Significance
Children raised in economically disadvantaged households face increased risks of poor health in adulthood, suggesting early origins of socioeconomic inequalities in health. In fact, maternal immune activity in response to stressful conditions during pregnancy has been found to play a key role in fetal brain development. Here we show that socioeconomic disadvantage is associated with lower concentrations of the pro-inflammatory cytokine IL-8 during the third trimester of pregnancy and, in turn, with offspring’s neurologic abnormalities during the first year of life. These results suggest stress–immune mechanisms as one potential pathophysiologic pathway involved in the early origins of population health inequalities.
Keywords: socioeconomic disadvantage, inflammation, gestation, neurodevelopment, epidemiology
Abstract
Children raised in economically disadvantaged households face increased risks of poor health in adulthood, suggesting that inequalities in health have early origins. From the child’s perspective, exposure to economic hardship may begin as early as conception, potentially via maternal neuroendocrine–immune responses to prenatal stressors, which adversely impact neurodevelopment. Here we investigate whether socioeconomic disadvantage is associated with gestational immune activity and whether such activity is associated with abnormalities among offspring during infancy. We analyzed concentrations of five immune markers (IL-1β, IL-6, IL-8, IL-10, and TNF-α) in maternal serum from 1,494 participants in the New England Family Study in relation to the level of maternal socioeconomic disadvantage and their involvement in offspring neurologic abnormalities at 4 mo and 1 y of age. Median concentrations of IL-8 were lower in the most disadvantaged pregnancies [−1.53 log(pg/mL); 95% CI: −1.81, −1.25]. Offspring of these pregnancies had significantly higher risk of neurologic abnormalities at 4 mo [odds ratio (OR) = 4.61; CI = 2.84, 7.48] and 1 y (OR = 2.05; CI = 1.08, 3.90). This higher risk was accounted for in part by fetal exposure to lower maternal IL-8, which also predicted higher risks of neurologic abnormalities at 4 mo (OR = 7.67; CI = 4.05, 14.49) and 1 y (OR = 2.92; CI = 1.46, 5.87). Findings support the role of maternal immune activity in fetal neurodevelopment, exacerbated in part by socioeconomic disadvantage. This finding reveals a potential pathophysiologic pathway involved in the intergenerational transmission of socioeconomic inequalities in health.
Children raised in conditions of economic hardship face higher risks of disease in adulthood and live shorter lives (1). Given that economic hardship influences health as early as the prenatal period (2), socioeconomic inequalities in health may become embedded early in a child’s development. However, the pathways through which early social and economic disadvantage influence health to create such disparities remain unclear (3, 4). Maternal stress responses during pregnancy may function as one potential pathway. Maternal reports of daily “hassles” during pregnancy—a means of estimating mothers’ exposure to everyday stressors—predicted lower scores on infant motor and mental development in the first year of life (5). More extreme stressors during pregnancy, such as loss of a close relative, are associated with severe neurologic deficits in offspring (6). These findings suggest that transmission of the increased burden of disease among socioeconomically disadvantaged individuals may involve maternal stress–immune–related mechanisms during pregnancy (7).
Here we investigate whether the social and economic environment disrupts maternal immune activity during pregnancy and, if so, whether there are implications for offspring neurodevelopment. To the extent that maternal immune activity is disrupted by social conditions during pregnancy, maternal immune dysregulation may represent a key pathway underlying adverse neurodevelopmental effects of prenatal social disadvantage (8).
Emerging evidence suggests that prenatal stressors can induce measurable inflammatory responses, and these responses are thought to play a major role in the fetal origins of disease (9). Coussons-Read and colleagues have shown that women who report high levels of psychosocial stressors, such as problematic relationships or finances, have higher levels of several proinflammatory molecules, including cytokines (IL-6, IL-1β, and TNF-α) and C-reactive protein (CRP), and lower levels of the anti-inflammatory cytokine IL-10 (10). Wright and colleagues reported increased IL-8 (also known as “CXCL8”) and TNF-α concentrations in cord blood following exposure to high levels of prenatal stressors (11).
Whether these responses have implications for offspring neurodevelopment remains unknown, although reports that show elevated risks for fetal brain injury, neuropsychiatric disorders, and cognitive deficits for children exposed to prenatal infection and intrauterine inflammation suggest that they do (12–18). Because both higher and lower levels of inflammatory markers are shown to be associated with adverse neuropsychiatric outcomes in the offspring (18–22), dysregulation of gestational immune activity, rather than mere suppression or up-regulation of the maternal immune system, may ultimately have the greatest impact on brain development in the offspring.
Here, we extend this work by investigating the associations between socioeconomic disadvantage during pregnancy and concentrations in third-trimester maternal serum of five proinflammatory and anti-inflammatory cytokines, IL-1β, IL-6, IL-8, TNF-α, and IL-10. These cytokines are the primary coactivators of hypothalamic–pituitary–adrenal (HPA) axis function and thus indicate signaling along stress–immune pathways (23, 24). Given the importance of understanding the roles of both proinflammatory and anti-inflammatory responses in fetal development, IL-10, which is considered anti-inflammatory, was included. Using data from a pregnancy cohort that collected extensive medical data throughout gestation, we were able to examine the influence of socioeconomic disadvantage on inflammatory molecules independent of the impact of maternal infection and other chronic conditions that are medically related to increases in inflammation.
Methods
Participants.
The study sample included 1,494 women enrolled during pregnancy (1959–1966) in the Massachusetts and Rhode Island cohorts of the Collaborative Perinatal Project (CPP) whose offspring were administered neurologic examinations at ages 4 mo and 1 y. The CPP, after obtaining informed consent from all participants (25), collected maternal serum samples during pregnancy and administered pediatric and neurologic assessments to offspring. Participants were selected for the current investigation on the basis of their offspring’s enrollment in the New England Family Study, a series of adult follow-up studies of CPP children (overseen by the Institutional Review Boards of Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health). We obtained prenatal serum samples acquired from mothers during early third trimester from the NIH repository (12, 13). Concentrations of maternal cytokines were assessed by multiplexed immunoassays and analyzed in relation to the level of maternal socioeconomic disadvantage during pregnancy and clinical ratings of neurologic development of offspring during the first year of life.
Prenatal Serologic Assays.
We assayed stored maternal serum for concentrations of five cytokines (IL-1β, IL-6, IL-8, TNF-α, and IL-10) that have receptor locations in stress circuitry brain regions (23). We preferentially selected samples drawn as close to the beginning of third trimester as available from the specimen repository, given the importance of this period for neurodevelopment and prior evidence that inflammation during mid to late gestation confers risk for offspring neuropsychiatric outcomes (26–28). Previous work using samples stored under similar conditions and for a similar length of time (>40 y) demonstrated the long-term stability of these cytokines (12, 13). Samples blinded with respect to maternal socioeconomic conditions and offspring neurodevelopment were randomly assessed using assay kits and reagents from a single lot, and all assays were completed within a 2-mo period. Maternal cytokine levels were analyzed using a multiplexed, bead-based immunoassay (Milliplex human cytokine panel MPXHCYTO-60K; Millipore) on a Luminex 3DTM detection platform (Luminex Corporation) (29). Assay sensitivities ranged from 0.1 to 0.4 pg/mL. Twenty-five microliters of each serum sample were diluted 1:1 in assay buffer and run with six serial dilutions (3.2–10,000 pg/mL) of cytokine standards, two commercial-quality control samples, one in-house plasma sample, and buffer only (background) on each 96-well plate (30). All samples, standards, and controls were run in duplicate in assays completed according to manufacturers’ protocols. Raw data (mean fluorescence intensity) were captured using Luminex xPONENT software (v.4.0.846.0); concentrations of immune factors in each sample were interpolated from standard curves in Milliplex Analyst (v.3.5.5.0) using a five-parameter, weighted, logistic regression curve equation. Measurements below the lower limit of detection of any analyte were recoded to the midpoint between zero and the limit of detection for that analyte. Samples initially yielding values at or above the upper limit of analyte detection were reassayed at multiple serial dilutions to bring concentrations into detectable range.
Clinical and Demographic Measures.
Maternal socioeconomic disadvantage.
We developed a measure of socioeconomic disadvantage using information collected from the social history interview administered to participants upon enrollment into the study, which we previously used to report associations with children’s neurologic abnormalities (31). The interview data combined information regarding (i) parental education (post-high school education, high school graduate, or non-high school graduate), (ii) income relative to the US poverty threshold (>150% of the poverty threshold, 100–150% of the poverty threshold, or < poverty threshold), (iii) parental occupation (nonmanual, manual, or unemployed), and (iv) family structure (both parents at home, single mother, or divorced/separated/widowed mother). Each of the four items was given a score of 0, 1, or 2, with higher scores indicating a higher degree of disadvantage. These scores were summed to produce a composite measure of low (0–2), medium (3–5), or high (≥6) socioeconomic disadvantage. More than 80% of highly disadvantaged families were characterized by parents with less than a high school degree, low household income, unemployed or manual occupations, and an absent father. In contrast, the majority of parents without high levels of socioeconomic disadvantage had a high school degree or greater education, high income, nonmanual occupation, and were married.
