Abstract
Recent advances in induced pluripotent stem cell (iPSC) technology have paved the way for the production of patient-specific neurons that are ideal for autologous cell replacement for treatment of neurodegenerative diseases. In the case of retinal degeneration and associated photoreceptor cell therapy, polymer scaffolds are critical for cellular survival and integration; however, prior attempts to materialize this concept have been unsuccessful in part due to the materials’ inability to guide cell alignment. In this work, we used two-photon polymerization to create 180 μm wide non-degradable prototype photoreceptor scaffolds with varying pore sizes, slicing distances, hatching distances and hatching types. Hatching distance and hatching type were significant factors for the error of vertical pore diameter, while slicing distance and hatching type most affected the integrity and geometry of horizontal pores. We optimized printing parameters in terms of structural integrity and printing time in order to create 1 mm wide scaffolds for cell loading studies. We fabricated these larger structures directly on a porous membrane with 3 μm diameter pores and seeded them with human iPSC-derived retinal progenitor cells. After two days in culture, cells nested in and extended neuronal processes parallel to the vertical pores of the scaffolds, with maximum cell loading occurring in 25 μm diameter pores. These results highlight the feasibility of using this technique as part of an autologous stem cell strategy for restoring vision to patients affected with retinal degenerative diseases.
Keywords: 3D Printing, two-photon polymerization, photoreceptors, retina, stem cells
Graphical Abstract
1. Introduction
Unlike amphibians and fish, which are able to rebuild their entire neural retina following injury [1–4], humans typically lose vision permanently when their photoreceptors are lost. In principle, blindness from degenerative human retinal diseases such as retinitis pigmentosa and atrophic age-related macular degeneration could be reversed if new retinal cells could be derived from stem cells and transplanted into the sub-retinal space. Extensive data suggest that stem cell-derived retinal progenitor and photoreceptor precursor cells may be useful for photoreceptor cell replacement and restoration of vision [5, 6]. However, traditional cell delivery methods typically result in significant cell loss (more than 95%) and limited cellular integration following transplantation [5, 7–9]. This is particularly true when investigators attempt sub-retinal transplants in late-stage retinal degenerative hosts that have sustained significant photoreceptor cell death and extensive gliosis [9]. For instance, in a rhodopsin null mouse model of retinal degeneration, the extent of integration is five times higher when recipients are 4 weeks of age compared to ten weeks [9].
We and others have hypothesized that the poor cell survival and integration associated with bolus injections of a single cell suspension are in large part due to the lack of physical support and substrate interactions afforded by this approach [10, 11]. Specifically, the act of mechanical and enzymatic dissociation that is required to generate a single cell suspension abruptly strips cells of their matrix and cell-cell contacts. Investigators have tried to overcome these issues by differentiating and transplanting cells on various scaffolds [10, 12, 13]. Such biomaterials can help shield cells from fluid-flow shear forces, maintain anatomical integrity, prevent apoptosis caused by a loss of adequate cell-matrix interactions and act as a physical support to hold cells in the appropriate location and orientation following implantation. For example, compared to a bolus cell injection, transplantation of retinal-progenitor-cells on porous poly-lactic-co-glycolic acid (PLGA) constructs results in a 10-fold increase in donor cell survival [14]. In addition, the presence of pores and ridges on polymer substrates appears to encourage attachment, differentiation and orientation of retinal progenitor and photoreceptor precursor cells [14–17].
As promising as these results are, initial scaffolds have been largely two-dimensional, designed to carry cells on their surface as a relatively unorganized mass. The normal human retinal outer nuclear layer has as many as 45,000 photoreceptor cells per mm2, tightly packed and aligned parallel to the path of light [18–20]. To most closely recapitulate this structure, an ideal cell delivery scaffold would provide a three-dimensional framework designed to promote optimal donor cell packing density, cell-cell interaction and orientation. Creating such a structure requires precise control of material geometry in 3D at a size scale at least an order of magnitude smaller than the cells themselves.
Recent advances in 3D printing have made fabrication of intricately structured biomaterials faster and easier than ever before. Specifically, 3D printers that use photopolymerization offer much higher resolution than other 3D printing approaches such as extrusion. However, even with the use of highly focused lasers, the photoinitiator absorbs light, and polymerization occurs, along the entire length of the beam, significantly limiting resolution and dramatically reducing applicability for fabrication of the type of scaffolds needed to rebuild the retina. Two-photon polymerization is an alternative approach that affords extremely high resolution. Using this technology, the simultaneous arrival of two photons, each carrying half of the total energy required to stimulate a polymerization event, can elevate the photoinitiator from the ground state to the excited state (Figure 1A) [21–24]. In order for two-photon polymerization to occur, the photons must arrive within 1 femtosecond of each other – an event very unlikely to occur anywhere except the focal point of the laser. This precision allows the printing of features as small as 50 nm, roughly three orders of magnitude smaller than other 3D printing systems [21–24]. Furthermore, because long wavelength light is less likely to scatter or become absorbed than its UV and visible counterparts, this technology can be used to polymerize thick structures and those that contain cells or proteins.
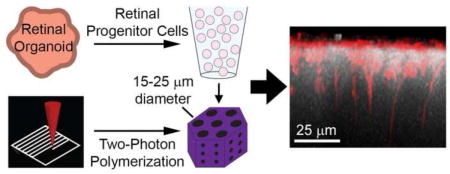
Figure 1. Design of retinal progenitor cell scaffolds.
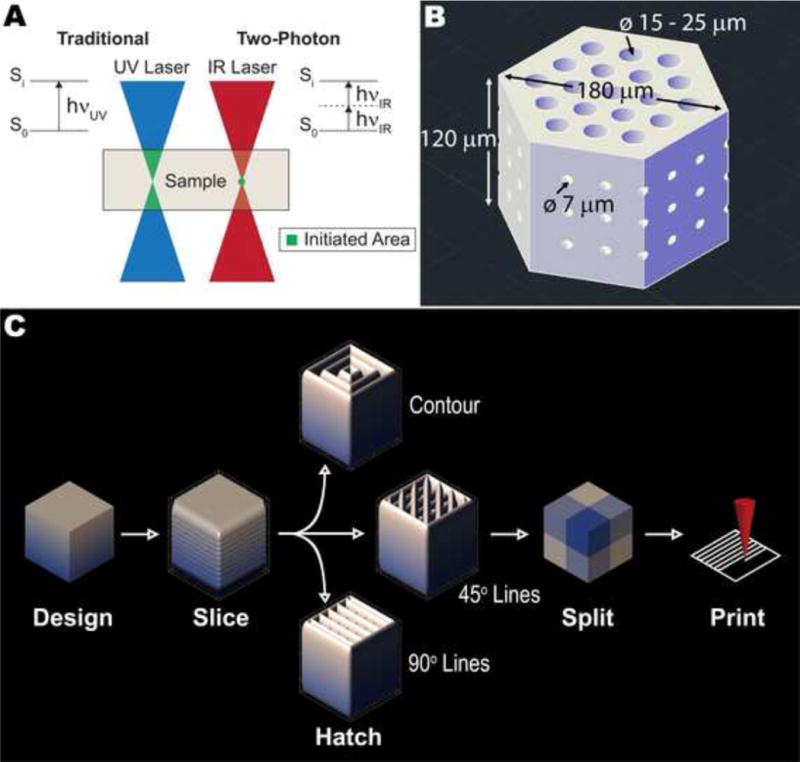
A) Schematic showing traditional photopolymerization versus two-photon polymerization. B) Electronic model of a basic photoreceptor support scaffold design with hexagonally packed vertical pores and small, interconnected horizontal pores. C) Design process for photoreceptor scaffolds: slicing distance, hatching distance, hatching type and splitting parameters are all user-defined.
