Significance
A most promising approach to boosting both efficiency and selectivity for electrochemical reduction of CO2 (CO2RR) is using Cu2O-based electrodes, and the surface Cu+ is believed to play an essential role that is totally unclear from both experiment and theory. We find that the surface Cu+ by itself actually deteriorates the performance of CO2RR. Instead we propose a Cu metal embedded in oxidized matrix (MEOM) model and show that it is synergy between surface Cu+ and surface Cu0 present in the MEOM model that improves significantly the kinetics and thermodynamics of both CO2 activation and CO dimerization, thereby boosting the efficiency and selectivity of CO2RR. The MEOM model serves as a unique platform for design of better electrocatalysts for CO2RR.
Keywords: electrochemical reduction of CO2, Cu metal embedded in oxidized matrix, density functional theory, CO2 activation, CO dimerization
Abstract
We propose and validate with quantum mechanics methods a unique catalyst for electrochemical reduction of CO2 (CO2RR) in which selectivity and activity of CO and C2 products are both enhanced at the borders of oxidized and metallic surface regions. This Cu metal embedded in oxidized matrix (MEOM) catalyst is consistent with observations that Cu2O-based electrodes improve performance. However, we show that a fully oxidized matrix (FOM) model would not explain the experimentally observed performance boost, and we show that the FOM is not stable under CO2 reduction conditions. This electrostatic tension between the Cu+ and Cu0 surface sites responsible for the MEOM mechanism suggests a unique strategy for designing more efficient and selective electrocatalysts for CO2RR to valuable chemicals (HCOx), a critical need for practical environmental and energy applications.
Electrochemical reduction of CO2 (CO2RR) to valuable chemicals is an essential strategy to achieve industrial-scale reduction of the carbon footprint under mild conditions and to provide a means of storing electrical power from intermittent renewable sources into stable chemical forms (1). Cu is the prototype electrocatalyst for CO2RR, because it is the only pure metal that delivers appreciable amounts of methane and ethylene plus minor alcohol products (2–7), but it suffers from high overpotentials and very significant hydrogen evolution reactions (HERs). Consequently, tremendous efforts are being made to develop more efficient and selective electrocatalysts, for example by surface modification (8) and by nanoparticle (9, 10) and nanowire (11) engineering.
We examine here the mechanism by which Cu2O-based electrodes are observed to improve both efficiency and selectivity for C2 products (12–15), which also suppresses HERs by severalfold. Because Cu2O is subject to reduction (back to Cu metal) under CO2RR conditions, the improved performance was initially attributed to Cu metal surface morphology (8, 16). But a more recent experiment (15) showed that Cu+ sites can survive on the Cu surface for the course of CO2RR. Importantly, a Cu sample that is first oxidized and then reduced using an H2 plasma leads to performance substantially worse than that of the oxidized sample, despite both having similarly roughened surfaces. This provides solid evidence that surface Cu+ plays an essential role in promoting the efficiency and selectivity of CO2RR. However, experiments have provided no clue about how surface Cu+ affects the mechanisms of CO2RR. Moreover, no previous theoretical efforts have elucidated its role.
To understand the promising results achieved with Cu2O-based electrodes, we investigated three distinct models aimed at unraveling the role of surface Cu+ in shaping the free energy profiles of two key steps for CO2RR. Here we carry out quantum mechanics (QM) calculations at constant potential by using our grand canonical methodology (17, 18) that uses the charge-asymmetric nonlocally determined local-electric (CANDLE) implicit solvation model (19) to achieve constant electrochemical potential (not constant number of electrons) within the framework of joint density functional theory (JDFT) (20, 21) (details in Computational Details). The three key steps we focus on are (i) CO2 activation, which we previously showed to be the rate-determining step (RDS) for CO production on pure Cu (22); (ii) CO dimerization, which we previously showed to be the RDS for forming C2 products from CO on pure Cu (17, 18); and (iii) C1 product formation, which we find to compete with C2 products for pure Cu.