Potential confounders: conditions during pregnancy associated with inflammation.
We constructed two summary measures of medical conditions that indicate the presence of elevated maternal immune activation. The first of these represents common conditions associated with acute inflammatory responses, primarily including infections during pregnancy. The second summary measure includes reports at enrollment or during pregnancy of conditions associated with chronic inflammation (e.g., asthma, diabetes). Data regarding these conditions were obtained from diagnostic summaries completed by study physicians at the time of enrollment, during pregnancy, and at the end of pregnancy (specific conditions are reported in Table S1).
Table S1.
Maternal conditions during pregnancy included in the summary measures of infection and chronic inflammatory condition
| Condition | Percent (n)* |
| Conditions associated with acute inflammation | 45.6 (681) |
| Cardiovascular conditions | |
| Fever 100.4 °F or above | 0 |
| Pulmonary conditions | |
| Tuberculosis (TBC), active | 0 |
| Positive sputum culture | 0 |
| Positive culture, other | 0 |
| Positive guinea pig inoculation | 0 |
| Pneumonia | 0.5 (7) |
| Positive culture | 0 |
| Viral and/or serologic evidence | 0.1 (1) |
| Chest x-ray evidence | 0.1 (2) |
| Venereal disease | |
| Syphilis | 0.3 (5) |
| Positive serology | 0.3 (4) |
| Positive cerebrospinal fluid | 0 |
| Positive Treponema immobilization test | 0 |
| Positive dark field | 0 |
| Gonorrhea | 0.1 (1) |
| Positive culture | 0.1 (1) |
| Positive smear | 0.1 (1) |
| Other | 0 |
| Urinary tract conditions | |
| Kidney, ureter, bladder infection | 6.1 (91) |
| Fever 100.4 °F or above | 0.8 (12) |
| Positive urine culture | 2.7 (40) |
| Pyuria (15 WBCs per high-power field) | 2.1 (32) |
| Gynecological conditions | |
| Vaginitis | 11.8 (176) |
| Gastrointestinal conditions | |
| Cholecystitis | 0.1 (2) |
| Hepatitis | 0 |
| Appendicitis | 0.2 (3) |
| Colitis, Ileitis | 0.2 (3) |
| Shock, septic | 0 |
| Infectious diseases during pregnancy | |
| Known or presumed viral | 6.7 (100) |
| Known or presumed bacterial | 3.8 (57) |
| Known or presumed parasitic | 0.3 (5) |
| Known or presumed fungal | 0.1 (2) |
| Type unknown | 0.3 (4) |
| Attenuated live vaccine, type | 9.1 (136) |
| Bacteriuria | 16.9 (253) |
| Autoimmune, allergic, and conditions associated with chronic inflammation | 12.9 (193) |
| Lifetime conditions | |
| Cardiovascular and blood conditions | |
| Rheumatic fever | 1.8 (27) |
| Thrombophlebitis | 0.1 (2) |
| Pulmonary conditions | |
| Bronchial asthma | 1.1 (16) |
| Sarcoidosis | 0 |
| Metabolic conditions | |
| Thyroid diseases | 0.8 (12) |
| Genito-urinary conditions | |
| Glomerulonephritis | 0 |
| Pregnancy conditions | |
| Cardiovascular conditions | |
| Rheumatic fever | 4.0 (59) |
| Pulmonary conditions | |
| Bronchial asthma | 2.8 (42) |
| Acute asthma | 0.1 (1) |
| Status asthmaticus | 0.1 (1) |
| Metabolic and endocrine conditions | |
| Diabetes mellitus | 1.3 (20) |
| Hypothyroidism | 3.2 (47) |
| Hyperthyroidism | 0.8 (12) |
| Urinary tract conditions | |
| Acute and chronic glomerulonephritis | 0.5 (8) |
| Gastrointestinal conditions | |
| Thrombosis and/or phlebitis, colitis, ileitis | 0.5 (7) |
Conditions with “0” prevalence in the sample were assessed in the Collaborative Perinatal Project but were not present among participants in the analytic sample.
Offspring neurologic abnormalities.
The presence of neurologic abnormalities at 4 mo and 1 y postnatal was established based on examinations conducted by a pediatrician with training in neurology or a neurologist with training in pediatrics. The 4-mo examination covered structural deformities, posturing, motor skills, and response to specific stimuli. Examination at 1 y included assessments of gait, locomotor activities, and muscle power; the presence of involuntary movements; and ratings of sensory and autonomic nervous system function. After completion of each neurologic examination, the physician reported a global impression of the child’s neurologic status as normal, suspicious, or abnormal. A rating of abnormal was given when examiners could make a definite or provisional diagnosis of a recognized syndrome or when any definite neurologic abnormality was identified. Children with physical stigmata often associated with (but not pathognomonic for) neurologic disorders, such as facial hemangiomas or spinal anomalies, were rated as suspicious. Children without these features were deemed normal. The present study compared children with either suspicious or abnormal ratings to children with normal ratings at ages 4 mo and 1 y.
Statistical Analysis.
Two sets of statistical analyses were performed: (i) associations between socioeconomic disadvantage and concentrations of the five inflammatory cytokines in prenatal serum using quantile regression; and (ii) associations of socioeconomic disadvantage and cytokine concentrations with neurologic outcomes in infancy using logistic regression.
Quantile regression models evaluated variations in maternal immune activity according to the level of maternal socioeconomic disadvantage. Quantile regression was used to detect differences between groups that may exist at the extremes of the distribution. These models estimated coefficients over the entire distribution of each cytokine from the fifth through 95th percentiles. We analyzed the five cytokines individually and subsequently analyzed concentrations of each proinflammatory cytokine (IL-1β, IL-6, IL-8, and TNF-α) relative to that of the anti-inflammatory cytokine IL-10 (e.g., ratio of each proinflammatory cytokine:IL-10). (Linear regressions of cytokine concentrations are presented in Tables S2 and S3 for comparison purposes.) All analyses were adjusted for maternal race/ethnicity and gestational age at sample collection. To estimate the effect of socioeconomic disadvantage on maternal immune activity independent of medically related increases in inflammation, we fitted models that controlled for maternal acute and chronic inflammatory conditions as well as models limited to women without any acute or chronic conditions associated with inflammation (n = 780).
Table S2.
Linear regression coefficients (and 95% CIs) from analyses of inflammatory cytokines in third-trimester maternal serum in relation to level of socioeconomic disadvantage
| Cytokine | Linear regression coefficient (95% CI) |
| IL-1β | |
| Socioeconomic disadvantage | |
| High | −0.40 (−0.85, 0.06) |
| Medium | −0.29 (−0.57, −0.02) |
| Low | Reference |
| IL-6 | |
| Socioeconomic disadvantage | |
| High | −0.33 (−0.71, 0.04) |
| Medium | −0.13 (−0.34, 0.08 |
| Low | Reference |
| IL-8 | |
| Socioeconomic disadvantage | |
| High | −1.39 (-1.74, −1.04) |
| Medium | −0.78 (−1.01, −0.55) |
| Low | Reference |
| TNF-α | |
| Socioeconomic disadvantage | |
| High | −0.12 (−0.33, 0.09) |
| Medium | −0.04 (−0.15, 0.07) |
| Low | Reference |
| IL-10 | |
| Socioeconomic disadvantage | |
| High | −0.18 (-0.43, 0.08) |
| Medium | −0.17 (−0.32, −0.03) |
| Low | Reference |
| IL-1β:IL-10 ratio | |
| Socioeconomic disadvantage | |
| High | −0.22 (−0.69, 0.25) |
| Medium | −0.12 (−0.38, 0.14) |
| Low | Reference |
| IL-6:IL-10 ratio | |
| Socioeconomic disadvantage | |
| High | −0.16 (−0.48, 0.17) |
| Medium | 0.04 (−0.14, 0.23) |
| Low | Reference |
| IL-8:IL-10 ratio | |
| Socioeconomic disadvantage | |
| High | −1.22 (−1.56, -0.88) |
| Medium | −0.60 (−0.85, -0.36) |
| Low | Reference |
| TNF-α:IL-10 ratio | |
| Socioeconomic disadvantage | |
| High | 0.06 (−0.20, 0.32) |
| Medium | 0.13 (−0.02, 0.28) |
| Low | Reference |
Natural log of cytokine concentrations (pg/mL) analyzed in separate linear regression models for each cytokine and cytokine ratio, also controlling for race/ethnicity and gestational age at prenatal serum sample collection.
Table S3.