In this study, we examine the feasibility of using two-photon polymerization to create 3D scaffolds for retinal progenitor cells. We quantitatively examined the effects of design parameters on fabrication time and structure fidelity, including the size and roundness of vertical and horizontal pores. To facilitate cell loading, we printed scaffolds directly on porous membrane substrates. We loaded human induced pluripotent stem cell (iPSC)-derived retinal progenitor cells (RPCs) into 3D scaffolds with interconnected horizontal pores and closely packed vertical pores of varying size. RPCs retained expression of the neural progenitor-specific protein TUJ1, nested in and aligned with scaffold vertical pores within two days in culture. These results pave the way for the design of scaffolds to deliver densely packed, correctly oriented retinal progenitor cells to the sub-retinal space. The developed scaffolds will also facilitate in vitro studies of photoreceptor cell behavior, disease pathogenesis and novel treatments for retinal degeneration.
2. Materials and Methods
2.1 Scaffold Design
Figure 1B shows the general design of the scaffolds. To closely recapitulate the packing of cells in the outer retina and minimize the amount of scaffold material present, we distributed vertical pores in a hexagonally packed pattern. Based on previous experience, we expected some degree of material shrinkage, so we selected three vertical pore sizes slightly larger than a range of typical retinal cell diameters: 15, 20 and 25 μm. We located each pore center 30 μm from its nearest neighbors in all directions. In order to facilitate the diffusion of nutrients and oxygen through the scaffold, we also included three vertical layers of interconnected horizontal pores with diameters of 7 μm that intersected the hexagonal scaffold in two directions. Prior to fabrication, we sliced, hatched and split each scaffold model (Figure 1C). For pre-fabrication parameter testing, we varied slicing distance (vertical layer-to-layer distance) between 0.5, 0.75 and 1.0 μm, hatching distance (line-to-line distance within each layer) between 0.2, 0.35 and 0.5 μm and hatching type between contour, lines with 45° offset for each layer and lines with 90° offset for each layer. During this parameter optimization, we selected a hexagonal prism width of 180 μm and height of 120 μm. We printed these small scaffolds in a 9×9 array, with each having a different combination of the parameters described. Our approach followed a full-factorial experimental design with four factors (pore size, slicing distance, hatching distance, hatching type), each with three levels. After parameter optimization, we fabricated scaffolds with varying pore size, a width of 1000 μm, height of 120 μm, arranged side by side and surrounded by a 20 μm thick wall with a diameter of 2400 μm and a height of 500 μm. We split each of these large structures into 250 μm × 250 μm × 50 μm segments for printing, which the software automatically stitched together. We used AutoCAD 2015 (Autodesk Inc., San Rafael, CA) to create all models and DeScribe (version 2.2.1, Nanoscribe GmbH, Eggenstein-Leopoldshafen, Germany) for slicing, hatching and splitting.
2.2 Scaffold Fabrication
To facilitate adhesion of the printed structure to the substrate, we functionalized ITO-coated glass (Nanoscribe GmbH) with polymerizable groups prior to its use for two-photon polymerization. Briefly, we exposed glass substrates with an ITO-coating facing oxygen plasma (Plasma Cleaner equipped with PlasmaFlo gas flow control, Harrick Plasma, Ithaca, NY) at an oxygen flow rate of 22.5 mL/min at 30 W radio frequency power for three minutes. Immediately after removing them from the plasma chamber, we submerged the substrates a 1% solution of coupling agent (3-(trimethoxysilyl)propyl methacrylate, Sigma-Aldrich, St. Louis, MO) in hexanes (Fisher Scientific, Waltham, MA) overnight. We then rinsed the glass substrates with hexanes, dried them and stored them in an airtight container at room temperature.
For each set of scaffolds, we confirmed the correct orientation of the glass substrate using the surface electrical resistance. We then secured the substrate in the sample holder and placed a droplet of IP-S photoresist (Nanoscribe GmbH) in the center of the substrate. We built the structures using a Nanoscribe Photonic Professional GT two-photon lithography system (Nanoscribe GmbH) in dip-in-laser lithography (DiLL) mode using a 25× objective (NA=0.8). We held the laser power, scanning speed and all other lithography parameters constant for all experiments. After fabrication, we removed the substrates from the sample holder, submerged them in propylene glycol monomethyl ether acetate (PGMEA, Sigma-Aldrich) for 20 minutes, and subsequently submerged them in isopropanol (Fisher Scientific) twice, each for 20 minutes.
We fabricated scaffolds intended for cell loading directly on porous cell culture membranes.[24] Briefly, we cut polycarbonate membrane inserts with 3μm pores (24 mm Transwell®, Corning, Corning, NY) such that they could be secured to the sample holder without obstructing its insertion into the instrument. We did not functionalize the membranes with polymerizable groups prior to their use. All other printing parameters and post-processing conditions were identical except that we substituted an additional isopropanol rinse for the PGMEA rinse (the polycarbonate membranes are soluble in PGMEA).
2.3 Scanning Electron Microscopy
After fabrication, we dried substrates and structures at room temperature overnight. We applied a gold-palladium coating using an argon beam K550 sputter coater (Emitech Ltd., Kent, England) and captured images using a Hitachi S-4800 SEM (Hitachi High-Technologies, Ontario, Canada) at an accelerating voltage of 1 kV. For parameter analysis, we analyzed vertical pores by capturing an image of each scaffold from directly above, whereas we tilted the sample stage at 30° for image capture of horizontal pores. To quantify the effect of parameters on model-to-structure fidelity, we processed images using ImageJ. Briefly, we created binary images using the software’s threshold algorithm such that the software recognized pores as dark regions. Thereafter, we measured the area (A), fitted ellipse dimensions [major axis (MjA) and minor axis] and roundness of each dark region using the ImageJ particle analysis function (for each structure, n = 19 for vertical pores, n = 6 for horizontal pores) where
| (1) |
For each vertical pore, we calculated the diameter using the average of the major and minor axes, and then calculated the diameter error by subtracting this value from the modeled pore diameter.
2.4 Retinal Progenitor Cell Generation and Seeding
To generate clinical-grade retinal progenitor cells we used our recently developed 3D-cGMP-differentiation protocol [25]. Briefly, we passaged, dissociated and resuspended individual iPSC colonies derived from a donor with normal ocular history in 3D differentiation medium [DMEM (Gibco, Thermo Fisher Scientific, Grand Island, NY), 10% heat-inactivated human serum (Innovative Research, Novi, MI), 20% CTS KnockOut Serum Replacement XenoFree medium (Gibco), 0.1 mM MEM non-essential amino acids (Gibco), 1 mM Sodium Pyruvate (Gibco), 0.1 mM 2-Mercaptoethanol (Gibco), 1% ECM (human type 1 and type 3 collagen/vitronectin/fibronectin, Advanced BioMatrix, Carlsbad, CA), 20 mM Y-27632 ROCK Inhibitor (EMD Milipore, Billerica, MA), 3 nMIWR1e (Cayman Chemical, Ann Arbor, MI), 3 M StemMACS CHIR (Miltenyi Biotec Inc., San Diego, CA), 100 nM SAG (Enzo Life Sciences, Farmingdale, NY)], and then plated the cells in a 96-well sphere forming plate (Corning) at 1×104 cells/well. We supplemented cells with fresh 3D differentiation medium every other day for 12 days to induce sphere formation. On day 12, we collected individual spheres, transferred them to 100 mm ultra-low adhesion plates and allowed them to differentiate for an additional week in 3D differentiation media. On day 18, we switched the sphere medium to neural retina medium 1 [CTS KnockOut DMEM/F12 (Gibco), 2 mM GlutaMAX supplement (Gibco) and CTS N-2 supplement (Cell Therapy Systems, Thermo Fisher Scientific)] to promote differentiation into retinal progenitor cells.
