Percutaneous microwave ablation of stage T1a renal cell carcinoma is an effective and safe treatment option at short- and intermediate-term follow-up.
Abstract
Purpose
To evaluate the effects of tumor complexity and technique on early and midterm oncologic efficacy and rate of complications for 100 consecutive biopsy-proved stage T1a renal cell carcinomas (RCCs) treated with percutaneous microwave ablation.
Materials and Methods
This HIPAA-compliant, single-center retrospective study was approved by the institutional review board. The requirement to obtain informed consent was waived. Ninety-six consecutive patients (68 men, 28 women; mean age, 66 years ± 9.4) with 100 stage T1a N0M0 biopsy-proved RCCs (median diameter, 2.6 cm ± 0.8) underwent percutaneous microwave ablation between March 2011 and June 2015. Patient and procedural data were collected, including body mass index, comorbidities, tumor histologic characteristics and grade, RENAL nephrometry score, number of antennas, generator power, and duration of ablation. Technical success, local tumor progression, and presence of complications were assessed at immediate and follow-up imaging. The Kaplan-Meier method was used for survival analyses.
Results
Technical success was achieved for all 100 tumors (100%), including 47 moderately and five highly complex RCCs. Median clinical and imaging follow-up was 17 months (range, 0–48 months) and 15 months (range, 0–44 months), respectively. No change in estimated glomerular filtration rate was noted after the procedure (P = .49). There were three (3%) procedure-related complications and six (6%) delayed complications, all urinomas. One case of local tumor progression (1%) was identified 25 months after the procedure. Three-year local progression-free survival, cancer-specific survival, and overall survival were 88% (95% confidence interval: 0.52%, 0.97%), 100% (95% confidence interval: 1.0%, 1.0%), and 91% (95% confidence interval: 0.51%, 0.99%), respectively.
Conclusion
Percutaneous microwave ablation is an effective and safe treatment option for stage T1a RCC, regardless of tumor complexity. Long-term follow-up is needed to establish durable oncologic efficacy and survival relative to competing ablation modalities and surgery.
© RSNA, 2017
Introduction
The detection of renal cell carcinoma (RCC) is increasing owing to an increase in the diagnosis of small asymptomatic renal masses with cross-sectional imaging (1). In patients with clinical stage T1a RCC (≤4 cm) who are candidates for surgery, surgery is the standard treatment—with increasing emphasis being given to nephron-sparing techniques (2,3). However, less invasive options such as active surveillance or percutaneous thermal ablation are increasingly being used as alternatives to surgery for selected patients (4,5). For patients who are poor surgical candidates, percutaneous thermal ablation is an option that may provide local control of the tumor with short convalescence, few serious complications, and the ability to preserve kidney function (2,5–8).
The two most widely applied thermal ablation methods for the treatment of RCC to date are radiofrequency (RF) ablation and cryoablation. Microwave ablation, like RF ablation, is a heat-based thermal ablation modality with an identical mechanism of cell death but several physical advantages for heat delivery (6,9). During the past decade, higher power microwave systems have been developed that offer potential advantages over older systems, but few studies have reported outcomes in patients with RCC treated with these modern microwave systems.
Historically, percutaneous thermal ablation was reserved for small (<3 cm) exophytic tumors arising from the posterior kidney. With increased use of ablation devices that harness thermal synergy and procedural strategies to displace nontarget anatomy, the size and location of successfully treated tumors has expanded (10). However, the rate of procedure-related complications has increased in step (11). Tumor complexity, a summation of renal tumor size, location, and depth, has been associated with complications and local recurrence after thermal ablation (12–14). The purpose of our study was to evaluate the effects of tumor complexity and technique on early and midterm oncologic efficacy and the rate of complications for 100 consecutive biopsy-proved stage T1a RCC tumors treated with percutaneous microwave ablation.
Materials and Methods
Patient Selection
This single-center retrospective study was compliant with the Health Insurance Portability and Accountability Act. The requirement to obtain informed consent was waived by the institutional review board.
One hundred biopsy-proved clinical stage T1a RCCs were treated in 96 patients (28 women, 68 men; mean age ± standard deviation, 66 years ± 9.4; mean body mass index, 32.2 kg/m2 ± 8.6) during 98 treatment sessions. The cohort of consecutive patients with previously untreated clinical stage T1a (≤4 cm) biopsy-proved RCC who underwent microwave ablation from March 2011, the inception of our microwave ablation program, to June 2015 was identified from an institutional database. Fifty-three patients (55 RCCs) were included in an earlier feasibility study (15). Our study includes a larger cohort, stratified analysis of technique effectiveness for low and moderate and/or high complexity tumors, and technical considerations to mitigate procedure-related complications. The decision for each patient to undergo microwave ablation was made in consensus by a team of subspecialty radiologists and urologists experienced in tumor ablation and kidney surgery, respectively. The decision to treat was based on age, comorbidities, histologic tumor characteristics, tumor size and location, and proximity of nontarget anatomy. Microwave ablation was performed by one of six radiologists (1–19 years of experience) and one of four urologists (1–15 years of experience).
