OVERVIEW
Fewer than 1 in 20 adult cancer patients enroll in cancer clinical trials. But although barriers to trial participation have been the subject of frequent study, the rate of trial participation has not changed substantially over time. Barriers to trial participation are structural, clinical, and attitudinal, and differ according to demographic and socioeconomic factors. In this paper, we characterize the nature of cancer clinical trial barriers, and we consider global and local strategies for reducing barriers. We also consider the specific case of adolescents with cancer, and show that the low rate of trial enrollment in this age group strongly correlates with limited improvements in cancer population outcomes compared to other age groups. Our analysis suggests that a clinical trial system that enrolls patients at higher rates produces treatment advances at a faster rate and corresponding improvements in cancer population outcomes. Viewed in this light, the issue of clinical trial enrollment is foundational, lying at the heart of the cancer clinical trial endeavor. Fewer barriers to trial participation would allow trials to be completed more quickly and would improve the generalizability of trial results. Moreover, increased accrual to trials is important to patients, since trials provide patients the opportunity to receive the newest treatments. In an era of increasing emphasis on a treatment decision-making process that incorporates the patient perspective, the opportunity for patients to choose trial participation for their care is vital.
INTRODUCTION
The path from initial development of a new cancer drug to diffusion of the new therapy into the cancer treatment community relies, crucially, on clinical trials, which represent the final step in evaluating the efficacy of new therapeutic approaches for malignancy. It has been repeatedly estimated that <5% of adult cancer patients enroll in cancer clinical trials.1,2 Conversely, the vast majority of adult cancer patients (>95%) do not participate in clinical trials, even though 70% of Americans are estimated to be inclined or very willing to participate in clinical trials.3 Thus a large gap exists between trial participation rates and the willingness of patients to participate, suggesting that barriers to trial participation are numerous and frequently insurmountable.
Barriers to trial participation have been the subject of frequent study, but the rate of trial participation has not changed substantially over time. The infrastructure around the conduct of clinical trials has been designed to anticipate a low, albeit steady, trial participation rate. The NCI’s cooperative group clinical trial treatment program caps enrollment for its funded groups at 17,000 total patients per year, representing 1% of the estimated 1.7 million new cancer diagnoses in the U.S. in 2015.4,5
To understand the impact of clinical trial participation on cancer population mortality and survival, one might imagine a counterfactual system in which the cancer clinical trial participation rate was much higher. Fortunately such a system already exists. Enrollment of children (<15 years old) to clinical trials has historically been much higher than for adult cancers (>50%).2,6,7 At the same time, mortality rates have for children have been decreasing since the 1970s, whereas for adults they have been decreasing only since the 1990s.8 The average reduction in the rate of mortality from 1975–1995 was 2.6% per year for those <20 years old.9 Interestingly, the reduction was weakest among older children (15–19 years; 2.0% per year), reflecting other studies which have found both lower trial enrollment for adolescents and young adults with cancer and lower rates of mortality reduction.10,11
These data are consistent with the idea that a clinical trial system that enrolls patients at higher rates produces treatment advances at a faster rate and concurrent survival increases and mortality reductions in the cancer population. In this context, the issue of clinical trial enrollment is viewed as foundational, lying at the heart of the cancer clinical trial endeavor.12 Therefore the identification of specific barriers to trial enrollment and efforts to remove such barriers represent critical research objectives for cancer investigators.
In this paper, we attempt, first, to characterize the specific barriers to cancer clinical trial participation. We consider as well the distinction between clinical trial enrollment between children and adolescents with cancer. As suggested above, these age-proximal patient groups provide a natural observational contrast illuminating the association between clinical trial enrollment rates and corresponding improvements in outcomes in the cancer population. We present original data to make this case. Finally, we examine global and local strategies to improve cancer clinical trial participation.
UNDERSTANDING BARRIERS TO CLINICAL TRIAL PARTICIPATION
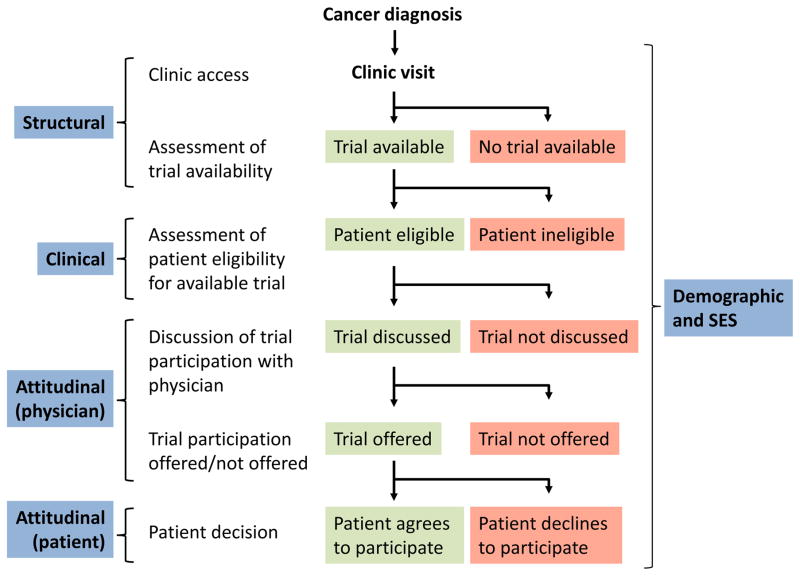
A patient’s decision about what cancer treatment to undergo is complex and deeply personal; the prospect of incorporating clinical trial treatment into a patient’s care adds another level of complexity. In this multi-factorial decision-making environment, patients may face several barriers to trial participation. As a guide to understanding the trial decision-making process, we present a simplified flow diagram (Figure 1) illustrating a representative pathway through which a patient may receive care. This model has been the basis for multiple studies examining barriers to clinical trial participation.13,14,15 The model supposes that after cancer diagnosis and a clinic visit, an assessment of trial availability is made to identify whether a trial exists at the institution for the patient’s histology and stage. If a trial is available, an evaluation of trial eligibility is made, and if eligible, a trial is discussed with the patient. The trial may then be offered to the patient, at which point the patient makes a decision about whether to participate. An important note is that under this model, patient attitudes toward clinical trial participation only come into play at the end of an otherwise long process.
Figure 1.
Model pathway of trial enrollment process
We have categorized barriers to trial participation as structural (especially, the absence of an available clinical trial), clinical (i.e., not meeting eligibility), attitudinal (with respect to both patients and physicians), and demographic and socioeconomic. It is recognized that there is greater fluidity between these categories than the model allows, but simplifications were made to facilitate discussion.
Structural Barriers
To participate in a clinical trial, patients must first have access to a cancer clinic. Access to a clinic can be influenced by many different structural factors such as transportation, travel costs, access to insurance, and availability of child care.16 Uninsured patients, in particular, present with later stage of disease and have worse cancer outcomes.17,18 To the extent that such patients present at their cancer diagnosis with a greater comorbid burden, their likelihood of eventually participating in a clinical trial will be lower.19
Once a patient has access to cancer care, a major structural barrier pertains to the availability of a clinical trial for the patient’s histology and stage. Multiple prospective studies of the cancer care decision-making process have examined the extent to which trials are unavailable for patients. Lara and colleagues prospectively tracked barriers to cancer clinical trials at the UCD cancer center from 1997–2000.20 Among patients considered for trial availability, 47% of patients had no trial available (Table 1). Javid and colleagues registered patients to a prospective survey study prior to their treatment decision regarding their cancer care at a diverse set of eight institutions. No trial was available for nearly half of the patients (46%).13 Together, these and earlier studies consistently show that once a patient has access to cancer care, the absence of an available clinical trial precludes participation for about half of all patients.14,21,22
Table 1.
Clinical trial enrollment patterns for multiple studies in the literature
| No. examined1 | Trial unavailable | Ineligible | Trial Participation rate | Did not participate | |
|---|---|---|---|---|---|
| Lara | 171 | 47% | 8% | 23% | 22% |
| Javid | 909 | 46% | 14% | 16% | 24% |
| Klabunde | 2339 | 60% | 16% | 7% | 17% |
| Begg | 3534 | 33% | 33% | 16% | 16% |
| Hunter | 44,156 | 60% | 18% | 8% | 14% |
| Average2 | 49% | 18% | 14% | 19% |
Assessed for trial availability.
Unweighted
Clinical Barriers
Even if a trial is available, patients may not be eligible. Studies have found that a common reason for patient ineligibility to available protocols is narrow eligibility criteria.3,14,21,22,23,24 Trial eligibility attempt to satisfy two opposing criteria. On the one hand, eligibility must be sufficiently narrow to produce a treatment effect that is approximately consistent across the cohort. On the other hand, eligibility should be sufficiently inclusive that the trial targets a meaningful population of patients to which a new treatment would apply.25 Eligibility criteria may also exclude patients due to safety concerns. In the event, trials are often criticized for having eligibility criteria that are too narrow, sacrificing generalizability.26 These exclusions also make trials less accessible for patients.
In the previously described prospective studies of trial barriers, ineligibility was identified as a reason for non-participation in 18% of all patients on average (Table 1).13,14,20,21,22,27 The dominant reason for ineligibility exclusions is likely exclusions due to comorbid conditions. One recent paper comprehensively catalogued the trial eligibility criteria for a set of 21 trials in diverse cancer settings.28 The authors found that the average number of eligibility criteria per trial was 16, 60% of which were related to comorbidity or performance status. Although pre-specified trial eligibility criteria that protect patient safety are crucial, it is also possible that certain kinds of exclusions are unnecessary. A recent report indicated trial eligibility criteria have increased in recent years for both academic group and pharmaceutical sponsored clinical trials.29 This trend not only renders trials less accessible for many patients, it may also limit the generalizability of trial results.
Physician Attitudes
As the agent linking patients to their cancer care, physicians play an obvious and vital role in clinical trial participation. A survey of oncologists in community cancer clinics found that most agreed that clinical trials provide high quality care (87%) and benefit enrolled patients (83%).30 But physicians face their own barriers to trial enrollment, so even if quality cancer care is assumed, physicians may treat otherwise eligible patients off-protocol with one arm of the trials, without actually entering the patient on the trial.31 Multiple earlier studies found physician decision or preference was the primary reason for non-participation in half of the patients for whom a protocol was available and the patient was eligible.21,22
A number of factors have been found to deter physician recommendation for trial participation. In their role of guiding patient care, physicians may have a strong inclination towards a specific treatment for a given patient.31,32,33 The prospective study by Javid and colleagues found that the nature of the study regimen was cited as a reason for not discussing a trial with eligible patients by 56% of physicians.13 Physicians are also frequently concerned that clinical trial participation can interfere with the physician-patient relationship.31,34,35 Randomization onto a phase III trial in particular subjects the treatment choice to uncertainty, and physicians may anticipate that the introduction of uncertainty will subvert patient confidence in the physician’s expertise, even if, as indicated by the existence of a randomized clinical trial, multiple treatments of potentially similar efficacy are available.
