas food intake of C75-treated mice was totally suppressed (Fig. 3B). Within 2 h of refeeding or C75 injection, plasma levels of ghrelin (total and octanoylated) decreased by ≈60% and ≈40%, respectively (Fig. 3C), despite the fact that C75-treated mice were in a fasted state. Consistent with the effect on secretion, C75 treatment or refeeding increased the level of ghrelin in stomach tissue (Fig. 3D). Regardless of the treatment imposed, most of the plasma ghrelin was in the des-octanoyl form, whereas most of that in the stomach tissue was octanoylated, suggesting that once octanoyl-ghrelin enters the blood deoctanoylation occurs rapidly. Because the stomach is the source of plasma ghrelin (10) and because ghrelin mRNA expression (Fig. 3E) is unaffected by C75, it seems that the C75-induced increase in tissue ghrelin was the result of a blockade of its secretion. The changes in tissue and plasma ghrelin caused by centrally administered C75 were independent of food intake, because they occurred despite a complete suppression of food intake (Fig. 3).
Fig. 3.
Effect of centrally administered C75 on blood and stomach ghrelin levels. (A) Treatment and analysis protocol (i.c.v., 10 μg of C75). (B) Food intake. The tissue content of total and active octanoyl-ghrelin and ghrelin mRNA were determined 2 h after C75 injection. (C) Ghrelin levels in plasma. (D) Ghrelin protein in stomach tissue. (E) Ghrelin mRNA level in stomach tissue. *, P < 0.01 vs. refed or C75-treated.
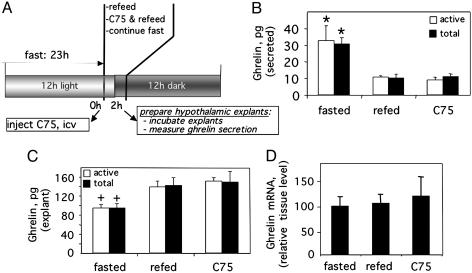
Centrally Administered C75 Blocks Secretion of Ghrelin by Hypothalamic Explants ex Vivo. To determine whether C75 blocks the secretion of ghrelin from hypothalamic neurons, which seems to be the case for the stomach, ex vivo experiments were conducted with hypothalamic explants from mice that had been given C75 by i.c.v. injection. Ghrelin secretion by hypothalamic explants from fasted donor mice was ≈3-fold greater (≈30 vs. 10 pg) than by explants from refed mice (Fig. 4B). C75, however, totally blocked the fasting-induced increase of ghrelin secretion even though C75-treated donor mice were in a fasted state. Consistent with the effect of C75 on secretion, the decrease of ghrelin in the hypothalamic explants (Fig. 4C) correlated closely with the increase of ghrelin secreted into the medium. There was no change in the expression of ghrelin during the course of the experiment as indicated by the constancy of ghrelin mRNA (Fig. 4D). Taken together these findings indicate that C75 blocks ghrelin secretion.
Fig. 4.
Effect of centrally administered C75 on the secretion of ghrelin by hypothalamic explants. (A) Treatment and analysis protocol. Mice were treated as described in the legend for Fig. 3, after which hypothalamic explants were prepared and incubated as described in Materials and Methods.(B) Amount of ghrelin secreted into the medium during the incubation. (C) Ghrelin content of explants at the end of incubation. (D) Relative ghrelin mRNA content of the hypothalamic tissue before incubation of the explants. *, P < 0.01 vs. refed or C75-treated; +, P < 0.05 vs. refed or C75-treated.