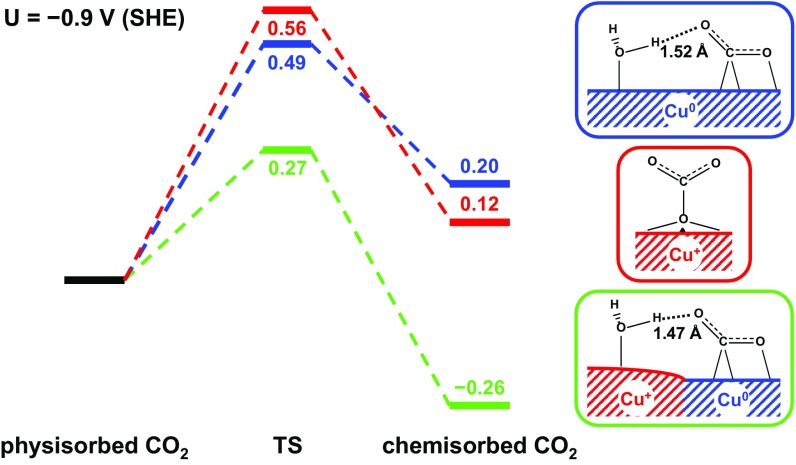
We find that the surface Cu+ by itself actually deteriorates the performance of CO2RR. Instead we show that it is synergy between surface Cu+ and surface Cu0 that improves significantly the kinetics and thermodynamics of both CO2 activation and CO dimerization, while making C1 unfavorable, thereby boosting the efficiency and selectivity of CO2RR. These results provide a unique concept for designing improved electrocatalysts. To illustrate this synergy we consider the case with an applied potential U = 0.9 V [referenced to standard hydrogen electrode (SHE)], which is where CO production reaches the peak and C2 production begins on the oxide electrode (15). The free energies at any other U can be calculated using Table S1.
Table S1.
Free energy barriers (G≠) and reaction free energies (G) for all key reactions
| Reaction | G≠, eV | G, eV |
| CO2(phys) = CO2(chem) on M | 0.5914 + 0.1117 U | 0.5021 + 0.3356 U |
| CO2(phys) = CO2(chem) on FOM | 0.6140 + 0.0593 U | 0.2508 + 0.1488 U |
| CO2(phys) = CO2(chem) on MEOM | 0.3636 + 0.1077 U | 0.0407 + 0.3392 U |
| 2CO = OCCO on M | 1.1664 + 0.0764 U | 1.0812 + 0.2407 U |
| 2CO = OCCO on FOM | 3.2747 + 0.1383 U | 2.8487 + 0.6696 U |
| 2CO = OCCO on MEOM | 0.8602 + 0.1688 U | 0.4670 + 0.3828 U |
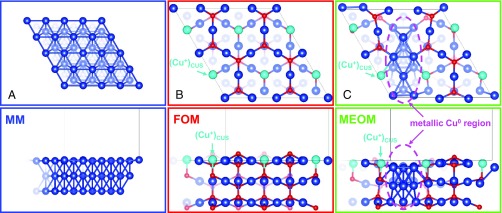
Fig. 1 shows the three surface models we used to probe the role of surface Cu+ in CO2RR. Fig. 1A shows the metallic matrix (MM), where the pristine Cu(111) surface serves as a reference model for pure MM that has only Cu0 on the surface Fig. 1B shows the fully oxidized matrix (FOM), where the stoichiometric nonpolar Cu2O(111) surface serves as a model for a FOM with only Cu+ on the surface. Here we find two types of Cu+ surface sites: (Cu+)CSS, a coordinatively saturated Cu+ site that is bonded to two O atoms, and (Cu+)CUS, a coordinatively unsaturated site that is bonded to only the one O atom directly below it (examples are marked in Fig. 1B). The (Cu+)CUS is believed to be the active site (ref. 23 and references therein). However, it has been suggested by both theory (24) and experiment (25) that such (Cu+)CUS sites are likely missing under oxygen-rich conditions (e.g., oxygen plasma treatment). More recent theoretical work (26) has shown that (Cu+)CUS sites are favored under CO2RR conditions at neutral pH. Here we focus on the role of active surface Cu+ in shaping the energetics and mechanisms of CO2RR, rather than stability. Fig. 1C shows metal embedded in oxidized matrix (MEOM), a partially reduced Cu2O(111) surface in which one-quarter of the surface is reduced. This serves as a conceptual model for our MEOM in which both Cu0 and Cu+ are present on the surface. We find that this leads to active (Cu+)CUS sites at the edge of metallic Cu0 regions that play an essential role in the enhanced activity. Our MEOM catalyst site mimics the case for CO2RR operation, where the majority of surface stays oxidized but some reduced regions are created.