After 30 days in culture, we manually isolated 15 spheres and transferred them to 1 mL of dissociation media [2 mg/mL papain (Sigma-Aldrich), 0.4 mg/mL DL-cysteine (Sigma-Aldrich) and 0.4 mg/mL bovine serum albumin (Sigma-Aldrich) in neurobasal media (Gibco) supplemented with 10 U/mL DNase 1 (Amplification grade, Invitrogen, Thermo Fisher Scientific)]. We incubated the solution at 37°C for 20 minutes and agitated by pipetting every five minutes. At the end of this time period, we added 1 mL fresh neural retina media 2 [2% B27 supplement (Life Technologies, Thermo Fisher Scientific), 1 mg/mL Primocin (Invivogen, San Diego, CA), 1 mM sodium pyruvate (Life Technologies), 2 mM GlutaMAX (Gibco), 5 μg/mL insulin (Sigma-Aldrich), 100 μg/mL transferrin (Sigma-Aldrich), 100 μg/mL human serum albumin (Sigma-Aldrich), 60 ng/mL progesterone (Sigma-Aldrich), 16 μg/mL putrescine (Sigma-Aldrich), 40 ng/mL sodium selenite (Sigma-Aldrich), 40 ng/mL thyroxine (Sigma-Aldrich), 40 ng/mL tri-iodothyronine (Sigma-Aldrich), 50 ng/mL BDNF (Life Technologies), 10 ng/mL CNTF (Life Technologies), 10 ng/mL hbFGF (EMD Millipore), 5 μM forskolin (Sigma-Aldrich) and 1% human serum (Sigma-Aldrich) in neurobasal media (Life Technologies)] to inactivate the papain, then we centrifuged the cells at 100g for 2 minutes, discarded the supernatant and resuspended the dissociated pellet in 100 μL fresh neural retina media 2 (yielding about 10,000 cells/μL). Our previous work showed that after thirty days in culture, retinal organoids contain a large fraction of cells that express the early retinal markers SOX2, PAX6, OTX2 and VSX2 [25]. Thus, for the purpose of this work, we assumed that cells dissociated from 30-day retinal organoids were retinal progenitor cells and assumed that they maintained this phenotype during the brief culture time described here.
We sterilized membrane-anchored scaffolds by submersion in 200-proof ethanol overnight, and then rinsed them using two washes of sterile PBS. One day prior to dissociation, we coated the membrane-anchored scaffolds with human laminin 521 [10 μg/mL rhLaminin-521 (Gibco) in 1× HBSS +/+ (Gibco)] overnight. After we removed excess laminin solution, we sequentially loaded cells onto the scaffolds as follows. We carefully added 3 μL of cell suspension to the center of the scaffolds then removed moisture from the opposite side of the membrane by absorption with a sterile cellulose swab (Weck-cel®, Beaver Visitec, Waltham, MA). In total, we added 90 μL of cell suspension to the scaffolds by repeating this process. We incubated freshly seeded scaffolds at 37°C and 5% CO2 for 30 minutes to allow the cells to attach. We then added excess neural retina media 2 to the culture dish and returned the seeded scaffolds to the incubator for 48 hours.
2.5 Immunocytochemistry
We analyzed cell localization within the scaffolds 2 days after seeding using immunocytochemistry. First we fixed samples for one hour in 4% paraformaldehyde (Sigma-Aldrich), rinsed three times with 1× PBS (Thermo Fisher Scientific) and blocked at room temperature for one hour in immunocytochemical blocking buffer [1× phosphate buffered saline (Thermo Fisher Scientific), 3% bovine serum albumin (Research Products International Corp., Mount Prospect, IL), 5% normal goat serum (Cell Signaling, Danvers, MA), 0.5% Triton X-100 (Sigma-Aldrich), and 0.2% NaN3 (sodium azide; Sigma-Aldrich)]. Next, we incubated samples overnight at 4°C in immunocytochemical blocking buffer containing rabbit anti-Tuj1 antibody (neuron-specific class III beta-tubulin; Sigma-Aldrich; Cat. No. T2200-200UL; 1:1000 dilution). The following day, we rinsed scaffolds in wash buffer [1× phosphate buffered saline (Thermo Fisher Scientific), 0.2% Tween® 20 (Sigma-Aldrich)] and incubated them in goat anti-rabbit 565 secondary antibody for 2 hours at room temperature in immunocytochemical blocking buffer, followed by additional rinses in wash buffer. We mounted labeled scaffolds in poly(vinyl alcohol) (PVA)-based mounting medium containing 1,4-Diazabicyclo[2.2.2]octane (DABCO) [100 μg/ml PVA (Sigma-Aldrich), 25% v/v glycerol (Sigma-Aldrich), 0.1M Tris-HCl (pH 8–8.5, Sigma-Aldrich), 25 μg/ml DABCO (Sigma-Aldrich) and 4′,6-Diamidino-2-phenylindole dihydrochloride (DAPI, Sigma-Aldrich; Cat. No. D9542; 1:10,000 dilution). We visualized labeled samples using a Leica TCS SPE DMi8 inverted confocal microscope system (Leica Microsystems, Wetzlar, Germany).
To quantify cell number, we collected four z-stack images at 1μm intervals spanning the full thickness (~100 μm) of each scaffold (pore sizes 15 μm, 20 μm and 25 μm) at 40× magnification. We manually counted the number of pores that contained at least one cell using Leica LAS X 3D Software (Leica Microsystems) and reported the average percentage of pores with cells.
2.6 Statistical Analyses
To evaluate the effect of various design and printing parameters on structure-to-design fidelity, we used a full factorial experimental design. The factors were: modeled pore size (3 levels: 15, 20 or 25 μm), slicing distance (3 levels: 0.5, 0.75 or 1.0 μm), hatching type (3 levels: contour lines, lines with 45° offset for each layer and lines with 90° offset for each layer) and hatching distance (3 levels: 0.2, 0.35 or 0.5 μm), resulting in a total of 81 unique conditions. The measured outcomes were vertical pore diameter (19 replicates per condition), horizontal pore major axis length (6 replicates per condition), horizontal pore roundness (6 replicates per condition) and printing time (one replicate per condition). We transformed each data set using Box-Cox transformations with optimal λ for that particular measured outcome. In each case, we confirmed a normal distribution of the data using standard regression analyses. We then used ANOVA (at a 95% confidence interval) to determine the significance of each factor and Tukey’s pairwise comparisons (at a 95% confidence interval) to ascertain differences between groups. We used Minitab® (version 17.1.0, Minitab Inc., State College, PA) for all statistical analyses and GraphPad Prism® (version 7.00, GraphPad Software, La Jolla, CA) to generate interval plots. An interval plot shows a factor’s tendency and variability across a range of conditions rather than focusing on a fixed set of conditions. Thus, differences seen in an interval plot carry more statistical power than any differences that might be observed at a fixed set of conditions.
For cell quantification, we did not assume that the data were normally distributed given the limited number of measurements (n=4). Thus, we used Kruskal-Wallis and Dunn’s multiple comparisons tests (both are non-parametric methods) at 95% confidence intervals to determine significance.
3. Results
To understand the effect of printing parameters on design-to-structure fidelity, we printed a series of 81 test scaffolds (Supplemental Figure 1). We fabricated each scaffold with a different combination of variables (pore size, slicing distance, hatching distance and hatching type) and measured the dimensions of their vertical and horizontal pores. Figure 2A provides an example of one such scaffold.
Figure 2. Vertical pores of small scaffolds.
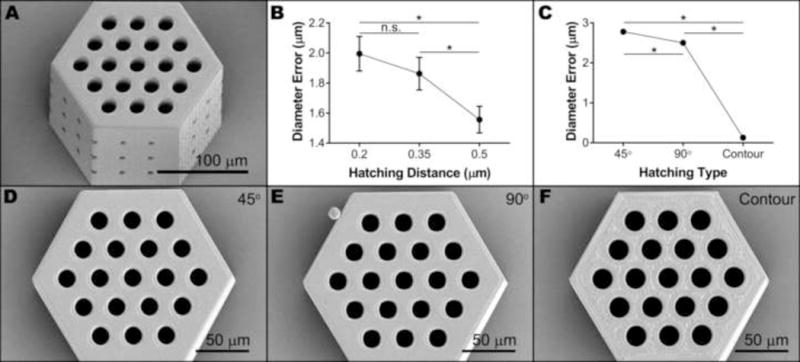
A) Representative SEM image of a small retinal progenitor cell scaffold used to determine design-to-structure fidelity. B–C) Interval plots showing the effect of hatching distance (B) and hatching type (C) on vertical pore diameter error. Each data point represents the mean (n = 513), while error bars represent the 95% confidence interval of the mean (*p < 0.05). D–F) Representative SEM images (taken from the top) of small scaffolds hatched using parallel lines alternating 45° (D) or 90° (E) between each layer or contour lines (F) with other factors held constant.
