Abstract
Alcohol consumption can lead to the increase in gut permeability and cause the translocation of bacteria-derived lipopolysaccharides from the gut to the liver, which subsequently activates immune responses. In this process, macrophages play a critical role and involve in the pathogenesis of alcoholic liver disease (ALD). To define the mechanism underpinning the function of macrophages, it is important to conduct extensive studies to further explicate the phenotypic diversity of macrophages in the context of ALD.
In this review, the role of hepatic macrophages in the pathogenesis of ALD is discussed.
INTRODUCTION
The pathogenesis of alcoholic liver disease (ALD) is complex.1,2 In addition to the direct cytotoxic and the reactive oxygen species (ROS)-mediated effects of alcohol1 and its metabolite, acetaldehyde,3–5 on hepatocytes, alcohol ingestion can activate the innate and adaptive immune responses,2,6 which can lead to alcohol-induced liver injury. Alcohol consumption can lead to the increase in gut permeability and cause the translocation of bacteria-derived lipopolysaccharides from the gut to the liver, which subsequently activates immune responses.1,2,7,8
Such responses by alcohol involves multiple cell types, including resident macrophages (Kupffer cells, KCs), natural killer cells, natural killer T cells, lymphocytes, neutrophils, and monocytes.6 The KC serves as the primary effector cell of the innate immune response within the liver9 and plays a key role in the early pathogenesis of alcohol-induced liver injury.10–12 As mentioned, the impairment in intestinal permeability by alcohol can lead to endotoxemia.1,2,7,8 Endotoxin can bind to CD14 (the KC endotoxin receptor) in association with toll-like receptor 4 to initiate intracellular signaling leading to alcohol-induced liver injury.11 Inactivation of KCs by gadolinium chloride ameliorates ALD seen with chronic ethanol administration using the Tsukamoto-French model.13 In addition to the KCs, another type of macrophages in the liver, known as infiltrating macrophages (IMs), can be differentiated from the circulating monocytes that are recruited into the liver tissues due to inflammatory reactions during liver injury.6
HETEROGENEITY OF MONOCYTES AND MACROPHAGES
Monocytes are circulating innate immune cells originated from the progenitor cells in the bone marrow.14,15 In humans and mice, monocytes can be divided, depending on the surface marker proteins, into two major subsets—classical and non-classical (see review in ref. 6 and table 1). In humans, all CD115+ monocytes are further divided into two major subsets based on their CD14 and CD16 expression, as well as on their expression of markers called CCR2 and CX3/CR1.6 The predominant classical subset, representing 90% of circulating monocytes, is characterized by the marker combination CD14hiCD16− and CCR2 +/CX3CR1lo. The less abundant non-classical monocyte subset can be subdivided into two groups: CD14dimCD16+ and CCR2 −/CX3CR1hi/CCR5hi (non-classical monocytes) or CD14hiCD16+ and CCR2−/CX3CR1hi/CCR5hi (intermediate monocytes, table 1).
Table 1.
Monocyte population of mouse and human as stratified by the protein surface markers (modified from ref. 6)
| Species | Subset | Markers | Function |
|---|---|---|---|
| Mouse | Classical | CD11b+ Ly6ChiCCR2+ CX3CR1− | Inflammatory effectors |
| Non-classical | CD11b+ Ly6CloCCR2− CX3CR1+ | Patrolling, tissue repair | |
| Human | Classical | CD14hiCD16− CCR2+ CX3CR1lo | Phagocytosis and inflammatory effectors |
| Intermediate | CD14hiCD16+ CCR2− CX3CR1hi | Inflammatory effectors | |
| Non-classical | CD14dimCD16+ CCR2− CX3CR1hi | Patrolling, antiviral role |
In mouse, the monocyte subsets include classical Ly6Chi monocytes, which are similar to the human CD14hiCD16− monocytes, and non-classical Ly6Clo monocytes, which are analogous to human CD14dimCD16+ monocytes.6,15 In human and mouse, these monocytes will circulate in the blood vessels until they are recruited to the organs in case of an injury or insult.6,15
Macrophages have a unique ability to alter their phenotypes and functions depending on tissue environmental factors, such as the presence of cytokines, growth factors, pathogen-associated molecular pattern molecules, and damage-associated molecular pattern molecules.6,15–17 This process leads to two macrophage phenotypes: M1 and M2 macrophages (figure 1). M1, proinflammatory macrophages, help mediate the initial defense against intracellular bacteria and viruses and response to a tissue injury. The M1 macrophages produce proinflammatory and stress mediators and cytokines, such as interleukin (IL)-1, tumor necrosis factor-α (TNF-α), interferon-γ, IL-12, IL-18, nitric oxide, and ROS.6,15 Once the infection or injury is controlled, macrophages differentiate into an anti-inflammatory, tissue-restorative phenotype in order to reign in excessive tissue-damaging inflammatory responses.6,15,18,19 These cells bear similarities to the alternatively activated macrophages (M2) and help promote the resolution of inflammation as well as tissue repair.6,15 The activation of circulating monocytes and accumulation of macrophages in the liver in the pathogenesis of ALD have not been fully elucidated.
