Significance
The majority of plant viruses are transmitted by insect vectors. Transovarial transmission of virus from female vectors to offspring can be very important in maintaining a source of infection and therefore has great epidemiological relevance. Identification of vector and virus components involved in transovarial transmission can lead to new strategies to combat virus spread. Here, we found that the specific interaction between viral coat protein and vector vitellogenin determines transovarial transmissibility of begomoviruses, which have caused great damage to agricultural production and are generally believed not to be transovarially transmitted by insect vectors. Our study gives valuable clues for designing strategies to block begomovirus transmission and provides insights into the evolution and global spread of begomoviruses.
Keywords: begomovirus, transovarial transmission, vector development, vitellogenin, whitefly
Abstract
The majority of plant viruses are transmitted by insect vectors between hosts, and transovarial transmission of viruses from vector parents to offspring has great significance to their epidemiology. Begomoviruses are transmitted by the whitefly Bemisia tabaci in a circulative manner and are maintained through a plant–insect–plant cycle. Other routes of begomovirus transmission are not clearly known. Here, we report that transovarial transmission from female whiteflies to offspring often happens for one begomovirus, Tomato yellow leaf curl virus (TYLCV), and may have contributed significantly to its global spread. We found that TYLCV entry of the reproductive organ of its vector mainly depended on the developmental stage of the whitefly ovary, and the transovarial transmission of TYLCV to offspring increased with whitefly adult age. The specific interaction between virus coat protein (CP) and whitefly vitellogenin (Vg) was vital for virus entry into whitefly ovary. When knocking down the expression of Vg, the entry of TYLCV into ovary was inhibited and the transovarial transmission efficiency decreased. In contrast, another begomovirus, Papaya leaf curl China virus (PaLCuCNV), CP did not interact with whitefly Vg, and PaLCuCNV could not be transovarially transmitted by whiteflies. We further showed that TYLCV could be maintained for at least two generations in the absence of virus-infected plants, and the adult progenies were able to infect healthy plants in both the laboratory and field. This study reports the transovarial transmission mechanism of begomoviruses, and it may help to explain the evolution and global spread of some begomoviruses.
Maternal transmission of microbes, including viruses, bacteria, microsporidia, and fungi, by arthropods is a universal phenomenon in nature (1–3). Of the ∼700 known plant viruses, more than 75% are dependent upon arthropod vectors for transmission between hosts, and some of them can be transmitted vertically from mother to offspring in a transovarial manner (4, 5). Because transovarial transmission controls virus dispersal in space and time and thus has great importance to virus epidemiology, it has received constant attention from entomologists and virologists (6, 7). However, despite its importance, transovarial transmission of plant viruses by insects remains uncommon. Depending on the mode of transmission, plant viruses are classified into four categories including nonpersistent, semipersistent, circulative-nonpropagative, and circulative-propagative (4). So far, only circulative-propagative plant viruses, such as reoviruses, rhabdoviruses, and tenuivirus, have been confirmed to be transovarially transmitted, because transovarial transmission usually requires the replication of viruses in the vector (8).
There are multiple barriers during the circulative transmission of plant and animal viruses, including midgut infection barrier, dissemination barrier, salivary gland escape barrier, and transovarial transmission barrier (9). Passage of viruses through these barriers requires specific interactions between virus and vector components (10). Identification of putative components could lead to new strategies to interdict viral spread. During the past decades, a number of virus and insect proteins involved in this transmission process have been identified in some virus–vector combinations (11–13). However, mechanisms underlying transovarial transmission of viruses have rarely been reported, especially for circulative-nonpropagative viruses, because this group of viruses is generally believed not able to replicate and transovarially transmit in their insect vectors.
Begomoviruses contain the largest known genus of ∼288 species of plant viruses and are generally known to be transmitted by the whitefly Bemisia tabaci in a circulative-nonpropagative manner (14, 15). The whitefly B. tabaci is now recognized as a complex containing at least 35 cryptic species (16, 17). During the past 30 y, the two most predominant and damaging species of the B. tabaci complex, Middle East Asia Minor 1 (MEAM1) and Mediterranean (MED), which have been commonly referred to as biotype B and biotype Q, respectively, have invaded many countries worldwide and displaced some indigenous whitefly species (17). With the global invasion of MEAM1 and MED whiteflies, many economic crops are at great risk of infection with begomoviruses (15, 18, 19). Among these viruses, Tomato yellow leaf curl virus (TYLCV) is one of the most devastating viral diseases and has spread to more than 50 countries and regions (20, 21). Interestingly, although most of the studies reported an absence or low frequency of transovarial transmission in begomoviruses, one case reported that TYLCV can be transovarially transmitted at high efficiency (22) (Table S1). Therefore, much remains to be learned about whether and how the begomoviruses are vertically transmitted.
Table S1.
A comprehensive comparison of the results of different studies on transovarial transmission of begomoviruses by whitefly Bemisia tabaci
| Viral DNA | Infectivity | ||||||
| Virus | Whitefly species | Whitefly age, DAE* | Source | Egg† | Nymph† | Adult† | Plant‡ |
| TYLCV | —§ | — | (35) | — | — | — | 0% |
| MYMV | — | — | (22) | — | — | — | 0% (0/15) |
| MYMV | — | — | (22) | — | — | — | 0% (0/70) |
| TLCV | — | — | (22) | — | — | — | 0% (0/195) |
| ACMV | — | — | (22) | — | — | — | 0% (0/45) |
| TYLCV | MEAM1 | 5–8 | (42) | 81% (46/57) | 37% (25/68) | 57% (46/81) | 10% (5/50) |
| TYLCV | MEAM1 | — | (38) | 0% (0/100) | 0% (0/100) | 0% (0/125) | 0% (0/106) |
| TYLCSV | MEAM1 | — | (38) | 9% (10/110) | 29% (32/110) | 2% (5/250) | 0% (0/118) |
| TYLCV | MEAM1 | 1 | (40) | 28% (11/40) | 8% (6/80) | 0% (0/40) | — |
| TYLCV | MED | 1 | (40) | 17% (13/75) | 15% (22/150) | 3% (2/75) | 0% (0/20) |
| TYLCCNV | MEAM1 | 1 | (40) | 19% (14/75) | 11% (17/150) | 0% (0/75) | 0% (0/20) |
| TYLCCNV | MED | 1 | (40) | 1% (1/75) | 1% (2/150) | 0% (0/75) | — |
| TYLCV | MEAM1 | 2 | (41) | 30% (30/100) | 11% (22/200) | 0% (0/100) | — |
| TYLCV | MED | 2 | (41) | 50% (50/100) | 11% (22/200) | 0% (0/100) | — |
| TYLCV | MEAM1 | — | (39) | — | — | 0% (0/340) | 0% (0/340) |
| TYLCV | MEAM1 | 1 | This study | 13% (8/60) | 8% (5/60) | 0% (0/60) | 0% (0/24) |
| TYLCV | MEAM1 | 11 | This study | 92% (55/60) | 68% (41/60) | 77% (46/60) | 21% (5/24) |
| TYLCV | MED | 1 | This study | 18% (11/60) | 5% (3/60) | 0% (0/24) | 0% (0/24) |
| TYLCV | MED | 11 | This study | 85% (51/60) | 77% (46/60) | 67% (40/60) | 33% (8/24) |
| PaLCuCNV | MEAM1 | 1 | This study | 0% (0/80) | 0% (0/80) | 0% (0/80) | — |
| PaLCuCNV | MEAM1 | 11 | This study | 0% (0/80) | 0% (0/80) | 0% (0/80) | — |
| PaLCuCNV | MED | 1 | This study | 0% (0/80) | 0% (0/80) | 0% (0/80) | — |
| PaLCuCNV | MED | 11 | This study | 0% (0/80) | 0% (0/80) | 0% (0/80) | — |
Days after eclosion.
Positive/tested.
Infected/tested.
Not mentioned or not tested.
In this study, we used TYLCV and Papaya leaf curl China virus (PaLCuCNV), a newly isolated begomovirus in China (23, 24), to investigate whether begomoviruses can be transovarially transmitted by their insect vectors and the virus or vector components involved in the transovarial transmission process. Our study demonstrated that TYLCV can be transovarially transmitted by the whitefly vector and that whitefly development is a key factor in determining transmission rate, whereas PaLCuCNV cannot. Furthermore, we found that specific interactions between viral CP and whitefly endogenous vitellogenin (Vg) are vital for virus to pass through the transovarial transmission barrier. Our study also revealed the importance of transovarial transmission of TYLCV in maintaining a source of virus in the absence of infected plant and its epidemiological relevance in the field, which may help to explain the worldwide spread and outbreak of this virus.
Results
TYLCV Entry in Whitefly Ovary at the Mature Stage of Ovarian Development.