Fig. 1.
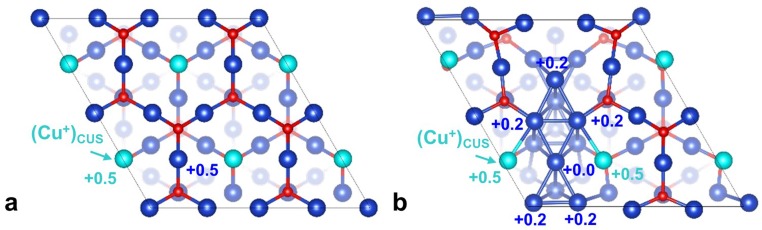
Top and side views of the three surface models. (A) A 4 4 Cu(111) surface, the model for a metallic matrix (MM). (B) A 2 2 Cu2O(111) surface, the model for fully oxidized matrix (FOM). (C) Metal embedded in oxidized matrix (MEOM) derived by reducing one-quarter of a 2 2 Cu2O(111) surface. Here Cu is dark blue, with the active (Cu+)CUS marked light blue, and red is O. Purple dashes mark the border between Cu0 and Cu+ regions. (Please refer to Fig. S7 for Bader charge analysis of surface Cu sites on FOM and MEOM models.)
Fig. S7.
Bader charges for representative sites on (A) FOM and (B) MEOM surfaces.
We focus here on the (111) surface orientation, because it is the most stable among Cu2O surfaces (27) and has the fastest kinetics for Cu surface oxidation (28) (thus the most likely oxide surface orientation from oxidation of Cu foil). In the experiment that directly compared the metal with oxide surfaces (15), the measured onset potentials for C2H4 production on Cu metal surfaces are 1.2 V to 1.1 V (this is the value for both electropolished and roughened surfaces obtained by first oxidizing and then reducing the Cu foil with hydrogen plasma), which are the same as the onset potentials measured on a Cu(111) single crystal electrode (1.2 V to 1.1 V), but very different from the value of 0.8 V to 0.6 V measured on Cu(100) (29, 30).
CO2 Activation
MM Model.
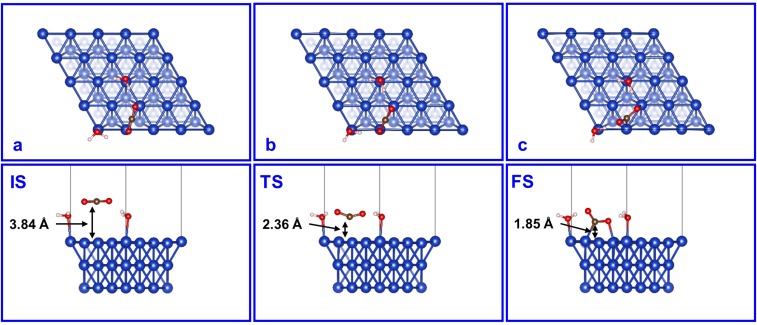
We find that physisorption of CO2 (CO2,phys) on the MM model leads to a noncovalent bond distance of 3.84 Å between the C atom of linear CO2 and the Cu surface (C-Cus; Fig. S1A), which is similar to our previous study (22). Forming chemisorbed bent CO2 (CO2,chem) from this CO2,phys involves a transition state (TS) that bends CO2 with C-Cus = 2.36 Å, and the resulting CO2,chem is asymmetrically adsorbed, with a surface CuO = 2.04 Å, whereas the second O atom pointing away from the surface to form a hydrogen bond (1.52 Å) to a surface H2O bonded to a nearby Cu0 (Fig. S1C and Fig. 2). On the MM model the activation free energy barrier is G≠ = 0.49 eV at 298 K, similar to the value (0.43 eV) for Cu(100) in our previous study (22).