After a Box-Cox transformation (optimal λ = 0.717052), vertical diameter error data were normally distributed (Supplemental Figure 2). The hatching distance and hatching type both significantly affected the difference between modeled and actual pore diameter (p < 0.001), while the modeled pore diameter and slicing distance did not (p = 0.057 and p = 0.205, respectively). The interval plot for hatching distance (Figure 2B) shows that pore diameter error decreased with increasing hatching distance, with the mean at a hatching distance of 0.5 μm (1.56 μm error) being statistically different from the means at the other two hatching distances. In other words, the vertical pore diameters of structures created with a hatching distance of 0.5 μm are more true to the model than those with smaller hatching distances. As for hatching type, the use of contour lines to fill each layer resulted in pores with twenty times less error than parallel lines (0.13 μm compared to 2.5 μm or 2.8 μm) regardless of their layer-to-layer orientation (Figure 2C). Hatching with parallel lines that alternated 45° between layers resulted in 0.3 μm more error than 90° – a significant but small effect compared to contour versus parallel lines. We also observed the effect of hatching type on vertical pore diameter qualitatively by examining micrographs of scaffolds with different hatching types and all other parameters held constant (Figure 2D–F). Compared to the smooth and continuous surfaces resulting from the use of parallel hatching lines (Figure 2D–E), the surface of contour-hatched structures appeared to have some small holes in the top layer, indicating incomplete filling of layers (Figure 2F).
As vertical pore size was not expected to affect horizontal pore dimension, for horizontal pore analysis vertical pore size was restricted to 20 μm. Horizontal pore roundness and major axis length data were normally distributed (Supplemental Figures 3 and 4, respectively) after Box-Cox transformations (optimal λ = 0.5 and −0.5, respectively). Slicing distance (p < 0.001) and hatching type (p < 0.001) but not hatching distance (p = 0.921) significantly influenced the roundness, which we used as a quantitative measure of horizontal pore integrity. Using a slicing distance of 1.0 μm resulted in significantly greater roundness than either 0.5 μm or 0.75 μm (Figure 3A). The use of contoured hatching lines caused a significantly lower roundness value than the use of parallel lines (Figure 3B). Hatching type was also a significant factor for horizontal pore major axis length, another quantitative measure of pore integrity. In this case, rotating the parallel line direction by 90° between layers resulted in significantly smaller major axes than rotating by 45° or using contour lines (Figure 3C). Line rotation by 45° between layers also resulted in significantly shorter axes than using contour lines. As with vertical pore diameter, we observed the effect of hatching type on horizontal pore dimensions qualitatively by examining micrographs of scaffolds with different hatching types and all other parameters held constant (Figure 3D–F). We also assessed horizontal pore integrity using bright-field microscopy. With few exceptions, we identified intact and interconnected horizontal pores in structures created using parallel lines, but were unable to do so in those created using contour lines (Figure 3G–I).
Figure 3. Horizontal pores of small scaffolds.
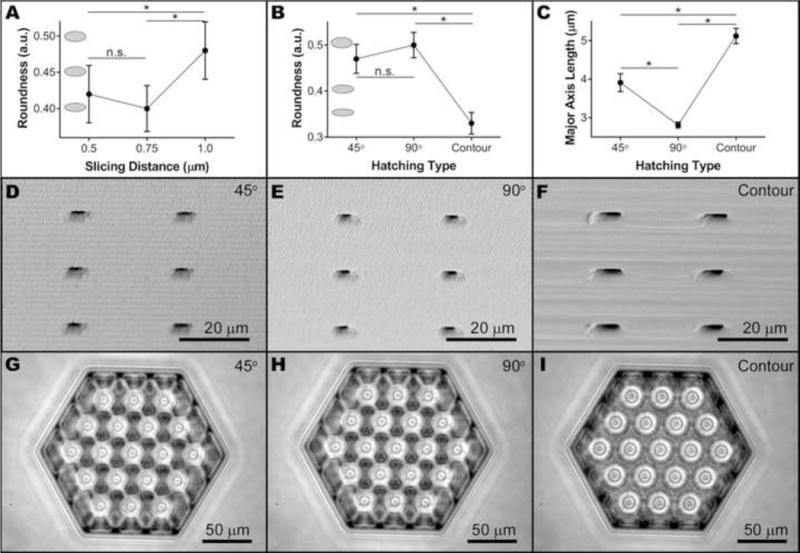
A–B) Interval plots showing the effect of slicing distance (A) and hatching type (B) on horizontal pore roundness. Grey ellipses represent the roundness at the corresponding value. C) Interval plot showing the effect of hatching type on horizontal pore major axis length. Each data point represents the mean (n = 162), while error bars represent the 95% confidence interval of the mean (*p < 0.05). Representative SEM (D–F, taken from the side) and bright field (G–I, taken from above) images of small scaffolds hatched using parallel lines alternating 45° (D,G) or 90° (E,H) between each layer or contour lines (F,I) with other factors held constant.
We recorded the time to print each small scaffold and used a Box-Cox transformation (optimal λ = −0.182244) to obtain normally distributed data (Supplemental Figure 5). Modeled pore size was not a significant factor for printing time (p = 0.528), but the three remaining factors were (all p < 0.001). As expected, printing time decreased with increasing slicing or hatching distance. In each case, use of the lowest value (0.5 μm for slicing distance and 0.2 μm for hatching distance) resulted in significantly slower printing times (Figure 4A–B). Hatching type was once again a significant factor; the use of contour lines caused significantly longer printing times than the use of parallel lines (Figure 4C). Thus, the statistical model indicated that we would achieve a minimum printing time (3.02 min.) by using a slicing distance of 1.0 μm, a hatching distance of 0.5 μm and hatching with parallel lines that rotate by 45° between layers. Theoretically, a slicing distance of 1.0 μm would maximize the roundness of horizontal pores while the use of 45° lines would maximize the major axis length without compromising horizontal pore integrity. Since a contoured hatching type was the only condition that resulted in vertical pore diameters close to the modeled values, we also expected a small amount of shrinkage (<15%) to occur when using these time-optimized conditions.
Figure 4. Printing time of small scaffolds.
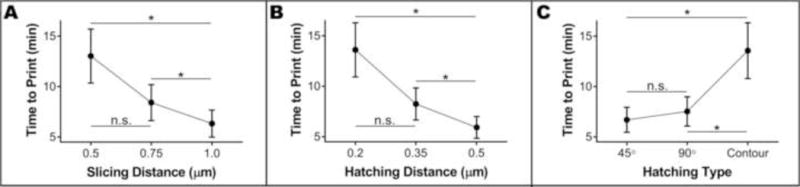
Interval plots showing the effect of slicing distance (A), hatching distance (B) and hatching type (C) on the time required to print small scaffolds. Each data point represents the mean (n = 27), while error bars represent the 95% confidence interval of the mean (*p < 0.05).