Figure 1.
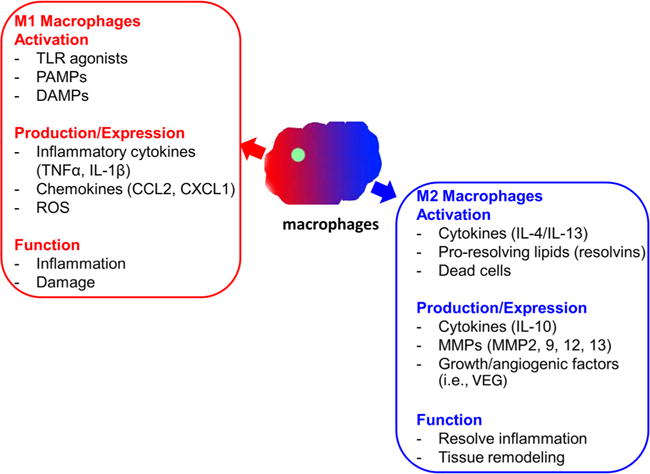
Proinflammatory and tissue-restorative macrophages. DAMP, damage-associated molecular pattern molecule; IL, interleukin; MMP, matrix metalloproteinase; PAMP, pathogen-associated molecular pattern molecule; ROS, reactive oxygen species; TLR, toll-like receptor; TNF-α, tumor necrosis factor-alpha; VEG, vascular endothelial growth factor.
ROLE OF HEPATIC MACROPHAGES IN ALD
To investigate the effects of alcohol on hepatic macrophage populations, C57BL/6J mice were fed an ethanol-containing Lieber-Decarli diet for 4 weeks. Interestingly, we observed a marked increase of IMs in the liver of ethanol-fed compared with pair-fed and naive mice. KCs express a high level of F4/80 and low level of CD11b, and they do not express Ly6C (F4/80hiCD11blowLy6C−). In contrast, IMs express a low level of F4/80 and high level of CD11b, and they are positive for Ly6C (F4/80IntCD11bhiLy6C+). It is important to note that two subsets of IMs with high and low expression levels of Ly6C were detected (Ly6Chi and Ly6Clow subsets). The Ly6Chi subset is mainly monocytes with horseshoe-shaped or kidney-shaped nuclei. On the other hand, the Ly6Clow population contains ∼30% neutrophils.20 It is very interesting that the IMs and KCs have different characteristics. For example, the expression levels of matrix metalloproteinase (MMP), such as MMP9 and MMP12, were increased significantly in IMs from ethanol-fed mice compared with KCs from pair-fed mice. MMP9 expression increased >10-fold in IMs, and MMP12 increased nearly 60-fold in KCs.20 The results suggest that IMs and KCs are involved in matrix remodeling during chronic ethanol exposure. We next examined the genes known to be associated with macrophages polarization.20 The M2 markers Ym1, Fizz1, and Mrc1 were induced significantly in IMs. However, Arg1, a typical M2 gene, is moderately upregulated in KCs but not in IMs.20 Genes known to be expressed as M1 markers, such as iNOS, IL-12p40, and CIITA, were also upregulated significantly in IMs. Lastly, IMs and KCs from ethanol-fed mice expressed elevated levels of proinflammatory cytokines, such as TNF-α, IL-1β, and IFN-γ.20 These data demonstrated that IMs express M1 and M2 markers, suggesting that there may be two subsets of IMs. In fact, our report showed the distinctive gene expression profiles between Ly6Chi and Ly6Clow IMs from ethanol-fed mice (see ref. 20 for more detail). Ly6Clow IMs exhibit an anti-inflammatory and tissue-protective phenotype; in contrast, Ly6Chi IMs exhibit a proinflammatory, tissue-damaging phenotype.
CONCLUSION
Taken together, chronic ethanol feeding induces the recruitment of two subsets of hepatic IMs, which play different or even opposite roles in regulating liver inflammation and repair. These findings may not only increase our understanding of the complex functions of macrophages in the pathogenesis of ALD but also help us to identify future novel therapeutic targets for the treatment of ALD.