Fig. S1.
Top and side views of (A) IS, (B) TS, and (C) FS for the CO2 activation on Cu MM. The marked distances are between the C atom of CO2 and the Cu surface. In the FS (chemisorbed CO2), Cu-C and Cu-O bond lengths are 2.05 Å and 2.04 Å, respectively.
Fig. 2.
Free energy profiles (at U = 0.9 V) for CO2 activation on the MM (blue), FOM (red), and MEOM (green) models, including the resulting chemisorbed CO2 structures. Note that FOM leads to a surface carbonate product.
FOM Model.
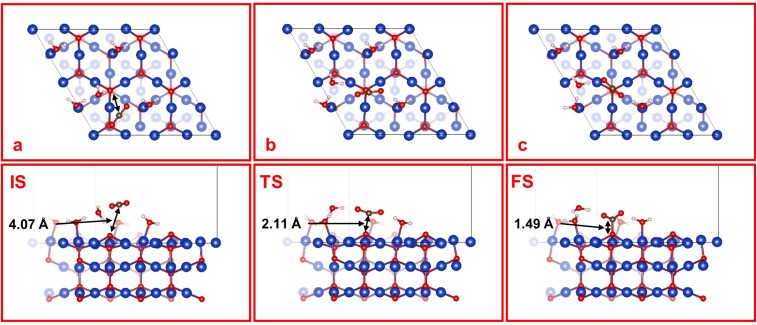
It was proposed (23) that in the FOM model CO2 can adsorb at the (Cu+)CUS site with a 2.09-Å bond of one O to (Cu+)CUS. However, exposed to the electrolyte, the (Cu+)CUS sites are mostly occupied by H2O molecules [strong electronic binding energy of E = 0.98 eV, much larger than that for CO2 (E = 0.31 eV)]. Thus, the initial structure for CO2 activation on the FOM model is still physisorption of CO2 with a 4.07-Å distance between the C atom of linear CO2 and Os, the closest surface O atom (Fig. S2A). We find a G≠ = 0.56 eV to convert this CO2,phys to a surface carbonate (Fig. 2), which can subsequently be released into the electrolyte, thereby reducing the FOM surface. Therefore, CO2 activation in the FOM model has a barrier 0.07 eV higher than the MM model and involves a different mechanism that does not lead to the key intermediate (chemisorbed CO2) for CO production. This indicates that the experimentally observed promotion of CO production using oxidized electrodes (12, 13, 15) cannot be explained with the presence only of surface Cu+.
Fig. S2.
Top and side views of (A) IS, (B) TS, and (C) FS for the CO2 activation on FOM. The marked distances are between the C atom of CO2 and the surface O atom that is bonded to CO2 in the FS. Note that a surface carbonate is formed in the FS.
MEOM Catalyst.
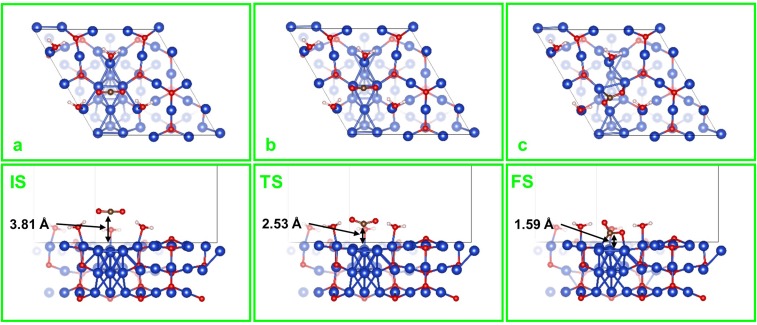
In contrast, the MEOM surface has a metallic Cu0 region bordered by the Cu+ oxide matrix. Here physisorbed CO2 is favored on top of the Cu0 region (Fig. S3A), and the activation of CO2 proceeds through a TS that bends CO2 just as in the MM case (Fig. S3B), leading to the asymmetrically chemisorbed CO2 on the Cu0 region. But now the free energy barrier is G≠ = 0.27 eV, which is 0.22 eV lower than for the MM model. Moreover, the chemisorbed CO2 is G = 0.26 eV more stable than physisorbed CO2 on the MEOM catalyst (Fig. 2).