Practical considerations may also influence physician’s willingness to participate in trials. Physicians often lack appropriate incentives to participate in clinical research.36 The time spent attending to the details of clinical trial enrollment and explaining clinical trials to patients can often be prohibitive for physicians.33 In addition, in a busy clinic environment, physicians may be less likely to refer patients to trials if they believe the process will be too time consuming.30,31,37 Oncologists who consider trial paperwork time consuming or who otherwise believe trial effort would be extra work are less likely to refer a patient to a clinical trial.37 Finally, some physicians find the requirement of obtaining informed consent to be problematic, even as nearly all agree that informed consent is necessary.30,31
Patient Attitudes toward Clinical Trials
Efforts to reduce structural, clinical, and physician barriers to trial participation are critical. However the ultimate decision regarding trial participation rests with the patient. Inevitably, the decision about whether to participate in a trial will reflect a patient’s personal preferences, which may also be influenced by family and close friends.38
Some proportion of patients are influenced by altruistic motivations.15 However the majority of patients are (appropriately) concerned primarily with finding the best possible treatment for their disease.15,39,40 In the absence of other barriers, a patient who believes that the best possible treatment option is to be found in a clinical trial is more likely to participate in that clinical trial.33
Patients have frequently reported being uneasy or fearful about the prospect of participating in a clinical trials.41 In some cases this could be due to a residual mistrust of medical science due to past abuses, such as the infamous Tuskegee Syphilis Study or the history of human experimentation with radiation following WWII.42,43 Attention in the last several decades to the process of rigorous consent may have reduced these fears, especially for younger generations of patients. Attention must also be paid to providing consent forms which are easy to read, since more complicated consent forms can themselves induce anxiety.44
More generally, a fear of experimentation may be expressed through a dislike of randomization.14,15,22,45,46,47 There is perhaps no stronger indication that a patient is about to undergo an experiment than the revelation that the patient will be randomly allocated to one of two or more treatments. Fear of randomization has been identified as the most commonly cited reason by patients for declining trial participation.15 Recognizing this, some physicians avoid the word “randomization”, relying instead on analogy to describe the randomization process, though this may lead to situations where patients sometimes do not understand they have been randomized.48,49
Patients are sometimes uneasy as well about the potential toxic effects of chemotherapy on trials, especially for the experimental therapies.38 Patients may already have a strong sense of the particular treatment they wish to receive after discussion with their physicians.33 Since trials sometimes require more frequent monitoring than non-trial care, getting to and from a cancer clinic has been indicated by many patients to be a reason for non-participation.15,20 Concern about how to pay for trials has been cited as a reason for non-participation among about a quarter of patients, despite the fact that the majority of states mandate that insurers cover the routine care costs of trials, as does Medicare.15 In a review by Ford et al, cost concerns were identified as the second most frequently indicated reason for non-participation in trials in the literature.41
Demographic and Socioeconomic Disparities
Demographic and socioeconomic disparities in trial enrollment can occur anywhere along the pathway from initial clinic visit until the patient ultimately makes their treatment decision. The most consistent and largest disparity pertains to age.1,15,50,51,52,53 Hutchins and colleagues found that patients in cooperative group trials were much less likely to be 65 or older than those in the US cancer population.50 Some evidence suggests that attitudinal barriers on the part of physicians play a role.13,54,55,56 In addition, older patients are likely to have higher comorbid burdens, inducing clinical exclusions.57,58 To the extent that trials seek to reflect the population of patients for whom new trial-proven treatments will be administered, better representation of older patients on trials is critical. Recognizing this, in the year 2000, Medicare was directed to cover the routine care costs of clinical trial participation for its patients.59 Unfortunately, the proportion of older patients on trials remains well below the expected rate.28,51
Evidence as to the association of race with trial participation is mixed. A study by Murthy and colleagues found that black patients were underrepresented in NCI sponsored breast, lung, colorectal, and prostate cancer clinical trials from 2000–2002.1,60 In contrast, in sequential studies within SWOG, a national clinical trials consortium, black patients were enrolled to trials in a representative fashion over an extended period of examination.28,50,53 This was confirmed in a sample of older breast cancer patients.61 Evidence has also been mixed for Hispanic patients.1,53 Even if enrollment of minorities is adequate in the treatment trial setting, enrollment of minority healthy volunteers for prevention trials has been decidedly more difficult, and has generated well-designed outreach programs for large individual trials.62,63,64,65 Given the increasingly diverse nature of the US population, continued attention to this issue is required.
Females may be somewhat underrepresented in the non-sex specific cancers, although the magnitude of the disparity is likely small.28,50,53 In addition, some evidence that any sex-disparity in clinical trial enrollment could be age-related, with older women less likely to enroll in trials than their younger counterparts.1 Such a pattern suggests generational differences in attitudes towards clinical research.
The examination of socioeconomic factors as a barrier to participation has historically been hindered by the lack of collection of patient-level SES data. This is unfortunate given the frequency with which the direct and indirect costs of trial participation have been cited as meaningful barriers.41 Two recent articles overcame this limitation. The first utilized a web-based survey to engage patients in their decision making process.15 Among numerous demographic and socioeconomic factors, patients with lower income (<50k) were 29% less likely to participate in trials than higher income patients. Utilizing data from a prospective barriers study, this observation was confirmed.13,66 Thus the evidence to date of income disparities in trial enrollment are fairly consistent.61 Given that trial treatment costs are not substantially different than non-trial treatment costs,67 this suggests that marginal direct costs play a role. A prior study of the impact of the year 2000 Medicare policy change on trial participation found that patients with Medicare plus private insurance participated at a higher rate following the year 2000 policy change, whereas patients with Medicare alone participated at rates similar to those prior to the policy change.53 This finding points to the prohibitive influence of copays and coinsurance for some patients.
EVIDENCE FOR THE BENEFIT OF CLINICAL TRIALS ON CANCER POPULATION OUTCOMES OBSERVED THROUGH THE RELATIVE LACK OF PROGRESS IN ADOLESCENTS AND YOUNG ADULTS
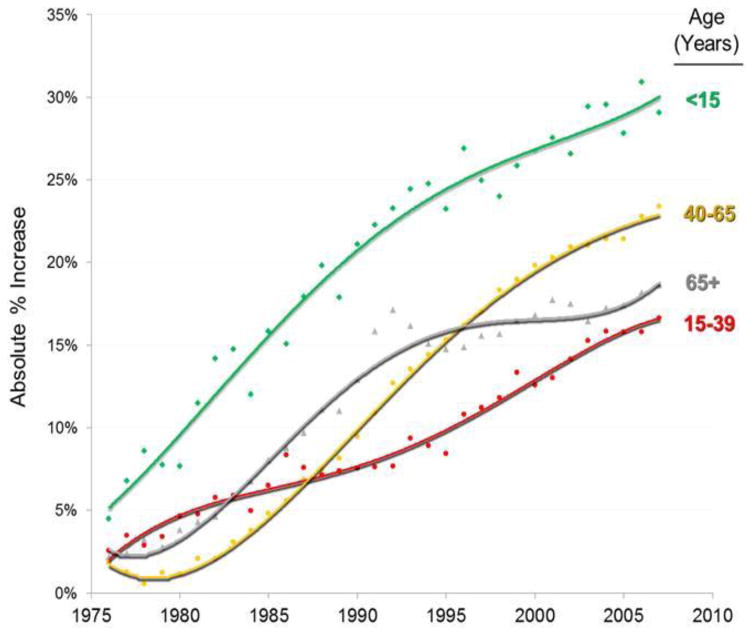
The potential barriers to trial enrollment that patients face are numerous. But just how important are clinical trials for progress against cancer? The answer to this question is crucial, since if trial participation is ultimately unrelated to cancer population survival gains, the issue of barriers to trial participation has little importance. To examine this, we studied the relationship between adolescents and young adults (AYAs) and cancer population outcomes over time. This is an ideal group in which to examine the impact of clinical trials given that since 1980, AYA patients have had a slower rate of cancer population survival improvement than younger and older age groups by 5% to 13% in absolute differences (Figure 2). Concomitantly, cancer has become the most frequent cause of death due to disease in AYAs.68 During the past decade (2000–2009), deaths due to cancer declined in all age groups except in young adults aged 20 to 29 years; in 25- to 29-year-olds, deaths due to cancer actually increased.69
Figure 2. Increase in Absolute Percentage of Annual 5-Year Cancer-Specific Survival Rates since 1973–1975 by Calendar Year and Age.
Baseline is 1973–1975 average. Kaposi sarcoma is excluded due to HIV/AIDS epidemic during the 1980s and early 1990s; thyroid cancer is excluded because of overdiagnosis and increasing survival inflation. Regressions are 4° polynomials. Data source is SEER 9 regions.75
The survival disparity between AYAs and other patients may be due in part to early achievements in improving survival for AYAs, after which resources were directed towards research in other age groups. Also, cancers for AYAs have potentially complex biological signatures that neither pediatric oncologists nor adult-treating medical and hematologist oncologists are accustomed to treating.70 AYAs are also less likely to have health insurance,71 especially prior to the advent of the Affordable Care Act, which could be associated with delays in diagnosis and compromises in optimum diagnostic and therapeutic interventions.72 At the same time, AYAs have had the lowest participation in clinical trials in absolute terms than any other major age group.10 The central issue then is to what extent lack of clinical trial activity in AYA cancer patients accounts for the relative lack of progress in improving cancer population outcomes.