Linear regression coefficients (and 95% CIs) from analyses of inflammatory cytokines in third-trimester maternal serum in relation to level of socioeconomic disadvantage (high or medium versus low), accounting for inflammatory risk factors present during gestation
| Cytokine | Adjusted model* | Restricted model† |
| IL-1β | ||
| Socioeconomic disadvantage | ||
| High | −0.38 (−0.84, 0.09) | −0.78 (−1.49, −0.07) |
| Medium | −0.30 (−0.57, −0.03) | −0.36 (−0.76, 0.03) |
| Low | Reference | Reference |
| Infection during pregnancy | 0.22 (−0.02, 0.46) | |
| Chronic inflammatory condition | −0.37 (−0.75, 0.01) | |
| IL-1β:IL-10 ratio | ||
| Socioeconomic disadvantage | ||
| High | −0.18 (−0.65, 0.29) | −0.18 (−0.65, 0.29) |
| Medium | −0.13 (−0.39, 0.14) | −0.19 (−0.57, 0.19) |
| Low | Reference | Reference |
| Infection during pregnancy | 0.36 (0.13, 0.59) | |
| Chronic inflammatory condition | −0.35 (−0.72, 0.02) | |
| IL-8 | ||
| Socioeconomic disadvantage | ||
| High | −1.43 (−1.78, −1.08) | −1.50 (−2.01, −0.99) |
| Medium | −0.80 (−1.03, −0.57) | −0.78 (−1.11, −0.45) |
| Low | Reference | Reference |
| Infection during pregnancy | 0.11 (−0.09, 0.31) | |
| Chronic inflammatory condition | −0.16 (−0.44, 0.13) | |
| IL-10 | ||
| Socioeconomic disadvantage | ||
| High | −0.19 (−0.44, 0.06) | −0.06 (−0.44, 0.33) |
| Medium | −0.17 (−0.32, -0.03) | −0.18 (−0.40, 0.04) |
| Low | Reference | Reference |
| Infection during pregnancy | −0.14 (−0.28, -0.01) | |
| Chronic inflammatory condition | −0.02 (−0.19, 0.16) | |
| IL-8:IL-10 ratio | ||
| Socioeconomic disadvantage | ||
| High | −1.23 (−1.57, −0.90) | −1.45 (−1.92, −0.98) |
| Medium | −0.63 (−0.87, −0.39) | −0.61 (−0.97, −0.25) |
| Low | Reference | Reference |
| Infection during pregnancy | 0.26 (0.04, 0.47) | |
| Chronic inflammatory condition | −0.14 (-0.44, 0.15) | |
Natural log of cytokine concentrations (pg/mL) analyzed in separate linear regressions for each cytokine or cytokine ratio, also controlling for race/ethnicity, gestational age at prenatal sample collection, and the maximum number of cigarettes smoked daily during pregnancy. Given their skewed distribution, mean differences in cytokine concentrations obtained from linear regression models may not fully capture the effects of covariates because a shift in the mean of a nonnormal variable does not necessarily imply a shift in the entire distribution.
Adjusted model estimated in the entire sample (n = 1,494), including adjustment for the presence of infections during pregnancy and medical conditions associated with chronic inflammation.
Restricted model estimated in the subsample of pregnancies (n = 714) in which no infection or no medical condition associated with chronic inflammation was present.
Finally, logistic regression analyses were used to investigate the extent to which socioeconomic differences in maternal immune activity during pregnancy affected offspring risk of neurologic abnormalities at 4 mo and 1 y. These analyses compared the odds ratios (ORs) for neurologic abnormalities across levels of socioeconomic disadvantage before and after adjustment for concentrations of cytokines in gestational serum (and formal tests of mediation as supplementary analyses).
Results
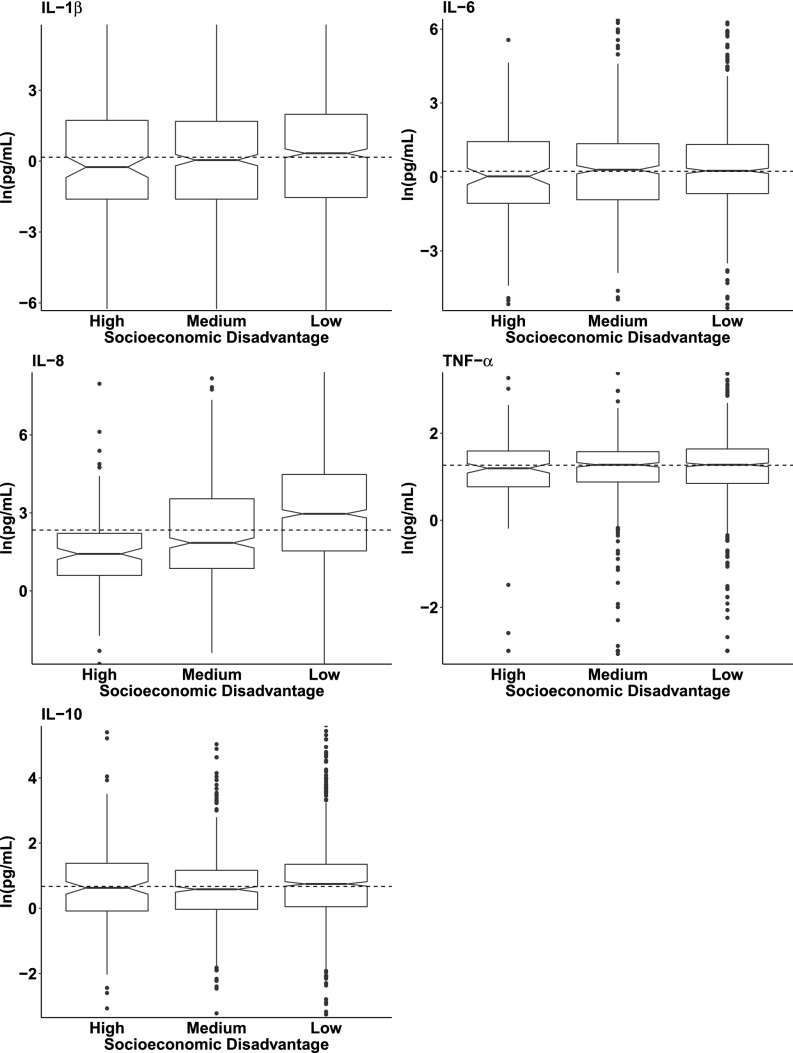
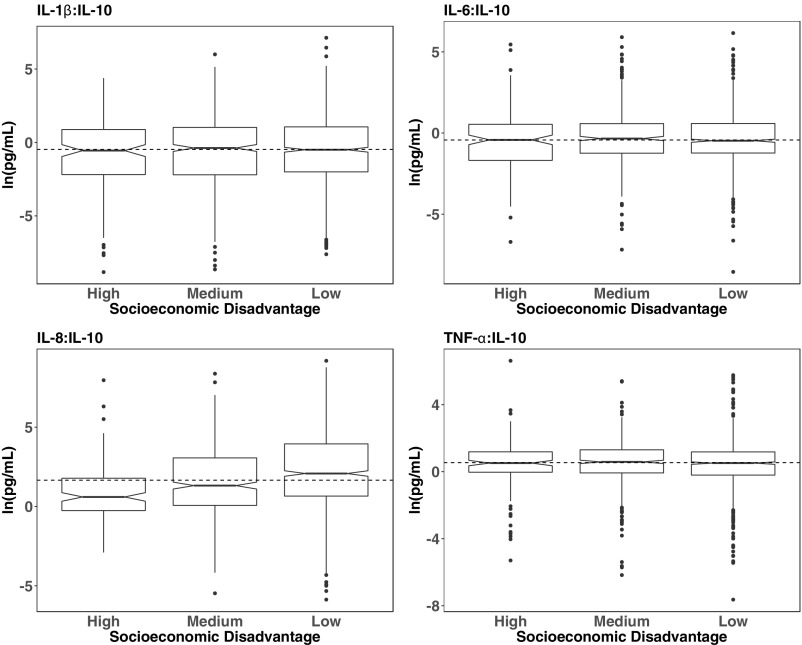
Characteristics of the women and their offspring are presented in Table 1. The sample was predominantly white (88.8%), and the mean age at enrollment into the CPP was 25.1 y (SD = 5.7 y). The median (interquartile range) gestational age at sample collection was 32 weeks (range, 30.5, 33.9 weeks); samples from all but 31 participants were collected during the third trimester. Approximately 10% of the sample was in the most socioeconomically disadvantaged category. Boxplots displaying the median and interquartile ranges of the five cytokines in maternal serum, stratified by level of maternal socioeconomic disadvantage, are presented in Fig. 1 (for each cytokine individually) and in Fig. S1 (for the ratio of each proinflammatory cytokine to IL-10). The median values of IL-8 and of the IL-8:IL-10 ratio decreased markedly with higher levels of socioeconomic disadvantage.
Table 1.