Fig. S3.
Top and side views of (A) IS, (B) TS, and (C) FS for the CO2 activation on MEOM. The marked distances are between the C atom of CO2 and the Cu metallic region surface. In the FS (chemisorbed CO2), Cu-C and Cu-O bond lengths are 2.02 Å and 2.04 Å, respectively.
This drastic improvement in both kinetics and thermodynamics for the MEOM catalyst is due to the presence of (Cu+)CUS sites that bind H2O molecules at the edge of the Cu0 region. This H2O molecule on the (Cu+)CUS site forms strong hydrogen bonds to the CO2, stabilizing both the TS and the final state (FS) (Fig. 2). This opens a channel in which the negative charge accumulated on the O atom of the CO2 during activation is distributed to the Cu+ region, thus stabilizing both the TS and the FS.
Summarizing, only this MEOM catalyst with both surface Cu0 regions (binds to activated CO2) and Cu+ (dilutes negative charge) sites has the ability to enable promotion of CO2 activation, with favorable kinetics and thermodynamics. This explains the experimental observation that both the onset potential and the peak Faradaic efficiency for CO production are improved for CO2RR on oxide-based electrodes (12, 13, 15). We propose that our MEOM catalyst might also provide the mechanism by which partially oxidized atomic cobalt layers improve formate production (31).
CO Dimerization
MM Model.
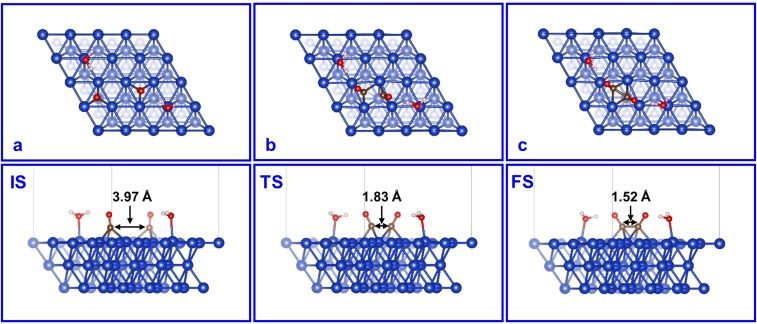
CO dimerization on the MM model has been thoroughly studied by us and others (17, 32). The initial structure of two well-separated adsorbed CO molecules (Fig. S4A) goes through a TS that tilts and draws the two COs close (Fig. S4B), with G≠ = 1.10 eV, to form an OCCO surface species with a 1.52-Å C-C bond (Fig. S4C).
Fig. S4.
Top and side views of (A) IS, (B) TS, and (C) FS for the CO dimerization on Cu MM. The marked distances are between the two C atoms of CO.
FOM Model.