Procedure
All procedures were performed in a computed tomography (CT) suite (Optima 580 W; GE Healthcare, Waukesha, Wis) with the patient under general anesthesia (92 of 96 patients, 96%) or deep conscious sedation (four of 96 patients, 4%). Immediately before the procedure, an indwelling bladder catheter was placed and a single dose of intravenous prophylactic antibiotics was administered to cover skin organisms (first-generation cephalosporin or clindamycin, weight-based dosing) according to the routine standard of care at our institution. Ultrasonography (US) (LOGIQ E9, GE Healthcare), CT fluoroscopy (Lightspeed 580, GE Healthcare), or a combination of both were used for antenna placement. A 2.45-GHz, gas-cooled microwave ablation system with 17-gauge antennas that can be powered simultaneously was used for all cases (Certus 140; NeuWave Medical, Madison, Wis). The LK (Liver-Kidney) microwave antenna was used before the introduction of the Precision PR antenna in early 2012. Relative to the LK antenna, Precision PR antennas create smaller and more spherical ablations at maximum power. There were no staged treatments and treatment intent was curative for all cases.
Hydrodisplacement was used in cases when nontarget anatomy was within 1 cm of the tumor or within the expected zone of ablation (16). For hydrodisplacement, faintly radiopaque (2% iohexol solution) normal saline was manually infused through an 18- or 20-gauge introducer placed between the tumor and nontarget anatomy until an adequate margin of safety was achieved. When placing multiple antennas and/or when CT was performed to confirm precise antenna location and proximity of nontarget anatomy, each antenna was “fixed” to the retroperitoneal tissues by using the cryogenic cooling system (17).
Antennas were typically placed with an initial goal of applying microwave power at maximum power for 5 minutes. The number of antennas used was dependent on the size of the index tumor, the volume of endophytic component, and the proximity of nontarget anatomy. In general, tumors 2.5 cm or smaller were treated with one antenna, tumors 2.5–3.5 cm were treated with two antennas, and tumors at least 3.5 cm were treated with three antennas. US or CT fluoroscopy was used for real-time monitoring of the ablation zone and its proximity to nontarget anatomy.
Contrast material–enhanced CT was performed immediately after the ablation procedure to evaluate technical success and assess for complications. For those with a contraindication to iodinated contrast material (six of 96 patients, 6%), postprocedure unenhanced CT in conjunction with contrast-enhanced US (Definity; Lantheus Medical Imaging, North Billerica, Mass) was used.
After the ablation procedure, all patients were admitted for observation according to the routine standard of care at our institution.
Data Collection and Analysis
Clinical, pathologic, and procedural data for each patient were collected from an institutional database by two authors (M.E.K. and S.A.W., with <1 year and 5 years of experience, respectively). Clinical data collected included patient age, sex, body mass index, Charlson comorbidity index, and estimated glomerular filtration rate (eGFR). The Charlson comorbidity index predicts 1-year mortality on the basis of a tiered scoring system of 22 health disorders (18). The eGFR was calculated by using the Chronic Kidney Disease Epidemiology Collaboration formula (19). The preprocedure eGFR was obtained within 6 months before the ablation procedure, and the postprocedure eGFR was obtained 6 months after the ablation procedure. Pathology data collected included tumor size, histologic characteristics, Fuhrman grade, and tumor complexity as assessed with the RENAL nephrometry score. The RENAL nephrometry score is a summation of radius (tumor size), endophytic or exophytic extent, nearness of the tumor relative to the renal sinus, anterior or posterior descriptor, and location of the tumor relative to the polar lines. A RENAL score of 4–6 corresponds to low tumor complexity; 7–9, moderate tumor complexity; and 10–12, high tumor complexity (20). Procedural data collected included type and number of microwave ablation antennas, duration of ablation, microwave generator output, use and volume of hydrodisplacement, and duration of hospitalization. Complications recorded within 30 days after ablation were classified according to the Clavien-Dindo system (21). Complications recorded after 30 days were described. Clinical follow-up and imaging with contrast-enhanced CT or contrast-enhanced magnetic resonance (MR) imaging were performed at target intervals of 3–6, 12, 18, and every 12 months after ablation. Two fellowship-trained abdominal radiologists (S.A.W. and T.J.Z., with 5 and 7 years of experience in tumor ablation) reviewed images in consensus for technical success, local tumor progression, metastatic disease, and complications. Established criteria were used to define treatment success (22).
Continuous features are summarized as means and standard deviations or medians and interquartile ranges (IQRs). Categoric data are summarized with frequency counts and percentages. A two-tailed paired t test was used to compare pre- and postprocedure eGFRs. The Kaplan-Meier method was used for survival analyses, which included local progression-free survival, cancer-specific survival, and overall survival. The duration of follow-up for progression-free survival was defined from the date of the microwave ablation to the date of last imaging follow-up. The duration of follow-up for cancer specific survival and overall survival was defined from the date of the microwave ablation (or first microwave ablation procedure for the two patients with two tumors treated in separate sessions) to the date of death or the date the patient was last known to be alive. P < .05 was considered indicative of a significant difference for all statistical tests.
Results
Patient and Procedure Data
Single-session treatments were performed for all tumors. Four patients had two tumors; both tumors were treated in a single session for two patients and during separate sessions for two patients. Clear cell RCC was the predominant RCC subtype (73%), followed by papillary (23%) and chromophobe (4%) RCC. Most RCCs were Fuhrman grade 1 (10%) or 2 (55%) at pathologic examination. The Fuhrman grade was not specified in 30 RCCs (30%). The mean pre- and postablation eGFR was 71.8 mL/min/1.73 m2 ± 22.4 and 68.7 mL/min/1.73 m2 ± 23.9, respectively. The median duration of hospitalization was 1 day (IQR, 1–1); three patients stayed longer than 1 day (range, 2–5 days). Clinical and pathologic characteristics are summarized in Table 1.