Descriptive characteristics of mothers during pregnancy and their offspring followed through age 1 (n = 1,494)
| Maternal or offspring characteristic | Percent (n) | Median (25th and 75th percentiles) |
| Demographic characteristics of participants | ||
| White race/ethnicity | 88.8% (1, 327) | |
| Maternal age at enrollment | 24.0 (21.0, 29.0) | |
| Level of socioeconomic disadvantage | ||
| High | 9.5% (142) | |
| Medium | 31.5% (471) | |
| Low | 59.0% (881) | |
| Medical conditions present during pregnancy | ||
| Infection | 45.6% (681) | |
| Condition associated with chronic inflammation | 12.9% (193) | |
| Inflammatory cytokines in maternal serum | ||
| Weeks of gestation at prenatal sample collection | 32.0 (30.5, 33.9) | |
| IL-1β concentration, pg/mL | 1.2 (0.2, 6.8) | |
| IL-6 concentration, pg/mL | 1.3 (0.5, 3.8) | |
| IL-8 concentration, pg/mL | 10.4 (3.2, 64.7) | |
| TNF-α concentration, pg/mL | 3.5 (2.3, 5.0) | |
| IL-10 concentration, pg/mL | 2.0 (1.0, 3.7) | |
| Offspring neurologic outcomes | ||
| Neurologic abnormalities at 4 mo (n = 1,262) | 16.2% (204) | |
| Neurologic abnormalities at 1 y (n = 1,367) | 9.4% (128) |
Fig. 1.
Distributions of five inflammatory cytokines in third-trimester maternal serum according to level of socioeconomic disadvantage (low, medium, or high). Box plots represent the distribution of each inflammatory cytokine in maternal serum, the center line indicates the median in each group, and notches around the center line indicate 95% CIs around the median. The dotted line indicates the sample median. (Outliers in the first and 99th percentiles are not shown.)
Fig. S1.
Distributions of pro:anti-inflammatory cytokine ratios in third-trimester maternal serum according to level of socioeconomic disadvantage (low, medium, or high). Box plots represent the distribution of each inflammatory cytokine in maternal serum; the center line indicates the median in each group, and notches around the center line indicate 95% CIs around the median. The dotted line indicates the sample median.
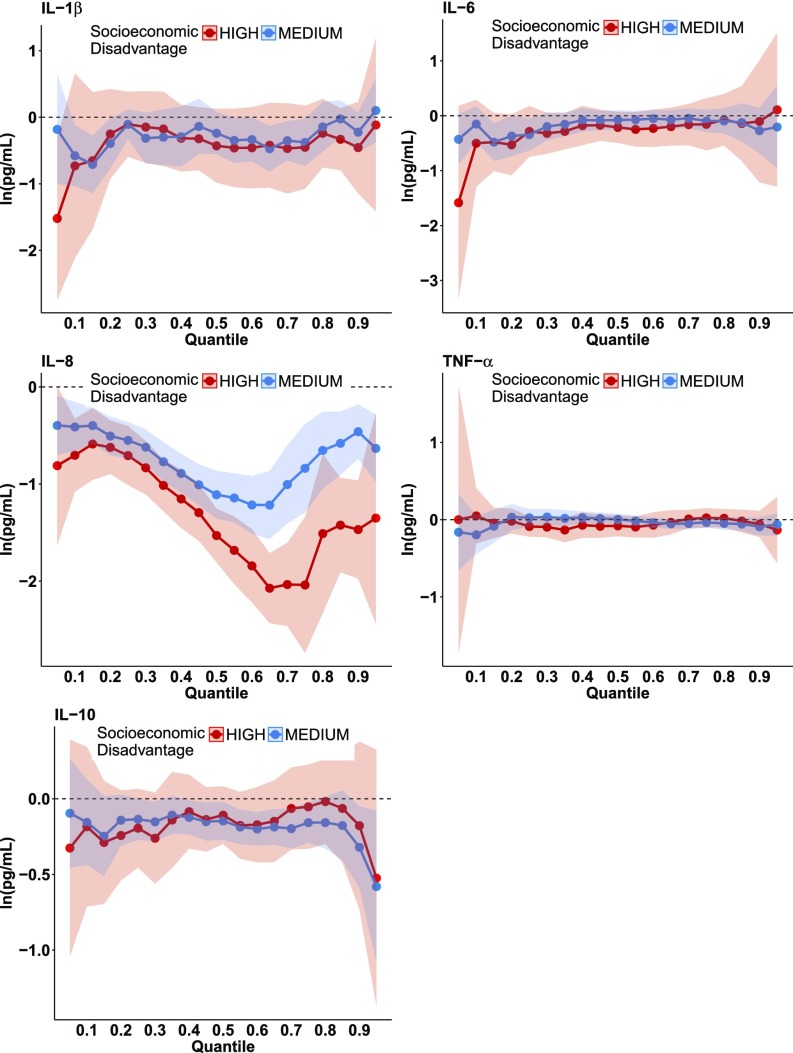
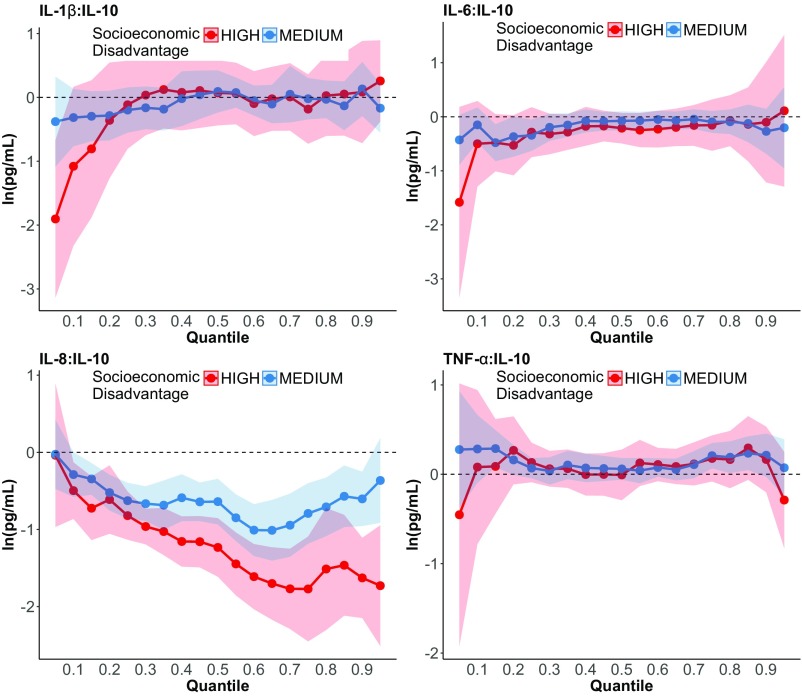
Quantile regression analyses showed that the most socioeconomically disadvantaged women had significantly lower concentrations of IL-8 than less disadvantaged women (Fig. 2). These differences were most pronounced in the upper half of the IL-8 distribution according to the quantile regression coefficient plot for IL-8 (Fig. 2, Middle Left) and the quantile regression coefficients in Table S4, which increased in magnitude from −0.62 at the 20th percentile to −1.51 at the 80th percentile. Concentrations of the IL-8:IL-10 ratio were also significantly lower among socioeconomically disadvantaged women (Fig. S2, Lower Left and Table S4). TNF-α, IL-6, and IL-1β were not significantly related to socioeconomic disadvantage and thus were not analyzed further. (Inferences were unchanged when using more conservative 99% CIs to account for the five cytokines analyzed.)
Fig. 2.
Results of quantile regression models of the association between socioeconomic disadvantage (high or medium vs. low) and the concentration of five inflammatory cytokines (on the logarithmic scale) in third-trimester maternal serum. Bands indicate 95% CIs.
Table S4.