All Cancers
The Cancer Therapy Evaluation Program (CTEP) of NCI’s Division of Cancer Therapy and Diagnosis has patient accrual data on Phase I, II and III cancer treatment trials conducted by the NCI cooperative groups and NCI-designated cancer centers. For this analysis, 371,302 patient entries during 1997–2009 were examined. In addition, we used cancer population data derived from the Surveillance, Epidemiology, and End Results registry, U.S. Census data, and joinpoint analyses to examine trends in U.S. cancer population estimates.73,74,75,76,77,78
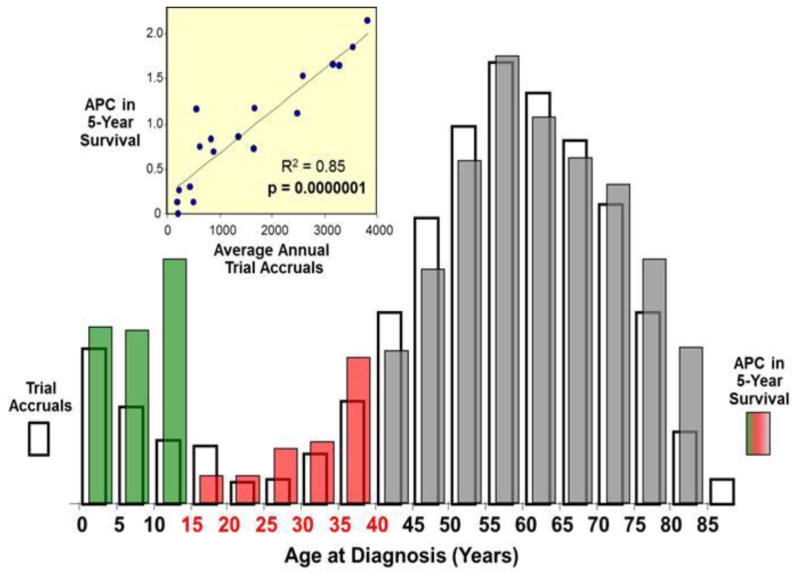
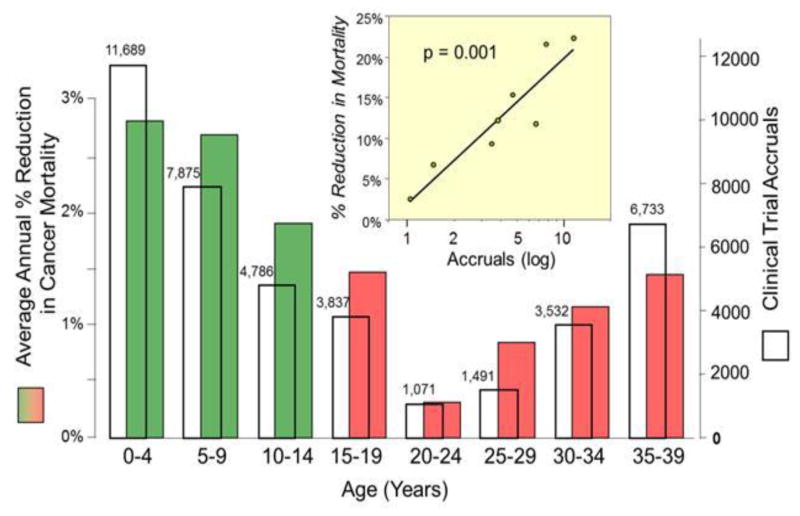
Figure 3 shows the relationship between the average percent change (APC) in the 5-year cancer-specific survival rate from 1985 to 1999 and the accrual rate to national cancer treatment trials during 2001–2006. Although this comparison is confounded by time, there was a nearly 1:1 correlation over the entire age range that was strongly statistically significant. Patients 15 to 34 years of age had the lowest APC in 5-year survival. A similar pattern was found with respect to cancer mortality as shown in Figure 4, which isolates those 0–40 years of age. Those 20–24 years of age had a particularly poor reduction in cancer mortality, as well as the lowest absolute number of clinical trial accruals. In both cases, the correlation between trial enrollment and, respectively, APC in 5-year survival and mortality is clearly evident and highly statistically significant (p<.001).
Figure 3. Comparison of Average Percent Change (APC) in the 5-Year Cancer Survival Rate and Treatment Trial Accruals, by 5-Year Age Intervals.
The open columns represent trial accruals during 2001–2006 and the colored bars the average percent change (APC) in 5-year relative survival rate of all invasive cancer except Kaposi sarcoma during 1985–1999. The red bars indicate the AYA age group. The inset compares the APC in 5-year survival rate with the treatment trial accruals. Accrual data from the NCI Cancer Therapy Evaluation Program (CTEP) were provided by Steve Friedman, Michael Montello, Troy Budd and Samantha Finnegan via the Freedom of Information Act. Survival data were obtained from SEER 9 Regions.75 Kaposi sarcoma is excluded from the survival statistic since the HIV/AIDS epidemic during the 1980s and early 1990s substantively altered the overall cancer survival rate in AYAs during those years.
Figure 4. Comparison of Average Percent Reduction in the Annual National Cancer Mortality Rate and Treatment Trial Accruals, by 5-Year Age Intervals, Age <40.
The open columns represent trial accruals during 2000–2006 and the colored bars the average percent reduction in national cancer mortality rate during 1990–1998. The red bars indicate the AYA age group. The inset compares the mortality rate reduction with the treatment trial accruals. Accrual data from the NCI Cancer Therapy Evaluation Program (CTEP) were provided by Steve Friedman, Michael Montello, Troy Budd and Samantha Finnegan via the Freedom of Information Act. Mortality data were obtained from the National Center for Health Statistics via the SEER program.78
Acute Lymphoblastic Leukemia
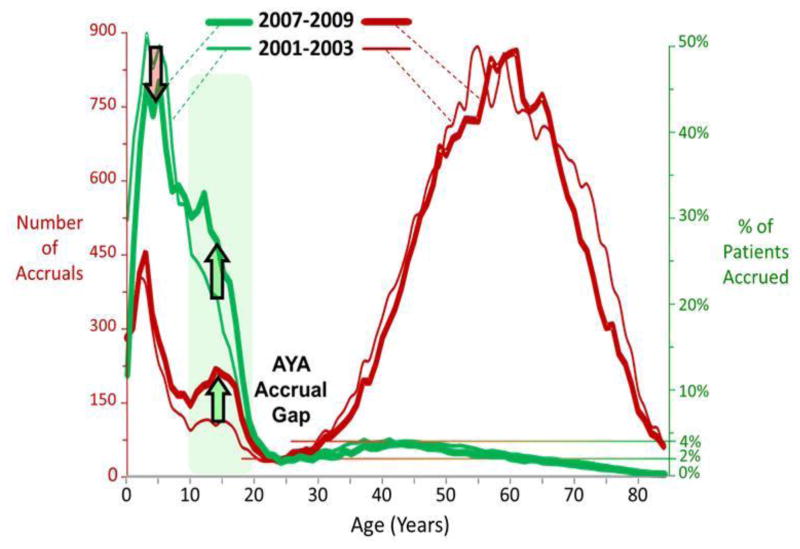
The greatest effort during the last decade to increase accruals in AYAs was directed at ALL, the most common pediatric cancer. New clinical trials in ALL specifically designed for AYAs were launched,79,80,81 the National Comprehensive Cancer Network released practice guidelines for ALL,82 and an increasing number of presentations and publications on the topic occurred at national meetings and appeared in the peer-reviewed medical literature.83,84,85,86,87,88,89 The effort was effective, with AYAs having the greatest accrual increase of all cancers in ALL, up to twice that of the cancer with the next greatest increase, AML.74,90 Perhaps in part for this reason, the only increase in either absolute accruals or accrual as a proportion of cases during the first decade of the 21st century occurred in patients between the ages of 10 and 20.[Figure 5]
Figure 5. Comparison of 2001–2003 and 2007–2009 for Annual Accruals to Treatment Trials sponsored by National Cancer Institute (NCI)-Sponsored Cooperative Groups and NCI-Designated Cancer Centers (red curves) and Accrual Proportion of All Patients in the U.S. with Invasive Cancer onto the Trials by 5-Year Age Intervals (green curves), by Single Years of Age.
The heavy curves represent 2007–2009 and the thin curves 2001–2003. Accrual data from the NCI Cancer Therapy Evaluation Program (CTEP) were provided by Steve Friedman, Michael Montello, Troy Budd and Samantha Finnegan via the Freedom of Information Act. Accrual proportion (%) was estimated from cancer incidence in SEER 9 regions and population data from the U.S. Census Bureau.73,75
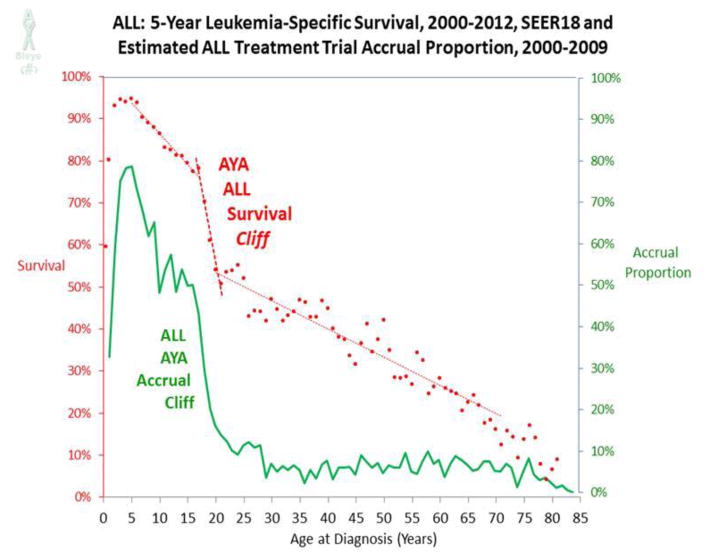
Hence, ALL was examined more closely for relationships between survival improvement and clinical trial participation. As shown in Figure 6, clinical trial accruals as a proportion of ALL cases in the U.S. during the period 2000–2009 drops precipitously for patients about 15 to 20 years. Figure 6 also shows the 5-year leukemia-specific survival rate for patients with ALL as a function of single year of age. Joinpoint analysis identified 2 inflections, ages 17 and 20, during which the 5-year survival rate decreased 23%. This “AYA ALL cliff” constituted 30% of the overall decline from 95% at age 5 to less than 20% at age 70. The “cliff patterns” for both accrual and survival are virtually superimposable, which strongly suggests they are related, although other factors, such as a switch from pediatric to adult treatment regimens, could also contribute.91
Figure 6. 5-year Leukemia-Specific Survival Rates in Patients with Acute Lymphoblastic Leukemia (ALL) Diagnosed during 2000–2012, and Estimated ALL Treatment Trial Accrual Proportion during 2000–2009, by Single Years of Age.
Each year of age was averaged from 2 consecutive years. Joinpoint analysis of survival data identified ages 17 and 20;74 linear regressions for survival data are for age ranges 5–17, 17–20, and 20–70 years. Survival data were obtained from SEER 18 regions.77 Accrual data from the NCI Cancer Therapy Evaluation Program (CTEP) were provided by Steve Friedman, Michael Montello, Troy Budd and Samantha Finnegan via the Freedom of Information Act. Accrual proportion (%) was estimated from cancer incidence in SEER 9 regions and population data from the U.S. Census Bureau.73,75
Clinical Trials Impact Summary
These data enable three fundamental conclusions. First, both survival prolongation and mortality reduction in patients with cancer are correlated with clinical trial activity. Second, the dependency of survival prolongation on treatment trial accrual has been apparent at all ages. Third, AYAs have had the least trial participation and the least survival prolongation and mortality reduction, particularly among patients 20 to 29 years of age.
It has been previously observed that the age-dependent rate in the reduction of deaths attributed to cancer in the United States is correlated with the age-dependent accrual of young adults to national cancer treatment trials during the same era.69 After suicide, cancer is the second leading cause of death due to disease in AYAs. More is needed to overcome the national AYA cancer death problem, beginning with increased clinical trial availability, access, referrals, participation and conduct. Fortunately, NCI-designated cancer centers are evaluating their own AYA referral patterns and clinical trial determinants92,93 and intergroup efforts are under way within the current organizational structure of the federal clinical trials enterprise, including the NCI’s National Clinical Trials Network (NCTN), to create novel opportunities for collaborative AYA oncology research among the pediatric and adult NCTN groups.94,95 As noted in England, age-specific biology, pharmacology, proteomics, genomics, clinician and patient behavior studies embedded within clinical trials are required to further improve survival for AYAs.96
GLOBAL AND LOCAL STRATEGIES TO IMPROVE CLINICAL TRIAL PARTICIPATION
We have illustrated the nature of clinical trial enrollment barriers and established the potential link between trial enrollment and improvements in cancer population survival. Efforts to improve trial enrollment of cancer patients are clearly needed. In this context, we propose the following global and local strategies to improve trial participation.