We used the optimized printing parameters described above to fabricate larger 1 mm wide scaffolds, with varying vertical pore diameters, to develop an efficient cell loading protocol (Figure 5A). The wall around the scaffolds, included to facilitate cell loading, adhered to the substrate well and was intact around its entire circumference (Figure 5B). Openings to the horizontal pores were apparent on each scaffold (Figure 5C), suggesting patency of the horizontal pores throughout the structure. Apart from minor pore formation issues alone the stitching plane (Figure 5D–F, arrows), vertical pores were well ordered and consistently sized (Figure 5G–I). Bright-field examination of these large scaffolds revealed their interconnected horizontal pores to span the full width of the scaffold (Figure 5J–L). The entire structure, including the three 1 mm hexagons and the surrounding wall, required 12 hours of fabrication time.
Figure 5. Large retinal progenitor cell scaffolds.
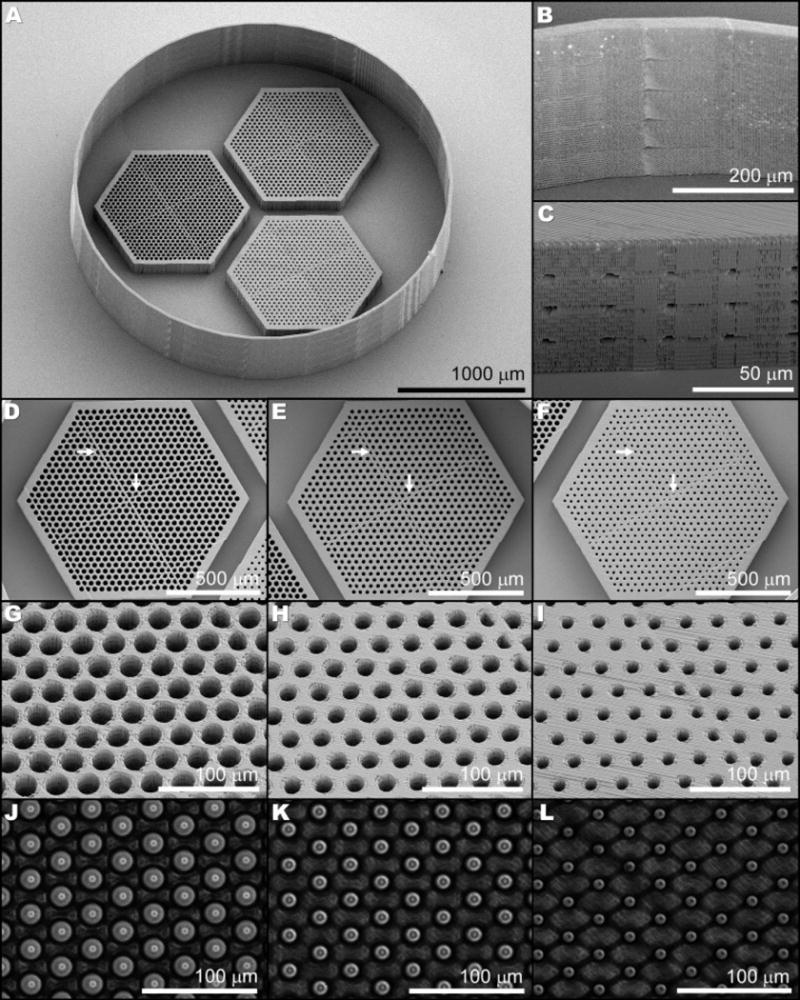
SEM (A–I) and phase contrast (J–L) images show the structural details of three juxtaposed scaffolds (A), each with a different vertical pore size (25 μm: D, G, J; 20 μm: E, H, K; and 15 μm: F, I, L) and intact horizontal pores (C, J–L) surrounded by a retaining wall (B).
When we attempted to seed these structures with human iPSC derived retinal progenitor cells, it became evident that when the structures were printed on glass, it limited the flow of fluid through the scaffolds, which in turn prevented efficient cell loading. We therefore needed to devise a strategy for printing on a porous substrate so that cells could be encouraged to enter the scaffolds using gentle fluid flow pressure (Figure 6A). In order for the printing software to consistently find the surface of the porous membrane, it was necessary for radial tension to be applied to the membrane during printing. When we satisfied this condition, the large scaffolds and surrounding wall adhered to the membrane and appeared to be of similar quality as those printed on glass (Figure 6B–E). The vertical pores of all sizes were free of debris (Figure 6C–E), indicating that cells would have a clear path to enter the scaffolds in the desired orientation.
Figure 6. Scaffolds printed on membrane substrates.
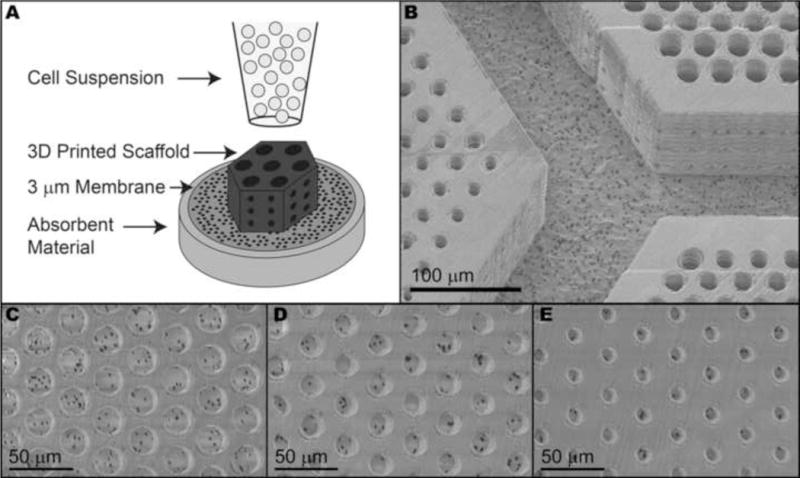
A) Schematic of the retinal progenitor cell loading strategy. Large photoreceptor cell scaffolds printed directly on a 3 μm porous membrane adhered to the membrane after processing (B). Regardless of size, vertical pores were clear of debris and unreacted monomer (C–E).
Two days after seeding these structures with retinal progenitor cells, we harvested and imaged the scaffolds (Figure 7A–C). Scaffolds with 25 μm pores exhibited the greatest percentage of pores with cells (94.3%): 34.3% more than those with 15 μm pores (p < 0.01) and 16.2% more than those with 20 μm pores (Figure 7D). Similarly, the percentage of cell-filled 20 μm pores was 18.1% more than the percentage of cell-filled 15 μm pores, suggesting a roughly linear relationship between pore size and cell loading. Regardless of pore size, retinal progenitor cells formed neuronal processes that extended into and aligned with vertical pores (Figure 7E). Some cell bodies were on top of the scaffold and had not yet entered the vertical pores at this time point, but many cell bodies had settled within the structured columns (Figure 7F–H).
Figure 7. Scaffolds loaded with human retinal progenitor cells.
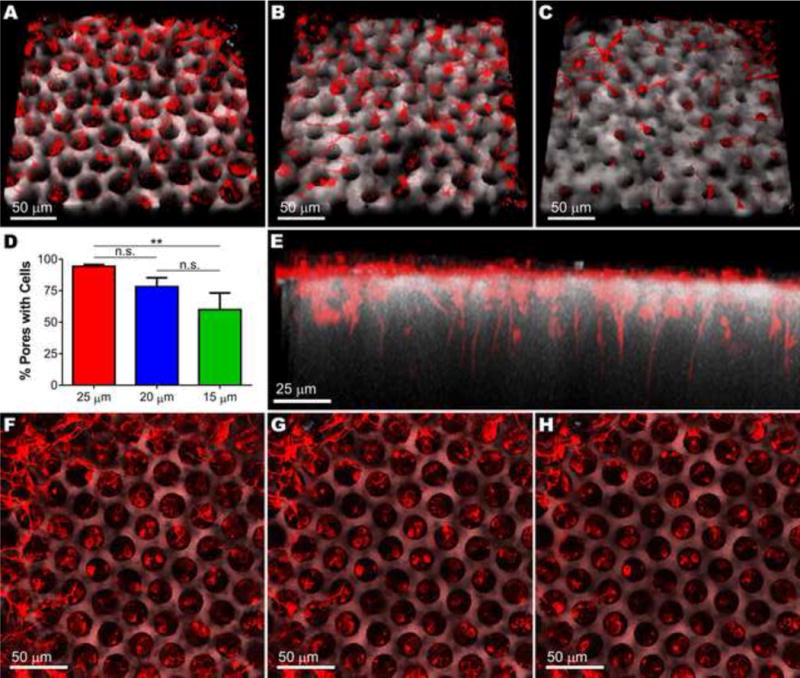
Two days after seeding, developing retinal neurons (TUJ1, red) on the surface of large photoreceptor cell scaffolds (autofluorescence, grey) had extended neuronal processes and migrated into scaffolds with 25 μm (A), 20 μm (B) or 15 μm (C) vertical pores. D) Average number of cells per pore (n = 4 z-stacks). Error bars represent standard deviation, **p < 0.01. E) Side view of retinal neurons settled in and aligned with 25 μm vertical pores. F–H) Sequential top-down images of retinal neurons on the surface of photoreceptor scaffolds and nestled in 25 μm pores.