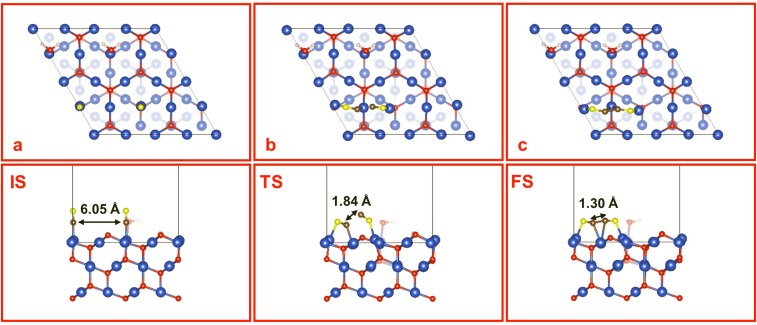
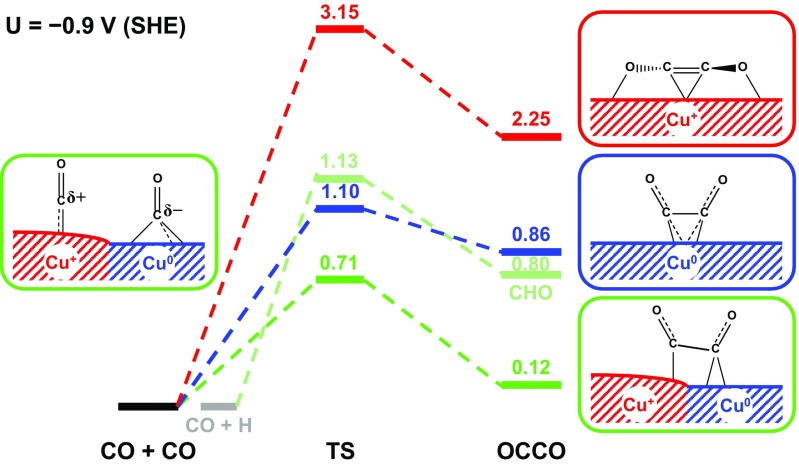
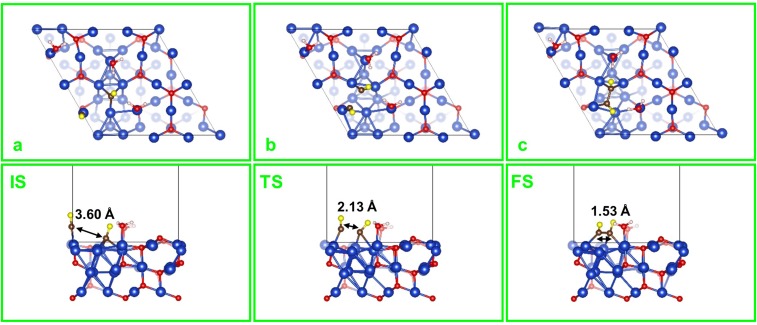
In contrast, CO dimerization in the FOM model takes a distinctly different path. A CO molecule introduced near the FOM surface (either direct or from CO2RR) binds to the (Cu+)CUS site by E = 1.62 eV, displacing the H2O (E = 0.98 eV). Thus, CO dimerization starts with two strongly adsorbed CO molecules on neighboring (Cu+)CUS sites (Fig. S5A), which proceeds through an asymmetric TS (Fig. S5B) that rotates both CO molecules to have O atoms bonded to the (Cu+)CUS sites (initially C atoms were bonded) with the C atom of one CO molecule bonded to the (Cu+)CSS site in between. The resulting OCCO surface species (Fig. S5C and Fig. 3) is formed with a C=C double bond (1.30 Å) with each C atom bonded to the middle (Cu+)CSS. This is the only stable CO dimer on the FOM surface, but the formation barrier is G≠ = 3.15 eV, and the product has a free energy unstable by G = 2.25 eV. Thus, the presence of only Cu+ at the active surface cannot explain the experimental observation that C2 products are promoted with oxidation-treated electrodes (13–15).
Fig. S5.
Top and side views of (A) IS, (B) TS, and (C) FS for the CO dimerization on FOM. The marked distances are between the two C atoms of CO. Note that the O atoms of CO are colored yellow for clarity.
Fig. 3.
Free energy profiles (at U = 0.9 V) of CO dimerization in the MM (blue), FOM (red), and MEOM (green) models and for CO hydrogenation to form surface CHO species in the MEOM model (gray green) at pH 7. Right shows resulting surface OCCO structures, whereas Left shows the initial structure on the MEOM model, which shows that the C atoms of the two COs on the Cu+ and Cu0 regions are positively and negatively charged, respectively, which assists the C-C coupling.
MEOM Catalyst.