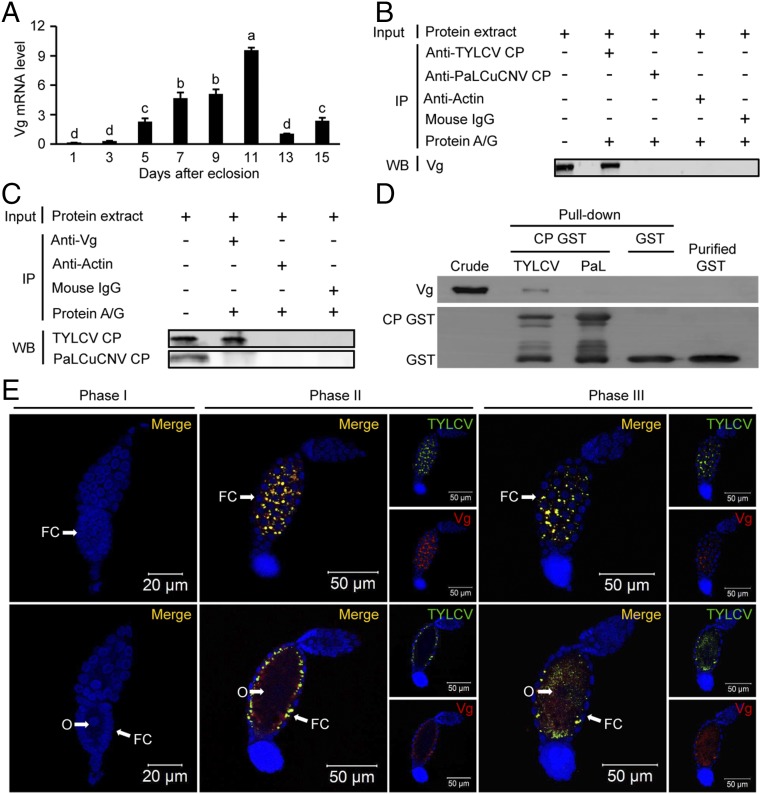
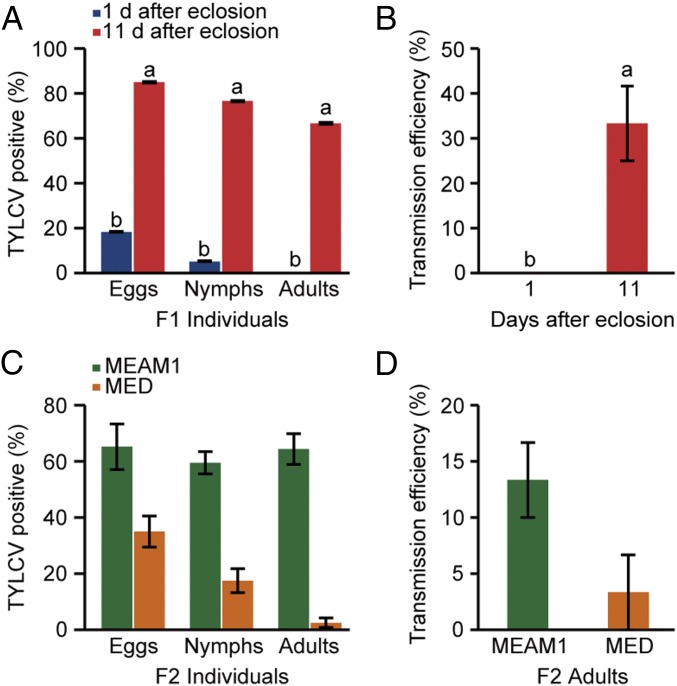
First, we observed the anatomy of ovary and ovariole at different developmental phases in female adult whiteflies, which has been reported by Guo et al. (25). Briefly, the ovary development could be classified into four stages according to whitefly development: stage I, freshly emerged whitefly; stage II, 1–2 d after eclosion (DAE); stage III, 3–10 DAE; and stage IV, 11–14 DAE (Fig. S1A). The ovariole development could be divided into four phases (phases I, II, III, and IV) based on oocyte yolk accumulation. The yolk content of oocytes gradually increased by phase (Fig. S1A) (25). Then, we investigated the localization of TYLCV and PaLCuCNV in ovaries of viruliferous MEAM1 adult whiteflies at different developmental stages. Interestingly, TYLCV virions were mainly located in ovaries of the more mature stages (stages III and IV), but not in ovaries of the less mature stages (stages I and II) (Fig. 1A), indicating that whitefly development may affect the transovarial transmission of TYLCV. To substantiate this observation, the transovarial transmission of TYLCV to vector offspring was examined using MEAM1 whiteflies at 1 or 11 DAE, respectively. The adult whiteflies at 1 DAE transmitted TYLCV to the eggs and nymphs of their progenies in a low frequency (8–13%), but no viral DNA was found in the adult progenies (Fig. 1B). Notably, the old mother whiteflies at 11 DAE vertically transmitted TYLCV to all developmental stages of their progeny efficiently (Fig. 1B). Virus transmission assays showed that none of the 24 healthy plants were infected by TYLCV when exposed to the adult progenies of 1 DAE viruliferous whiteflies, whereas 5 out of 24 plants were infected after inoculation by adult offspring of 11 DAE viruliferous whiteflies (Fig. 1C). Our data indicate that the age of whitefly is responsible for transovarial transmission efficiency of TYLCV.
Fig. S1.
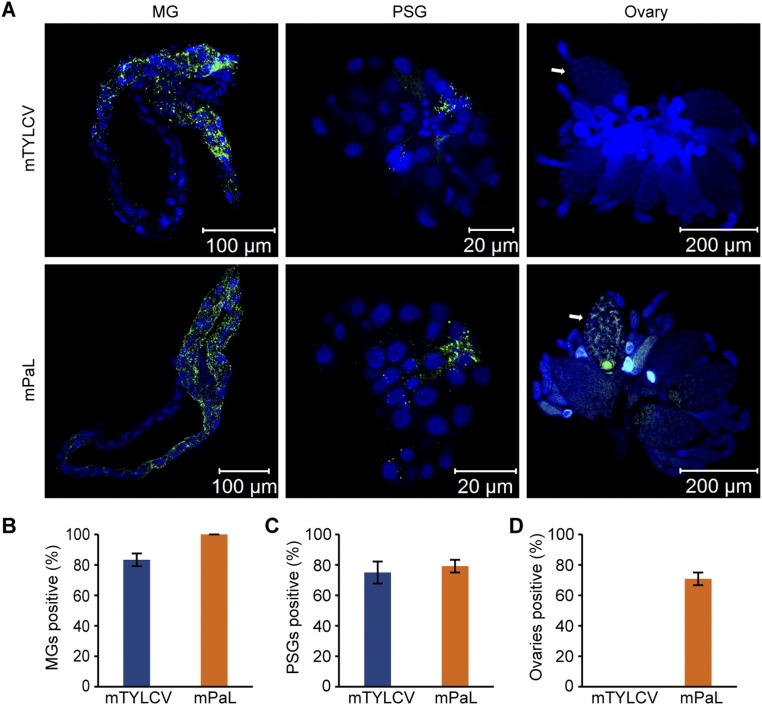
Ovary morphology of the whitefly B. tabaci and localization of virus in different tissues of MEAM1 whitefly. (A) Ovary (upper lane) of whiteflies at different developmental stages and oocyte (lower lane) at different developmental phases. Stage I, freshly emerged whitefly; stage II, 1–2 DAE; stage III, 3–10 DAE; and stage IV, 11–14 DAE. There was no yolk protein in phase I oocyte. The yolk content of oocyte gradually increased at phases II, III, and IV. (Scale bar, 0.10 mm.) (B) Localization of PaLCuCNV in ovaries of MEAM1 whiteflies at different developmental stages. Green, anti-PaLCuCNV CP. Blue (DAPI), cell nucleus. (C–E) The proportion of TYLCV or PaLCuCNV (PaL)-positive midguts (MGs) (C), primary salivary glands (PSGs) (D), and ovaries (E). MGs (n = 24), PSGs (n = 24), and ovary (n = 24). Mean ± SEM from three experiments. P < 0.05 (one-way ANOVA, LSD test). White arrows indicate mature oocytes in ovaries. Red arrows indicate dissected ovariole.
Fig. 1.
Transovarial transmission of two begomoviruses in MEAM1 whitefly. (A) Detection of TYLCV virions in ovaries of viruliferous MEAM1 adult whiteflies at different developmental stages by immunofluorescence. (Upper panels) Immunostaining of TYLCV CP in different stages of ovaries. (Lower panel) Percentage of ovaries at different stages infected by TYLCV. Stage I, freshly emerged whitefly (n = 30); stage II, 1–2 DAE (n = 30); stage III, 3–10 DAE (n = 30); and stage IV, 11–14 DAE (n = 30). (B) Transovarial transmission of TYLCV to offspring via ovum by MEAM1 whitefly. Virus DNA was traced in individual eggs (n = 60), nymphs (n = 60), or adults (n = 60) in the offspring produced by viruliferous adults at 1 or 11 DAE. (C) The infectivity of two groups of adults that developed from eggs produced by viruliferous whiteflies at 1 (n = 24) or 11 (n = 24) DAE. (D and E) Quantitative analysis of TYLCV and PaLCuCNV (PaL) DNA in whole bodies (D) and ovaries (E) of MEAM1 adult whiteflies following a 48-h acquisition access period (AAP) on virus-infected tomato plants starting at 1 and 11 DAE. (F) Localization of TYLCV or PaLCuCNV (PaL) in midguts (MGs), primary salivary glands (PSGs), and ovaries of viruliferous whiteflies at 11 DAE. (A and F) TYLCV and PaLCuCNV virions were detected by use of a mouse anti-CP monoclonal antibody and goat anti-mouse IgG labeled with Dylight 488 (green) secondary antibody. Cell nucleus was stained with DAPI (blue). White arrows indicate mature oocyte. (A–E) Mean ± SEM from three experiments. P < 0.05 [one-way ANOVA, least significant difference (LSD) test].