Overall Strategies
In 2010, the National Cancer Institute and the American Society of Clinical Oncology sponsored a Cancer Trial Accrual Symposium to provide recommendations for trial recruitment. Summary recommendations centered on the patient, community, physician/provider, and site.97 This symposium led to many recommendations at each level consistent with the overarching view that “one size does not fit all” when it comes to recruitment to clinical trials. The organizers noted in particular that although clinical trial enrollment barriers have been extensively studied, “Few rigorously conducted studies have tested interventions to address challenges to clinical trials accrual.”97 In this broader context, the National Cancer Institute’s AccrualNet website provides strategies, tools, and other resources to support clinical trials.98
Global strategies
At the beginning of this paper, we delineated many of the specific challenges to clinical trial enrollment. Given the need to accrue large numbers of patients in a shortened timeline and the increased complexity of U.S.-based clinical trials, academic and industry sponsors are increasingly exploring regions outside of the United States to conduct trials, including in less developed regions of the world.
In the view of Barrios and colleagues, the globalization of clinical trial research is unavoidable.99 In a wide ranging review, they propose the following solutions to some of the challenges of clinical trial globalization:
Harmonize and share standards and goals for product safety, quality, and efficacy.
Use finite resources more efficiently, share knowledge, and optimize inspection resources.
Engage global partners to advance regulatory science and public health solutions.
Implement risk-based monitoring and clinical inspection.
Incorporate error reduction strategies and consider regional variations in the standards of care and their impact on trial results.
Decentralize laboratories in favor of developing regional expertise to provide “carry-over” benefits after trial completion.
Streamline and advance bio-bank regulatory issues.
Invest in research and evidence-based cancer care relevant to each region, including cancer registries and clinical trial infrastructure.
In the authors’ view, the globalization of clinical research “is vital to speed up availability of life-saving medicines throughout the world”.99 In the setting of a domestic clinical trial system that has been described as being in a “state of crisis”, this view has added weight.12
The importance of differing cultural, scientific, ethical, governmental, and logistical issues in each region must be considered.100 One key ethical issue is whether or not the study drug will be available at the end of the clinical trial. Availability may be impacted by local religious customs concerning contraceptive studies and ethical guidelines limiting pediatric clinical trials. An additional ethical issue in low resource countries involves whether to develop local resources for testing or to use international vendors for that purpose. Although developing local resources provides an often needed benefit, this could involve higher costs and may also be deterred by local shipping laws and other logistical barriers.
An excellent example of global recruitment is the START trial.101 START (Stimulating Targeted Antigenic Response To NSCLC) is a multi-center, phase III, randomized, double-blind placebo-controlled trial of the cancer vaccine tecemotide in non-small cell lung cancer subjects with unresectable stage III disease sponsored. The trial is sponsored by Merck KGaA and EMD Serono, Inc, in 275 study centers in 33 countries worldwide. A continuous series of strategies was implemented for patient recruitment and retention throughout the life of the trial.102 These strategies were referred to as “the START global patient recruitment and retention continuum,” with the overarching purpose of raising awareness of the START trial and keeping it in the forefront in physician communities and in the local START sites to increase patient enrollment and retention. The strategies target physicians, research staff, local sites, patients, and the local community. Physicians were provided START informational calls (sometimes physician-to-physician), visits, and meetings, and START information at national oncology meetings. Research staff received START education, and recruitment tools including motivational videos. The local sites were offered additional site funding for accelerated recruitment and START educational teleconferencing. Patients received holiday and thank you cards and patient START educational materials. The local community was reached through local media outreach, public awareness advertisements, and engagement of local site liaisons. Enrollment to the trial ran from 2007 through 2011 and reached full accrual 1513 patients randomized, indicating a highly successful recruitment effort.
Domestic trials may also partner with international collaborators to augment trial enrollment. The SWOG Selenium and Vitamin E Cancer Prevention Trial (SELECT) for prostate cancer prevention used similar recruitment strategies as the START trial. The SELECT trial enrolled over 35,000 men in the United States, Canada, and Puerto Rico, and completed enrollment two years ahead of schedule.62,63 On a smaller accrual scale, the contributions of the International Breast Cancer Study Group, in collaboration with the cooperative groups of the NCI, provided necessary accrual to a trial evaluating ovarian failure in premenopausal women with breast cancer.103
Local Strategies
Social Media
Increasingly, social media platforms provide an opportunity to communicate about clinical trials with potential trial researchers and participants.104 The Quorum Review IRB offered considerations for a plan to use social media in research.105,106 These considerations are to:
Provide a rationale for the application of social media to the target population.
Address the privacy and confidentiality concerns of the social media applications to be used.
All communications should be vetted for sensitivity and potential for harm, even if the content does not require IRB approval.
Provide a summary statement regarding the social media account intended uses.
If user generated content is allowed, which is essential to creating a robust online community, close monitoring is required for patient protection and study integrity.
Moreover, predetermined, IRB approved responses to anticipated user generated content should be available to allow “immediate” responses when needed, and links to the study website should be included to allow for integration of study platforms.
The FDA has provided no specific guidance on the use of social media in clinical research.107 The Recruitment Information Sheet states that in the case of direct advertising, the information and mode of communication should be reviewed by the IRB for evidence of coercion or implication of benefits to participation. The Office of the Inspector General has released guidance regarding the use of clinical trial websites, indicating that IRB approval of a clinical trial listing, if limited to selected basic information, is not required.108
InVentiv Health, a contract research organization, has provided specific plans to assist researchers to plan digital recruitment campaigns.[Table 2]109 Their plans are premised on the idea that recruitment for global clinical trials is area ripe for re-engineering. More broadly, Facebook has often been listed as the social media of choice; of the 73% of online US adults using social media, 71% use Facebook. Often researchers using Facebook attempt to recruit from the initial audience prior to forming a relationship. A better approach may be to first grow and engage your audience first before patients are recruited.110
Table 2.
Proposed steps to plan digital recruitment campaigns[inVentiv2013]
| Steps for strategy development | Steps for campaign implementation |
|---|---|
| Audit digital channels and social media sites to determine where the targeted patients/caregivers can be found | Place ads with key messages and a call to action on the selected sites and social media platforms |
| Monitor existing online chats on the area of research interest to understand the issues and concern to patients/caregivers and listen for “patient speak” so it can be replicated in your messages | Link banner or pop-up ads to search engine results for targeted health-related searches |
| Map the recruitment messages to the targeted patients/caregivers and appropriate channels | Partner with advocacy groups and place trial ads on these groups’ online sites |
| Identify key bloggers, etc. who could eventually raise awareness and support trial participation | Engage key opinion leaders as online ambassadors to raise awareness of the trial |
| Develop the creative concepts for advertising and test the messaging and imagery for effectiveness |
Strategies to Address Demographic and Socioeconomic Barriers
Elderly Recruitment
Patients with concomitant illnesses are often excluded from trials to ensure safety and to isolate the cancer as the primary source of morbidity in the patient. Unfortunately this has the effect of excluding many patients from trials, especially older patients with a greater comorbid burden.28,57,58 Further, trials typically exclude patients with prior cancers, even as the population of cancer survivors in the U.S. is growing, currently numbering around 15 million.111 In this context, one strategy to remove barriers trials would be to remove unnecessary eligibility criteria. Such an approach would improve access to trials, especially for older patients, and – since histology and stage explain the vast majority of variation in cancer outcomes, rather than comorbid conditions – would result in only limited loss of power to test the efficacy of new treatments.28 One study estimated that if protocol exclusions related to organ-system abnormalities and functional status were relaxed, participation of older patients in clinical trials would approach 60%, in line with cancer population rates.51
Researchers should also consider increasing the number of trials targeted to older patients, with due consideration to potential safety issues.112 Several trials found no more toxicity in elderly patients in chemotherapy containing trials than in younger patients when patients are appropriately selected.113,114 However when chemotherapy was given to patients >=80 years, high risks of hospitalization or treatment discontinuation due to toxicity (even with frequent dose modifications) were observed.115 The International Society of Geriatric Oncology recommends the use of comprehensive geriatric assessment (CGA) in cancer patients older than 70.116,117 The CGA is time consuming, often leading to physician abandonment. Fortunately CGA time requirements have led to the development of prescreening tools used to determine whether full screening with CGA is required, though there are inconsistent results regarding the validity of these tools.118,119,120 More generally, it is important to develop prediction models capable of estimating risk of chemotherapy for octogenarians and nonagenarians with regards to toxicity and hospitalization.115 A comprehensive approach to the evaluation of the older cancer patient considers the residence, fitness, and an interdisciplinary team to provide individualized care.121
Strategies to Address Socioeconomic Barriers
If marginal direct costs are prohibitive for some patients, then measures to cover these costs would remove a critical barrier to enrollment. One approach would be to cover the excess costs of clinical trials for all patients, since even in an insured population, co-pays and co-insurance have been shown to deter clinical trial participation.53 Another potential approach would be to provide payments to patients. In the U.S., the practice of paying patients for trial participation is widespread, but also contentious, highly variable, and lacking in general guidance.122 One concern is that a payment inducement might alter a subject’s assessment of potential risks or impair their judgment, although there is little evidence that payment inducements do or do not do this.123,124 A careful calibration of the size of any monetary incentive would be necessary to avoid undue influence.125
Measures to address socioeconomic disparities in recruitment may have a preferentially beneficial impact on minority patients. For the SELECT trial, several strategies specifically addressed patients with low socioeconomic status.61,62 SELECT provided funds to sites semiannually to offset travel expenses and meals in addition to providing patient retention items. Larger supplemental site grants were awarded to fifteen SELECT sites with potential to increase minority recruitment through a competitive award mechanism. These additional funds were most commonly used to provide additional staff time for minority recruitment. Sites also provided reimbursement for food and/or transportation costs expended to participate in the trial.62
DISCUSSION
Both patients and physicians have been found to regard clinical trial participation as a positive approach to cancer care.3 Despite this, the complexity of the enrollment process and the potential barriers faced by patients have combined to make a successful clinical trial enrollment a rare event. Clinical trials are the key step in advancing new treatments from the research setting to the cancer care clinic. Therefore, a thorough understanding the nature of trial enrollment patterns and barriers to enrollment is of paramount importance. The literature indicates that structural barriers preclude patient participation in trials for half of all cancer patients. Among patients for whom a trial is available, about half (or a quarter of all patients) are excluded due to eligibility issues with trial exclusion criteria. The remaining patients are sometimes not offered the chance to participate due to physician concerns, or decline due to patient concerns. Structural, clinical, and attitudinal barriers to trials can differ according to some important factors, especially age. In the end, only a small portion of adult cancer patients participate in trials, <5%, a rate that has remained fairly constant over decades.