4. Discussion
Successful transplantation of retinal progenitor cells into the sub-retinal space of subjects with end-stage retinal degeneration will likely require the use of biocompatible structures with specific sub-micron 3D structure to guide cell orientation and packing density similar to that of the native tissue. In this study, we used two-photon polymerization to create such structures and determined the effect of various printing parameters on design-to-structure fidelity and printing time. We loaded two-photon polymerized scaffolds with retinal progenitor cells and determined the effect of scaffold pore size on cell loading. This study opens the door to using two-photon polymerization to create scaffolds with tunable properties, especially microstructure, for the study and treatment of various neurodegenerative diseases.
Understanding the relationship between modeled and actual pore diameter is important to the scaffold design process. Since we found the diameter error to be constant with varying modeled pore size, we can use our statistical model to calculate an expected error based on the hatching distance and type, and then confidently counteract the effect of shrinkage by designing pores of any size that are slightly larger in diameter than the desired final pore size. Since slicing occurred on a plane running parallel to vertical pore cross-sections, it is not surprising that slicing distance did not affect the vertical pore diameter. Conversely, hatching occurs within the same plane as the vertical pore cross-sections. Thus, we speculate that as hatching distance increases, there is more void space in the layer, resulting in less polymer infringement on otherwise empty pore space (Figure 2B). Furthermore, printing a straight line that transverses a pore requires brief shuttering of the laser, which leads to the 2–3 μm diameter error noted in Figure 2C. Since contour hatching uses single lines to trace around pores, no shuttering is required and thus, there is a smaller contribution to dimensional errors than analogous printing using parallel lines. This precision occurs at the expense of layer filling. The small gaps observed in scaffolds printed using contour hatching (Figure 2F) may represent structural weaknesses. In general, the presence of such unpredictable pores in tissue scaffolds could lead to changes in nutrient diffusion or material degradation that could be difficult to control.
The ability to easily create horizontal pores within a scaffold is one of the primary advantages to using two-photon polymerization instead of traditional templating or lithography techniques. Thus, characterizing the fidelity and integrity of these pores is important for probing the practicality of two-photon polymerization in human retinal engineering. We used the horizontal pore roundness as an indicator of structural integrity; a lower roundness value corresponded to collapse of the horizontal pore and vice-versa. Given the limitations of the instrument used to collect images and the angle at which horizontal pores exit the scaffold, a perfect roundness value (1.0) was not possible, so we only used this metric relatively. Slicing, which occurs within the same plane as horizontal pore cross-sections, significantly affected horizontal pore roundness (Figure 3A). We theorize that the greater void volume between layers that occurs with increased slicing distance results in less polymer encroachment on potential pore space compared to smaller slicing distances. As hatching distance does not affect the plane containing horizontal pore cross-sections, it is not a significant factor for horizontal pore roundness. On the other hand, hatching type is a significant factor for major axis length and horizontal pore roundness. As with the vertical pore diameter error, we hypothesize that shuttering of the laser affects horizontal pore major axis length. Contoured hatching, which requires no shuttering, resulted in major axes closest to the modeled size (Figure 3C). There was also a significant difference between 45° and 90° parallel line hatching (Figure 3C). When printing lines shift direction by 45° at each new layer, they are more often close to being in phase with the horizontal pores (which run at 60° and 120°) than if they shift by 90° at each new layer. The closer the printing lines are to being in phase with horizontal pores, the less laser shuttering contributes to deviation from the modeled dimensions. We theorize this to be the source of the significantly greater major axis length in 45° line hatched scaffolds compared to those hatched with 90° lines. Hatching type also affects horizontal pore roundness, with contoured hatching lines causing significantly lower roundness than parallel lines (Figure 3B). This result is another indication that the use of contour lines negatively affects scaffold structural integrity. In fact, bright-field microscopy of contour-hatched scaffolds (Figure 3I) revealed that their horizontal pores did not extend completely through the scaffold or had collapsed after printing. Paired with the general concerns about the structural integrity stated above, this result indicates that contour lines are not an appropriate choice of hatching type for printing scaffolds intended to support transplanted retinal progenitor cells.
Our goal is to create cell delivery scaffolds that, when loaded with cells, can be used use to rebuild the human macula, an approximately 5 mm diameter portion of the retina responsible for central vision. Determining how printing parameters affect printing time is an important consideration for realizing this goal; reducing the printing time of 180 μm scaffolds by just one minute would translate into a 16-hour reduction in printing time when fabricating 5 mm scaffolds. We found that increasing the slicing or hatching distance reduces printing time (Figure 4A–B), most likely because a scaffold of the same volume contains fewer printed lines. Conversely, intricate stage movements, which are required for hatching with contour lines, increases printing time relative to the more simplistic approach of hatching with parallel lines (Figure 4C). We used our statistical model for printing time to determine that the minimum printing time for a small scaffold is 3.02 minutes. Assuming that the relationship between scaffold volume and printing time is linear, we can use the time-optimized printing parameters to create a full-size scaffold (roughly 5 mm in diameter) in less than 2 days. This period is reasonable given that it takes much longer than this to differentiate the cells needed to seed such a graft [25–27]. Furthermore, our results indicate that at these conditions, large scaffolds will have intact horizontal pores and that tuning the size of vertical pores will not significantly affect the margin of error for pore size. We confirmed that scaling up to a larger scaffold size does not interfere with vertical or horizontal pore integrity by using the time-optimized conditions to create 1 mm scaffolds (Figure 5). Slight structural distortions from ‘stitching’ together scaffold segments only affected a small percentage of the vertical pores and did not affect the interconnectivity of horizontal pores.
Over the last decade, several groups have developed scaffolds for retinal cell transplantation with mild success. Two-dimensional scaffolds with randomly distributed pores remain a popular investigatory option; solvent casting, electrospinning and molecular templating are all readily available and relatively simple fabrication techniques [11, 28–32]. However, the structures fabricated with any of these methods fail to provide proper vertical guidance to transplanted cells. To try to overcome this limitation, several groups have created scaffolds with vertical channels using phase separation or traditional microfabrication techniques. In the case of phase separation, pore sizes and distributions are difficult to control in tandem with other properties [14, 33]. Although traditional microfabrication techniques offer precise control of pore size and distribution, resolution can limit pore size and spacing [17, 34–36]. In addition, even if one uses microfabrication to form sub-cellular structures, creating a truly three-dimensional scaffold with horizontal pores for nutrient diffusion in addition to vertical channels is challenging [37, 38]. Furthermore, the efficiency of traditional microfabrication is usually dependent on the use of silicon oxide or silicone rubber templates, meaning that one cannot easily adjust the overall scaffold design after they create the template. Two-photon polymerization is a new approach to creating scaffolds with closely packed vertical channels for cell loading and horizontal pores for nutrient diffusion.