Whitefly Ovary Is a Selective Barrier That Blocks the Transovarial Transmission of PaLCuCNV.
Interestingly, PaLCuCNV was never detected in the ovary of viruliferous whiteflies (Fig. S1B) or in any developmental stage of the progeny produced by viruliferous MEAM1 whiteflies at 1 or 11 DAE (Table S2). Virus accumulation experiment revealed that, after a 48-h access acquisition period (AAP), the abundance of TYLCV in the whole bodies of adults was comparable to that of PaLCuCNV, at both 1 and 11 DAE (Fig. 1D). However, the abundance of TYLCV in ovaries of whiteflies at 11 DAE was severalfold that of whiteflies at 1 DAE, whereas for PaLCuCNV, the abundance of the virus was low in ovaries of adult whiteflies at both 1 and 11 DAE (Fig. 1E). We next performed immunofluorescence staining assays to detect the two viruses in midguts (MGs), primary salivary glands (PSGs), and ovaries of whiteflies at 11 DAE, and found that all MGs were TYLCV-positive or PaLCuCNV-positive; and 96% PSGs were TYLCV-positive, and 88% were PaLCuCNV-positive, indicating that both TYLCV and PaLCuCNV can reach MG and PSG effectively. However, although 79% of tested ovaries were TYLCV-positive, none of the ovaries was PaLCuCNV-positive (Fig. 1F and Fig. S1 C–E). These observations clearly imply that the failure of transovarial transmission of PaLCuCNV is due to nonpenetration of ovary rather than to inefficient virus intake.
Table S2.
Transovarial transmission of PaLCuCNV via ovum of MEAM1 or MED whitefly at 1 and 11 DAE
| Whitefly species | DAE | Eggs* | Nymphs* | Adults* |
| MEAM1 | 1 | 0/80 | 0/80 | 0/80 |
| 11 | 0/80 | 0/80 | 0/80 | |
| MED | 1 | 0/80 | 0/80 | 0/80 |
| 11 | 0/80 | 0/80 | 0/80 |
Positive/tested.
Interaction Between Viral CP and Vector Vg Is Vital for Virus Entry into Whitefly Ovary.
The above results indicate that virus entry of whitefly ovary mainly relies on viral type and whitefly developmental stages. As Vg plays a critical role in yolk formation and oogenesis (26–29), we assumed that Vg is involved in the entry of TYLCV into ovary. Interestingly, the expression level of Vg was positively correlated with the transovarial transmission efficiency of TYLCV (Fig. 2A). We then tested whether TYLCV CP or PaLCuCNV CP interacts with Vg in vivo by coimmunoprecipitation. Both anti-TYLCV CP antibody and anti-Vg antibody coimmunoprecipitated the other interacting proteins, whereas neither anti-PaLCuCNV CP antibody nor anti-Vg antibody coimmunoprecipitated the other proteins (Fig. 2 B and C). In vitro pull-down assay also confirmed that whitefly endogenous Vg was coeluted with GST-fused TYLCV CP, but not with GST-fused PaLCuCNV CP (Fig. 2D). These findings suggest that the specific interaction between viral CP and whitefly Vg is vital for virus entry of ovary.
Fig. 2.
Vg is involved in TYLCV entry into whitefly oocyte. (A) The expression patterns of Vg in adult female whiteflies at different developmental stages. Mean ± SEM of three experiments. P < 0.05 (one-way ANOVA, LSD test). (B and C) Coimmunoprecipitation (CO-IP) of Vg with anti-CP monoclonal antibody (B) and vice versa (C) in whitefly crude extracts. (D) B. tabaci endogenous Vg coeluted with GST-fused TYLCV CP but not with GST-fused PaLCuCNV (PaL) CP. (E) Localization of TYLCV and Vg in follicular cells (upper lane) and oocytes (lower lane) of ovarioles at different developmental phases. Cell nucleus was stained with DAPI (blue). For phases II and III, both Vg (red) and CP (green) are shown. Yellow color indicates the overlay of red and green. FC, follicular cells; O, oocyte.
Next, we traced the entry process of Vg and TYLCV into oocytes of viruliferous whitefly at 11 DAE. The ovariole of the whitefly is mainly composed of three parts: the tropharium, segregating oocyte, and vitellarium (30). The oocyte, located in the center of vitellarium, is surrounded by a single layer of follicular cells on its surface and has a nutritive cord connected with the tropharium (30) (Fig. S2A). We did not detect TYLCV virions in phase I ovarioles of viruliferous whiteflies when Vg was not detected in the ovarioles (Fig. 2E and Fig. S2B). TYLCV virions were first detected in the space between follicular cells of phase II ovarioles, when Vg began to accumulate in the space between follicular cells and just started to be absorbed into oocytes (Fig. 2E and Fig. S2B). Then TYLCV virions were detected not only in the space between follicular cells but also in the oocytes of phase III ovarioles, when massive Vg accumulated in the space between follicular cells and was rapidly absorbed into the oocytes (Fig. 2E and Fig. S2B). Moreover, TYLCV and Vg colocalized with each other in both the space between follicular cells and oocytes (Fig. 2E). These results suggest that the entry of TYLCV virions into the oocyte shares the same route of Vg transport into the oocyte.
Fig. S2.
Localization of Vg in ovarioles of whitefly. (A) Ovariole structure of whitefly. (B) Localization of Vg in ovarioles at different developmental phases. Phase I, Vg was not detected in the ovariole. Phase II, Vg began to accumulate in the space between follicular cells and just started to be absorbed into oocyte. Phase III, massive Vg accumulated in the space between follicular cells and was absorbed into the oocyte. F-actin was stained with phalloidin (red). Vg was detected by use of a mouse anti-Vg monoclonal antibody and goat anti-mouse IgG labeled with Dylight 549 (red) secondary antibody. Cell nucleus was stained with DAPI (blue). FC, follicular cells; O, oocyte; So, segregating oocyte; T, tropharium; Tn, trophocytes nucleus; V, vitellarium.
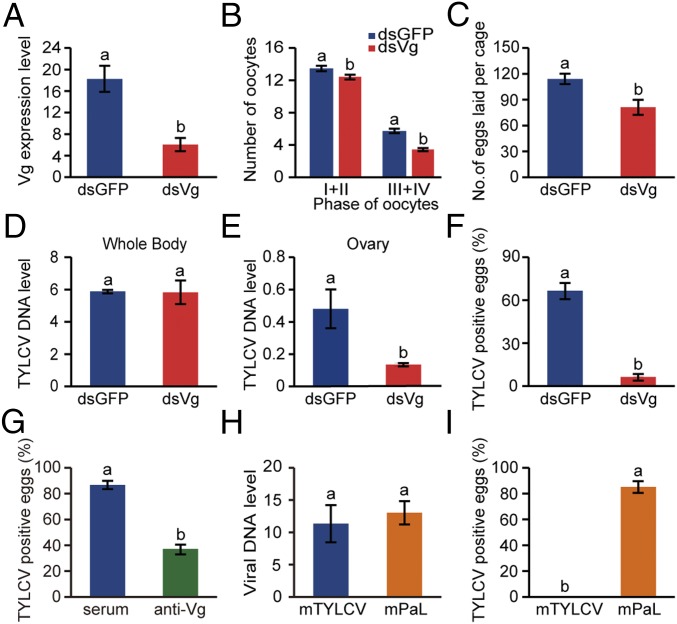
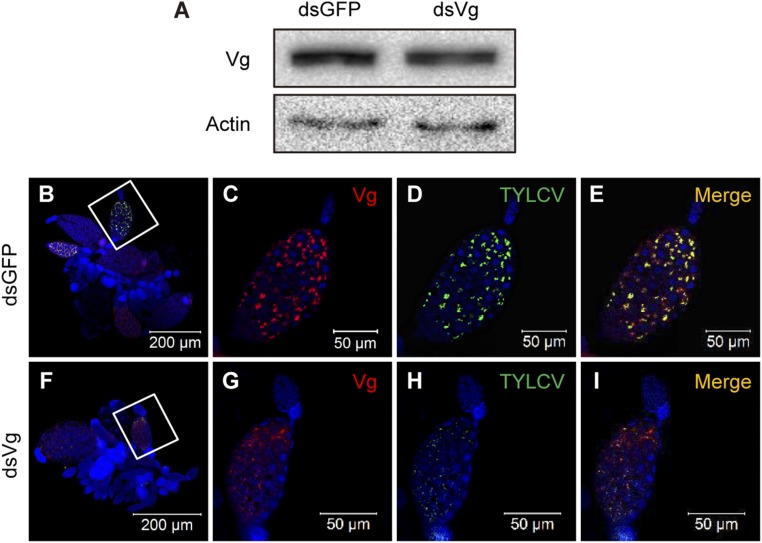
To verify the role of Vg in transovarial transmission of TYLCV, we knocked down the expression of Vg using RNA interference (RNAi). Compared with whiteflies fed with dsGFP (control), Vg mRNA level decreased by 67% in the group fed with dsVg (Fig. 3A) and Vg protein level also decreased (Fig. S3A). As the expression of Vg was decreased after ingestion of dsVg, the maturation of ovaries dissected from the treated whiteflies was delayed (Fig. 3B). Meanwhile, the number of eggs laid by female whiteflies after dsVg treatment decreased by 29% compared with the control (Fig. 3C). After inoculation on TYLCV-infected plants for 48 h, the whiteflies had similar level of virus in the whole bodies when they had been treated with dsVg or dsGFP (Fig. 3D); however, the abundance of TYLCV DNA decreased by 73% in the ovaries of dsVg-treated whiteflies compared with that of the control (Fig. 3E). Moreover, the overall TYLCV and Vg signals in dsVg-treated ovaries were lower than those of the control (Fig. S3 B–I), suggesting that knockdown of Vg expression reduced yolk accumulation into oocytes and impeded the entry of TYLCV into the ovary of whitefly. Most importantly, the frequency of TYLCV transmission to eggs was reduced by 90% compared with the control group (Fig. 3F). Furthermore, the transmission rate of TYLCV to eggs by the adults decreased by 57% after oral ingestion of anti-Vg antibody, which would bind to Vg and therefore inhibit CP–Vg interaction, compared with preimmune serum (control)-treated group (Fig. 3G).