Table 1.
Patient and Tumor Characteristics
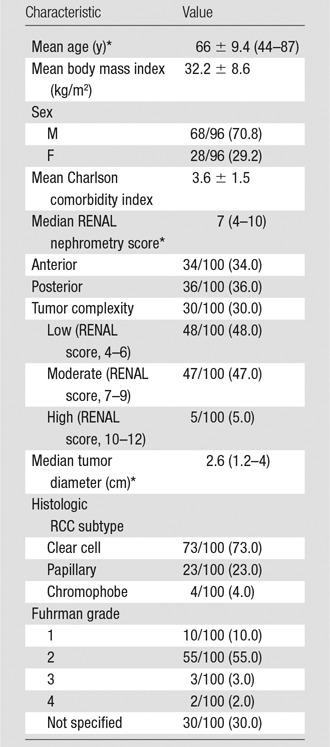
Note.—Except where indicated, data are raw data, with percentages in parentheses.
*Numbers in parentheses are the range.
The median tumor diameter was 2.6 cm (range, 1.2–4 cm; standard deviation, 0.8 cm), and the median RENAL nephrometry score was 7 (range, 4–10). According to the RENAL score, there were 48 low complexity, 47 moderate complexity, and five high complexity tumors. The median diameter of low (2.6 cm), moderate (2.6 cm), and highly complex (2.5 cm) RCCs was similar (P = .2; standard deviation, 0.77 cm). RENAL nephrometry scores are summarized in Table 1. A mean volume of 519 mL (range, 60–1000 mL) of normal saline or 5% dextrose in water was used for hydrodisplacement in 33 of the 96 patients (34.4%).
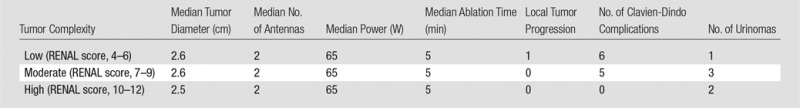
The median number of antennas used was two (IQR, 1–2). The PR antenna was used in 94 of the 100 tumors (94%) and the LK antenna was used in six (6%). One, two, and three antennas were used in 35, 48, and 17 ablations, respectively. The median tumor diameter when one, two, and three antennas were used was 1.9 cm (IQR, 1.5–2 cm), 2.8 cm (IQR, 2.5–3.1 cm), and 3.5 cm (IQR, 3.4–3.7 cm), respectively. The median duration of power application was 5 minutes (IQR, 5–6), and the median generator output was 65 W (IQR, 65–65 W)—maximum power for a PR antenna. There was no significant difference in the number of antennas (median: 2 vs 2 vs 2; range: 1–3 vs 1–2 vs 2–3), generator output (median: 65 W vs 65 W vs 65 W; range: 50–113 W vs 65–65 W vs 65–65 W), or time (median: 5 minutes vs 5 minutes vs 5 minutes; range: 3–20 minutes vs 5–6 minutes vs 5–6 minutes) between low, moderate, and high tumor complexity, respectively, when stratified according to RENAL score (P = .81, standard deviation = 0.72; P = .21, standard deviation = 10.0; P = .43, standard deviation = 2.15, respectively). Ablation protocol and clinical results are summarized in Tables 2 and 3.
Table 2.
Ablation Protocol and Clinical Results

Note.—There were 100 tumors in the entire cohort. Except where indicated, data are raw data, with percentages in parentheses.
*Numbers in parentheses are the range.
†Numbers in parentheses are the IQR.
Table 3.
Tumor Complexity: Ablation Protocol and Clinical Results
Follow-up
Technical success was achieved in all 100 tumors (100%). The median duration of clinical follow-up was 17 months (IQR, 8–28 months). Six patients were lost to follow-up and excluded from survival analysis. The median duration of imaging follow-up was 15 months (IQR, 6–20 months), with 90 patients (93.8%) receiving follow-up imaging in addition to immediate postablation contrast-enhanced CT and/or contrast-enhanced US. Four patients have had at least 3 years of imaging follow-up, 14 have had 2–3 years, 39 (41 tumors) have had 1–2 years, and 39 (41 tumors) have had less than 1 year. Results stratified according to imaging follow-up are summarized in Table 4.
Table 4.
Results according to Follow-up Imaging Time
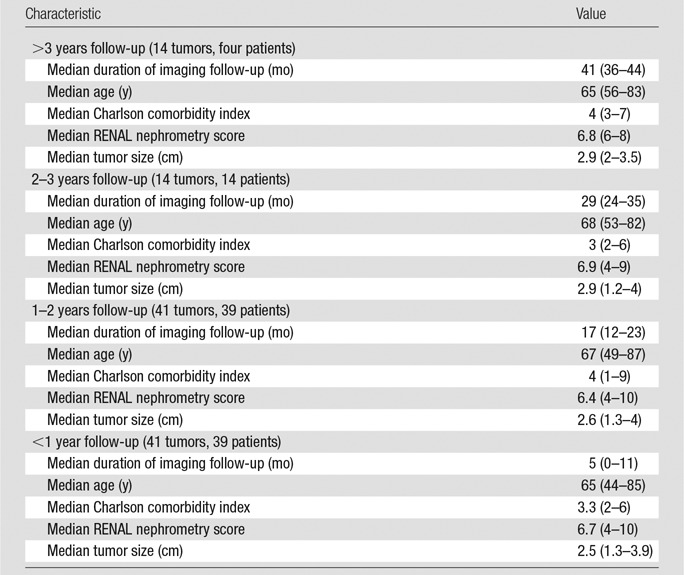
Note.—Numbers in parentheses are the range.