Because two-photon polymerization is a direct fabrication technique and does not require the use of multi-step templating, one can readily alter design parameters such as overall scaffold dimensions or pore geometry, diameter and spacing, by simply adjusting the 3D model and reprocessing in silico (i.e. repeating the slicing and hatching computational procedures). For example, the 120 μm thickness used for scaffolds in this study is likely too large to easily be accommodated by the human sub-retinal space. In future studies, reducing this thickness to precisely 100 μm (average thickness of the human photoreceptor cell layer being replaced) would take less than a day of computing. Although the optimal scaffold dimensions, including thickness, for sub-retinal compatibility remain to be determined, they will likely depend on a multitude of factors such as available surgical instrumentation, ultimate scaffold mechanical properties and the desired quantity of cells to be transplanted. In addition to structure design versatility, two-photon polymerization can also be applied to a wide variety of chemistries, including biodegradable polymers. Theoretically, a custom resist need only contain a photoinitiator capable of producing free radicals when exposed to half the specified laser wavelength and a monomer or prepolymer that is susceptible to free radical polymerization. A large body of work describes methods for adding susceptible groups to various synthetic and naturally occurring biopolymers, while an increasing number of commercial and academic cohorts are identifying and creating two-photon photoinitiators. However, given the relative novelty of two-photon polymerization in general and especially as a technique in biomedical applications, identifying useable combinations of polymer and initiator constitutes a significant time and resources investment. Thus, using a readily available resin that is optimized for two-photon polymerization to establish the feasibility of such a technique for the specified purpose is an important prerequisite to its application to custom, degradable resists.
For example, one of the primary challenges to implementing a two-photon polymerization strategy for production of 3D tissue grafts is the ability to efficiently load scaffolds following fabrication. Since fluid, and the cells suspended within it, follow the path of least resistance, it was not surprising that cells plated on top of scaffolds printed on glass flowed off the scaffold and onto the surrounding substrate instead of entering the pores. To encourage fluid flow through the scaffold, and in turn efficient scaffold loading, we printed scaffolds directly on a porous membrane (Figure 6). We found that larger pores (i.e. 25 μm) were more conducive to cell loading (Figure 7), most likely because the path of a cell moving into a pore was less obstructed than in scaffolds with smaller pores (i.e. 15 μm). In other words, the wider the pores, the more likely cells moving along the pressure gradient (caused by media absorption through the membrane) are to enter a pore rather than stay on the scaffold surface or roll away from the structure.
In this study, we examined the effect of pore size, slicing distance, hatching distance and hatching type on the structural outcomes of two-photon polymerized cell delivery scaffolds. By tuning these parameters, we were able to optimize both printing time and design-to-structure fidelity. We successfully loaded scaffolds with three pore sizes with retinal progenitor cells, which settled into and aligned with vertical pores. We can now use these prototype scaffolds to better understand the cues that affect photoreceptor cell orientation and development. Furthermore, the results described here lay the groundwork for using two-photon polymerization to create biodegradable cell delivery scaffolds in the future with tunable structure, elastic modulus and degradation time. These structures, combined with human iPSC derived retinal progenitor cells can produce autologous retinal cell grafts that represent a promising therapeutic for those suffering from late-stage retinal degeneration.
Supplementary Material
Significance Statement.
Cell replacement therapy is an important goal for investigators aiming to restore neural function to those suffering from neurodegenerative disease. Cell delivery scaffolds are frequently necessary for the success of such treatments, but traditional biomaterials often fail to facilitate the neuronal orientation and close packing needed to recapitulate the in vivo environment. Here, we use two-photon polymerization to create prototype cell scaffolds with densely packed vertical pores for photoreceptor cell loading and small, interconnected horizontal pores for nutrient diffusion. This study offers a thorough characterization of how two-photon polymerization parameters affect final structural outcomes and printing time. Our findings demonstrate the feasibility of using two-photon polymerization to create scaffolds that can align neuronal cells in 3D and are large enough to be used for transplantation. In future work, these scaffolds could comprise biodegradable materials with tunable microstructure, elastic modulus and degradation time; a significant step towards a promising treatment option for those suffering from late-stage neurodegeneration, including retinal degenerative blindness.
Acknowledgments
The authors gratefully acknowledge financial support from the National Institute of Health (1 R01 024605-01), Research to Prevent Blindness (RPB) and the International Retinal Research Foundation (IRRF) (Catalyst Award), Fight for Sight (postdoctoral fellowship), the Howard F. Ruby Endowment for Human Retinal Engineering and the Stephen A. Wynn Foundation. We performed scanning electron microscopy (NIH Shared Instrumentation Grant 1 S10 RR022498-01) at the University of Iowa Central Microscopy Facility, a core resource supported by the Vice President for Research and Economic Development, the Holden Comprehensive Cancer Center and the Carver College of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centanin L, Wittbrodt J. Retinal neurogenesis. Development. 2014;141(2):241–244. doi: 10.1242/dev.083642. [DOI] [PubMed] [Google Scholar]
- 2.Hitchcock P, Ochocinska M, Sieh A, Otteson D. Persistent and injury-induced neurogenesis in the vertebrate retina. Progress in retinal and eye research. 2004;23(2):183–194. doi: 10.1016/j.preteyeres.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Saito T, Kaneko Y, Maruo F, Niino M, Sakaki Y. Study of the Regenerating Newt Retina by Electrophysiology and Immunohistochemistry (Bipolar-Specific and Cone-Specific Antigen Localization) J Exp Zool. 1994;270(6):491–500. doi: 10.1002/jez.1402700602. [DOI] [PubMed] [Google Scholar]
- 4.Stenkamp DL. Neurogenesis in the fish retina. Int Rev Cytol. 2007;259:173–+. doi: 10.1016/S0074-7696(06)59005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacLaren RE, Pearson RA, MacNeil A, Douglas RH, Salt TE, Akimoto M, Swaroop A, Sowden JC, Ali RR. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444(7116):203–7. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- 6.Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell stem cell. 2009;4(1):73–9. doi: 10.1016/j.stem.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klassen H, Sakaguchi DS, Young MJ. Stem cells and retinal repair. Progress in retinal and eye research. 2004;23(2):149–81. doi: 10.1016/j.preteyeres.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Ballios BG, Cooke MJ, Donaldson L, Coles BLK, Morshead CM, van der Kooy D, Shoichet MS. A Hyaluronan-Based Injectable Hydrogel Improves the Survival and Integration of Stem Cell Progeny following Transplantation. Stem Cell Rep. 2015;4(6):1031–1045. doi: 10.1016/j.stemcr.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barber AC, Hippert C, Duran Y, West EL, Bainbridge JW, Warre-Cornish K, Luhmann UF, Lakowski J, Sowden JC, Ali RR, Pearson RA. Repair of the degenerate retina by photoreceptor transplantation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(1):354–9. doi: 10.1073/pnas.1212677110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hynes SR, Lavik EB. A tissue-engineered approach towards retinal repair: scaffolds for cell transplantation to the subretinal space. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2010;248(6):763–78. doi: 10.1007/s00417-009-1263-7. [DOI] [PubMed] [Google Scholar]