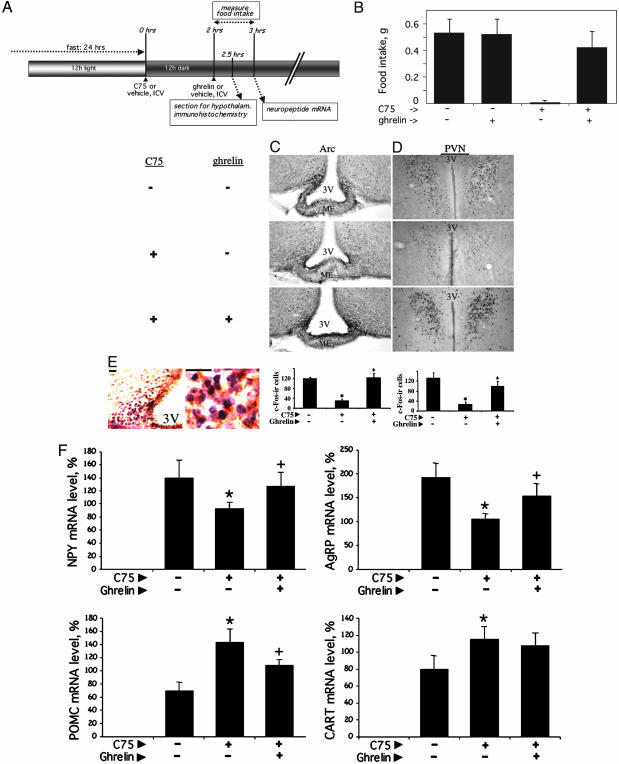
Centrally Administered Ghrelin Reverses the Effect of C75 on Food Intake and Hypothalamic Neuronal c-Fos and Neuropeptide mRNA Expression. The studies described above raised the question of whether inhibition of ghrelin secretion by C75 is responsible for its inhibition of food intake. Were this the case, ghrelin should reverse, i.e., bypass, downstream signaling events and restore food intake. Using the protocol illustrated in Fig. 5A, we found centrally administered ghrelin to almost completely reverse the anorexic effect of C75 (Fig. 5B). Ghrelin itself had no effect on food intake of control animals that had been fasted and then refed (Fig. 5A). These findings suggest that the anorexic effect of C75 depends on ghrelin action. Previous studies (19) showed that the central administration of NPY can also reverse the anorexic effect of C75.
Fig. 5.
Effect of central administration of ghrelin on C75-induced changes in food intake and hypothalamic neuronal activity and neuropeptide mRNA expression. (A) Treatment and analysis protocol. The preliminary treatment of the mice was similar to that described in the legend for Fig. 3. (B) Food intake was measured 2–3 h after the i.c.v. administration of 10 μg of C75, i.e., 1 h after i.c.v. administration of 0.2 μg of ghrelin. (C and D) Immunostaining and quantitation of c-Fos in the Arc and PVN. (Magnification: ×10 objective). 3V, third ventricle; ME, median eminence. Bar graphs show the quantification of c-Fos staining (n = 4) in the Arc and PVN. Values are the mean ± SEM. Differences between treatment groups in each region were assessed by Student's t test. *, P < 0.01 vs. none; +, P < 0.01 vs. C75. (E) Brain sections in the region of Arc in the hypothalamus and nuclear c-Fos [3,3′-diaminobenzidine (DAB) plus nickel, shown in purple] and cytoplasmic NPY (DAB, shown in brown) in the Arc. (Scale bars, 20 μm.) (F) Expression of neuropeptide mRNAs in the hypothalamus. *, P < 0.01 vs. none; +, P < 0.05 vs. C75.
To identify the hypothalamic nuclei responsible for the effects of C75 and ghrelin, brain sections from mice subjected to the treatment protocol (Fig. 5A) were immunostained for c-Fos, an indicator of neuronal activation. As shown in Fig. 5, C75 rapidly (in 2.5 h or less) blocked the fasting-induced expression of c-Fos, both in the Arc (Fig. 5C) and the paraventricular nucleus (PVN) (Fig. 5D). Expression of c-Fos in the PVN is most likely due to signaling from the Arc, because the PVN is richly supplied by axons projecting from the Arc (18). Consistent with the finding that ghrelin reverses the reduction of food intake caused by C75 (Fig. 5B), ghrelin also reverses the reduction of c-Fos staining of neurons in the Arc and PVN caused by C75 (Fig. 5 C and D). The c-Fos-staining neurons in the Arc responsible for these effects seem to be primarily NPY/AgRP neurons, as evidenced by the fact that c-Fos and NPY antibodies costain the same subset of neurons in the Arc (Fig. 5E). The fact that NPY/AgRP neurons are affected by ghrelin treatment is consistent with the fact, as discussed above, that ghrelin-producing neurons interact with NPY/AgRP neurons (15).
Experiments were also conducted to determine the effect of centrally administered ghrelin on C75-induced changes in the expression of the orexigenic and anorexigenic neuropeptide mRNAs in the hypothalamus. As previously reported (21, 26), C75 reduces the expression of the orexigenic neuropeptide (NPY and AgRP) mRNAs and increases the expression of the anorexigenic neuropeptide (POMC and CART) mRNAs in the hypothalami of fasted mice (Fig. 5F). Centrally administered ghrelin rapidly reversed the C75-induced effects on hypothalamic neuropeptide expression (Fig. 5F) as it did the C75-induced effects on food intake (Fig. 5B) and activation of neurons in the Arc and PVN (as shown by c-Fos expression) (Fig. 5 C and D).