Quantile regression coefficients (and 95% CIs) from analyses of inflammatory cytokines in third-trimester maternal serum in relation to level of socioeconomic disadvantage (high or medium versus low)
| Cytokine | 20th percentile | 50th percentile | 80th percentile |
| IL-1β | |||
| Socioeconomic disadvantage | |||
| High | −0.25 (−0.93, 0.42) | −0.43 (−0.98, 0.13) | −0.24 (−0.75, 0.38) |
| Medium | −0.39 (−0.77, -0.02) | −0.24 (−0.56, 0.08) | −0.14 (−0.52, 0.23) |
| Low | Reference | Reference | Reference |
| IL-6 | |||
| Socioeconomic disadvantage | |||
| High | −0.53 (−1.08, 0.03) | −0.21 (−0.49, 0.07) | −0.07 (−0.54, 0.40) |
| Medium | −0.37 (−1.08, 0.03) | −0.08 (−0.25, 0.10) | −0.09 (−0.32, 0.14) |
| Low | Reference | Reference | Reference |
| IL-8 | |||
| Socioeconomic disadvantage | |||
| High | −0.62 (−0.90, -0.35) | −1.53 (−1.81, −1.25) | −1.51 (−2.23, −0.69) |
| Medium | −0.51 (−0.70, -0.31) | −1.11 (−1.36, −0.86) | −0.65 (−1.05, −0.26) |
| Low | Reference | Reference | Reference |
| TNF-α | |||
| Socioeconomic disadvantage | |||
| High | −0.02 (−0.18, 0.14) | −0.08 (−0.21, 0.06) | 0.02 (−0.10, 0.14) |
| Medium | 0.33 (−0.14, 0.20) | 0.01 (−0.07, 0.08) | −0.05 (−0.13, 0.04) |
| Low | Reference | Reference | Reference |
| IL-10 | |||
| Socioeconomic disadvantage | |||
| High | −0.24 (−0.54, 0.06) | −0.11 (−0.30, 0.08) | −0.02 (−0.30, 0.26) |
| Medium | −0.14 (−0.31, 0.03) | −0.15 (−0.27, -0.02) | −0.16 (−0.33, 0.01) |
| Low | Reference | Reference | Reference |
| IL-1β:IL-10 | |||
| Socioeconomic disadvantage | |||
| High | −0.36 (−1.27, 0.55) | 0.07 (−0.44, 0.58) | 0.03 (−0.60, 0.66) |
| Medium | −0.29 (−0.67, 0.10) | 0.09 (−0.24, 0.42) | −0.03 (−0.33, 0.26) |
| Low | Reference | Reference | Reference |
| IL-6:IL-10 | |||
| Socioeconomic disadvantage | |||
| High | −0.38 (−0.80, 0.03) | −0.23 (−0.58, 0.11) | 0.06 (−0.43, 0.54) |
| Medium | −0.04 (−0.30, 0.22) | 0.11 (−0.09, 0.30) | 0.10 (−0.20, 0.41) |
| Low | Reference | Reference | Reference |
| IL-8:IL-10 | |||
| Socioeconomic disadvantage | |||
| High | −0.61 (−1.06, -0.17) | −1.23 (−1.61, -0.85) | −1.51 (−2.30, -0.72) |
| Medium | −0.52 (−0.75, -0.29) | −0.64 (−0.93, -0.34) | −0.71 (−1.09, -0.33) |
| Low | Reference | Reference | Reference |
| TNF-α:IL-10 | |||
| Socioeconomic disadvantage | |||
| High | 0.27 (−0.11, 0.65) | −0.01 (−0.29, 0.28) | 0.16 (−0.16, 0.49) |
| Medium | 0.16 (−0.01, 0.33) | 0.06 (−0.06, 0.18) | 0.19 (0.01, 0.37) |
| Low | Reference | Reference | Reference |
Natural log of cytokine concentrations (pg/mL) analyzed in quantile regressions, also controlling for race/ethnicity and gestational age when prenatal serum was obtained.
Fig. S2.
Results of quantile regression models of the association between socioeconomic disadvantage (high or medium vs. low) and the ratio of pro:anti-inflammatory cytokines (on the logarithmic scale) in third-trimester maternal serum. Bands indicate 95% CIs.
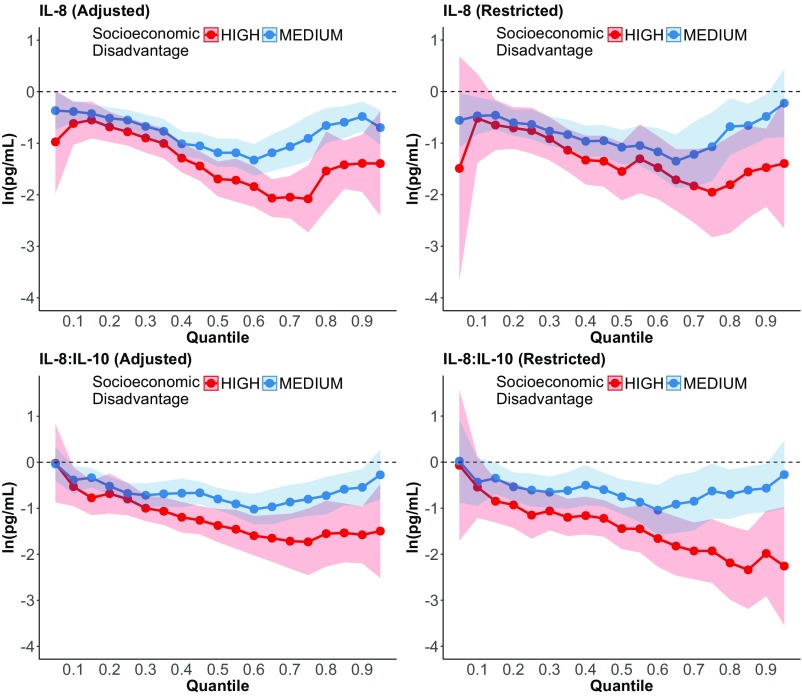
Associations between socioeconomic disadvantage and the concentration of IL-8 in prenatal serum were not affected by the presence of infectious or other medical conditions associated with inflammation. When adjusted for the presence of any infection or chronic inflammatory condition during pregnancy, a significant association between socioeconomic disadvantage and IL-8 remained in the quantile regressions (Fig. S3, Left). In analyses that removed women with any acute or chronic conditions associated with inflammation, results were unchanged, i.e., greater socioeconomic disadvantage was associated with lower concentration of IL-8 and lower IL-8 relative to IL-10 (Fig. S3, Right).
Fig. S3.
Results of quantile regression models of the association between socioeconomic disadvantage (high or medium vs. low) and the concentration of IL-8 and IL-8 relative to IL-10 (IL-8:IL-10) in third-trimester maternal serum. (Left) Data adjusted for the presence of maternal conditions associated with inflammation. (Right) Women with conditions associated with inflammation have been removed from the analytic sample. Bands indicate 95% CIs.
Finally, we examined the association among maternal socioeconomic disadvantage, IL-8 levels, and offspring neurologic outcomes during the first year of life. Greater maternal socioeconomic disadvantage was associated with a 4.6-fold higher odds of offspring neurologic abnormalities at 4 mo (CI: 2.84, 7.48) and twofold higher odds of offspring neurologic abnormalities at 1 y (CI: 1.08, 3.90) (Table 2). The magnitude of these associations was reduced by one-quarter to one-third in models that included concentrations of IL-8 and IL-10 (model 2 for each outcome, in which the corresponding ORs for neurologic abnormalities were 3.09 at 4 mo and 1.56 at 1 y) and the IL-8:IL-10 ratio (model 3 for each outcome, in which the corresponding ORs for neurologic abnormalities were 3.47 at 4 mo and 1.71 at 1 y). These analyses also revealed that lower IL-8 relative to the concentration of IL-10 was associated with substantially elevated risks of neurologic abnormalities at 4 mo (OR: 4.47; CI: 2.53, 7.87) and 1 y (OR: 2.36; CI: 1.25, 4.45). Results of formal analyses of mediation are provided in Table S5; these analyses provide estimates of indirect effects, that is, the proportion of the effect of socioeconomic disadvantage on children’s neurologic outcomes that is the result of variation in gestational immune activity. Exposure to high vs. low socioeconomic disadvantage was associated with a 1.36 (CI: 1.20, 1.53) higher odds of neurologic abnormalities at 4 mo and 1.22 (CI: 1.07, 1.37) higher odds of neurologic abnormalities at 1 y as a result of fetal exposure to maternal cytokines.
Table 2.
Effects of socioeconomic disadvantage and inflammatory cytokines in third-trimester maternal serum on offspring neurologic abnormalities at age 4 mo and 1 y
| Neurologic abnormalities at 4 mo, OR (CI) | Neurologic abnormalities at 1 y, OR (CI) | |||||
| Predictor | Model 1* | Model 2† | Model 3† | Model 1* | Model 2† | Model 3† |
| Socioeconomic disadvantage | ||||||
| High | 4.61 (2.84, 7.48) | 3.09 (1.83, 5.19) | 3.47 (2.06, 5.82) | 2.05 (1.08, 3.90) | 1.56 (0.80, 3.02) | 1.71 (0.90, 3.25) |
| Medium | 2.60 (1.86, 3.65) | 2.10 (1.47, 3.00) | 2.26 (1.60, 3.20) | 1.82 (1.23, 2.69) | 1.66 (1.13, 2.44) | 1.66 (1.12, 2.46) |
| Low | Reference | Reference | Reference | Reference | Reference | Reference |
| IL-8 quintile | ||||||
| First | 7.67 (4.05, 14.49) | 2.92 (1.46, 5.87) | ||||
| Second | 5.25 (2.76, 10.01) | 2.96 (1.50, 5.84) | ||||
| Third | 3.09 (1.55, 6.18) | 2.90 (1.44, 5.84) | ||||
| Fourth | 1.95 (0.96, 3.96) | 1.52 (0.72, 3.24) | ||||
| Fifth | Reference | Reference | ||||
| IL-10 quintile | ||||||
| First | 0.58 (0.34, 1.00) | 0.82 (0.44, 1.54) | ||||
| Second | 0.97 (0.59, 1.60) | 0.62 (0.32, 1.22) | ||||
| Third | 0.70 (0.40, 1.12) | 0.66 (0.35, 1.22) | ||||
| Fourth | 0.60 (0.35, 1.12) | 0.90 (0.49, 1.67) | ||||
| Fifth | Reference | Reference | ||||
| IL-8:IL-10 ratio quintile | ||||||
| First | 4.47 (2.53, 7.87) | 2.36 (1.25, 4.45) | ||||
| Second | 4.09 (2.28, 7.34) | 2.16 (1.14, 4.12) | ||||
| Third | 2.20 (1.20, 4.05) | 1.99 (1.04, 3.82) | ||||
| Fourth | 1.22 (0.63, 2.37) | 1.27 (0.68, 1.95) | ||||
| Fifth | Reference | Reference | ||||
Results of logistic regression analyses in which the dependent variable is the presence of clinician-rated neurologic abnormalities in offspring at 4 mo or 1 y. The sample size for the analysis of neurologic abnormalities at 4 mo = 1,262, at 1 y = 1,367.