Oncologic Efficacy
There was a single treatment failure (one of 100 RCCs, 1%) in which local tumor progression was identified 25 months after the procedure. This patient had been temporarily lost to follow-up and imaging was not performed 10–25 months after the procedure, during which time the tumor recurred locally. The patient underwent radical nephrectomy and is currently alive without evidence of local tumor progression or metastatic disease. Histologic findings from the percutaneous biopsy before the procedure demonstrated clear cell RCC, Fuhrman grade 2. However, the tumor was upgraded to Fuhrman grade 4 with rhabdoid features at explant histologic examination.
To date, there have been no instances of progression to metastatic disease and no RCC-related deaths. Three patients in this series have died of unrelated causes: one from a myocardial infarction at 5 months after the procedure, one from lymphoma at 9 months, and one from a gastrointestinal bleed at 39 months. Three-year local progression-free, cancer-specific, and overall survival were 88% (95% confidence interval: 0.52, 0.97), 100% (95% confidence interval: 1.0, 1.0), and 91% (95% confidence interval: 0.51, 0.99), respectively.
Complications
There were 11 (11%) complications within 30 days of the procedure: five Clavien-Dindo grade I complications, three grade II complications, one grade III complication, and two grade IV complications. There were no grade V complications. Three complications were directly related to the ablation procedure. A retroperitoneal hematoma necessitating transfusion developed after anticoagulation was resumed in a patient with chronic pulmonary emboli (RENAL score, 6). Two patients (RENAL score, 6 and 8) developed hematuria necessitating bladder irrigation and discharge with an indwelling bladder catheter. The remaining complications included corneal abrasion, urinary retention, urinary tract infection, prerenal azotemia, viral prodrome, asymptomatic bradycardia, myocardial infarction, and stroke.
Six late complications developed during imaging follow-up. Six asymptomatic urinomas were detected in six patients a mean of 173.5 days (range, 112–209 days) after ablation. Three urinomas were associated with renal cortex volume loss and resulted in partial or diffuse renal atrophy (Fig 1). There was no change in renal cortex volume in the other three patients (Fig 2). The decrease in pre- and postprocedure eGFR in these patients, from a mean of 57.9 mL/min/1.73 m2 ± 26.9 to a mean of 44 mL/min/1.73 m2 ± 20.4, was not clinically or statistically significant (P = .5).
Figure 1a:

(a) Axial contrast-enhanced T1-weighted fat-saturation MR image shows endophytic 2.5-cm clear cell RCC (arrow) (RENAL score, 8). (b) Axial oblique unenhanced CT scan obtained after placement of two microwave applicators with US guidance. Both applicators were placed to point at renal sinus. Tip of one applicator (arrowhead) terminates in renal sinus. (c) Coronal T2-weighted MR image obtained 24 months after microwave ablation. Urinoma (arrow) surrounds index ablation (arrowhead). Inadvertent placement of applicator tips into renal sinus caused calyceal injury and thermal injury to urothelium, resulting in infundibular stricture.
Figure 2a:
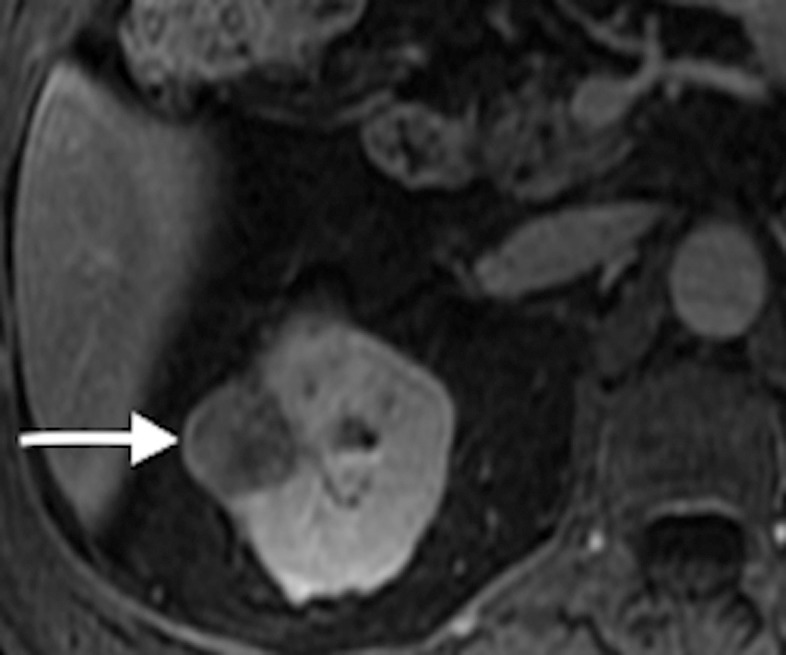
(a) Axial contrast-enhanced T1-weighted fat-saturation MR image shows endophytic 2.5-cm papillary RCC (arrow) (RENAL score, 7). (b) Two microwave applicators (arrow) were placed across base of RCC, tangential to renal sinus, with US guidance. (c) Axial oblique unenhanced CT scan helps confirm precise placement of applicator (arrow) tangential to renal sinus. Placement of applicators tangential to renal sinus mitigates risk of calyceal injury and urinoma formation.