Fig. 3.
The role of Vg and viral CP in transovarial transmission of virus. (A) Vg mRNA levels after feeding with dsRNAs. (B and C) dsVg treatment delayed whitefly ovary development (B) and reduced egg production (C) of whitefly. For B, sample sizes were 30 females per treatment, and for C, nine replicates per treatment (five females/replicate). (D and E) TYLCV DNA levels in whole bodies (D) and ovaries (E) of whiteflies after feeding with dsRNAs. (F) dsVg treatment reduced transovarial transmission of TYLCV to eggs. (G) Oral ingestion of anti-Vg antibody decreased transmission rate of TYLCV to eggs. (H) The viral DNA level of mutant TYLCV (mTYLCV) and mutant PaLCuCNV (mPaL) in whitefly whole bodies. (I) Transovarial transmission rate of mTYLCV and mPaL via ovum by MEAM1 whitefly. We detected 45–80 eggs for F, G, and I. (A–I) Mean ± SEM of three independent experiments. P < 0.05 (one-way ANOVA, LSD test).
Fig. S3.
Knocking down the expression of Vg reduced yolk accumulation into oocytes and impeded entry of TYLCV into the ovary. (A) Vg protein levels after feeding with dsRNAs. (B–I) Localization of TYLCV and Vg in ovaries of dsGFP (B–E)- or dsVg (F–I)-treated whiteflies after a 48-h AAP on TYLCV-infected plants. C–E are the magnification of the boxed area in B, and G–I are the magnification of boxed area in F. TYLCV virions were detected by use of a rabbit anti-CP polyclonal antibody and goat anti-rabbit IgG labeled with Dylight 488 (green) secondary antibody. Vg was detected by use of a mouse anti-Vg monoclonal antibody and goat anti-mouse IgG labeled with Dylight 549 (red) secondary antibody. Cell nucleus was stained with DAPI (blue). Yellow color indicates the overlay of red and green.
To examine the role of viral CP in transovarial transmission, we exchanged partial CP sequence of TYLCV with that of PaLCuCNV (Fig. S4A), and observed the transovarial transmission of the mutated TYLCV (mTYLCV) and mutated PaLCuCNV (mPaL) by whiteflies at 11 DAE. Both mTYLCV and mPaL induced typical disease symptoms after agroinoculated into tomato plants (Fig. S4B). After a 48-h AAP, the whiteflies had a similar amount of mTYLCV and mPaL in their whole bodies (Fig. 3H). However, mTYLCV could not be detected in eggs of viruliferous whiteflies, whereas mPaL was transmitted to the eggs in a high frequency (85%) (Fig. 3I). Immunofluorescence analysis of viruliferous whiteflies at 11 DAE showed that nearly 90% of the tested MGs and 80% of the tested PSGs were mTYLCV- and mPaL-positive. For the ovaries, none of the tested samples was mTYLCV-positive, whereas 71% were mPaL-positive (Fig. S5). Overall, these results demonstrate that specific interactions between viral CP and whitefly Vg determine the transovarial transmission of TYLCV.
Fig. S4.
Comparison of amino acid sequence of TYLCV and PaLCuCNV coat protein (CP) and typical disease symptoms of viruses used in this study. (A) Comparison of amino acid sequence of TYLCV and PaLCuCNV (PaL) CP. Alignments were done by GeneDoc. The different regions are indicated in red. The exchanged region is indicated by a blue underline. (B) Typical disease symptoms of TYLCV, mutant TYLCV (mTYLCV), PaLCuCNV (PaL), and mutant PaLCuCNV (mPaL).
Fig. S5.
Localization of mTYLCV or mPaLCuCNV in different tissues of MEAM1 whitefly. (A) Localization of mutant TYLCV (mTYLCV) or mutant PaLCuCNV (mPaL) in midguts (MGs), primary salivary glands (PSGs), and ovaries of viruliferous whiteflies at 11 DAE. Mutant TYLCV (mTYLCV) and mutant PaLCuCNV (mPaL) virions were detected by use of a mouse anti-CP monoclonal antibody and goat anti-mouse IgG labeled with Dylight 488 (green) secondary antibody. Cell nucleus was stained with DAPI (blue). (B–D) The proportion of mutant TYLCV (mTYLCV) or mutant PaLCuCNV (mPaL)-positive MGs (n = 24) (B), PSGs (n = 24) (C), and ovaries (n = 24) (D). Mean ± SEM from three experiments. P < 0.05 (one-way ANOVA, LSD test). White arrows indicate mature oocytes.
Transovarial Transmission of TYLCV and PaLCuCNV by MED Whiteflies.
To examine whether this phenomenon also exists in other whitefly species, we further investigated the transovarial transmission of TYLCV and PaLCuCNV by MED whiteflies, which is another invasive species of the B. tabaci complex (17). MED whiteflies of 1 DAE transmitted TYLCV only to the eggs and nymphs of their progeny; however, whiteflies of 11 DAE transmitted TYLCV to eggs, nymphs, and adults of their progeny efficiently (67–85%) (Fig. 4A). Whereas 8 out of 24 tested plants were infected with TYLCV after inoculation by adult progenies of 11 DAE viruliferous MED whiteflies, no viral DNA was detected after inoculation by progenies of viruliferous MED whiteflies at 1 DAE (Fig. 4B). Similarly, PaLCuCNV was never detected in the progeny of viruliferous MED whiteflies at both 1 and 11 DAE (Table S2). Immunostaining showed that both TYLCV and PaLCuCNV can invade the MG and PSG of MED whiteflies. As for the ovaries, nearly 50% of the tested were TYLCV-positive, whereas none was PaLCuCNV-positive (Fig. S6 A–D). Furthermore, Vg and TYLCV CP colocalized in mature ovarioles of viruliferous MED whiteflies (Fig. S6E), suggesting that Vg of MED whitefly is also involved in the entry of ovary by TYLCV.
Fig. 4.
Transovarial transmission of TYLCV via ovum by MED and MEAM1 whiteflies. (A) Virus DNA was traced in individual eggs (n = 60), nymphs (n = 60), and adults (n = 60) in the offspring produced by viruliferous MED adults at 1 and 11 DAE. (B) The infectivity of first-generation adults that developed from eggs deposited by viruliferous MED adult whiteflies at 1 or 11 DAE; 1 DAE (n = 24) and 11 DAE (n = 24). (C) Transovarial transmission of TYLCV to F2 progeny by F1 adults of either MEAM1 or MED at 11 DAE, which developed from eggs deposited on cotton by F0 viruliferous whiteflies at 11 DAE. Eggs (n = 80), nymphs (n = 80), and adults (n = 80). (D) The infectivity of F2 adults that developed from eggs deposited on cotton by F1 adults at 11 DAE, which themselves developed from eggs deposited on cotton by F0 viruliferous whiteflies at 11 DAE. MEAM1 (n = 30) and MED (n = 30). (A–D) Mean ± SEM of three experiments. P < 0.05 (one-way ANOVA, LSD test).
Fig. S6.
Localization of viruses and Vg in different tissues of MED whiteflies. (A–D) Localization of TYLCV or PaLCuCNV (PaL) in midguts (MGs) (n = 24), primary salivary glands (PSGs) (n = 24), and ovaries (n = 24) of viruliferous MED whiteflies at 11 DAE. For A, TYLCV and PaL virions were detected by use of a mouse anti-CP monoclonal antibody and goat anti-mouse IgG labeled with Dylight 488 (green) secondary antibody. White arrows indicate mature oocytes. For B–D, mean ± SEM from three experiments. P < 0.05 (one-way ANOVA, LSD test). (E) TYLCV and Vg colocalized with each other in ovariole of MED whitefly. TYLCV virions were detected by use of a rabbit anti-CP polyclonal antibody and goat anti-rabbit IgG labeled with Dylight 488 (green) secondary antibody. Vg was detected by use of a mouse anti-Vg monoclonal antibody and goat anti-mouse IgG labeled with Dylight 549 (red) secondary antibody. Cell nucleus was stained with DAPI (blue). Yellow color indicates the overlay of red and green.
TYLCV Can Be Maintained for at Least Two Generations by Whitefly Vector.