Increasing accrual to clinical trials is important for multiple reasons. Faster accrual would allow trials to be conducted more quickly. The predominant reason that trials fail to complete is poor accrual.126 These failures represent a lost investment on the part of funding agencies. Moreover, when clinical trials close because of failure to accrue, non-financial costs are also incurred. Patients have exposures to study drugs with potential or realized adverse events. Patients and research staff may experience psychological effects such as loss of trust and morale. Informed consent documents rare rarely include the risk of closure due to lack of study participation, despite the fact that about 1 in 4 randomized phase III trials have just such an outcome.126
The more rapid completion of trials would allow new treatments to be developed more quickly. We have shown data indicating a compelling relationship between the incidence of clinical trial enrollments and improvements in cancer population survival outcomes. Our focus was a natural observational contrast between AYAs and other age groups with cancer. We found a slower rate of progress in AYAs compared to younger and older patients, which underscores the need to increase the number of clinical trials available to AYAs with cancer and their participation in them. By extension, this observation also points to the need to increase trial enrollment for patients of any age group, or any demographic group, since this could have a beneficial impact on increasing survival and reducing mortality from cancer.
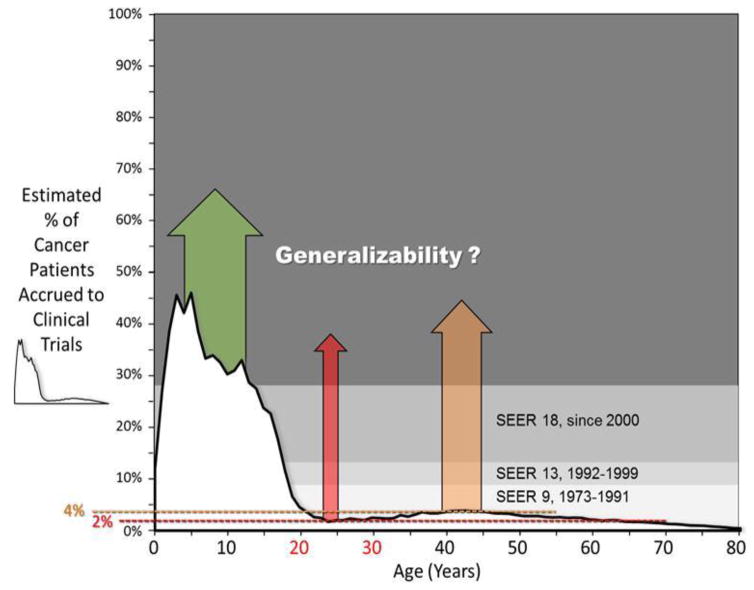
Another important reason to increase clinical trial accrual is to improve the generalizability of clinical trial results. Figure 7 illustrates how the vast majority of cancer patients of all ages – but especially those over about 15 years of age – do not participate in clinical trials.127 However we have made the case that there is a strong correlation between trial participation and cancer population survival improvements. Thus trial results must generalize to non-trial patients, at least to some degree. But they may not do so in an efficient manner, and cancer population survival gains may be lost in the process. Under this rubric, greater participation leads to greater generalizability which leads to better cancer population outcomes. Cancer trial samples, in particular, are usually younger, healthier, and perhaps wealthier than the typical non-trial cancer patient. To the extent that trials are more inclusive with respect to comorbid or other conditions, adequately represent the demographic makeup of the U.S., and are easier to pay for, the generalizability of trials would likely improve.
Figure 7. Estimated Treatment Trial Accrual Proportion of Patients Diagnosed with Cancer during 2008–2010 by Single Year of Age and the History of SEER Representation of the United States Population.
Modified from Bleyer A.127 Accrual data from the NCI Cancer Therapy Evaluation Program (CTEP) were provided by Steve Friedman, Michael Montello, Troy Budd and Samantha Finnegan via the Freedom of Information Act. Accrual proportion (%) was estimated from cancer incidence in SEER 9, SEER13, and SEER18 regions and population data from the U.S. Census Bureau.73,75,76,77
Finally, increased accrual to trials is important to patients, since trials provide opportunity to receive the newest treatments. The principle of equipoise posits that a properly designed treatment trial tests a new or modified form of therapy that is not known to have that benefit (otherwise the trial would not be justified in conducting). On the other hand, in addition to access to the newest treatments, subjects who participate in clinical trials have other potential advantages, such as access to potentially less expensive therapies (if the agent is provided at no or reduced cost to the patient), to teams of professional dedicated to the patient’s care, and to care that is strictly directed by a protocol. Moreover, in an era of increasing emphasis on a treatment decision-making process that incorporates the patient perspective, the opportunity for patients to choose trial participation for their care should not be hindered by unnecessary barriers. In the end, the potential benefits of trial participation will be shared by patients, researchers, and future generations.
Key Points.
Although barriers to trial participation have been the subject of frequent study, the rate of trial participation has not changed substantially over time.
Barriers to trial participation are structural, clinical, and attitudinal, and differ according to demographic and socioeconomic factors.
An analysis of the specific case of adolescents with cancer illustrates how a clinical trial system that enrolls patients at higher rates produces treatment advances at a faster rate and corresponding improvements in cancer population outcomes.
Fewer barriers to trial participation would allow trials to be completed more quickly and would improve the generalizability of trial results, but crucially as well, increased accrual to trials is important to patients, since trials provide patients the opportunity to receive the newest treatments.
In an era of increasing emphasis on a treatment decision-making process that incorporates the patient perspective, the opportunity for patients to choose trial participation for their care is vital.
References
- 1.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004 Jun 9;291(22):2720–6. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 2.Tejeda HA, Green SB, Trimble EL, Ford L, High JL, Ungerleider RS, Friedman MA, Brawley OW. Representation of African-Americans, Hispanics, and whites in National Cancer Institute cancer treatment trials. J Natl Cancer Inst. 1996 Jun 19;88(12):812–6. doi: 10.1093/jnci/88.12.812. [DOI] [PubMed] [Google Scholar]
- 3.Comis RL, Miller JD, Aldige’ CR, Krebs L, Stoval E. Public attitudes toward participation in cancer clinical trials. J Clin Oncol. 2003 Mar 1;21(5):830–5. doi: 10.1200/JCO.2003.02.105. [DOI] [PubMed] [Google Scholar]
- 4.National Cancer Institute. [Accessed January 13, 2016];The NCTN Budget. Available online at: http://www.cancer.gov/research/areas/clinical-trials/nctn/budget#1.
- 5.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975–2012. National Cancer Institute; Bethesda, MD: Apr, 2015. http://seer.cancer.gov/csr/1975_2012/, based on November 2014 SEER data submission, posted to the SEER web site. [Google Scholar]
- 6.Bond MC, Pritchard S. Understanding clinical trials in childhood cancer. Paediatr Child Health. 2006 Mar;11(3):148–50. [PMC free article] [PubMed] [Google Scholar]
- 7.Hunger SP, Lu X, Devidas M, Camitta BM, Gaynon PS, Winick NJ, Reaman GH, Carroll WL. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J Clin Oncol. 2012 May 10;30(14):1663–9. doi: 10.1200/JCO.2011.37.8018. Epub 2012 Mar 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohler BA, Sherman RL, Howlader N, Jemal A, Ryerson AB, Henry KA, Boscoe FP, Cronin KA, Lake A, Noone AM, Henley SJ, Eheman CR, Anderson RN, Penberthy L. Annual Report to the Nation on the Status of Cancer, 1975–2011, Featuring Incidence of Breast Cancer Subtypes by Race/Ethnicity, Poverty, and State. J Natl Cancer Inst. 2015 Mar 30;107(6):djv048. doi: 10.1093/jnci/djv048.Print2015Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ries LAG, Smith MA, Gurney JG, et al., editors. National Cancer Institute, SEER Program. Bethesday, MD: 1999. Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995. NIH Pub. No. 99-4649. [Google Scholar]
- 10.Bleyer A, Budd T, Montello M. Adolescents and young adults with cancer: the scope of the problem and criticality of clinical trials. Cancer. 2006 Oct 1;107(7 Suppl):1645–55. doi: 10.1002/cncr.22102. [DOI] [PubMed] [Google Scholar]
- 11.Ferrari A, Bleyer A. Participation of adolescents with cancer in clinical trials. Cancer Treat Rev. 2007 Nov;33(7):603–8. doi: 10.1016/j.ctrv.2006.11.005. Epub 2007 Jan 23. [DOI] [PubMed] [Google Scholar]
- 12.Institute of Medicine. A national cancer clinical trials system for the 21st century: Reinvigorating the NCI Cooperative Group Program. Washington, DC: The National Academies Press; 2010. [PubMed] [Google Scholar]
- 13.Javid SH, Unger JM, Gralow JR, Moinpour CM, Wozniak AJ, Goodwin JW, Lara PN, Jr, Williams PA, Hutchins LF, Gotay CC, Albain KS. A prospective analysis of the influence of older age on physician and patient decision-making when considering enrollment in breast cancer clinical trials (SWOG S0316) Oncologist. 2012;17(9):1180–90. doi: 10.1634/theoncologist.2011-0384. Epub 2012 Jun 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klabunde CN, Springer BC, Butler B, White MS, Atkins J. Factors influencing enrollment in clinical trials for cancer treatment. South Med J. 1999 Dec;92(12):1189–93. doi: 10.1097/00007611-199912000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Unger JM, Hershman DL, Albain KS, Moinpour CM, Petersen JA, Burg K, Crowley JJ. Patient income level and cancer clinical trial participation. J Clin Oncol. 2013 Feb 10;31(5):536–42. doi: 10.1200/JCO.2012.45.4553. Epub 2013 Jan 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rivers D, August EM, Sehovic I, Lee Green B, Quinn GP. A systematic review of the factors influencing African Americans’ participation in cancer clinical trials. Contemp Clin Trials. 2013 Jul;35(2):13–32. doi: 10.1016/j.cct.2013.03.007. Epub 2013 Apr 1. [DOI] [PubMed] [Google Scholar]
- 17.Roetzheim RG, Pal N, Tennant C, Voti L, Ayanian JZ, Schwabe A, Krischer JP. Effects of health insurance and race on early detection of cancer. J Natl Cancer Inst. 1999 Aug 18;91(16):1409–15. doi: 10.1093/jnci/91.16.1409. [DOI] [PubMed] [Google Scholar]
- 18.Ward E, Halpern M, Schrag N, Cokkinides V, DeSantis C, Bandi P, Siegel R, Stewart A, Jemal A. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008 Jan-Feb;58(1):9–31. doi: 10.3322/CA.2007.0011. Epub 2007 Dec 20. [DOI] [PubMed] [Google Scholar]
- 19.Robbins AS, Pavluck AL, Fedewa SA, Chen AY, Ward EM. Insurance status, comorbidity level, and survival among colorectal cancer patientsage 18 to 64 years in the National Cancer Data Base from 2003 to 2005. J Clin Oncol. 2009 Aug 1;27(22):3627–33. doi: 10.1200/JCO.2008.20.8025. Epub 2009 May 26. [DOI] [PubMed] [Google Scholar]