Discussion
The present investigation shows that the effects of C75 administered i.c.v. are not only felt locally within the hypothalamus, e.g., by the Arc, but are also rapidly(in 2 h or less) transmitted to peripheral tissues, including the stomach, where ghrelin secretion is affected (Fig. 3). Recent studies in this laboratory (S.H.C. and M.D.L., unpublished results) show that the C75 signal is also rapidly transmitted peripherally to skeletal muscle, where energy metabolism is affected. Thus, when administered centrally, C75 triggers changes in neuropeptide expression in the hypothalamus, the effects of which are rapidly transmitted, probably via the sympathetic nervous system (SNS), to peripheral tissues. In the case of skeletal muscle, C75 administered i.c.v. rapidly up-regulates expression of UCP3 and PGC1α, a transcriptional coactivator for the peroxisome proliferator-activated receptor γ (PPARγ) and the UCP3 gene. These events correlate closely with the activation of whole-body fatty acid oxidation (S.H.C. and M.D.L., unpublished results).
The central administration of C75, an inhibitor of fatty acid synthase (FAS), provokes an immediate increase in the concentration of its substrate, malonyl-CoA, in the hypothalamus concomitant with reciprocal changes in the expression of orexigenic and anorexigenic neuropeptides that cause suppression of food intake (21). Conversely, inhibition of acetyl-CoA carboxylase (ACC), which catalyzes the formation of malonyl-CoA, reverses the effects of C75 (19, 21). These and other indirect lines of evidence (27–29) have implicated malonyl-CoA as a participant in the energy-sensing system that mediates changes in the expression of these hypothalamic neuropeptides, notably the down-regulation of the orexigens NPY and AgRP and the up-regulation of the anorexigens POMC and α-melanocyte-stimulating hormone (αMSH). The present investigation adds ghrelin, an orexigenic neuropeptide, to the list of neuropeptides affected by C75 and changes in the energy status. Central administration of C75, rather than affecting the expression of ghrelin, however, seems to suppress the fasting-induced secretion of ghrelin, both in the hypothalamus (Fig. 4) and peripherally in the stomach (Fig. 3). Thus, i.c.v. injection of C75 into fasted mice inhibited ghrelin secretion by hypothalamic explants prepared from C75-treated animals. We suggest that ghrelin plays an intermediary role between malonyl-CoA and the circuitry that affects the expression of these neuropeptides.
Recent studies by Cowley et al. (15) showed that axons of ghrelin-producing neurons are disposed proximally to NPY axons in the Arc, their efferents making synaptic contacts with NPY/AgRP neurons. It was found that ghrelin binds primarily to the presynaptic terminals of these neurons. Presumably, ghrelin binds to GH secretagogue receptors (GHS-Rs) to increase the activity of such terminals of NPY axons to increase the secretion of GABA and possibly of NPY and AgRP. This altered GABA and neuropeptide release would most likely alter the activity of POMC neurons and the anorexic effects of POMC. Such synaptic GABAergic contacts with NPY-containing nerve terminals would seem to be sufficient for the modulation of the activity of postsynaptic POMC neurons. We suggest that at least part of the anorexic effect of C75 may be elicited through this type of circuitry. Centrally administered C75 rapidly inactivates NPY neurons in the Arc as indicated by the rapid suppression of c-Fos expression in these neurons (which costain with NPY) (Fig. 5 C–E). Furthermore, these events correlate closely with the onset of appropriate changes in the expression of the downstream orexigenic and anorexigenic neuropeptide mRNAs, notably those encoding NPY, AgRP, and POMC (Fig. 5F).
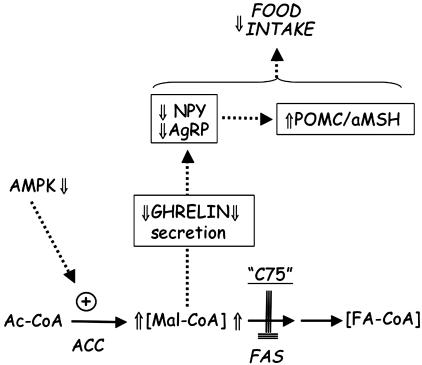
Recent studies (27–29) have also shed light on the upstream events that link changes in energy status to hypothalamic malonyl-CoA concentration. The activity of ACC, which catalyzes malonyl-CoA formation and regulates fatty acid synthesis (30–32), increases in the fed state (particularly a carbohydrate meal) and decreases in the fasting state (33). These fluctuations in energy status produce corresponding changes in the hypothalamic malonyl-CoA level (21). ACC and, therefore, malonyl-CoA seem to function as intermediaries in the AMP kinase energy-sensing system (reviewed in ref. 34). Thus, an increase in energy status, such as that which occurs upon feeding, increases blood glucose and insulin levels and decreases [AMP]/[ATP], which inactivates AMP kinase. Because ACC is a substrate of AMP kinase, this cascade promotes dephosphorylation and, thereby, activation of ACC and an increased [malonyl-CoA] (21, 27, 29). Elevated hypothalamic [malonyl-CoA] caused either by such physiological conditions or by pharmacological treatment with C75 seems to alter the secretion of ghrelin and expression of hypothalamic neuropeptides that produce anorexia (21). A model that brings together the upstream and downstream events described above is shown in Fig. 6
Fig. 6.