On the MEOM surface, CO also adsorbs on the (Cu+)CUS site (CO@Cu+), more stable by 0.48 eV than on the metallic Cu0 region (CO@Cu0).With MEOM, CO dimerization from two neighboring CO@Cu+ is the same in nature as that on the FOM surface, so it would lead to the same G≠ (3.15 eV) as for the FOM. However, CO dimerization starting with CO@Cu0 and a neighboring CO@Cu+ (Fig. S6A) has a modest barrier of G≠ = 0.71 eV to form the OCCO surface species, in which the two C atoms are still bonded to the Cu+ and Cu0 regions (Fig. S6C), leading to G = 0.12 eV (Fig. 3). The favorable energetics of this C-C coupling can be understood by noting that the C atom of CO@Cu+ is positively charged (Mulliken charge of +0.11) whereas the C atom of CO@Cu0 is negatively charged (Mulliken charge of 0.31) due to back donation. Thus, the attractive electrostatics between the two Cs assists C-C bond formation. It is this favorable dimerization process on the MEOM model that improves both kinetics and thermodynamics of the RDS for C2 products, compared with the traditional MM model (Fig. 3). Thus, we propose that promotion of C2 products for oxidation-treated electrodes arises from the MEOM surface via the mechanism described above (13–15).
Fig. S6.
Top and side views of (A) IS, (B) TS, and (C) FS for the CO dimerization on MEOM. The marked distances are between the two C atoms of CO. Note that the O atoms of CO are colored yellow for clarity.
The major C2 products from CO2RR have been reported to be either ethylene (13, 15) or ethanol (14), where the major difference in these experiments is the pH (neutral pH for ethylene and basic pH for ethanol). We have shown recently (18) that the energetics of surface water determine the selectivity of alcohol vs. hydrocarbon products. We found that at neutral pH it is favorable for surface water to donate a proton for dehydroxylation to form hydrocarbon products. Whereas in basic pH the ability of surface water to dehydroxylate the surface species is suppressed (because the product OH is less favorable), favoring instead the alcohol product (ethanol).
C1 Pathways
Next we consider the possible pathways for forming C1 with the MEOM surface. Here we expect CO@Cu+ and H@Cu0. Interestingly, the COH pathway previously proposed by us (17, 33) is an unreasonable option, because COH@Cu+ is higher than CHO@Cu+ by G = 1.86 eV. This also eliminates the CO-COH pathway for C2 products we previously proposed (17). On the other hand, the CHO pathway has a reasonable free energy barrier of G≠ = 1.13 eV (Fig. 3) at neutral pH, which is still significantly higher than the G≠ = 0.71 eV for C-C coupling with CO@Cu+ and CO@Cu0. Consequently the stability of CO@Cu+ (which is more resistant to hydrogenation) blocks the C1 products. This selectivity for C2 over C1 is intrinsic and not due to the external local high-pH effect as speculated previously (13, 15).
Notes on the MEOM Catalyst
The MEOM concept is in fact a synergistic metal and oxidized matrix cocatalyst, with both ingredients directly participating in catalysis. Thus, for the MEOM model to be effective, it is necessary for the metal surface to be level with the oxidized matrix surface so that the surface species can interact via proper geometries. Therefore, a suitable scheme to generate the MEOM catalyst is deriving the metal directly from the oxidized matrix surface as in our construction of the MEOM model, which is also consistent with the current experimental strategy. This scheme can be naturally extended to starting with a mixed oxidized matrix (e.g., Cu2O/Ag2S) for alternative oxidized matrices.
Summary
We present the MEOM model for a partially oxidized Cu surface and show that this model leads to plausible mechanisms to explain the experimental findings that CO2RR can be made more efficient and selective, using oxidized electrodes. However, MEOM requires that we only partially oxidize the surface. This MEOM model presents a unique guideline for design of improved CO2RR electrocatalysts. In contrast to previous speculations, we find that the active surface Cu+ sites alone do not improve the efficiencies of CO2RR and indeed deteriorate the efficiency. Instead the synergy between active surface Cu+ and Cu0 regions present in the MEOM model is responsible for improving significantly the kinetics and thermodynamics of both CO2 activation and CO dimerization while impeding C1 pathways, the key steps for efficiency and selectivity of CO2RR.
Based on our MEOM model, we conclude that the oxidized matrix (Cu2O) is unstable under CO2RR working conditions. We find that the role of the Cu2O is mainly electrostatic in diluting the negative charge built up on the CO2 as it transitions from physisorbed to chemisorbed structures, which in turn makes the C atom of CO positively charged. This MEOM model suggests alternative oxidized matrices (like Ag2S) could also deliver similar electrostatic contributions, leading to much improved electrochemical stabilities.