Next, we examined whether TYLCV could be vertically transmitted from the first generation to the following generations. We conducted transovarial transmission assays with the first-generation adults, which developed from eggs deposited on cotton by viruliferous MEAM1 and MED whiteflies at 11 DAE. We found that the first-generation adults could transmit TYLCV to the second-generation progenies through ovum, with 59–65% for MEAM1 whiteflies and 3–35% for MED whiteflies (Fig. 4C). Moreover, the second-generation adults were able to transmit TYLCV to healthy plants, with 4 out of 30 (13%) test plants being TYLC-positive for MEAM1 whiteflies and 1 out of 30 (3%) test plants being TYLCV-positive for MED whiteflies (Fig. 4D). Additionally, field cage trials and field sampling provided evidence of transovarial transmission of TYLCV by MEAM1 and MED and its relevance to TYLCV epidemiology in the field (Fig. S7 and Table S3).
Fig. S7.
Infection of tomato plants in field cages by first-generation adults that developed from eggs deposited on cotton by viruliferous MEAM1 and MED adult whiteflies. Data are mean (±SEM) percentages of TYLCV-infected plant of three replicates.
Table S3.
Field investigation of transovarial transmission of TYLCV by whiteflies
Positive/tested.
Discussion
Vertical transmission of viruses from female parent to offspring is an important strategy for viruses to speed up transmission, and is recognized to play a significant role in viral epidemics (2, 3). Transovarial transmission of plant viruses has received constant attention since 1933 (5–7). However, most of the reports were focused on circulative-propagative plant viruses, such as reoviruses, rhabdoviruses, or tenuiviruses (31–34). Even so, some research groups have investigated the transovarial transmission of circulative-nonpropagative begomoviruses in the past 50 y. However, whether begomoviruses can be transmitted transovarially is still in great controversy. Although most of the studies reported absence of transovarial transmission (35–39), several studies reported the presence of transovarial transmission, but the efficiencies varied considerably between studies (40–42) (Table S1). Our study proved that TYLCV can be effectively transmitted via ovary of whitefly adults of elder age, but not by whitefly adults of very young age (Fig. 1). Our findings explained why different results were obtained in previous experiments on transovarial transmission of TYLCV in the last half century, as the age of whiteflies used in those experiments was different. The only report that showed a very high rate of TYLCV transovarial transmission actually used 5–8 DAE whiteflies (42), whereas the other two studies with very low frequency used newly emerged whiteflies (40, 41). In addition, not all begomoviruses can be transovarially transmitted (Table S1); therefore, the virus strains used in different experiments may also contribute to the discrepancy.
Vg, the precursor of major yolk protein in most oviparous species and invertebrates, is synthesized mainly by the fat body and secreted into hemolymph, from where it is absorbed into the growing oocytes by receptor-mediated endocytosis, and finally serving as nutrition for developing embryos (28, 43, 44). Here, we discovered that the specific interaction between viral CP and whitefly Vg determines the transovarial transmission specificity of begomoviruses, and that the binding between TYLCV CP and Vg is important for TYLCV to overcome the transovarial transmission barriers (Figs. 2 and 3). TYLCV CP and Vg may bind together in the hemolymph and then be transported into the oocyte via the existing transportation systems for Vg. In contrast, another begomovirus PaLCuCNV could not be transovarially transmitted by whitefly, and PaLCuCNV CP did not interact with whitefly Vg (Fig. 2 and Table S2). When we replaced partial CP sequence of PaLCuCNV with that of TYLCV, the mutated PaLCuCNV became transovarially transmissible and mutated TYLCV became nontransmissible (Fig. 3I and Fig. S5). Thus, this part of CP sequence determines transovarial transmissibility. However, there are 33 different amino acids in the exchanged fragment (Fig. S4A). To identify the essential amino acids that are responsible for transovarial transmission, more virus CP mutants with single-amino acid substitution could be generated to test their transovarial transmission and interactions with Vg. Previous study has shown that the transovarial transmission of a propagative RNA virus (Rice stripe virus) is mediated by Vg of its insect vector (31). Moreover, the Babesia parasite in Haemaphysaalis longicornis and the ZAM virus in Drosophila melanogaster are dependent on the Vg receptor when transmitted into the oocyte (45, 46). Therefore, the existing transovarial transportation systems for absorbing nutrients in the oviparous species, including insects, may be a common way used by DNA virus, RNA virus, and parasites for their maternal transmission. Given the great significance of vertical transmission for microbe ecology, evolution, and epidemiology, more investigations on this mechanism among other microbe–host associations are needed.
TYLCV, one of the world’s most devastating pathogens of tomato, is considered to have spread from the Middle East or the Eastern Mediterranean to the world since the 1980s, and the dissemination process is probably still ongoing (47, 48). The global invasion of MEAM1 and MED whitefly together with the movement of infected plant material are believed to be the two main factors that are attributed to the rapid spread of TYLCV worldwide (49, 50). In our study, both MEAM1 and MED whitefly can transovarially transmit TYLCV to their progeny efficiently, and the virus can be maintained for at least two generations by whiteflies in the absence of a host plant (Figs. 1B and 4). Moreover, the adult progenies that developed on virus nonhost plant can infect healthy plants in the field (Fig. S7). With an almost invisible size, viruliferous eggs laid by virus-infected whiteflies on plants can be transported for long distance with plants by human activities. Thus, the transovarial transmission may also contribute significantly to the global spread of TYLCV as well as the outbreak of TYLCV disease in the field.
Methods
Transmission of Virus via Ovum by Whitefly.
Viruliferous MEAM1 and MED adult whiteflies at different developmental stages or under different treatments were collected in groups of 10 each (female/male = 1:1). For each replicate of a treatment, a group of insects was placed on the lower surface of a leaf of cotton plant, a nonhost plant of TYLCV and PaLCuCNV, enclosed in a leaf clip cage. The adults were left on the leaf to feed, mate, and oviposit for 72 h at 26–31 °C, 40–60% relative humidity, and a photoperiod of 14:10 h (light/dark). Then, all adults were removed and five eggs were collected from each replicate using disposable sterilized needles (one needle for each individual) and stored at –20 °C for detection of virus DNA subsequently. The remaining eggs were left on the leaf to develop. Eighteen and 25 d later, five nymphs and five adults were collected from each replicate, using disposable sterilized needles (one needle for each individual) and stored at −20 °C for detection of virus DNA subsequently.
Transmission of TYLCV to Plants by Adult Progenies.
For indoor transmission assays, the adult progenies of viruliferous whiteflies were collected in groups of 10 (female/male = 1:1) and each group was used to inoculate one uninfected tomato plant. The inoculation was performed on the top second leaf of the plant at the 3–4 true-leaf stage (∼3 wk after sowing) for a 48-h inoculation access period, using a leaf clip cage. Twenty-four uninfected tomato plants were used for the first-generation adults and 30 for the second-generation adults. The plants were then sprayed with imidacloprid at a concentration of 20 mg/L to kill all of the whitefly adults and eggs, and maintained until symptoms had developed.
Coimmunoprecipitation Assay.
Immunoprecipitation was performed using protein G-Sepharose 4 Fast Flow (catalog no. 17-0405-03; GE Healthcare) according to the manufacturer’s instructions. Whitefly protein extracts were prepared in cell lysis buffer (20 mM Tris⋅HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 20 mM β-glycerophosphate, 10 mM NaF, 1 mM PMSF, 1 mM sodium orthovanadate, 10 mg/mL leupeptin, 2 mg/mL aprotinin, 1 mM EDTA). Monoclonal antibodies anti-CP (51), anti-Vg (52), or anti-Actin (catalog no. E021020-02; EarthOx) and their corresponding preimmune sera (catalog no. A7028; Beyotime) as controls were incubated with whitefly protein extracts for 10 h at 4 °C, followed by incubating with protein G-Sepharose beads for an additional 2 h at 4 °C. After washing five times with lysis buffer, immunoprecipitated proteins were eluted by boiling in PAGE buffer for 5 min, and then resolved by SDS/PAGE. Each immunodetection was repeated at least three times. Incubation with antibodies was followed by ECL Plus Detection (catalog no. 170-5060; Bio-Rad). Protein G-Sepharose beads bound to anti-Actin antibody or preimmune sera were used as negative controls.
GST Pull-Down Assay.
The fragments of TYLCV and PaLCuCNV CPs were amplified and cloned into pGEX-6p-1 for fusion with GST. Primers are listed in Table S4. All recombinant proteins were expressed in Escherichia coli strain Rosetta and purified. The GST-tag–fused protein, TYLCV CP-GST or PaLCuCNV CP-GST, was bound to glutathione Sepharose beads (catalog no. 17-5132-01; GE Healthcare) for 3 h at 4 °C, and the mixtures were centrifuged for 5 min at 100 × g, and the supernatants were discarded. The nonviruliferous whitefly soluble protein extracts or the His-tag fusion proteins were added to the beads and incubated for 2 h at 4 °C. After being centrifuged and washed five times with PBS, the beads-bound proteins were eluted by boiling in PAGE buffer for 5 min, and then the proteins were separated by SDS/PAGE gel electrophoresis and detected by anti-Vg antibody.
Table S4.