Possible events leading to decreased secretion of ghrelin and suppression food intake. AMPK, AMP kinase; Ac-CoA, acetyl-CoA; FA-CoA, fatty acetyl-CoA; Mal-CoA, malonyl-CoA.
Acknowledgments
We thank Dr. M. Molliver (Department of Neuroscience, The Johns Hopkins University School of Medicine) for technical advice. This work was supported by the Yamanouchi Pharmaceutical Co., Ltd.
Author contributions: Z.H. and M.D.L. designed research; Z.H., S.H.C., G.v.H., and J.W. performed research; Z.H., S.H.C., G.v.H., and M.D.L. analyzed data; and Z.H. wrote the paper.
Abbreviations: GH, growth hormone; Arc, arcuate nucleus; NPY, neuropeptide Y; AgRP, agouti-related protein; i.c.v., intracerebroventricular(ly); POMC, proopiomelanocortin; PVN, paraventricular nucleus; ACC, acetyl-CoA carboxylase.
References
- 1.Kojima, M., Hosoda, H., Date, Y., Nakazato, M., Matsuo, H. & Kangawa, K. (1999) Nature 402, 656–660. [DOI] [PubMed] [Google Scholar]
- 2.Tschop, M., Smiley, D. L. & Heiman, M. L. (2000) Nature 407, 908–913. [DOI] [PubMed] [Google Scholar]
- 3.Nakazato, M., Murakami, N., Date, Y., Kojima, M., Matsuo, H., Kangawa, K. & Matsukura, S. (2001) Nature 409, 194–198. [DOI] [PubMed] [Google Scholar]
- 4.Cowley, M. A. & Grove, K. L. (2004) Endocrinology 145, 2604–2606. [DOI] [PubMed] [Google Scholar]
- 5.Chen, H. Y., Trumbauer, M. E., Chen, A. S., Weingarth, D. T., Adams, J. R., Frazier, E. G., Shen, Z., Marsh, D. J., Feighner, S. D., Guan, X. M., et al. (2004) Endocrinology 145, 2607–2612. [DOI] [PubMed] [Google Scholar]
- 6.Date, Y., Nakazato, M., Murakami, N., Kojima, M., Kangawa, K. & Matsukura, S. (2001) Biochem. Biophys. Res. Commun. 280, 904–907. [DOI] [PubMed] [Google Scholar]
- 7.Broglio, F., Arvat, E., Benso, A., Gottero, C., Prodam, F., Granata, R., Papotti, M., Muccioli, G., Deghenghi, R. & Ghigo, E. (2002) Isr. Med. Assoc. J. 4, 607–613. [PubMed] [Google Scholar]
- 8.Hosoda, H., Kojima, M., Matsuo, H. & Kangawa, K. (2000) Biochem. Biophys. Res. Commun. 279, 909–913. [DOI] [PubMed] [Google Scholar]
- 9.Masuda, Y., Tanaka, T., Inomata, N., Ohnuma, N., Tanaka, S., Itoh, Z., Hosoda, H., Kojima, M. & Kangawa, K. (2000) Biochem. Biophys. Res. Commun. 276, 905–908. [DOI] [PubMed] [Google Scholar]
- 10.Konturek, S. J., Konturek, J. W., Pawlik, T. & Brzozowski, T. (2004) J. Physiol. Pharmacol. 55, 137–154. [PubMed] [Google Scholar]
- 11.Date, Y., Murakami, N., Toshinai, K., Matsukura, S., Niijima, A., Matsuo, H., Kangawa, K. & Nakazato, M. (2002) Gastroenterology 123, 1120–1128. [DOI] [PubMed] [Google Scholar]
- 12.Cummings, D. E. & Schwartz, M. W. (2003) Annu. Rev. Med. 54, 453–471. [DOI] [PubMed] [Google Scholar]
- 13.Cowley, M. A. (2003) Eur. J. Pharmacol. 480, 3–11. [DOI] [PubMed] [Google Scholar]
- 14.Ellacott, K. L. & Cone, R. D. (2004) Recent Prog. Horm. Res. 59, 395–408. [DOI] [PubMed] [Google Scholar]
- 15.Cowley, M. A., Smith, R. G., Diano, S., Tschop, M., Pronchuk, N., Grove, K. L., Strasburger, C. J., Bidlingmaier, M., Esterman, M., Heiman, M. L., et al. (2003) Neuron 37, 649–661. [DOI] [PubMed] [Google Scholar]