Figure 1b:

(a) Axial contrast-enhanced T1-weighted fat-saturation MR image shows endophytic 2.5-cm clear cell RCC (arrow) (RENAL score, 8). (b) Axial oblique unenhanced CT scan obtained after placement of two microwave applicators with US guidance. Both applicators were placed to point at renal sinus. Tip of one applicator (arrowhead) terminates in renal sinus. (c) Coronal T2-weighted MR image obtained 24 months after microwave ablation. Urinoma (arrow) surrounds index ablation (arrowhead). Inadvertent placement of applicator tips into renal sinus caused calyceal injury and thermal injury to urothelium, resulting in infundibular stricture.
Figure 1c:
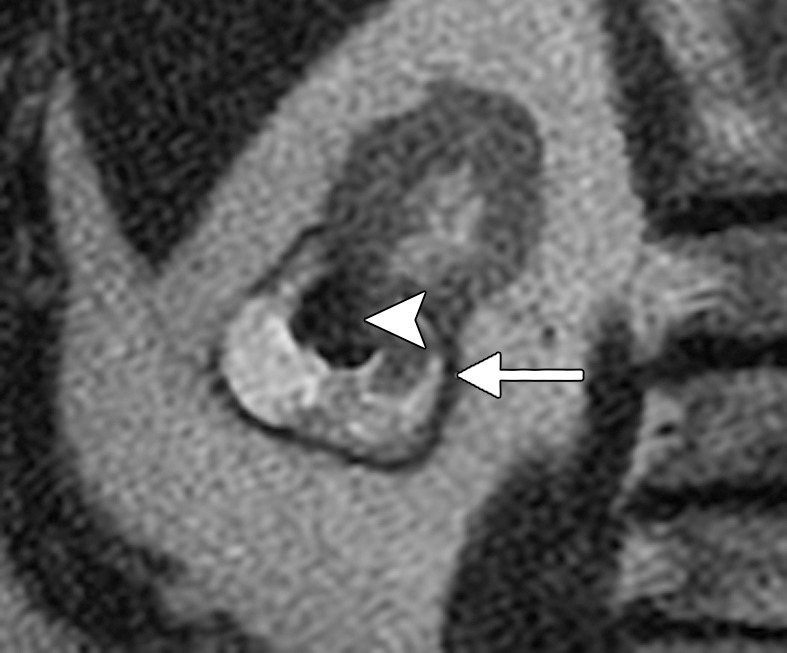
(a) Axial contrast-enhanced T1-weighted fat-saturation MR image shows endophytic 2.5-cm clear cell RCC (arrow) (RENAL score, 8). (b) Axial oblique unenhanced CT scan obtained after placement of two microwave applicators with US guidance. Both applicators were placed to point at renal sinus. Tip of one applicator (arrowhead) terminates in renal sinus. (c) Coronal T2-weighted MR image obtained 24 months after microwave ablation. Urinoma (arrow) surrounds index ablation (arrowhead). Inadvertent placement of applicator tips into renal sinus caused calyceal injury and thermal injury to urothelium, resulting in infundibular stricture.
Figure 2b:

(a) Axial contrast-enhanced T1-weighted fat-saturation MR image shows endophytic 2.5-cm papillary RCC (arrow) (RENAL score, 7). (b) Two microwave applicators (arrow) were placed across base of RCC, tangential to renal sinus, with US guidance. (c) Axial oblique unenhanced CT scan helps confirm precise placement of applicator (arrow) tangential to renal sinus. Placement of applicators tangential to renal sinus mitigates risk of calyceal injury and urinoma formation.
Figure 2c:
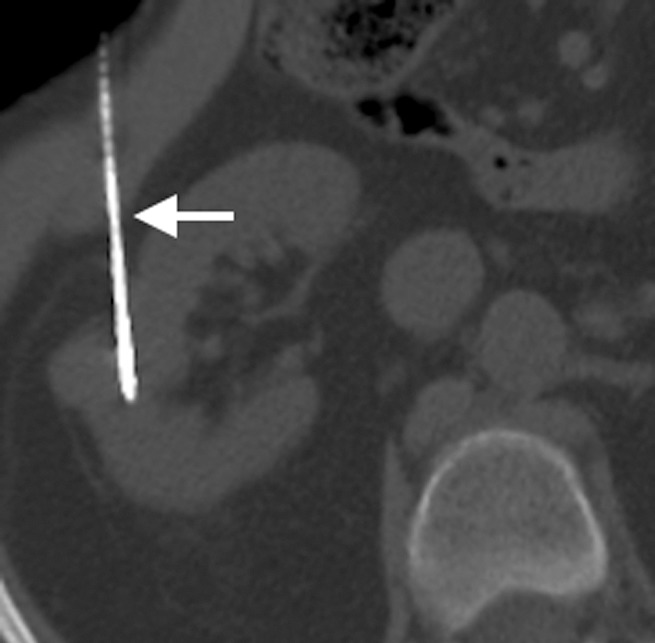
(a) Axial contrast-enhanced T1-weighted fat-saturation MR image shows endophytic 2.5-cm papillary RCC (arrow) (RENAL score, 7). (b) Two microwave applicators (arrow) were placed across base of RCC, tangential to renal sinus, with US guidance. (c) Axial oblique unenhanced CT scan helps confirm precise placement of applicator (arrow) tangential to renal sinus. Placement of applicators tangential to renal sinus mitigates risk of calyceal injury and urinoma formation.