Primers used in this study
| Primer name* | Sequence, 5′–3′† | Length, bp | Purpose |
| TYLCV-F | CAGAGCAGTTGATCATG | 682 | TYLCV DNA detection |
| TYLCV-R | ATCGAAGCCCTGATATCCCCCGTGG | ||
| PaLCuCNV-F | TAGTCATTTCCACTCCCGC | 645 | PaLCuCNV DNA detection |
| PaLCuCNV-R | TGATTGTCATACTTCGCAGC | ||
| mTYLCV-F | CAGAGCAGTTGATCATG | 679 | Mutant TYLCV DNA detection |
| mTYLCV-R | ATCGAAGCCCTGATATCCCCCGTGG | ||
| mPaLCuCNV-F | CCTGATGTTCCTCGTGGTT | 408 | Mutant PaLCuCNV DNA detection |
| mPaLCuCNV-R | CCTGTTCCTTCATTCCAGAG | ||
| QTYLCV(mTYLCV)-F | GAAGCGACCAGGCGATATAA | 189 | qPCR for TYLCV (mutant TYLCV) |
| QTYLCV(mTYLCV)-R | GGAACATCAGGGCTTCGATA | ||
| QPaL(mPaL)-F | GACCCGCCGATATAGTCATT | 109 | qPCR for PaLCuCNV (mutant PaLCuCNV) |
| QPaL(mPaL)-R | GTTTGTGACGAGGACAGTGG | ||
| QVg-F | ACAAGTCTCCGACGCCGAAG | 185 | qRT-PCR for Vg |
| QVg-R | TTGACATCGGCTTTACGGCA | ||
| Qβ-Actin-F | TCTTCCAGCCATCCTTCTTG | 173 | q(RT)-PCR for β-Actin |
| Qβ-Actin-R | CGGTGATTTCCTTCTGCATT | ||
| Vg-RNAi-F | T7-ACATCGTCAAGGCCACCAA | 451 | Vg dsRNA synthesis |
| Vg-RNAi-R | T7-TAGAGCTGGAACTAGATGAG | ||
| gfp-RNAi-F | T7-CTCGTGACCACCCTGACCTAC | 314 | dsgfp synthesis |
| gfp-RNAi-R | T7-GTTCACCTTGATGCCGTTCTT | ||
| PaL CP-GST-F | CGGGATCCATGTCGAAGCGACCCGCCG | 790 | Expression of PaL CP in E. coli |
| PaL CP-GST-R | CGGAATTCTTAATTCTGAACAGAATCA | (BamHI and EcoRI sites are underlined) | |
| TYLCV CP-GST-F | CGGGATCCATGTCGAAGCGACCAGGCGA | 793 | Expression of TYLCV CP in E. coli |
| TYLCV CP-GST-R | CGGAATTCTTAATTTGATATTGAATCAT | (BamHI and EcoRI sites are underlined) |
Additional primer sequences are available upon request.
T7, 5′-TAATACGACTCACTATAGG-3′.
Gene Silencing by Oral Ingestion of dsRNA.
RNA silencing was performed as previously described (53). Briefly, dsRNAs were diluted into 15% (wt/vol) sucrose solution at the concentration of 300 ng/μL. Approximately 100 adult whiteflies at 2 DAE were released into each feeding chamber. The tube was incubated in an insect-rearing room for 48 h. Subsequently, whiteflies were transferred to TYLCV-infected tomato plants and maintained for 48 h, and then RNA was extracted from 20 female individuals to examine the Vg mRNA level, and total proteins were extracted from 30 female individuals to examine the Vg protein level after dsRNA treatment. The remaining insects were used for quantitative assays and virus transmission tests. Each set of experiment was repeated three times.
Immunofluorescence Assay.
For virus localization, ovaries, MGs, and PSGs of female whiteflies at required developmental stages were dissected after a 48-h AAP on virus-infected plants. For Vg antibody staining, ovaries of whiteflies at 11 DAE were dissected and ovarioles were dispersed from ovaries. The specimens were fixed in 4% paraformaldehyde for 1 h at room temperature, and washed in PBST containing 0.1% Triton X-100 three times for 1 h each. Then the specimens were blocked in PBST containing 1% BSA (catalog no. A3828; MultiSciences Biotech) for 2 h at room temperature, followed by incubation with anti-Vg monoclonal antibody (1:500) or anti-CP monoclonal (1:500) or polyclonal antibody (1:500) in PBST containing 1% BSA overnight at 4 °C and then with goat anti-mouse (1:500) or goat anti-rabbit (1:500) secondary antibody labeled with Dylight 488 (catalog no. LK-GAM4882; MultiSciences Biotech) or Dylight 549 (catalog no. LK-GAR5492; MultiSciences Biotech) in PBST containing 1% BSA for 1 h at room temperature after extensive washing. The nucleus was stained with 100 nM 4′,6-diamidino-2-phenylindole (DAPI) (catalog no. ab104139; Abcam) in PBST for 2 min at room temperature.
SI Text
Insects, Viruses, and Plants.
Two cryptic species of the Bemisia tabaci whitefly complex, Middle East Asia Minor 1 (MEAM1) (mitochondrial cytochrome oxidase I; GenBank accession no. GQ332577.1) and Mediterranean (MED) (mitochondrial cytochrome oxidase I; GenBank accession no. GQ371165), were reared on cotton plants (Gossypium hirsutum L. cv. Zhemian 1793) in insect-proof cages at 26 °C (±1 °C) under a photoperiod of 14:10 h (light/dark) and relative humidity of 50% (±10%). The purity of the cultures was monitored every three generations using the random amplified polymorphic DNA-PCR technique and the sequence of mitochondrial cytochrome oxidase I gene, which has been used widely to differentiate B. tabaci genetic groups (17). Clones of TYLCV isolate SH2 (GenBank accession no. AM282874.1) and PaLCuCNV isolate HeNZM1 (GenBank accession no. FN256260.1) were agroinoculated into plants of tomato (Solanum lycopersicum L. cv. Hezuo903). Uninfected tomato plants were grown in insect-proof greenhouses under controlled temperature at 25 ± 3 °C and natural lighting supplemented with artificial lights for 14 h a day from 0600 to 2000 hours.
q(RT)-PCR Analysis.
For viral DNA quantification, total DNA was extracted using previously described methods (54). Whitefly adults or tissue samples were ground in 40 μL of ice-cold lysis buffer (5 mM Tris⋅HCl, pH 8.4, 0.5 mM EDTA, 0.5% Nonidet P-40, and 1 mg/mL proteinase K) and then were incubated at 65 °C for 2 h, and then 100 °C for 10 min. The supernatants were kept at −20 °C.
For Vg expression level measurement, total RNA was first isolated using TRIzol reagent (catalog no. 15596018; Ambion), and then cDNAs were produced with random primers using the PrimeScript RT reagent kit with gDNA Eraser (catalog no. RR047A; TaKaRa). q(RT)-PCR was performed using an ABI Prism 7500 Fast Real-Time PCR system (Applied Biosystems) with SYBR Premix Ex TaqTM II (catalog no. RR820A; TaKaRa) and the primers are shown in Table S4. For each reaction, 0.8 μL of each primer (10 mM), 6.4 μL of nuclease-free water, and 10 μL of SYBR Premix Ex Taq were added, in a total volume of 20 μL. The q(RT)-PCR protocol was 95 °C for 30 s, followed by 40 cycles of at 95 °C for 5 s and 60 °C for 30 s. A negative control (nuclease-free water) was included throughout the experiments to detect contamination and to determine the degree of dimer formation. The results (threshold cycle values) of the q(RT)-PCR assays were normalized to the expression level of B. tabaci β-actin gene. The relative abundance of viral DNA or relative expression level of Vg was calculated using the 2−ΔCt method.
dsRNA Preparation.
dsRNA specific to Vg of B. tabaci (GenBank accession no. GU332720) or GFP was synthesized using the AmpliScribe T7-Flash Transcription Kit (catalog no. ASF3507; Epicentre), following the manufacturer’s instructions. Briefly, the DNA template for dsRNA synthesis was amplified with primers containing the T7 RNA polymerase promoter at both ends (Table S4), and the purified DNA template (200 ng) was then used to generate dsRNAs. Subsequently, the synthesized dsRNA was purified via phenol-chloroform precipitation and resuspended in nuclease-free water, and the concentration of dsRNA was quantified with a NanoDrop 2000 (Thermo Fisher Scientific). Finally, the quality and size of the dsRNAs were further verified via electrophoresis in a 1% agarose gel.
Determination of Ovary Development.
Two days after eclosion (DAE), adult whiteflies were treated with dsRNAs for 48 h. After treatments, the insects were transferred to cotton plants to feed for another 48 h. Then ovaries (n = 30) were dissected from female whiteflies and observed under a Zeiss Stemi 2000-C stereomicroscope for counting the number of oocytes at different development phases.
Observation of Whitefly Fecundity.
Whiteflies at 2 DAE were treated with dsRNAs for 48 h. After treatments, the insects were collected in groups of 10 per replicate (female/male = 1:1). For each replicate of a treatment, the 10 insects was placed on the lower surface of a leaf of a cotton plant, a nonhost plant of TYLCV, enclosed in a leaf clip cage. The adults were left on the leaf to feed, mate, and oviposit for 72 h at 26–31 °C, 40–60% relative humidity, and a photoperiod of 14:10 h (light/dark). Then, all adults were removed and the number of eggs was counted under a Zeiss Stemi 2000-C stereomicroscope.
Oral Ingestion of Vg Antibodies.