- 11.Worthington KS, Green BJ, Rethwisch M, Wiley LA, Tucker BA, Guymon CA, Salem AK. Neuronal Differentiation of Induced Pluripotent Stem Cells on Surfactant Templated Chitosan Hydrogels. Biomacromolecules. 2016;17(5):1684–1695. doi: 10.1021/acs.biomac.6b00098. [DOI] [PubMed] [Google Scholar]
- 12.Yao J, Tao SL, Young MJ. Synthetic Polymer Scaffolds for Stem Cell Transplantation in Retinal Tissue Engineering. Polymers-Basel. 2011;3(2):899–914. [Google Scholar]
- 13.Treharne AJ, Grossel MC, Lotery AJ, Thomson HA. The chemistry of retinal transplantation: the influence of polymer scaffold properties on retinal cell adhesion and control. The British journal of ophthalmology. 2011;95(6):768–73. doi: 10.1136/bjo.2010.184002. [DOI] [PubMed] [Google Scholar]
- 14.Tomita M, Lavik E, Klassen H, Zahir T, Langer R, Young MJ. Biodegradable polymer composite grafts promote the survival and differentiation of retinal progenitor cells. Stem cells. 2005;23(10):1579–88. doi: 10.1634/stemcells.2005-0111. [DOI] [PubMed] [Google Scholar]
- 15.Lim JM, Byun S, Chung S, Park TH, Seo JM, Joo CK, Chung H, Cho DI. Retinal pigment epithelial cell behavior is modulated by alterations in focal cell-substrate contacts. Investigative ophthalmology & visual science. 2004;45(11):4210–6. doi: 10.1167/iovs.03-1036. [DOI] [PubMed] [Google Scholar]
- 16.Tezcaner A, Hicks D. In vitro characterization of micropatterned PLGA-PHBV8 blend films as temporary scaffolds for photoreceptor cells. Journal of biomedical materials research Part A. 2008;86(1):170–81. doi: 10.1002/jbm.a.31600. [DOI] [PubMed] [Google Scholar]
- 17.Tao S, Young C, Redenti S, Zhang Y, Klassen H, Desai T, Young MJ. Survival, migration and differentiation of retinal progenitor cells transplanted on micro-machined poly(methyl methacrylate) scaffolds to the subretinal space. Lab on a chip. 2007;7(6):695–701. doi: 10.1039/b618583e. [DOI] [PubMed] [Google Scholar]
- 18.Chui TYP, Song H, Burns SA. Individual variations in human cone photoreceptor packing density: Variations with refractive error. Investigative ophthalmology & visual science. 2008;49(10):4679–4687. doi: 10.1167/iovs.08-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chui TYP, Song HX, Clark CA, Papay JA, Burns SA, Elsner AE. Cone Photoreceptor Packing Density and the Outer Nuclear Layer Thickness in Healthy Subjects. Investigative ophthalmology & visual science. 2012;53(7):3545–3553. doi: 10.1167/iovs.11-8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song HX, Chui TYP, Zhong ZY, Elsner AE, Burns SA. Variation of Cone Photoreceptor Packing Density with Retinal Eccentricity and Age. Investigative ophthalmology & visual science. 2011;52(10):7376–7384. doi: 10.1167/iovs.11-7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bratton D, Yang D, Dai JY, Ober CK. Recent progress in high resolution lithography. Polym Advan Technol. 2006;17(2):94–103. [Google Scholar]
- 22.Zhang YL, Chen QD, Xia H, Sun HB. Designable 3D nanofabrication by femtosecond laser direct writing. Nano Today. 2010;5(5):435–448. [Google Scholar]
- 23.Sun HB, Kawata S. Two-photon photopolymerization and 3D lithographic microfabrication. Adv Polym Sci. 2004;170:169–273. [Google Scholar]
- 24.Greiner AM, Jackel M, Scheiwe AC, Stamow DR, Autenrieth TJ, Lahann J, Franz CM, Bastmeyer M. Multifunctional polymer scaffolds with adjustable pore size and chemoattractant gradients for studying cell matrix invasion. Biomaterials. 2014;35(2):611–619. doi: 10.1016/j.biomaterials.2013.09.095. [DOI] [PubMed] [Google Scholar]
- 25.Wiley LA, Burnight ER, DeLuca AP, Anfinson KR, Cranston CM, Kaalberg EE, Penticoff JA, Affatigato LM, Mullins RF, Stone EM, Tucker BA. cGMP production of patient-specific iPSCs and photoreceptor precursor cells to treat retinal degenerative blindness. Sci Rep. 2016;6:30742. doi: 10.1038/srep30742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong XF, Gutierrez C, Xue T, Hampton C, Vergara MN, Cao LH, Peters A, Park TS, Zambidis ET, Meyer JS, Gamm DM, Yau KW, Canto-Soler MV. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat Commun. 2014;5 doi: 10.1038/ncomms5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinha D, Phillips J, Phillips MJ, Gamm DM. Mimicking Retinal Development and Disease With Human Pluripotent Stem Cells. Investigative ophthalmology & visual science. 2016;57(5) doi: 10.1167/iovs.15-18160. [DOI] [PubMed] [Google Scholar]
- 28.Tucker BA, Redenti SM, Jiang C, Swift JS, Klassen HJ, Smith ME, Wnek GE, Young MJ. The use of progenitor cell/biodegradable MMP2-PLGA polymer constructs to enhance cellular integration and retinal repopulation. Biomaterials. 2010;31(1):9–19. doi: 10.1016/j.biomaterials.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Cai S, Smith ME, Redenti SM, Wnek GE, Young MJ. Mouse Retinal Progenitor Cell Dynamics on Electrospun Poly(epsilon-Caprolactone) J Biomat Sci-Polym E. 2012;23(11):1451–1465. doi: 10.1163/092050611X584388. [DOI] [PubMed] [Google Scholar]
- 30.Lawley E, Baranov P, Young M. Hybrid vitronectin-mimicking polycaprolactone scaffolds for human retinal progenitor cell differentiation and transplantation. J Biomater Appl. 2015;29(6):894–902. doi: 10.1177/0885328214547751. [DOI] [PubMed] [Google Scholar]
- 31.Kundu J, Michaelson A, Talbot K, Baranov P, Young MJ, Carrier RL. Decellularized retinal matrix: Natural platforms for human retinal progenitor cell culture. Acta Biomater. 2016;31:61–70. doi: 10.1016/j.actbio.2015.11.028. [DOI] [PubMed] [Google Scholar]
- 32.Worthington KS, Wiley LA, Guymon CA, Salem AK, Tucker BA. Differentiation of Induced Pluripotent Stem Cells to Neural Retinal Precursor Cells on Porous Poly-Lactic-co-Glycolic Acid Scaffolds. J Ocul Pharmacol Th. 2016;32(5):310–316. doi: 10.1089/jop.2015.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lavik EB, Klassen H, Warfvinge K, Langer R, Young MJ. Fabrication of degradable polymer scaffolds to direct the integration and differentiation of retinal progenitors. Biomaterials. 2005;26(16):3187–96. doi: 10.1016/j.biomaterials.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 34.Redenti S, Neeley WL, Rompani S, Saigal S, Yang J, Klassen H, Langer R, Young MJ. Engineering retinal progenitor cell and scrollable poly(glycerol-sebacate) composites for expansion and subretinal transplantation. Biomaterials. 2009;30(20):3405–3414. doi: 10.1016/j.biomaterials.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steedman MR, Tao SL, Klassen H, Desai TA. Enhanced differentiation of retinal progenitor cells using microfabricated topographical cues. Biomed Microdevices. 2010;12(3):363–369. doi: 10.1007/s10544-009-9392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sodha S, Wall K, Redenti S, Klassen H, Young MJ, Tao SL. Microfabrication of a Three-Dimensional Polycaprolactone Thin-Film Scaffold for Retinal Progenitor Cell Encapsulation. J Biomat Sci-Polym E. 2011;22(4–6):443–456. doi: 10.1163/092050610X487738. [DOI] [PubMed] [Google Scholar]
- 37.Redenti S, Tao S, Yang J, Gu P, Klassen H, Saigal S, Desai T, Young MJ. Retinal tissue engineering using mouse retinal progenitor cells and a novel biodegradable, thin-film poly(e-caprolactone) nanowire scaffold. J Ocul Biol Dis Infor. 2008;1(1):19–29. doi: 10.1007/s12177-008-9005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao J, Ko CW, Baranov PY, Regatieri CV, Redenti S, Tucker BA, Mighty J, Tao SL, Young MJ. Enhanced Differentiation and Delivery of Mouse Retinal Progenitor Cells Using a Micropatterned Biodegradable Thin-Film Polycaprolactone Scaffold. Tissue Eng Pt A. 2015;21(7–8):1247–1260. doi: 10.1089/ten.tea.2013.0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