- 20.Lara PN, Jr, Higdon R, Lim N, Kwan K, Tanaka M, Lau DH, Wun T, Welborn J, Meyers FJ, Christensen S, O’Donnell R, Richman C, Scudder SA, Tuscano J, Gandara DR, Lam KS. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol. 2001 Mar 15;19(6):1728–33. doi: 10.1200/JCO.2001.19.6.1728. [DOI] [PubMed] [Google Scholar]
- 21.Begg CB, Zelen M, Carbone PP, McFadden ET, Brodovsky H, Engstrom P, Hatfield A, Ingle J, Schwartz B, Stolbach L. Cooperative groups and community hospitals. Measurement of impact in the community hospitals. Cancer. 1983 Nov 1;52(9):1760–7. doi: 10.1002/1097-0142(19831101)52:9<1760::aid-cncr2820520934>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 22.Hunter CP, Frelick RW, Feldman AR, Bavier AR, Dunlap WH, Ford L, Henson D, Macfarlane D, Smart CR, Yancik R, et al. Selection factors in clinical trials: results from the Community Clinical Oncology Program Physician’s Patient Log. Cancer Treat Rep. 1987 Jun;71(6):559–65. [PubMed] [Google Scholar]
- 23.McCusker J, Wax A, Bennett JM. Cancer patient accessions into clinical trials. Am J Clin Oncol (CCT) 1982;5:227–36. doi: 10.1097/00000421-198204000-00072. [DOI] [PubMed] [Google Scholar]
- 24.Simon MS, Brown DR, Du W, LoRusso P, Kellogg CM. Accrual to breast cancer clinical trials at a university-affiliated hospital in metropolitan Detroit. Am J Clin Oncol. 1999 Feb;22(1):42–6. doi: 10.1097/00000421-199902000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Green S, Benedetti J, Crowley J. Clinical Trials in Oncology. 2. Boca Raton, FL: CRC Press; 2003. [Google Scholar]
- 26.Newhouse JP, McClellan M. Econometrics in outcomes research: The use of instrumental variables. Annu Rev Public Health. 1998;19:17–34. doi: 10.1146/annurev.publhealth.19.1.17. [DOI] [PubMed] [Google Scholar]
- 27.Langford AT, Resnicow K, Dimond EP, Denicoff AM, Germain DS, McCaskill-Stevens W, Enos RA, Carrigan A, Wilkinson K, Go RS. Racial/ethnic differences in clinical trial enrollment, refusal rates, ineligibility, and reasons for decline among patients at sites in the National Cancer Institute’s Community Cancer Centers Program. Cancer. 2014 Mar 15;120(6):877–84. doi: 10.1002/cncr.28483. Epub 2013 Dec 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Unger JM, Barlow WE, Martin DP, Ramsey SD, Leblanc M, Etzioni R, Hershman DL. Comparison of survival outcomes among cancer patients treated in and out of clinical trials. J Natl Cancer Inst. 2014 Mar;106(3):dju002. doi: 10.1093/jnci/dju002. Epub 2014 Mar 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clisant S, Clermont A, Adenis A, Penel N. Inflation in the number of eligibility criteria for industry-sponsored phase II cancer clinical trial: illustration over a 20-year period. Contemp Clin Trials. 2012 May;33(3):459. doi: 10.1016/j.cct.2012.02.009. Epub 2012 Feb 16. [DOI] [PubMed] [Google Scholar]
- 30.Somkin CP, Altschuler A, Ackerson L, Geiger AM, Greene SM, Mouchawar J, Holup J, Fehrenbacher L, Nelson A, Glass A, Polikoff J, Tishler S, Schmidt C, Field T, Wagner E. Organization barriers to physician participation in cancer clinical trials. Am J Manag Care. 2005 Jul;11(7):413–21. [PubMed] [Google Scholar]
- 31.Benson AB, 3rd, Pregler JP, Bean JA, Rademaker AW, Eshler B, Anderson K. Oncologists’ reluctance to accrue patients onto clinical trials: an Illinois Cancer Center study. J Clin Oncol. 1991 Nov;9(11):2067–75. doi: 10.1200/JCO.1991.9.11.2067. [DOI] [PubMed] [Google Scholar]
- 32.Melisko ME, Hassin F, Metzroth L, Moore DH, Brown B, Patel K, Rugo HS, Tripathy D. Patient and physician attitudes toward breast cancer clinical trials: developing interventions based on understanding barriers. Clin Breast Cancer. 2005 Apr;6(1):45–54. doi: 10.3816/CBC.2005.n.008. [DOI] [PubMed] [Google Scholar]
- 33.Prescott RJ, Counsell CE, Gillespie WJ, Grant AM, Russell IT, Kiauka S, Colthart IR, Ross S, Shepherd SM, Russell D. Factors that limit the quality, number and progress of randomised controlled trials. Health Technol Assess. 1999;3(20):1–143. [PubMed] [Google Scholar]
- 34.Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participationin randomized controlled trials: a systematic review. J Clin Epidemiol. 1999 Dec;52(12):1143–56. doi: 10.1016/s0895-4356(99)00141-9. [DOI] [PubMed] [Google Scholar]
- 35.Taylor KM, Margolese RG, Soskolne CL. Physicians’ reasons for not entering eligible patients in a randomized clinical trial of surgery for breast cancer. N Engl J Med. 1984 May 24;310(21):1363–7. doi: 10.1056/NEJM198405243102106. [DOI] [PubMed] [Google Scholar]
- 36.Institute of Medicine. Transforming Clinical Research in the United States: Challenges and Opportunities: Workshop Summary. Washington, DC: The National Academies Press; 2010. [PubMed] [Google Scholar]
- 37.Siminoff LA, Zhang A, Colabianchi N, Sturm CM, Shen Q. Factors that predict the referral of breast cancer patients onto clinical trials by their surgeons and medical oncologists. J Clin Oncol. 2000 Mar;18(6):1203–11. doi: 10.1200/JCO.2000.18.6.1203. [DOI] [PubMed] [Google Scholar]
- 38.Ellis PM, Butow PN, Tattersall MH, Dunn SM, Houssami N. Randomized clinical trials in oncology: understanding and attitudes predict willingness to participate. J Clin Oncol. 2001 Aug 1;19(15):3554–61. doi: 10.1200/JCO.2001.19.15.3554. [DOI] [PubMed] [Google Scholar]
- 39.Cassileth BR, Lusk EJ, Miller DS, Hurwitz S. Attitudes toward clinical trials among patients and the public. JAMA. 1982 Aug 27;248(8):968–70. [PubMed] [Google Scholar]
- 40.Daugherty C, Ratain MJ, Grochowski E, Stocking C, Kodish E, Mick R, Siegler M. Perceptions of cancer patients and their physicians involved in phase I trials. J Clin Oncol. 1995 May;13(5):1062–72. doi: 10.1200/JCO.1995.13.5.1062. [DOI] [PubMed] [Google Scholar]
- 41.Ford JG, Howerton MW, Lai GY, Gary TL, Bolen S, Gibbons MC, Tilburt J, Baffi C, Tanpitukpongse TP, Wilson RF, Powe NR, Bass EB. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008 Jan 15;112(2):228–42. doi: 10.1002/cncr.23157. Review. [DOI] [PubMed] [Google Scholar]
- 42.Jones JH. Bad Blood: The Tuskegee Syphilis Experiment. New York: Free Press; 1993. [Google Scholar]
- 43.Lederer S. Subjected to Science: Human Experimentation in America after the Second World War. Baltimore: Johns Hopkins University Press; 1995. [PubMed] [Google Scholar]
- 44.Coyne CA, Xu R, Raich P, Plomer K, Dignan M, Wenzel LB, Fairclough D, Habermann T, Schnell L, Quella S, Cella D. Randomized, controlled trial of an easy-to-read informed consent statement for clinical trial participation: a study of the Eastern Cooperative Oncology Group. J Clin Oncol. 2003 Mar 1;21(5):836–42. doi: 10.1200/JCO.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 45.Jenkins V, Fallowfield L. Reasons for accepting or declining to participate in randomized clinical trials for cancer therapy. Br J Cancer. 2000 Jun;82(11):1783–8. doi: 10.1054/bjoc.2000.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Llewellyn-Thomas HA, McGreal MJ, Thiel EC, Fine S, Erlichman C. Patients’ willingness to enter clinical trials: measuring the association with perceived benefit and preference for decision participation. Soc Sci Med. 1991;32(1):35–42. doi: 10.1016/0277-9536(91)90124-u. [DOI] [PubMed] [Google Scholar]
- 47.McQuellon RP, Muss HB, Hoffman SL, Russell G, Craven B, Yellen SB. Patient preferences for treatment of metastatic breast cancer: a study of women with early-stage breast cancer. J Clin Oncol. 1995 Apr;13(4):858–68. doi: 10.1200/JCO.1995.13.4.858. [DOI] [PubMed] [Google Scholar]
- 48.Jenkins VA, Fallowfield LJ, Souhami A, Sawtell M. How do doctors explain randomized clinical trials to the patients? Eur J Cancer. 1999 Aug;35(8):1187–93. doi: 10.1016/s0959-8049(99)00116-1. [DOI] [PubMed] [Google Scholar]
- 49.Hietanen P, Aro AR, Holli K, Absetz P. Information and communication in the context of a clinical trial. Eur J Cancer. 2000 Oct;36(16):2096–104. doi: 10.1016/s0959-8049(00)00191-x. [DOI] [PubMed] [Google Scholar]
- 50.Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999 Dec 30;341(27):2061–7. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 51.Lewis JH, Kilgore ML, Goldman DP, Trimble EL, Kaplan R, Montello MJ, Housman MG, Escarce JJ. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003 Apr 1;21(7):1383–9. doi: 10.1200/JCO.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 52.Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004 Nov 15;22(22):4626–31. doi: 10.1200/JCO.2004.02.175. [DOI] [PubMed] [Google Scholar]
- 53.Unger JM, Coltman CA, Jr, Crowley JJ, Hutchins LF, Martino S, Livingston RB, Macdonald JS, Blanke CD, Gandara DR, Crawford ED, Albain KS. Impact of the year 2000 Medicare policy change on older patient enrollment to cancer clinical trials. J Clin Oncol. 2006 Jan 1;24(1):141–4. doi: 10.1200/JCO.2005.02.8928. Epub 2005 Dec 5. [DOI] [PubMed] [Google Scholar]
- 54.Kemeny MM, Peterson BL, Kornblith AB, Muss HB, Wheeler J, Levine E, Bartlett N, Fleming G, Cohen HJ. Barriers to clinical trial participation by older women with breast cancer. J Clin Oncol. 2003 Jun 15;21(12):2268–75. doi: 10.1200/JCO.2003.09.124. [DOI] [PubMed] [Google Scholar]
- 55.Kornblith AB, Kemeny M, Peterson BL, Wheeler J, Crawford J, Bartlett N, Fleming G, Graziano S, Muss H, Cohen HJ Cancer and Leukemia Group B. Survey of oncologists’ perceptions of barriers to accrual of older patients with breast carcinoma to clinical trials. Cancer. 2002 Sep 1;95(5):989–96. doi: 10.1002/cncr.10792. [DOI] [PubMed] [Google Scholar]
- 56.Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol. 2005 May 1;23(13):3112–24. doi: 10.1200/JCO.2005.00.141. [DOI] [PubMed] [Google Scholar]
- 57.Havlik RJ, Yancik R, Long S, Ries L, Edwards B. The National Institute on Aging and the National Cancer Institute SEER collaborative study of comorbidity and early diagnosis of cancer in the elderly. Cancer. 1994 Oct 1;74(7 Suppl):2101–6. doi: 10.1002/1097-0142(19941001)74:7+<2101::aid-cncr2820741718>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 58.Yancik R. Cancer burden in the aged: an epidemiologic and demographic overview. Cancer. 1997 Oct 1;80(7):1273–83. [PubMed] [Google Scholar]
- 59.Medicare Coverage ~ Clinical Trials: Final National Coverage Decision. Available online at: https://www.cms.gov/Medicare/Coverage/ClinicalTrialPolicies/downloads/finalnationalcoverage.pdf.