Adult whiteflies at 5–8 DAE were collected and fed with anti-Vg antibodies (1:200) or mouse preimmune serum (1:200, control) in 15% (wt/vol) sucrose solution using membrane feeding device. Approximately 100 adult whiteflies were released into each feeding chamber, and the feeding device was incubated in an insect-rearing room for 48 h. Subsequently, whiteflies were transferred to TYLCV-infected tomato plants for a 48-h AAP, and then collected in group of 10 for each replicate (female/male = 1:1) and used to examine transovarial transmission of TYLCV.
Engineering Infectious Clones of CP Mutant Viruses.
Procedures for construction of infectious clones of mutated TYLCV (mTYLCV) and mutated PaLCuCNV (mPaL) were performed as previously described (55, 56). Briefly, a 423-bp fragment (positions 241–663) of the TYLCV CP gene was replaced with a 420-bp fragment (positions 241–660) of the PaLCuCNV CP gene by overlap extension PCR (Fig. S4A for details of the replaced region). The fidelity of mTYLCV and mPaL was examined by Sanger sequencing.
Image Acquisition and Processing.
Fluorescence images were acquired using a Zeiss LSM 710 confocal microscope. Nuclear DNA was visualized using a 405-nm laser for excitation; the Dylight 488-labeled antibody and Dylight 549-labeled antibody were visualized using a 488-nm laser and a 561-nm laser for excitation, respectively. Maximum intensity projection images were generated using the ZEN 2010 digital imaging system.
Transovarial Transmission of TYLCV on Tomato Plants in Field Cages.
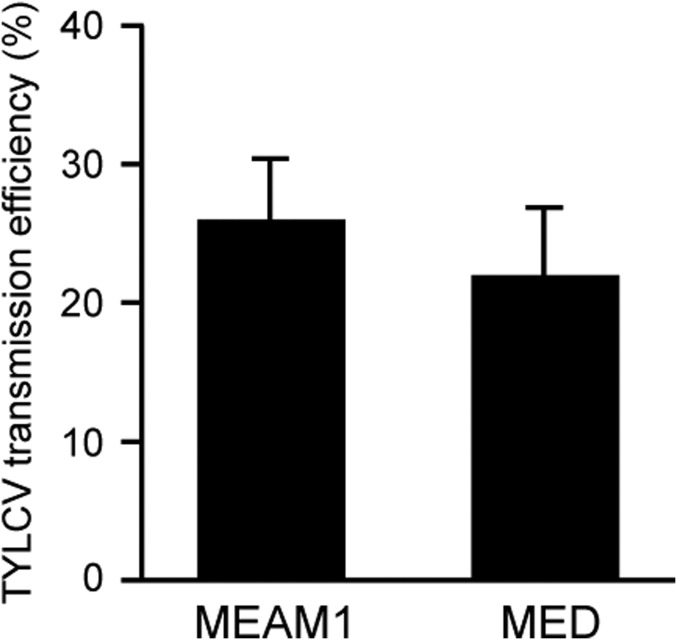
From April to June 2016, we conducted a viral transmission trial under field cage condition using adults that developed from eggs laid by viruliferous whiteflies on cotton, a nonhost plant of TYLCV. For either MEAM1 or MED, we first set up three cages with 120 mesh gauze (proven to be inaccessible to whitefly adults) in the field, with the size 1.3 m × 1.3 m × 1.3 m. In each of the field cages, we planted 30 tomato seedlings. Meanwhile, we set up three cohorts of either MEAM1 and MED in the laboratory, each cohort with one cotton plant. We released 50 viruliferous female adults and 50 viruliferous male adults into each cage and allowed them to mate, feed, and oviposit on the cotton plant for 3 d. We then removed all of the adults and reared the eggs to adults on the cotton plant. Twenty-five days later, we collected ∼500 adults (females and males mixed) from each cage and released them into a field cage for virus inoculation. The tomato plants at the time of whitefly release were at 3–4 true-leaf stage. One month later, we collected one young leaf from each of the tomato plants in the cages and diagnosed for TYLCV infection. In the three cages inoculated with adult progeny of viruliferous MEAM1 whiteflies, on average 26% of the plants were infected by TYLCV, whereas in the three cages inoculated with adult progeny of viruliferous MED whiteflies, on average 22% of the plants were infected by TYLCV (Fig. S7).
Field Sampling of TYLCV Infection of Whitefly Progeny on Cotton.
In May 2016, on a farm in a Hangzhou suburb, we found a plot of cotton grown next to a plot of tomato that was infected by TYLCV. We also found that the cotton plants were infested by whiteflies. We assumed that if whitefly eggs/nymphs on cotton were infected by a begomovirus, they were likely progeny of viruliferous whiteflies from the diseased tomato plants nearby, because cotton is a nonhost of all of the begomoviruses we had recorded in this region. We randomly collected 48 whitefly eggs and 48 whitefly nymphs from cotton plants along the edge of the cotton plot (within 5 m from the edge of the virus-infected tomato) and brought them back to the laboratory. Using the molecular methods presented in the main text, we found that all of the eggs and nymphs were of MEAM1, and 16 of the 48 eggs (33%) and 11 of the 48 nymphs (23%) were viruliferous with TYLCV (Table S3).
Statistics.
Data were presented as mean ± SEM of three independent biological replicates, unless otherwise noted. Statistical analysis was performed using SPSS (version 13). Means were compared using one-way ANOVA followed by least significant difference (LSD) test at the P < 0.05 significance level. No statistical methods were used to predetermine the sample size.
Acknowledgments
We thank Prof. Gong-Ying Ye for providing whitefly Vg antibody. Financial support for this study was provided by the National Natural Science Foundation of China (31390421) and the National Key Research and Development Program (2016YFC1200601).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1701720114/-/DCSupplemental.
References
- 1.Moya A, Peretó J, Gil R, Latorre A. Learning how to live together: Genomic insights into prokaryote-animal symbioses. Nat Rev Genet. 2008;9:218–229. doi: 10.1038/nrg2319. [DOI] [PubMed] [Google Scholar]
- 2.Lequime S, Paul RE, Lambrechts L. Determinants of arbovirus vertical transmission in mosquitoes. PLoS Pathog. 2016;12:e1005548. doi: 10.1371/journal.ppat.1005548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tesh RB, Chaniotis BN, Johnson KM. Vesicular stomatitis virus (Indiana serotype): Transovarial transmission by phlebotomine sandlies. Science. 1972;175:1477–1479. doi: 10.1126/science.175.4029.1477. [DOI] [PubMed] [Google Scholar]
- 4.Gray SM, Banerjee N. Mechanisms of arthropod transmission of plant and animal viruses. Microbiol Mol Biol Rev. 1999;63:128–148. doi: 10.1128/mmbr.63.1.128-148.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hogenhout SA, Ammar D, Whitfield AE, Redinbaugh MG. Insect vector interactions with persistently transmitted viruses. Annu Rev Phytopathol. 2008;46:327–359. doi: 10.1146/annurev.phyto.022508.092135. [DOI] [PubMed] [Google Scholar]
- 6.Fukushi T. Transmission of the virus through the eggs of an insect vector. Proc Imp Acad (Tokyo) 1933;9:457–460. [Google Scholar]
- 7.Pedro SA, Abelman S, Tonnang HE. Predicting rift valley fever inter-epidemic activities and outbreak patterns: Insights from a stochastic host-vector model. PLoS Negl Trop Dis. 2016;10:e0005167. doi: 10.1371/journal.pntd.0005167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mims CA. Vertical transmission of viruses. Microbiol Rev. 1981;45:267–286. doi: 10.1128/mr.45.2.267-286.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray S, Cilia M, Ghanim M. Circulative, “nonpropagative” virus transmission: An orchestra of virus-, insect-, and plant-derived instruments. Adv Virus Res. 2014;89:141–199. doi: 10.1016/B978-0-12-800172-1.00004-5. [DOI] [PubMed] [Google Scholar]
- 10.Brown JK, Czosnek H. Whitefly transmission of plant viruses. Adv Bot Res. 2002;36:65–100. [Google Scholar]
- 11.Blanc S, Uzest M, Drucker M. New research horizons in vector-transmission of plant viruses. Curr Opin Microbiol. 2011;14:483–491. doi: 10.1016/j.mib.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 12.DeBlasio SL, et al. Insights into the polerovirus-plant interactome revealed by coimmunoprecipitation and mass spectrometry. Mol Plant Microbe Interact. 2015;28:467–481. doi: 10.1094/MPMI-11-14-0363-R. [DOI] [PubMed] [Google Scholar]
- 13.Liu W, et al. Proteomic analysis of interaction between a plant virus and its vector insect reveals new functions of Hemipteran cuticular protein. Mol Cell Proteomics. 2015;14:2229–2242. doi: 10.1074/mcp.M114.046763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosen R, et al. Persistent, circulative transmission of begomoviruses by whitefly vectors. Curr Opin Virol. 2015;15:1–8. doi: 10.1016/j.coviro.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Gilbertson RL, Batuman O, Webster CG, Adkins S. Role of the insect supervectors Bemisia tabaci and Frankliniella occidentalis in the emergence and global spread of plant viruses. Annu Rev Virol. 2015;2:67–93. doi: 10.1146/annurev-virology-031413-085410. [DOI] [PubMed] [Google Scholar]
- 16.Boykin LM. Bemisia tabaci nomenclature: Lessons learned. Pest Manag Sci. 2014;70:1454–1459. doi: 10.1002/ps.3709. [DOI] [PubMed] [Google Scholar]
- 17.De Barro PJ, Liu SS, Boykin LM, Dinsdale AB. Bemisia tabaci: A statement of species status. Annu Rev Entomol. 2011;56:1–19. doi: 10.1146/annurev-ento-112408-085504. [DOI] [PubMed] [Google Scholar]
- 18.Navas-Castillo J, Fiallo-Olivé E, Sánchez-Campos S. Emerging virus diseases transmitted by whiteflies. Annu Rev Phytopathol. 2011;49:219–248. doi: 10.1146/annurev-phyto-072910-095235. [DOI] [PubMed] [Google Scholar]