Discussion
The results of our study demonstrate that percutaneous microwave ablation of clinical stage T1a RCC is safe, with promising early and midterm oncologic outcomes. There was minimal impact on renal function and few ablation-related complications. The results of our study add to those from the study by Yu et al (23), who compared early and midterm oncologic efficacy of percutaneous microwave ablation to open radical nephrectomy. However, we achieved 100% technical success in single-session ablations for the entire cohort, whereas 14 tumors in the study by Yu et al required repeat treatment in a second session to achieve a similar rate of technical success (23). A potential explanation for our single-session success may relate to differences between multiple and single microwave applicator ablations. The simultaneous use of multiple microwave antennas creates thermal and electromagnetic synergy when the antennas are controlled to have coherent phase. This synergy generates larger, confluent ablations that are more likely to achieve temperature extremes able to overcome perfusion-mediated tissue cooling (24). Whereas Yu et al performed all ablations with a single applicator, we generally used two applicators for tumors between 2.5 and 3.5 cm and three applicators for those larger than 3.5 cm.
Unlike with RF ablation and cryoablation, the technical success of microwave ablation was not influenced by tumor complexity. Factors associated with RF ablation and cryoablation treatment failures included larger tumor diameters and three of the anatomic components of the RENAL nephrometry scores: (a) endophytic and/or exophytic property, (b) nearness to the collecting system, and (c) location relative to the polar lines (12). RF ablation is known to have limited efficacy for tumors larger than 3 cm, for tumors with a significant endophytic component, and/or for tumors near the collecting system. McClure et al (13) showed a significantly higher rate of treatment failure for tumors 3.5 cm and larger and for tumors that were not of low complexity (RENAL score, 4–6). Schmit et al (12) and Camacho et al (14) showed that treatment failure with RF ablation and cryoablation was associated with RENAL scores greater than 7.6 and 8, respectively. Herein, we report a 100% rate of single-session treatment success for the entire cohort, including 34 (34%) tumors ranging in size from 3.0–4.0 cm and 47 moderately and five highly complex renal tumors. Furthermore, the number of antennas used, generator output, and duration of ablation was similar for low, moderate, and high tumor complexity. This suggests that microwave ablation of clinical stage T1a RCC is more straightforward than RF ablation and cryoablation, where the operator must consider tumor diameter and tumor complexity when planning and performing tumor ablation.
The one patient who experienced local tumor progression had a 2.6-cm Fuhrman grade 2 clear cell RCC and a RENAL score of 6, which corresponds to a low tumor complexity. After salvage radical nephrectomy, the tumor was upgraded to Fuhrman grade 4 RCC with rhabdoid features, a histologically aggressive RCC. Tumor histologic characteristics rather than technique likely explained the cause of the treatment failure. Histologic grading of RCC from renal mass biopsy is a well-known limitation, with concordance rates at extirpation ranging from 43% to 80% (25). Importantly, higher grade RCCs (Fuhrman grade III or IV) and RCCs with aggressive histologic characteristics (sarcomatoid or rhabdoid features) may require a larger margin when performing thermal ablation or may be more suited for surgical management.
The complication profile in our study is similar to that of previous RF ablation and microwave studies (23,26,27). Most Clavien-Dindo complications were low grade (I or II) and causally unrelated to the ablation procedure. However, we did identify six asymptomatic urinomas during postprocedure surveillance. These six urinomas occurred early in our microwave experience during the treatment of 29 endophytic and partially endophytic RCCs (median RENAL score, 8.5). In all patients who developed urinomas, microwave antennas had been placed directly into the renal sinus. Three patients developed renal cortical loss similar to that previously described, presumably the result of calyceal strictures (26). Importantly, there was minimal impact on global renal function. Before recognition of this complication, microwave antennas were placed so that the tips pointed toward the collecting system. We hypothesize that the mechanism of urinoma formation was related to the tips of applicators being inadvertently placed too deep, where they terminated in the collecting system. This provided a direct pathway for thermal injury to the urothelium, leading to infundibular stricture and retrograde collection of urine along the applicator track into the retroperitoneum. We have modified our procedural technique to avoid collecting system puncture by placing antennas tangential to the renal sinus, perpendicular to the long axis of the calyx. After modifying our technique to avoid placement into the renal sinus, no urinomas have been identified in the subsequent 35 endophytic and partially endophytic RCCs (median RENAL score, 8.5).
The results of our study add to the growing body of literature suggesting that stage T1a RCC can be successfully treated with percutaneous thermal ablation by using a variety of different ablation modalities. Comparison between modalities is difficult because of the large number of devices within a specific ablation modality as well as the lack of studies directly comparing each modality within a single center with the same operators. Given these limitations, our results with microwave ablation compare favorably with those of other retrospective single-center reports of RF ablation and cryoablation for similar tumor sizes and follow-up. Large RF cohort studies with similar patient and tumor characteristics to those of this study have showed a rate of local control of 90% or less, compared with the 99% rate we report herein (13,14,26–28). Furthermore, the local progression-free survival rate in cryoablation studies ranged from 77% to 98% during a mean follow-up of 20–41 months, and these rates are lower than the rate in this study and other microwave reports from Asia (14,29–31).