Model 1 also adjusts for the presence of infections and medical conditions associated with chronic inflammation, maternal smoking during pregnancy, race/ethnicity, and offspring sex.
Models 2 and 3 additionally adjust for gestational age at prenatal sample collection.
Table S5.
Mediation analyses of the indirect effects of maternal socioeconomic disadvantage on offspring neurologic abnormalities at 4 mo and 1 y of age, via maternal immune activity (IL-8:IL-10) in the third trimester
| Mediation analysis | Neurologic abnormalities at 4 mo, OR (CI) | Neurologic abnormalities at 1 y, OR (CI) |
| Total effects of socioeconomic disadvantage on neurologic abnormalities (from Table 2, model 1) | ||
| High | 4.61 (2.84, 7.48) | 2.05 (1.08, 3.90) |
| Medium | 2.60 (1.86, 3.65) | 1.82 (1.23, 2.69) |
| Low | Reference | Reference |
| Indirect effects of socioeconomic disadvantage on neurologic abnormalities via IL-8:IL-10* | ||
| High | 1.36 (1.20, 1.53) | 1.22 (1.07, 1.37) |
| Medium | 1.17 (1.08, 1.26) | 1.10 (1.03, 1.17) |
| Low | Reference | Reference |
| Direct effects of socioeconomic disadvantage on neurologic abnormalities not via IL-8:IL-10† | ||
| High | 3.47 (1.76, 5.17) | 1.70 (0.62, 2.78) |
| Medium | 2.28 (1.52, 3.05) | 1.67 (1.02, 2.33) |
| Low | Reference | Reference |
| Effect of IL-8:IL-10 on neurologic abnormalities | 0.79 (0.73, 0.85) | 0.86 (0.79, 0.93) |
| Linear regression of IL-8:IL-10 on socioeconomic disadvantage, B (CI) | ||
| High | −1.37 (-1.73, −1.01) | −1.31 (−1.67, -0.95) |
| Medium | −0.68 (-0.94, −0.41) | −0.60 (−0.85, -0.35) |
| Low | Reference | Reference |
These analyses quantify and provide formal tests of the extent to which differences in the risk of neurologic abnormalities at 4 mo and 1 y across levels of socioeconomic disadvantage are caused by differences in fetal exposure to inflammatory cytokines, indicated by the concentration of IL-8 relative to IL-10 in maternal serum midgestation accounts. Mediation analyses, implemented in MPLUS (55), quantify both direct and indirect effects using two regression models: (i) a logistic regression of neurologic abnormalities on IL-8:IL-10 (entered as a continuous variable), socioeconomic disadvantage, and covariates and (ii) a linear regression of IL-8:IL-10 on socioeconomic disadvantage and covariates. Under a set of no unmeasured confounding assumptions described by VanderWeele and Vansteelandt, the coefficients in these two equations can be used to derive the natural indirect effect of socioeconomic disadvantage on neurologic abnormalities via IL-8:IL-10 (i.e., the expected change in the odds of neurologic abnormalities associated with a change in fetal exposure to inflammatory cytokines, holding socioeconomic disadvantage constant); and the natural direct effect of socioeconomic disadvantage on neurologic abnormalities (i.e., the expected change in the odds of neurologic abnormalities associated with a change in maternal socioeconomic disadvantage, not via pathways involving fetal cytokine exposure) (56). Given a three-category measure of maternal socioeconomic disadvantage, two indirect effects are estimable, for the high vs. low and medium vs. low contrasts of disadvantage.
Exposure to high vs. low socioeconomic disadvantage was associated with a 1.36 higher odds of neurologic abnormalities at 4 mo and 1.22 higher odds of neurologic abnormalities at 1 y as a result of fetal exposure to maternal cytokines.
Exposure to high vs. low socioeconomic disadvantage was associated with a 3.47 higher odds of neurologic abnormalities at 4 mo and 1.70 higher odds of neurologic abnormalities at 1 y, not via variations in fetal exposure to maternal cytokines.
Discussion
Socioeconomic disadvantage during pregnancy has been shown to affect offspring brain development significantly (31). We investigated whether this effect is caused in part by variations in maternal immune activity associated with maternal socioeconomic disadvantage, given its association with adverse pregnancy outcomes and offspring cognitive, neurologic, and psychiatric conditions (17, 18, 32). We found that greater maternal socioeconomic disadvantage was associated with significantly lower concentrations of the proinflammatory cytokine IL-8 during the third trimester and with a lower ratio of IL-8 to the anti-inflammatory cytokine, IL-10. These associations were independent of maternal medical conditions known to induce dysregulation of immune responses. Moreover, lower concentrations of IL-8 in maternal serum were associated with the presence of offspring neurologic abnormalities during early life (at ages 4 mo and 1 y). Lower IL-8 also accounted for a significant proportion of the association between maternal socioeconomic disadvantage and offspring neurologic abnormalities.
Limitations.
Although study procedures were standardized throughout the CPP, we cannot rule out measurement error or the possibility that measurement error was correlated with parental socioeconomic circumstances. That is, there is the possibility that physicians would be more likely to rate children from socioeconomically disadvantaged families as neurologically abnormal. However, a major CPP objective was to study children’s neurological development using a systematic, comprehensive assessment across all 12 study sites. Further, although the CPP was conducted between 1959 and 1974, there is evidence to support the generalizability of this historic study to contemporary circumstances, because the socioeconomic assessments were validated at the time the CPP was conducted and have been related to adult physical and mental health outcomes (33, 34).
It is possible that analytes were subject to degradation because the samples were drawn in the 1960s. However, importantly, such degradation would not have been greater in the samples obtained from socioeconomically disadvantaged pregnancies, because storage conditions and duration of storage did not differ across groups. It is possible that cytokines degraded at different rates. Cytokine degradation would have attenuated associations toward the null and thus could be one explanation for not having found associations involving four of the five cytokines analyzed. That said, we note that cytokine concentrations in the CPP samples are within the same ranges as those in samples obtained from participants in more recent pregnancy cohorts (35, 36).
The time of day of blood collection was not recorded; the lack of information on the circadian rhythm in the cytokines could have attenuated associations toward the null. We also could not evaluate effects involving trimester-specific inflammation, which should be pursued in future studies. Finally, it has been argued that cytokines drawn from maternal serum rather than cord blood are indirect rather than direct indicators of fetal exposure. However, there is evidence that maternal prenatal cytokines correlate with offspring cytokines at 2 mo postnatal (37).
Prenatal Stress and Maternal Immune Functioning.
The inverse relationship of high socioeconomic disadvantage and maternal cytokine levels may be explained, in part, by the finding in numerous studies that high levels of glucocorticoids (as may occur with a stress challenge or chronic exposure to stressors) tend to suppress inflammatory responses (24). In particular, glucocorticoids are known to reduce concentrations of IL-8, a potent neutrophil chemoattractant and activator, by accelerating the decay of messenger RNA coding for IL-8 (24, 38).
Although physiologic responses to stressors are typically attenuated during a healthy pregnancy (39), our findings suggest that socioeconomic disadvantage could precipitate dysregulation of the mother’s ability to regulate a healthy response to stressful conditions. In this case, stress-induced elevations of glucocorticoids may be exaggerated and/or more persistent in socioeconomically disadvantaged pregnancies, thereby resulting in greater immunosuppression and lower concentrations of IL-8, as observed in this study. The association between stressors and inflammation during pregnancy likely depends on gestational timing, given the importance of pro- and anti-inflammatory cytokine balance. In early pregnancy, the immune balance is skewed toward activation, given that inflammation has been found to be essential for implantation (40–42). In contrast, the attenuated inflammatory responses of later pregnancy are thought to represent adaptations that protect the fetus from rejection by the maternal immune system (39).