- 60.Stewart JH, Bertoni AG, Staten JL, Levine EA, Gross CP. Participation in surgical oncology clinical trials: gender-, race/ethnicity-, and age-based disparities. Ann Surg Oncol. 2007 Dec;14(12):3328–34. doi: 10.1245/s10434-007-9500-y. Epub 2007 Aug 8. [DOI] [PubMed] [Google Scholar]
- 61.Gross CP, Filardo G, Mayne ST, Krumholz HM. The impact of socioeconomic status and race on trial participation for older women with breast cancer. Cancer. 2005 Feb 1;103(3):483–91. doi: 10.1002/cncr.20792. [DOI] [PubMed] [Google Scholar]
- 62.Cook ED, Moody-Thomas S, Anderson KB, Campbell R, Hamilton SJ, Harrington JM, Lippman SM, Minasian LM, Paskett ED, Craine S, Arnold KB, Probstfield JL. Minority recruitment to the Selenium and Vitamin E Cancer Prevention Trial (SELECT) Clin Trials. 2005;2(5):436–42. doi: 10.1191/1740774505cn111oa. [DOI] [PubMed] [Google Scholar]
- 63.Cook ED, Arnold KB, Hermos JA, McCaskill-Stevens W, Moody-Thomas S, Probstfield JL, Hamilton SJ, Campbell RD, Anderson KB, Minasian LM. Impact of supplemental site grants to increase African American accrual for the Selenium and Vitamin E Cancer Prevention Trial. Clin Trials. 2010 Feb;7(1):90–9. doi: 10.1177/1740774509357227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fouad MN, Corbie-Smith G, Curb D, Howard BV, Mouton C, Simon M, Talavera G, Thompson J, Wang CY, White C, Young R. Special populations recruitment for the Women’s Health Initiative: successes and limitations. Control Clin Trials. 2004 Aug;25(4):335–52. doi: 10.1016/j.cct.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 65.Moinpour CM, Atkinson JO, Thomas SM, Underwood SM, Harvey C, Parzuchowski J, Lovato LC, Ryan AM, Hill MS, Deantoni E, Gritz ER, Thompson IM, Jr, Coltman CA., Jr Minority recruitment in the prostate cancer prevention trial. Ann Epidemiol. 2000 Nov;10(8 Suppl):S85–91. doi: 10.1016/s1047-2797(00)00185-x. [DOI] [PubMed] [Google Scholar]
- 66.Unger JM, Gralow JR, Albain KS, Ramsey SD, Hershman DL. Patient Income Level and Cancer Clinical Trial Participation: A Prospective Survey Study. JAMA Oncol. 2016 Jan 1;2(1):137–9. doi: 10.1001/jamaoncol.2015.3924. No abstract available. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goldman DP, Berry SH, McCabe MS, Kilgore ML, Potosky AL, Schoenbaum ML, Schonlau M, Weeks JC, Kaplan R, Escarce JJ. Incremental treatment costs in national cancer institute-sponsored clinical trials. JAMA. 2003 Jun 11;289(22):2970–7. doi: 10.1001/jama.289.22.2970. [DOI] [PubMed] [Google Scholar]
- 68.National Center for Health Statistics. [Accessed on September 13, 2010]; Available at: http://www.cdc.gov/nchs.
- 69.Bleyer A. Potential favorable impact of the Affordable Care Act of 2010 on cancer in young adults in the United States. The Cancer J. 2010;16(6):563–573. doi: 10.1097/PPO.0b013e3181ff6509. [DOI] [PubMed] [Google Scholar]
- 70.Bleyer A, Barr R, Hayes-Lattin B, Thomas D, Ellis C, Anderson B. The distinctive biology of cancer in adolescents and young adults. Nature Reviews Cancer. 2008;8(4):288–298. doi: 10.1038/nrc2349. [DOI] [PubMed] [Google Scholar]
- 71.Bleyer WA, Barr R, Keohan ML. ASCO 2001 Educational Book. Alexandria, VA: ASCO; 2001. Adolescents and young adults with cancer: what will it take to improve outcome; pp. 125–140. [Google Scholar]
- 72.Martin S, Ulrich C, Munsell M, Taylor S, Lange G, Bleyer A. Delays in cancer diagnosis in underinsured young adults and older adolescents. Oncologist. 2007 Jul;12(7):816–24. doi: 10.1634/theoncologist.12-7-816. [DOI] [PubMed] [Google Scholar]
- 73. [Accessed January 28, 2016];National Intercensal Estimates (2000–2010) http://www.census.gov/popest/data/intercensal/national/nat2010.html.
- 74.Joinpoint Regression Program, Version 4.1.1. Statistical Research and Applications Branch, National Cancer Institute; Aug, 2014. [Accessed January 15, 2016]. http://surveillance.cancer.gov/joinpoint. [Google Scholar]
- 75.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2014 Sub (1973–2012) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2013 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2015, based on the November 2014 submission.
- 76.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 13 Regs Research Data, Nov 2014 Sub (1992–2012) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2013 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2015, based on the November 2014 submission.
- 77.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2014 Sub (1973–2012 varying) - Linked To County Attributes - Total U.S., 1969–2013 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2015, based on the November 2014 submission.
- 78.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Mortality - All COD, Aggregated With State, Total U.S. (1969–2012) <Katrina/Rita Population Adjustment>, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2015. Underlying mortality data provided by NCHS (www.cdc.gov/nchs).
- 79.Breitenbach K, Stock W. Intergroup Trial C10403: A Pediatric Treatment Approach to Improve Outcomes in Adolescents and Young Adults with Acute Lymphoblastic Leukemia. J Adolesc Young Adult Oncol. 2011 Jun;1(2):107–108. doi: 10.1089/jayao.2011.1511. [DOI] [PubMed] [Google Scholar]
- 80. [Accessed January 28, 2016];Chemotherapy with liposomal cytarabine CNS prophylaxis for adult acute lymphoblastic leukemia & lymphoblastic lymphoma. https://clinicaltrials.gov/ct2/show/NCT02043587?term=acute+lymphoblastic+leukemia+wieduwilt&rank=1.
- 81.DeAngelo DJ, Stevenson KE, Dahlberg SE, et al. Long-term outcome of a pediatric-inspired regimen used for adults aged 18–50 years with newly diagnosed acute lymphoblastic leukemia. Leukemia. 2015 Mar;29(3):526–534. doi: 10.1038/leu.2014.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bleyer A. How NCCN guidelines can help young adults and older adolescents with cancer and the professionals who care for them. J Natl Compr Canc Netw. 2012;10(9):1065–1071. doi: 10.6004/jnccn.2012.0112. [DOI] [PubMed] [Google Scholar]
- 83.Dombret H, Cluzeau T, Huguet F, Boissel N. Pediatric-like therapy for adults with ALL. Curr Hematol Malig Rep. 2014 Jun;9(2):158–164. doi: 10.1007/s11899-014-0210-9. [DOI] [PubMed] [Google Scholar]
- 84.Hallböök H, Gustafsson G, Smedmyr B, et al. Treatment outcome in young adults and children >10 years of age with acute lymphoblastic leukemia in Sweden: a comparison between a pediatric protocol and an adult protocol. Cancer. 2006 Oct 1;107(7):1551–61. doi: 10.1002/cncr.22189. [DOI] [PubMed] [Google Scholar]
- 85.Hayakawa F, Sakura T, Yujiri T, et al. Markedly improved outcomes and acceptable toxicity in adolescents and young adults with acute lymphoblastic leukemia following treatment with a pediatric protocol: a phase II study by the Japan Adult Leukemia Study Group. Blood Cancer J. 2014 Oct;4(10):e252. doi: 10.1038/bcj.2014.72. Published online 2014 Oct 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hocking J, Schwarer AP, Gasiorowski R, et al. Excellent outcomes for adolescents and adults with acute lymphoblastic leukemia and lymphoma without allogeneic stem cell transplant: the FRALLE-93 pediatric protocol. Leuk Lymphoma. 2014 Dec;55(12):2801–2807. doi: 10.3109/10428194.2014.894191. [DOI] [PubMed] [Google Scholar]
- 87.Ram R, Wolach O, Vidal L, et al. Adolescents and young adults with acute lymphoblastic leukemia have a better outcome when treated with pediatric-inspired regimens: systematic review and meta-analysis. Am J Hematol. 2012 May;87(5):472–478. doi: 10.1002/ajh.23149. [DOI] [PubMed] [Google Scholar]
- 88.Ribera JM, Oriol A, Sanz MA, et al. Comparison of the results of the treatment of adolescents and young adults with standard-risk acute lymphoblastic leukemia with the Programa Español de Tratamiento en Hematología pediatric-based protocol ALL-96. J Clin Oncol. 2008 Apr 10;26(11):1843–1849. doi: 10.1200/JCO.2007.13.7265. [DOI] [PubMed] [Google Scholar]
- 89.Usvasalo A, Räty R, Knuutila S, Vettenranta K, et al. Acute lymphoblastic leukemia in adolescents and young adults in Finland. Haematologica. 2008 Aug;93(8):1161–1168. doi: 10.3324/haematol.12466. [DOI] [PubMed] [Google Scholar]
- 90.Stock W, La M, Sanford B, et al. What determines the outcomes of adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? Blood. 2008;112(5):1646–1654. doi: 10.1182/blood-2008-01-130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Douer D, DeAngelo DJ, Advani A, Arellano M, Litzow M, Damon L, Kovacsovics T, Luger S, Seibel N, Bleyer A. Applying pediatric therapeutic strategies to adults with acute lymphoblastic leukemia and lymphoma. II. Comparison with adult treatment regimens, including hyper-CVAD. Amer Oncol & Hemat Rev. 2014:47–53. [Google Scholar]
- 92.Collins CL, Malvar J, Hamilton AS, Deapen DM, Freyer DR. Case-linked analysis of clinical trial enrollment among adolescents and young adults at a National Cancer Institute-Designated comprehensive cancer center. Cancer. 2015 Dec 15;121(24):4398–4406. doi: 10.1002/cncr.29669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sender L, Zabokrtsky KB. Adolescent and young adult patients with cancer: a milieu of unique features. Nat Rev Clin Oncol. 2015 Aug;12(8):465–480. doi: 10.1038/nrclinonc.2015.92. [DOI] [PubMed] [Google Scholar]