Our study has several limitations. The retrospective, single-center design is a limitation and may be biased by institutional expertise. In addition, the median follow-up interval is relatively short for the assessment of durable oncologic efficacy, but this limitation is unavoidable with new technology and is similar to that in early reports of RF ablation and cryoablation for the treatment of RCC (28,31). Although we have shown that microwave is associated with very high technical success when treating moderate or highly complex stage T1a RCC, longer follow-up and direct comparison studies between ablation modalities and surgery are needed. One limitation that deserves added emphasis is the use of a single microwave device, which may not be fully representative of other microwave devices on the market. Despite differences in technology, our results do align closely with those of the largest studies from Asia, where there was a very low rate of local tumor progression for similar sized tumors (23,29,30). Whether these results are generalizable to other microwave systems remains to be seen. We emphasize caution in extrapolating our results across all microwave ablation devices and all centers.
Percutaneous microwave ablation of stage T1a RCC is an effective and safe treatment option for low, moderate, and high complexity RCC in short- and midterm follow-up. Continued follow-up to establish long-term oncologic efficacy and comparison to competing ablation modalities and surgery appear warranted.
Advances in Knowledge
■ Tumor size and complexity do not affect treatment efficacy and complications during microwave ablation of stage T1a renal cell carcinoma (RCC), and the single-session technical success rate was 100% (100 of 100 RCCs) for the entire cohort, including 34 tumors ranging from 3.0 to 4.0 cm and 47 moderately and five highly complex renal tumors; the number of antennas used (P = .81), generator output (P = .21), and duration of ablation (P = .43) was similar for low, moderate, and high complexity RCCs.
■ The median tumor diameter when one, two, and three microwave antennas were used was 1.9, 2.8, and 3.5 cm, respectively.
■ Microwave applicators should be placed tangential to the renal sinus to mitigate the risk of urinoma formation.
Implications for Patient Care
■ Microwave ablation of clinical stage T1a RCC (≤4.0 cm) is safe, with promising early and midterm oncologic outcomes.
■ Our study adds to a growing body of literature suggesting that stage T1a RCC can be successfully treated with percutaneous thermal ablation by using a variety of ablation modalities.
Received March 10, 2016; revision requested May 22; revision received September 30; accepted September 30; final version accepted November 16.
C.L.B. is supported by the National Cancer Institute (R01 CA142737).
Disclosures of Conflicts of Interest: M.E.K. disclosed no relevant relationships. E.J.A. disclosed no relevant relationships. T.J.Z. disclosed no relevant relationships. S.B. disclosed no relevant relationships. M.G.L. Activities related to the present article: received grants from Ethicon and Philips. Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships. S.Y.N. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a paid consultant for Boston Scientific. Other relationships: disclosed no relevant relationships. J.L.H. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a paid consultant for Neuwave Medical; spouse is a co-founder of Accure Medical; is a stockholder in Elucent Medical. Other relationships: disclosed no relevant relationships. C.L.B. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: received personal fees from Neuwave Medical and Symple Surgical. Other relationships: disclosed no relevant relationships. F.T.L. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a board member for Histosonics; is a paid consultant for Ethicon; has grants/grants pending from Histosonics; institution has a patent with Medtronic/Covidien; receives royalties from Medtronic/Covidien; institution receives royalties from Medtronic/Covidien; has stock/stock options in Elucent and Histosonics. Other relationships: disclosed no relevant relationships. S.A.W. disclosed no relevant relationships.