The effects of glucocorticoids on immune activity are dynamic and bidirectional (43). Although acute exposure to glucocorticoids is typically associated with suppressed immune responses, chronic exposure is thought to enhance proinflammatory responses, potentially by inducing glucocorticoid resistance (9, 24). Our study focused on a multifaceted, chronic exposure—socioeconomic disadvantage—that was likely to remain stable throughout the entire pregnancy. In contrast, other studies of the association of prenatal stressors with glucocorticoid levels focused on more acute stressors, which might vary to a greater degree across pregnancy (44, 45). It is noteworthy that only IL-8 was found to be suppressed in the face of the complex, chronic stressors posed to the pregnancy through socioeconomic disadvantage. We speculate that in the context of glucocorticoid resistance, such as is thought to occur with prolonged exposures to stressful conditions and persistently high levels of glucocorticoids (46), maternal IL-1β, IL-6, and TNF-α might escape the immunosuppressive effects of glucocorticoids. In contrast, IL-8 may remain sensitive to glucocorticoid suppression through an as-yet-undefined mechanism.
Immune Programming of Offspring Brain Health.
Although some studies reported that high levels of proinflammatory cytokines during pregnancy were associated with worse outcomes in offspring [e.g., cerebral palsy (19), schizophrenia (21), and autism (22)], we recently reported that lower levels of maternal cytokines were associated with higher risk of depression and psychosis among female offspring (12, 13). These studies are consistent with work by Kaukola and colleagues, who reported lower concentrations of IL-6 in cord blood associated with neurologic abnormalities at age 2 (47), and by Von Ehrenstein and colleagues, who reported that higher concentrations of IFN-γ and TNF-α in cord blood were protective against poor cognitive outcomes at age 7 y (18).
Our analyses point to IL-8, an angiogenic factor with important roles in normal uterine and placental physiology, including vascular remodeling and permeability of the fetal–maternal unit (48, 49). In the third trimester, the time point at which the samples for analyses here were drawn, IL-8 is up-regulated via activation of the prokineticin1 receptor (PKR1) for endothelial cell chemotaxis and proliferation (48). In animal models, increased glucocorticoid exposure early in the third trimester has been reported to inhibit the expression of the PKR1 ligand, prokineticin1 (PK1), resulting in decreased placental vascularization (50) and, consequently, deficits in fetal neurodevelopment (51). Furthermore, the receptor for IL-8, CXCR2, is present in the human fetal brain and plays a role in synaptogenesis (52). Deficits in IL-8 signaling through CXCR2 therefore may disrupt the normal trafficking of neuronal processes and the formation of appropriate synaptic contacts during CNS development (53). These IL-8/CXCR2 signaling abnormalities could be potential mechanisms underlying the observed neurologic disturbances in the offspring of the socioeconomically disadvantaged pregnancies.
Although the directions of associations between gestational immune activity and offspring neurodevelopmental and neuropsychiatric outcomes vary across studies, there is consistent evidence that prenatal stress adversely affects neurodevelopment (8, 28). For example, in the national CPP sample, socioeconomic disadvantage assessed during pregnancy conferred an elevated risk for neurologic abnormalities through early childhood (31). Here we show that the involvement of socioeconomic disadvantage in the increased risk of neurologic abnormalities at 4 mo and 1 y was attributable, in part, to socioeconomically driven variations in gestational immune activity. We thus suggest that one pathway through which social disadvantage affects the next generation involves immune signaling (17). Our observations suggesting that gestational stress may suppress IL-8 need to be replicated and assessed within a broader panel of inflammatory and stress response molecules.
The generally larger associations of socioeconomic disadvantage and gestational IL-8 with neurologic abnormalities at 4 mo than at 1 y may be an indication that such effects become weaker over time, perhaps as a result of developmental plasticity or the possibility that such effects are mitigated by postnatal environment (54). That said, studies showing associations between gestational inflammation and offspring psychopathology in adulthood suggest that if effects do weaken over time in the context of early development, underlying vulnerability may resurface later as the brain and its connectivity mature and frank illness is expressed (12, 21, 23).
The importance of the early childhood environment for long-term health has been well established (1). Here, we provide further evidence for the role of the social environment during pregnancy on the neurodevelopment of offspring. Our findings point to gestational maternal immune activity as one potential pathway contributing to the intergenerational transmission of socioeconomic inequalities in health. Given the long-term associations between health at the beginning of life and adult health and longevity, investment in maternal health during pregnancy, irrespective of the mechanisms involved, would likely reduce the intergenerational transmission of health inequalities.
Acknowledgments
We thank Anne Remington, MA, JoAnn Donatelli, PhD, Harlyn Aizley, EdM, and Jennifer Walch, EdM, for data collection and study coordination; Kathleen McGaffigan, MS, for her expertise in data management and statistical analysis; Wai Wong and Noel Pura, MS, for technical support and data management; and Natalie Slopen, ScD, for comments on an earlier version of this manuscript. This work was supported by Grant P50 MH082679 from the Office for Research on Women’s Health and National Institute of Mental Health (NIMH) (Specialized Centers of Research) (to J.M.G.), NIMH and National Heart, Lung and Blood Institute Grant R01 MH074679 (to J.M.G.), NIMH Grant R01 MH087544 (to S.E.G.), and the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1617698114/-/DCSupplemental.
References
- 1.Braveman P, Barclay C. Health disparities beginning in childhood: A life-course perspective. Pediatrics. 2009;124:S163–S175. doi: 10.1542/peds.2009-1100D. [DOI] [PubMed] [Google Scholar]
- 2.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Talge NM, Neal C, Glover V. Early Stress, Translational Research and Prevention Science Network: Fetal and Neonatal Experience on Child and Adolescent Mental Health Antenatal maternal stress and long-term effects on child neurodevelopment: How and why? J Child Psychol Psychiatry. 2007;48:245–261. doi: 10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beydoun H, Saftlas AF. Physical and mental health outcomes of prenatal maternal stress in human and animal studies: A review of recent evidence. Paediatr Perinat Epidemiol. 2008;22:438–466. doi: 10.1111/j.1365-3016.2008.00951.x. [DOI] [PubMed] [Google Scholar]
- 5.Huizink AC, Robles de Medina PG, Mulder EJ, Visser GH, Buitelaar JK. Stress during pregnancy is associated with developmental outcome in infancy. J Child Psychol Psychiatry. 2003;44:810–818. doi: 10.1111/1469-7610.00166. [DOI] [PubMed] [Google Scholar]
- 6.Li J, et al. Prenatal stress and cerebral palsy: A nationwide cohort study in Denmark. Psychosom Med. 2009;71:615–618. doi: 10.1097/PSY.0b013e3181a56ca1. [DOI] [PubMed] [Google Scholar]
- 7.Johnson SB, Riley AW, Granger DA, Riis J. The science of early life toxic stress for pediatric practice and advocacy. Pediatrics. 2013;131:319–327. doi: 10.1542/peds.2012-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Donnell K, O’Connor TG, Glover V. Prenatal stress and neurodevelopment of the child: Focus on the HPA axis and role of the placenta. Dev Neurosci. 2009;31:285–292. doi: 10.1159/000216539. [DOI] [PubMed] [Google Scholar]
- 9.Howerton CL, Bale TL. Prenatal programing: At the intersection of maternal stress and immune activation. Horm Behav. 2012;62:237–242. doi: 10.1016/j.yhbeh.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coussons-Read ME, Okun ML, Nettles CD. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain Behav Immun. 2007;21:343–350. doi: 10.1016/j.bbi.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Wright RJ, et al. Prenatal maternal stress and cord blood innate and adaptive cytokine responses in an inner-city cohort. Am J Respir Crit Care Med. 2010;182:25–33. doi: 10.1164/rccm.200904-0637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilman SE, et al. Prenatal immune programming of the sex-dependent risk for major depression. Transl Psychiatry. 2016;6:e822. doi: 10.1038/tp.2016.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstein JM, et al. Prenatal maternal immune disruption and sex-dependent risk for psychoses. Psychol Med. 2014;44:3249–3261. doi: 10.1017/S0033291714000683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elovitz MA, et al. Intrauterine inflammation, insufficient to induce parturition, still evokes fetal and neonatal brain injury. Int J Dev Neurosci. 2011;29:663–671. doi: 10.1016/j.ijdevneu.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonakait GM. The effects of maternal inflammation on neuronal development: Possible mechanisms. Int J Dev Neurosci. 2007;25:415–425. doi: 10.1016/j.ijdevneu.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 16.Brown AS, et al. Prenatal exposure to maternal infection and executive dysfunction in adult schizophrenia. Am J Psychiatry. 2009;166:683–690. doi: 10.1176/appi.ajp.2008.08010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagberg H, Gressens P, Mallard C. Inflammation during fetal and neonatal life: Implications for neurologic and neuropsychiatric disease in children and adults. Ann Neurol. 2012;71:444–457. doi: 10.1002/ana.22620. [DOI] [PubMed] [Google Scholar]