- 19.Legg JP, et al. Biology and management of Bemisia whitefly vectors of cassava virus pandemics in Africa. Pest Manag Sci. 2014;70:1446–1453. doi: 10.1002/ps.3793. [DOI] [PubMed] [Google Scholar]
- 20.Czosnek H. In: Tomato Yellow Leaf Curl Virus Disease: Management, Molecular Biology, Breeding for Resistance. Czosnek H, editor. Springer; Dordrecht, The Netherlands: 2007. [Google Scholar]
- 21.Scholthof KBG, et al. Top 10 plant viruses in molecular plant pathology. Mol Plant Pathol. 2011;12:938–954. doi: 10.1111/j.1364-3703.2011.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Accotto GP, Sardo L. Transovarial transmission of begomoviruses in Bemisia tabaci. In: Stansly PA, Naranjo SE, editors. Bemisia: Bionomics and Management of a Global Pest. Springer; Dordrecht, The Netherlands: 2009. pp. 339–348. [Google Scholar]
- 23.Wang X, Xie Y, Zhou X. Molecular characterization of two distinct begomoviruses from Papaya in China. Virus Genes. 2004;29:303–309. doi: 10.1007/s11262-004-7432-1. [DOI] [PubMed] [Google Scholar]
- 24.Guo T, et al. Comparison of transmission of Papaya leaf curl China virus among four cryptic species of the whitefly Bemisia tabaci complex. Sci Rep. 2015;5:15432. doi: 10.1038/srep15432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo JY, Ye GY, Dong SZ, Liu SS. An invasive whitefly feeding on a virus-infected plant increased its egg production and realized fecundity. PLoS One. 2010;5:e11713. doi: 10.1371/journal.pone.0011713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raikhel AS, Dhadialla TS. Accumulation of yolk proteins in insect oocytes. Annu Rev Entomol. 1992;37:217–251. doi: 10.1146/annurev.en.37.010192.001245. [DOI] [PubMed] [Google Scholar]
- 27.Sappington TW, Raikhel AS. Molecular characteristics of insect vitellogenins and vitellogenin receptors. Insect Biochem Mol Biol. 1998;28:277–300. doi: 10.1016/s0965-1748(97)00110-0. [DOI] [PubMed] [Google Scholar]
- 28.Snigirevskaya ES, Raikhel AS. Receptor-mediated endocytosis of yolk proteins in insect oocytes. In: Raikhel AS, Sappington TW, editors. Reproductive Biology of Invertebrates: Progress in Vitellogenesis. Science Publishers, Inc.; Enfield, NH: 2005. pp. 199–228. [Google Scholar]
- 29.Tufail M, Takeda M. Molecular characteristics of insect vitellogenins. J Insect Physiol. 2008;54:1447–1458. doi: 10.1016/j.jinsphys.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Guo JY, Wan FH, Ye GY. Oogenesis in the Bemisia tabaci MEAM1 species complex. Micron. 2016;83:1–10. doi: 10.1016/j.micron.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Huo Y, et al. Transovarial transmission of a plant virus is mediated by vitellogenin of its insect vector. PLoS Pathog. 2014;10:e1003949. doi: 10.1371/journal.ppat.1003949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ammar D, Tsai CW, Whitfield AE, Redinbaugh MG, Hogenhout SA. Cellular and molecular aspects of rhabdovirus interactions with insect and plant hosts. Annu Rev Entomol. 2009;54:447–468. doi: 10.1146/annurev.ento.54.110807.090454. [DOI] [PubMed] [Google Scholar]
- 33.Falk BW, Tsai JH. Biology and molecular biology of viruses in the genus Tenuivirus. Annu Rev Phytopathol. 1998;36:139–163. doi: 10.1146/annurev.phyto.36.1.139. [DOI] [PubMed] [Google Scholar]
- 34.Nault LR, Ammar ED. Leafhopper and planthopper transmission of plant viruses. Annu Rev Entomol. 1989;34:503–529. [Google Scholar]
- 35.Cohen S, Nitzany F. Transmission and host range of the Tomato yellow leaf curl virus. Phytopathology. 1966;56:1127–1131. [Google Scholar]
- 36.Ioannou N. Yellow leaf curl and other virus diseases of tomato in Cyprus. Plant Pathol. 1985;34:428–434. [Google Scholar]
- 37.Dubern J. Transmission of African cassava mosaic geminivirus by the whitefly (Bemisia tabaci) Trop Sci. 1994;34:82–91. [Google Scholar]
- 38.Bosco D, Mason G, Accotto GP. TYLCSV DNA, but not infectivity, can be transovarially inherited by the progeny of the whitefly vector Bemisia tabaci (Gennadius) Virology. 2004;323:276–283. doi: 10.1016/j.virol.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 39.Becker N, et al. Rapid accumulation and low degradation: Key parameters of Tomato yellow leaf curl virus persistence in its insect vector Bemisia tabaci. Sci Rep. 2015;5:17696. doi: 10.1038/srep17696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J, et al. Low frequency of horizontal and vertical transmission of two begomoviruses through whiteflies exhibits little relevance to the vector infectivity. Ann Appl Biol. 2010;157:125–133. [Google Scholar]
- 41.Pan H, et al. Rapid spread of tomato yellow leaf curl virus in China is aided differentially by two invasive whiteflies. PLoS One. 2012;7:e34817. doi: 10.1371/journal.pone.0034817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghanim M, Morin S, Zeidan M, Czosnek H. Evidence for transovarial transmission of tomato yellow leaf curl virus by its vector, the whitefly Bemisia tabaci. Virology. 1998;240:295–303. doi: 10.1006/viro.1997.8937. [DOI] [PubMed] [Google Scholar]
- 43.Schwabl H. Yolk is a source of maternal testosterone for developing birds. Proc Natl Acad Sci USA. 1993;90:11446–11450. doi: 10.1073/pnas.90.24.11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cho KH, Cheon HM, Kokoza V, Raikhel AS. Regulatory region of the vitellogenin receptor gene sufficient for high-level, germ line cell-specific ovarian expression in transgenic Aedes aegypti mosquitoes. Insect Biochem Mol Biol. 2006;36:273–281. doi: 10.1016/j.ibmb.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 45.Boldbaatar D, et al. Tick vitellogenin receptor reveals critical role in oocyte development and transovarial transmission of Babesia parasite. Biochem Cell Biol. 2008;86:331–344. doi: 10.1139/o08-071. [DOI] [PubMed] [Google Scholar]
- 46.Brasset E, et al. Viral particles of the endogenous retrovirus ZAM from Drosophila melanogaster use a pre-existing endosome/exosome pathway for transfer to the oocyte. Retrovirology. 2006;3:25. doi: 10.1186/1742-4690-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lefeuvre P, et al. The spread of tomato yellow leaf curl virus from the Middle East to the world. PLoS Pathog. 2010;6:e1001164. doi: 10.1371/journal.ppat.1001164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mabvakure B, et al. Ongoing geographical spread of tomato yellow leaf curl virus. Virology. 2016;498:257–264. doi: 10.1016/j.virol.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 49.Díaz-Pendón JA, et al. Tomato yellow leaf curl viruses: Ménage à trois between the virus complex, the plant and the whitefly vector. Mol Plant Pathol. 2010;11:441–450. doi: 10.1111/j.1364-3703.2010.00618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seal SE, vandenBosch F, Jeger MJ. Factors influencing begomovirus evolution and their increasing global significance: Implications for sustainable control. Crit Rev Plant Sci. 2006;25:23–46. [Google Scholar]
- 51.Wu JX, Shang HL, Xie Y, Shen QT, Zhou XP. Monoclonal antibodies against the whitefly-transmitted tomato yellow leaf curl virus and their application in virus detection. J Integr Agric. 2012;11:263–268. [Google Scholar]
- 52.Guo JY, et al. Enhanced vitellogenesis in a whitefly via feeding on a begomovirus-infected plant. PLoS One. 2012;7:e43567. doi: 10.1371/journal.pone.0043567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang LL, et al. The autophagy pathway participates in resistance to tomato yellow leaf curl virus infection in whiteflies. Autophagy. 2016;12:1560–1574. doi: 10.1080/15548627.2016.1192749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeBarro PJ, Driver F. Use of RAPD PCR to distinguish the B biotype from other biotypes of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) Aust J Entomol. 1997;36:149–152. [Google Scholar]
- 55.Wei J, et al. Specific cells in the primary salivary glands of the whitefly Bemisia tabaci control retention and transmission of begomoviruses. J Virol. 2014;88:13460–13468. doi: 10.1128/JVI.02179-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cui X, Tao X, Xie Y, Fauquet CM, Zhou X. A DNAβ associated with tomato yellow leaf curl China virus is required for symptom induction. J Virol. 2004;78:13966–13974. doi: 10.1128/JVI.78.24.13966-13974.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]