- 94.Freyer DR, Felgenhauer J, Perentesis J. COG Adolescent and Young Adult Oncology Discipline Committee. Children’s Oncology Group’s 2013 blueprint for research: adolescent and young adult oncology. Pediatr Blood Cancer. 2013 Jun;60(6):1055–8. doi: 10.1002/pbc.24431. Epub 2012 Dec 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Weiss AR, Nichols CR, Freyer DR. Enhancing adolescent and young adult oncology research within the National Clinical Trials Network: Rationale, progress, and emerging strategies. Semin Oncol. 2015 Oct;42(5):740–747. doi: 10.1053/j.seminoncol.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stark D, Bowen D, Dunwoodie E, Feltbower R, Johnson R, Moran A, Stiller C, O’Hara C. Survival patterns in teenagers and young adults with cancer in the United Kingdom: Comparisons with younger and older age groups. Eur J Cancer. 2015 Nov;51(17):2643–2654. doi: 10.1016/j.ejca.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 97.Denicoff AM1, McCaskill-Stevens W, Grubbs SS, Bruinooge SS, Comis RL, Devine P, Dilts DM, Duff ME, Ford JG, Joffe S, Schapira L, Weinfurt KP, Michaels M, Raghavan D, Richmond ES, Zon R, Albrecht TL, Bookman MA, Dowlati A, Enos RA, Fouad MN, Good M, Hicks WJ, Loehrer PJ, Sr, Lyss AP, Wolff SN, Wujcik DM, Meropol NJ. The National Cancer Institute-American Society of Clinical Oncology Cancer Trial Accrual Symposium: summary and recommendations. J Oncol Pract. 2013 Nov;9(6):267–76. doi: 10.1200/JOP.2013.001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.AccrualNet. Strategies, tools, and resources to support accrual to clinical trials. Available at: https://accrualnet.cancer.gov/
- 99.Barrios CH, Werutsky G, Martinez-Mesa J. The global conduct of cancer clinical trials: challenges and opportunities. American Society of Clinical Oncology Education Book. 2015:e132–e139. doi: 10.14694/EdBook_AM.2015.35.e132. [DOI] [PubMed] [Google Scholar]
- 100.Tierney G, Sieher S. Key strategies for effective globalization of clinical trials. Pharmaceutical Outsourcing. 2013 Sep 25; [Google Scholar]
- 101.Butts C, Socinski MA, Mitchell P, Thatcher N, Havel L, Krzakowski M, Nawrocki S, Ciuleanu TE, Bosquée L, Trigo JM, Spira A, Tremblay L, Nyman J, Ramlau R, Helwig C, Falk MH, Shepherd FA. START: A phase III study of L-BLP25 cancer immunotherapy for unresectable stage III non-small cell lung cancer. American Society of Clinical Oncology - 49th Annual Meeting; 2013; Abstr No. 7500. [Google Scholar]
- 102.Gaumond B, Ferrande L, Houde J, Braman M, Olchowka K, Batelaan JH, Koh P, Molnar R. Global patient recruitment continuum for a phase III non-small cell lung cancer clinical trial. NCI - ASCO Cancer Trial Accrual Symposium: Science and Solutions 2010. Track 3: Site Leadership, Infrastructure and Operations; Abstract Page 20. [Google Scholar]
- 103.Moore HC, Unger JM, Phillips KA, Boyle F, Hitre E, Porter D, Francis PA, Goldstein LJ, Gomez HL, Vallejos CS, Partridge AH, Dakhil SR, Garcia AA, Gralow J, Lombard JM, Forbes JF, Martino S, Barlow WE, Fabian CJ, Minasian L, Meyskens FL, Jr, Gelber RD, Hortobagyi GN, Albain KS POEMS/S0230 Investigators. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Engl J Med. 2015 Mar 5;372(10):923–32. doi: 10.1056/NEJMoa1413204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Frandsen M, Walters J, Ferguson SG. Exploring the viability of using online social media advertising as a recruitment method for smoking cessation clinical trials. Nicotine Tob Res. 2014 Feb;16(2):247–51. doi: 10.1093/ntr/ntt157. Epub 2013 Oct 14. [DOI] [PubMed] [Google Scholar]
- 105.Lamberti MJ, Kush R, Kubick W, Henderson C, Hinkson B, Kamenju P, Getz K. An examination of eClinical Technology usage and CDISC standard adoption. Therapeutics Innovation and Regulatory Science. 2015 May 19; doi: 10.1177/2168479015586003. [DOI] [PubMed] [Google Scholar]
- 106.Top considerations for using social media in research- it all starts with a plan. Quorum Review IRB. Downloaded 2/15/2015 emailed 2/15/16
- 107.Recruiting Study Subjects - Information Sheet. 2016 Jan 25; www.fda.gov/regulatoryinformation/guidances/ucm126428.htm Downloaded 2/15/2016.
- 108.Office of Inspector General: Clinical Trial Web Sites. A promising tool to foster informed consent. http://oig.hhs.gov/oei/reports/oei-01-97-00198.pdf.
- 109.Whitepaper E-Recruiting: using digital platforms, social, media, and mobile technologies to improve clinical trial enrollment. Ventiv Health Clinical Division; Oct 14, 2013. [Google Scholar]
- 110.Gossen R. SoCRA. Jul 9, 2014. Great facebook pages for research sites. Engage and recruit clinical research participants. PDF of presentation. [Google Scholar]
- 111.American Cancer Society. Cancer Treatment and Survivorship Facts & Figures 2014–2015. Atlanta: American Cancer Society; 2014. [Google Scholar]
- 112.Denson AC, Mahipal A. Participation of the elderly population in clinical trials: barriers and solutions. Cancer Control. 2014 Jul;21(3):209–14. doi: 10.1177/107327481402100305. [DOI] [PubMed] [Google Scholar]
- 113.Chen H, Cantor A, Meyer J, Beth Corcoran M, Grendys E, Cavanaugh D, Antonek S, Camarata A, Haley W, Balducci L, Extermann M. Can older cancer patients tolerate chemotherapy? A prospective pilot study. Cancer. 2003 Feb 15;97(4):1107–14. doi: 10.1002/cncr.11110. [DOI] [PubMed] [Google Scholar]
- 114.Mariano C, Franl M, Pope J, Wong L, Lim HJ, Lohrisch C. Comparison of toxicity experienced by older versus younger patients enrolled in breast cancer clinical trials. Clin Breast Cancer. 2015 Feb;15(1):73–9. doi: 10.1016/j.clbc.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 115.Sud S, Lai P, Zhang T, Clemons M, Wheatley-Price P. Chemotherapy in the oldest old: The feasibility of delivering cytotoxic therapy to patients 80 years old and older. J Geriatr Oncol. 2015 Sep;6(5):395–400. doi: 10.1016/j.jgo.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 116.Extermann M, Aapro M, Bernabei R, Cohen HJ, Droz JP, Lichtman S, Mor V, Monfardini S, Repetto L, Sorbye L, Topinkova E. Task Force on CGA of the International Society of Geriatric Oncology. Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG) Crit Rev Oncol Hematol. 2005 Sep;55(3):241–52. doi: 10.1016/j.critrevonc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 117.Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007 May 10;25(14):1824–31. doi: 10.1200/JCO.2007.10.6559. [DOI] [PubMed] [Google Scholar]
- 118.Hamaker ME, Jonker JM, de Rooij SE, Vos AG, Smorenburg CH, van Munster BC. Frailty screening methods for predicting outcome of a comprehensive geriatric assessment in elderly patients with cancer: a systematic review. Lancet Oncol. 2012 Oct;13(10):e437–44. doi: 10.1016/S1470-2045(12)70259-0. [DOI] [PubMed] [Google Scholar]
- 119.Kellen E, Bulens P, Deckx L, Schouten H, Van Dijk M, Verdonck I, Buntinx F. Identifying an accurate pre-screening tool in geriatric oncology. Crit Rev Oncol Hematol. 2010 Sep;75(3):243–8. doi: 10.1016/j.critrevonc.2009.12.002. Epub 2010 Jan 13. [DOI] [PubMed] [Google Scholar]
- 120.Luciani A, Ascione G, Bertuzzi C, Marussi D, Codecà C, Di Maria G, Caldiera SE, Floriani I, Zonato S, Ferrari D, Foa P. Detecting disabilities in older patients with cancer: comparison between comprehensive geriatric assessment and vulnerable elders survey-13. J Clin Oncol. 2010 Apr 20;28(12):2046–50. doi: 10.1200/JCO.2009.25.9978. Epub 2010 Mar 22. [DOI] [PubMed] [Google Scholar]
- 121.Balducci L, Colloca G, Cesari M, Gambassi G. Assessment and treatment of elderly patients with cancer. Surg Oncol. 2010;19:117–123. doi: 10.1016/j.suronc.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 122.Grady C. Payment of clinical research subjects. J Clin Invest. 2005 Jul;115(7):1681–7. doi: 10.1172/JCI25694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bernstein M. Payment of research subjects involved in clinical trials is unethical. Journal of Neuro-Oncology. 2003;63:223–224. doi: 10.1023/a:1024211909893. [DOI] [PubMed] [Google Scholar]
- 124.Grady C, Dickert N, Jawetz T, Gensler G, Emanuel E. An analysis of U.S. practices of paying research participants. Contemp Clin Trials. 2005 Jun;26(3):365–75. doi: 10.1016/j.cct.2005.02.003. Epub 2005 Mar 28. [DOI] [PubMed] [Google Scholar]
- 125.U.S. Department of Health and Human Services. Federal Policy for the Protection of Human Subjects (‘Common Rule’) Available at: http://www.hhs.gov/ohrp/humansubjects/commonrule/
- 126.Unger JM, Barlow WE, Ramsey SD, LeBlanc M, Blanke CD, Hershman DL. The scientific impact of positive and negative phase 3 cancer clinical trials. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2015.6487. In press. [DOI] [PMC free article] [PubMed]
- 127.Bleyer A. In and out, good and bad news, of generalizability of SWOG treatment trial results. JNCI. 2014 Mar;106(3):dju027. doi: 10.1093/jnci/dju027. Epub 2014 Mar 13. [DOI] [PubMed] [Google Scholar]