- 18.von Ehrenstein OS, et al. Child intellectual development in relation to cytokine levels in umbilical cord blood. Am J Epidemiol. 2012;175:1191–1199. doi: 10.1093/aje/kwr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon BH, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000;182:675–681. doi: 10.1067/mob.2000.104207. [DOI] [PubMed] [Google Scholar]
- 20.Ellman LM, et al. Structural brain alterations in schizophrenia following fetal exposure to the inflammatory cytokine interleukin-8. Schizophr Res. 2010;121:46–54. doi: 10.1016/j.schres.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown AS, et al. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am J Psychiatry. 2004;161:889–895. doi: 10.1176/appi.ajp.161.5.889. [DOI] [PubMed] [Google Scholar]
- 22.Brown AS, et al. Elevated maternal C-reactive protein and autism in a national birth cohort. Mol Psychiatry. 2014;19:259–264. doi: 10.1038/mp.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldstein JM, Handa RJ, Tobet SA. Disruption of fetal hormonal programming (prenatal stress) implicates shared risk for sex differences in depression and cardiovascular disease. Front Neuroendocrinol. 2014;35:140–158. doi: 10.1016/j.yfrne.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Connor TM, O’Halloran DJ, Shanahan F. The stress response and the hypothalamic-pituitary-adrenal axis: From molecule to melancholia. QJM. 2000;93:323–333. doi: 10.1093/qjmed/93.6.323. [DOI] [PubMed] [Google Scholar]
- 25.Hardy JB. The Collaborative Perinatal Project: Lessons and legacy. Ann Epidemiol. 2003;13:303–311. doi: 10.1016/s1047-2797(02)00479-9. [DOI] [PubMed] [Google Scholar]
- 26.Entringer S, et al. Attenuation of maternal psychophysiological stress responses and the maternal cortisol awakening response over the course of human pregnancy. Stress. 2010;13:258–268. doi: 10.3109/10253890903349501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer U, et al. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glover V, O’Connor TG, O’Donnell K. Prenatal stress and the programming of the HPA axis. Neurosci Biobehav Rev. 2010;35:17–22. doi: 10.1016/j.neubiorev.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Vignali DA. Multiplexed particle-based flow cytometric assays. J Immunol Methods. 2000;243:243–255. doi: 10.1016/s0022-1759(00)00238-6. [DOI] [PubMed] [Google Scholar]
- 30.Martins TB. Development of internal controls for the Luminex instrument as part of a multiplex seven-analyte viral respiratory antibody profile. Clin Diagn Lab Immunol. 2002;9:41–45. doi: 10.1128/CDLI.9.1.41-45.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chin-Lun Hung G, et al. Socioeconomic disadvantage and neural development from infancy through early childhood. Int J Epidemiol. 2015;44:1889–1899. doi: 10.1093/ije/dyv303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coussons-Read ME, Okun ML, Schmitt MP, Giese S. Prenatal stress alters cytokine levels in a manner that may endanger human pregnancy. Psychosom Med. 2005;67:625–631. doi: 10.1097/01.psy.0000170331.74960.ad. [DOI] [PubMed] [Google Scholar]
- 33.Loucks EB, et al. Education and coronary heart disease risk associations may be affected by early-life common prior causes: A propensity matching analysis. Ann Epidemiol. 2012;22:221–232. doi: 10.1016/j.annepidem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilman SE, Kawachi I, Fitzmaurice GM, Buka SL. Family disruption in childhood and risk of adult depression. Am J Psychiatry. 2003;160:939–946. doi: 10.1176/appi.ajp.160.5.939. [DOI] [PubMed] [Google Scholar]
- 35.Cheslack-Postava K, et al. Maternal serum cytokine levels and risk of bipolar disorder. Brain Behav Immun. 2017;63:108–114. doi: 10.1016/j.bbi.2016.07.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferguson KK, Meeker JD, McElrath TF, Mukherjee B, Cantonwine DE. Repeated measures of inflammation and oxidative stress biomarkers in preeclamptic and normotensive pregnancies. Am J Obstet Gynecol. 2017;216:527.e1–527.e9l. doi: 10.1016/j.ajog.2016.12.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Djuardi Y, et al. Determinants of the relationship between cytokine production in pregnant women and their infants. PLoS One. 2009;4:e7711. doi: 10.1371/journal.pone.0007711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cunha FQ, Cacini AT, Ferreira SH. Inhibition of the release of a neutrophil chemotactic factor from macrophages partially explains the anti-inflammatory action of glucocorticoids. Agents Actions. 1986;17:314–317. doi: 10.1007/BF01982632. [DOI] [PubMed] [Google Scholar]
- 39.Christian LM. Psychoneuroimmunology in pregnancy: Immune pathways linking stress with maternal health, adverse birth outcomes, and fetal development. Neurosci Biobehav Rev. 2012;36:350–361. doi: 10.1016/j.neubiorev.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Challis JR, et al. Inflammation and pregnancy. Reprod Sci. 2009;16:206–215. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- 41.Curry AE, et al. Maternal plasma cytokines in early- and mid-gestation of normal human pregnancy and their association with maternal factors. J Reprod Immunol. 2008;77:152–160. doi: 10.1016/j.jri.2007.06.051. [DOI] [PubMed] [Google Scholar]
- 42.Palm M, Axelsson O, Wernroth L, Larsson A, Basu S. Involvement of inflammation in normal pregnancy. Acta Obstet Gynecol Scand. 2013;92:601–605. doi: 10.1111/aogs.12093. [DOI] [PubMed] [Google Scholar]
- 43.Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: Implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16:300–317. doi: 10.1159/000216188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Christian LM, Franco A, Glaser R, Iams JD. Depressive symptoms are associated with elevated serum proinflammatory cytokines among pregnant women. Brain Behav Immun. 2009;23:750–754. doi: 10.1016/j.bbi.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coussons-Read ME, et al. The occurrence of preterm delivery is linked to pregnancy-specific distress and elevated inflammatory markers across gestation. Brain Behav Immun. 2012;26:650–659. doi: 10.1016/j.bbi.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen S, et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci USA. 2012;109:5995–5999. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaukola T, et al. Population cohort associating chorioamnionitis, cord inflammatory cytokines and neurologic outcome in very preterm, extremely low birth weight infants. Pediatr Res. 2006;59:478–483. doi: 10.1203/01.pdr.0000182596.66175.ee. [DOI] [PubMed] [Google Scholar]
- 48.Denison FC, Battersby S, King AE, Szuber M, Jabbour HN. Prokineticin-1: A novel mediator of the inflammatory response in third-trimester human placenta. Endocrinology. 2008;149:3470–3477. doi: 10.1210/en.2007-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maldonado-Pérez D, et al. Prokineticin 1 modulates IL-8 expression via the calcineurin/NFAT signaling pathway. Biochim Biophys Acta. 2009;1793:1315–1324. doi: 10.1016/j.bbamcr.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hewitt DP, Mark PJ, Waddell BJ. Glucocorticoids prevent the normal increase in placental vascular endothelial growth factor expression and placental vascularity during late pregnancy in the rat. Endocrinology. 2006;147:5568–5574. doi: 10.1210/en.2006-0825. [DOI] [PubMed] [Google Scholar]
- 51.Bronson SL, Bale TL. The Placenta as a Mediator of Stress Effects on Neurodevelopmental Reprogramming. Neuropsychopharmacology. 2016;41:207–218. doi: 10.1038/npp.2015.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Filipovic R, Jakovcevski I, Zecevic N. GRO-alpha and CXCR2 in the human fetal brain and multiple sclerosis lesions. Dev Neurosci. 2003;25:279–290. doi: 10.1159/000072275. [DOI] [PubMed] [Google Scholar]
- 53.Semple BD, Kossmann T, Morganti-Kossmann MC. Role of chemokines in CNS health and pathology: A focus on the CCL2/CCR2 and CXCL8/CXCR2 networks. J Cereb Blood Flow Metab. 2010;30:459–473. doi: 10.1038/jcbfm.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bergman K, Sarkar P, Glover V, O’Connor TG. Maternal prenatal cortisol and infant cognitive development: Moderation by infant-mother attachment. Biol Psychiatry. 2010;67:1026–1032. doi: 10.1016/j.biopsych.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muthén B, Asparouhov T. Causal Effects in Mediation Modeling: An Introduction With Applications to Latent Variables. Struct Equ Modeling. 2014;22:12–23. [Google Scholar]
- 56.VanderWeele TJ, Vansteelandt S. Conceptual issues concerning mediation, interventions and composition. Stat Interface. 2009;2:457–468. [Google Scholar]