Abbreviations:
- eGFR
- estimated glomerular filtration rate
- IQR
- interquartile range
- RCC
- renal cell carcinoma
- RF
- radiofrequency
References
- 1.Lightfoot N, Conlon M, Kreiger N, et al. Impact of noninvasive imaging on increased incidental detection of renal cell carcinoma. Eur Urol 2000;37(5):521–527. [DOI] [PubMed] [Google Scholar]
- 2.Lucas SM, Stern JM, Adibi M, Zeltser IS, Cadeddu JA, Raj GV. Renal function outcomes in patients treated for renal masses smaller than 4 cm by ablative and extirpative techniques. J Urol 2008;179(1):75–79; discussion 79–80. [DOI] [PubMed] [Google Scholar]
- 3.Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol 2009;182(4):1271–1279. [DOI] [PubMed] [Google Scholar]
- 4.Khiatani V, Dixon RG. Renal ablation update. Semin Intervent Radiol 2014;31(2):157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venkatesan AM, Wood BJ, Gervais DA. Percutaneous ablation in the kidney. Radiology 2011;261(2):375–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brace CL. Microwave tissue ablation: biophysics, technology, and applications. Crit Rev Biomed Eng 2010;38(1):65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagstaff P, Ingels A, Zondervan P, de la Rosette JJ, Laguna MP. Thermal ablation in renal cell carcinoma management: a comprehensive review. Curr Opin Urol 2014;24(5):474–482. [DOI] [PubMed] [Google Scholar]
- 8.Larcher A, Meskawi M, Valdivieso R, et al. Comparison of renal function detriments after local tumor ablation or partial nephrectomy for renal cell carcinoma. World J Urol 2016;34(3):383–389. [DOI] [PubMed] [Google Scholar]
- 9.Laeseke PF, Lee FT, Jr, Sampson LA, van der Weide DW, Brace CL. Microwave ablation versus radiofrequency ablation in the kidney: high-power triaxial antennas create larger ablation zones than similarly sized internally cooled electrodes. J Vasc Interv Radiol 2009;20(9):1224–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atwell TD, Farrell MA, Callstrom MR, et al. Percutaneous cryoablation of large renal masses: technical feasibility and short-term outcome. AJR Am J Roentgenol 2007;188(5):1195–1200. [DOI] [PubMed] [Google Scholar]
- 11.Atwell TD, Carter RE, Schmit GD, et al. Complications following 573 percutaneous renal radiofrequency and cryoablation procedures. J Vasc Interv Radiol 2012;23(1):48–54. [DOI] [PubMed] [Google Scholar]
- 12.Schmit GD, Thompson RH, Kurup AN, et al. Usefulness of R.E.N.A.L. nephrometry scoring system for predicting outcomes and complications of percutaneous ablation of 751 renal tumors. J Urol 2013;189(1):30–35. [DOI] [PubMed] [Google Scholar]
- 13.McClure TD, Chow DS, Tan N, Sayre JA, Pantuck AJ, Raman SS. Intermediate outcomes and predictors of efficacy in the radiofrequency ablation of 100 pathologically proven renal cell carcinomas. J Vasc Interv Radiol 2014;25(11):1682–1688; quiz 1689. [DOI] [PubMed] [Google Scholar]
- 14.Camacho JC, Kokabi N, Xing M, et al. R.E.N.A.L. (Radius, exophytic/endophytic, nearness to collecting system or sinus, anterior/posterior, and location relative to polar lines) nephrometry score predicts early tumor recurrence and complications after percutaneous ablative therapies for renal cell carcinoma: a 5-year experience. J Vasc Interv Radiol 2015;26(5):686–693. [DOI] [PubMed] [Google Scholar]
- 15.Moreland AJ, Ziemlewicz TJ, Best SL, et al. High-powered microwave ablation of T1a renal cell carcinoma: safety and initial clinical evaluation. J Endourol 2014;28(9):1046–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SJ, Choyke LT, Locklin JK, Wood BJ. Use of hydrodissection to prevent nerve and muscular damage during radiofrequency ablation of kidney tumors. J Vasc Interv Radiol 2006;17(12):1967–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knavel EM, Hinshaw JL, Lubner MG, et al. High-powered gas-cooled microwave ablation: shaft cooling creates an effective stick function without altering the ablation zone. AJR Am J Roentgenol 2012;198(3):W260–W265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol 2009;182(3):844–853. [DOI] [PubMed] [Google Scholar]
- 21.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240(2):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria—a 10-year update. Radiology 2014;273(1):241–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu J, Liang P, Yu XL, et al. US-guided percutaneous microwave ablation versus open radical nephrectomy for small renal cell carcinoma: intermediate-term results. Radiology 2014;270(3):880–887. [DOI] [PubMed] [Google Scholar]
- 24.Laeseke PF, Lee FT, Jr, van der Weide DW, Brace CL. Multiple-antenna microwave ablation: spatially distributing power improves thermal profiles and reduces invasiveness. J Interv Oncol 2009;2(2):65–72. [PMC free article] [PubMed] [Google Scholar]
- 25.Blute ML, Jr, Drewry A, Abel EJ. Percutaneous biopsy for risk stratification of renal masses. Ther Adv Urol 2015;7(5):265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gervais DA, Arellano RS, McGovern FJ, McDougal WS, Mueller PR. Radiofrequency ablation of renal cell carcinoma. II. Lessons learned with ablation of 100 tumors. AJR Am J Roentgenol 2005;185(1):72–80. [DOI] [PubMed] [Google Scholar]
- 27.Psutka SP, Feldman AS, McDougal WS, McGovern FJ, Mueller P, Gervais DA. Long-term oncologic outcomes after radiofrequency ablation for T1 renal cell carcinoma. Eur Urol 2013;63(3):486–492. [DOI] [PubMed] [Google Scholar]
- 28.Breen DJ, Rutherford EE, Stedman B, et al. Management of renal tumors by image-guided radiofrequency ablation: experience in 105 tumors. Cardiovasc Intervent Radiol 2007;30(5):936–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guan W, Bai J, Liu J, et al. Microwave ablation versus partial nephrectomy for small renal tumors: intermediate-term results. J Surg Oncol 2012;106(3):316–321. [DOI] [PubMed] [Google Scholar]
- 30.Yu J, Zhang G, Liang P, et al. Midterm results of percutaneous microwave ablation under ultrasound guidance versus retroperitoneal laparoscopic radial nephrectomy for small renal cell carcinoma. Abdom Imaging 2015;40(8):3248–3256. [DOI] [PubMed] [Google Scholar]
- 31.Atwell TD, Farrell MA, Leibovich BC, et al. Percutaneous renal cryoablation: experience treating 115 tumors. J Urol 2008;179(6):2136–2140; discussion 2140–2141. [DOI] [PubMed] [Google Scholar]