Acknowledgments
Synopsis from the symposia entitled ‘Emerging new mechanism in alcoholic liver disease’; which was presented at the Experimental Biology 2015 meeting in San Diego, California, USA. The meeting is supported by the American Federation for Medical Research (AFMR).
Funding: This work was supported by U01AA021723 (to CJ), VA Merit Award 1I01CX000361, NIH U01AA021840, US DOD W81XWH-12-1-0497 (to SL).
Footnotes
Competing interests: None declared.
Provenance and peer review; Commissioned; externally peer reviewed.
References
- 1.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–85. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao B, Seki E, Brenner DA, et al. Innate immunity in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2011;300:G516–25. doi: 10.1152/ajpgi.00537.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marmier S, Dentin R, Daujat-Chavanieu M, et al. Novel role for ChREBP in the control of ethanol metabolism and susceptibility to binge drinking. Hepatology. 2015;62:1086–100. doi: 10.1002/hep.27778. [DOI] [PubMed] [Google Scholar]
- 4.Kwon HJ, Won YS, Park O, et al. Aldehyde dehydrogenase 2 deficiency ameliorates alcoholic fatty liver but worsens liver inflammation and fibrosis in mice. Hepatology. 2014;60:146–57. doi: 10.1002/hep.27036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhong W, Zhang W, Li Q, et al. Pharmacological activation of aldehyde dehydrogenase 2 by Alda-1 reverses alcohol-induced hepatic steatosis and cell death in mice. J Hepatol. 2015;62:1375–81. doi: 10.1016/j.jhep.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ju C, Mandrekar P. Macrophages and alcohol-related liver inflammation. Alcohol Res. 2015;37:251–62. [PMC free article] [PubMed] [Google Scholar]
- 7.Chen P, Stärkel P, Turner JR, et al. Dysbiosis-induced intestinal inflammation activates tumor necrosis factor receptor I and mediates alcoholic liver disease in mice. Hepatology. 2015;61:883–94. doi: 10.1002/hep.27489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bala S, Marcos M, Gattu A, et al. Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PLoS One. 2014;9:e96864. doi: 10.1371/journal.pone.0096864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagy LE. Recent insights into the role of the innate immune system in the development of alcoholic liver disease. Exp Biol Med (Maywood) 2003;228:882–90. doi: 10.1177/153537020322800803. [DOI] [PubMed] [Google Scholar]
- 10.Nagy LE. Stabilization of tumor necrosis factor-alpha mRNA in macrophages in response to chronic ethanol exposure. Alcohol. 2004;33:229–33. doi: 10.1016/j.alcohol.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Wheeler MD. Endotoxin and Kupffer cell activation in alcoholic liver disease. Alcohol Res Health. 2003;27:300–6. [PMC free article] [PubMed] [Google Scholar]
- 12.Wheeler MD, Kono H, Yin M, et al. The role of Kupffer cell oxidant production in early ethanol-induced liver disease. Free Radic Biol Med. 2001;31:1544–9. doi: 10.1016/s0891-5849(01)00748-1. [DOI] [PubMed] [Google Scholar]
- 13.Hines IN, Wheeler MD. Recent advances in alcoholic liver disease III. Role of the innate immune response in alcoholic hepatitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G310–14. doi: 10.1152/ajpgi.00094.2004. [DOI] [PubMed] [Google Scholar]
- 14.Das A, Sinha M, Datta S, et al. Monocyte and macrophage plasticity in tissue repair and regeneration. Am J Pathol. 2015;185:2596–606. doi: 10.1016/j.ajpath.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ju C, Tacke F. Hepatic macrophages in homeostasis and liver diseases: from pathogenesis to novel therapeutic strategies. Cell Mol Immunol. 2016;13:316–27. doi: 10.1038/cmi.2015.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He C, Carter AB. The metabolic prospective and redox regulation of macrophage polarization. J Clin Cell Immunol. 2015;6:371. doi: 10.4172/2155-9899.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Motwani MP, Gilroy DW. Macrophage development and polarization in chronic inflammation. Semin Immunol. 2015;27:257–66. doi: 10.1016/j.smim.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181:3733–9. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- 19.Noël W, Raes G, Hassanzadeh Ghassabeh G, et al. Alternatively activated macrophages during parasite infections. Trends Parasitol. 2004;20:126–33. doi: 10.1016/j.pt.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Wang M, You Q, Lor K, et al. Chronic alcohol ingestion modulates hepatic macrophage populations and functions in mice. J Leukoc Biol. 2014;96:657–65. doi: 10.1189/jlb.6A0114-004RR. [DOI] [PMC free article] [PubMed] [Google Scholar]