- 16.Kohno, D., Gao, H. Z., Muroya, S., Kikuyama, S. & Yada, T. (2003) Diabetes 52, 948–956. [DOI] [PubMed] [Google Scholar]
- 17.Chan, C. B. & Cheng, C. H. (2004) Mol. Cell. Endocrinol. 214, 81–95. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz, M. W., Woods, S. C., Porte, D., Jr., Seeley, R. J. & Baskin, D. G. (2000) Nature 404, 661–671. [DOI] [PubMed] [Google Scholar]
- 19.Loftus, T. M., Jaworsky, D. E., Frehywot, G. L., Townsend, C. A., Ronnett, G. V., Lane, M. D. & Kuhajda, F. P. (2000) Science 288, 2379–2381. [DOI] [PubMed] [Google Scholar]
- 20.Kumar, M. V., Shimokawa, T., Nagy, T. R. & Lane, M. D. (2002) Proc. Natl. Acad. Sci. USA 99, 1921–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu, Z., Cha, S. H., Chohnan, S. & Lane, M. D. (2003) Proc. Natl. Acad. Sci. USA 100, 12624–12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Date, Y., Kojima, M., Hosoda, H., Sawaguchi, A., Mondal, M. S., Suganuma, T., Matsukura, S., Kangawa, K. & Nakazato, M. (2000) Endocrinology 141, 4255–4261. [DOI] [PubMed] [Google Scholar]
- 23.Cha, S. H., Hu, Z. & Lane, M. D. (2004) Biochem. Biophys. Res. Commun. 317, 301–308. [DOI] [PubMed] [Google Scholar]
- 24.Gao, S. & Lane, M. D. (2003) Proc. Natl. Acad. Sci. USA 100, 5628–5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murakami, N., Hayashida, T., Kuroiwa, T., Nakahara, K., Ida, T., Mondal, M. S., Nakazato, M., Kojima, M. & Kangawa, K. (2002) J. Endocrinol. 174, 283–288. [DOI] [PubMed] [Google Scholar]
- 26.Shimokawa, T., Kumar, M. V. & Lane, M. D. (2002) Proc. Natl. Acad. Sci. USA 99, 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim, E. K., Miller, I., Aja, S., Landree, L. E., Pinn, M., McFadden, J., Kuhajda, F. P., Moran, T. H. & Ronnett, G. V. (2004) J. Biol. Chem. 279, 19970–19976. [DOI] [PubMed] [Google Scholar]
- 28.Landree, L. E., Hanlon, A. L., Strong, D. W., Rumbaugh, G., Miller, I. M., Thupari, J. N., Connolly, E. C., Huganir, R. L., Richardson, C., Witters, L. A., et al. (2004) J. Biol. Chem. 279, 3817–3827. [DOI] [PubMed] [Google Scholar]
- 29.Minokoshi, Y., Alquier, T., Furukawa, N., Kim, Y. B., Lee, A., Xue, B., Mu, J., Foufelle, F., Ferre, P., Birnbaum, M. J., Stuck, B. J. & Kahn, B. B. (2004) Nature 428, 569–574. [DOI] [PubMed] [Google Scholar]
- 30.Moss, J. & Lane, M. D. (1971) Adv. Enzymol. Relat. Areas Mol. Biol. 35, 321–442. [DOI] [PubMed] [Google Scholar]
- 31.Wakil, S. (1989) Biochemistry 28, 4523–4530. [DOI] [PubMed] [Google Scholar]
- 32.Winder, W. W., Wilson, H. A., Hardie, D. G., Rasmussen, B. B., Hutber, C. A., Call, G. B., Clayton, R. D., Conley, L. M., Yoon, S. & Zhou, B. (1997) J. Appl. Physiol. 82, 219–225. [DOI] [PubMed] [Google Scholar]
- 33.Hardie, D. G. (1989) Prog. Lipid Res. 28, 117–146. [DOI] [PubMed] [Google Scholar]
- 34.Hardie, D. G. & Pan, D. A. (2002) Biochem. Soc. Trans. 30, 1064–1070. [DOI] [PubMed] [Google Scholar]