Computational Details.
The calculations were first performed with the VASP package (34–36), using the Perdew–Burke–Ernzerhof (PBE) flavor (37) of DFT and the projector augmented wave (PAW) method (38) to account for core–valence interactions. The kinetic energy cutoff for plane-wave expansions was set to 400 eV, and reciprocal space was sampled by a -centered Monkhorst–Pack scheme with a grid of . The Cu(111) and Cu2O(111) surface slabs were constructed with three layers (bottom layer fixed), using the experimental lattice parameters of 3.615 Å and 4.2745 Å, respectively, with vacuum layers of at least 15 Å.
The convergence criteria are eV and eV energy differences for solving for the electronic wavefunction for local minima [initial states (ISs) and FSs] and TSs, respectively. The Methfessel–Paxton smearing of second order with a width of 0.1 eV was applied. All IS, TS, and FS geometries (atomic coordinates) are converged to within eV/Å for maximal components of forces. The TS search was conducted by using the climbing-image nudged elastic band (CI-NEB) method (39) to generate initial guess geometries, followed by the dimer method (40) to converge to the saddle points.
Zero-point energy (ZPE), enthalpy, and entropy contributions to free energies at room temperature (298.15 K) were calculated from vibrational modes of surface species, which were computed with the finite difference approach. Note that very low-frequency modes were obtained in some cases, because the explicit water molecules are not properly constrained by the hydrogen-bonding network present in water bulk. Such low-frequency modes can cause unphysically large entropy contributions, so they were reset to a threshold value of 60 cm−1, corresponding to the acoustic translational mode of the six-member rings in water bulk (41, 42).
For the CO hydrogenation step where the surface H model was used to locate the TS, the IS was referenced back to the H+(H3O+/H2O) + e− pair through the free energy difference between the surface H and H2(g), based on the half-cell reactions,
Thus, the pH effect is introduced into the free energy profile with the reference. In addition to vibrational contributions, the translational and rotational contributions to the free energy of H2(g) were included, assuming the ideal gas model.
The explicit constant electrochemical potential () calculations with the implicit CANDLE solvation model (19) were performed upon all IS, TS, and FS geometries, using JDFTx (43). The Garrity–Bennett–Rabe–Vanderbilt (GBRV) (44) ultrasoft pseudopotentials (USPP) were used, with a plane-wave cutoff of 544 eV (20 a.u.). All other settings are similar to those in VASP calculations. The ionic screening of net charges resulting from the constant condition was achieved with cation (0.1 M K+) and anion (0.1 M F−) components in the fluid model (21) under the JDFT framework (20). The algorithm used by JDFTx variationally minimizes the grand free energy at fixed electron chemical potential with respect to Kohn–Sham orbitals (45), fluid bound charge, and an auxiliary Hamiltonian for the occupations (46). Previously we found that the relative free energies (barriers G≠ and reaction energies G) are linearly dependent on the applied potential U for U < ∼2 V [vs. standard hydrogen electrode (SHE)] (17), so the U dependence of all G≠ and G was calculated assuming a linear relationship between U = 0.0 V and 1.2 V. Note that here all Us are referenced to SHE.
Acknowledgments
This research was supported by the Joint Center for Artificial Photosynthesis, a Department of Energy (DOE) Energy Innovation Hub, supported through the Office of Science of the US DOE under Award DE-SC0004993. This work used the computational resources of Zwicky (at California Institute of Technology).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1702405114/-/DCSupplemental.
References
- 1.Zhu DD, Liu JL, Qiao SZ. Recent advances in inorganic heterogeneous electrocatalysts for reduction of carbon dioxide. Adv Mater. 2016;28:3423–3452. doi: 10.1002/adma.201504766. [DOI] [PubMed] [Google Scholar]
- 2.Hori Y, Kikuchi K, Suzuki S. Production of CO and CH4 in electrochemical reduction of CO2 at metal electrodes in aqueous hydrogencarbonate solution. Chem Lett. 1985;14:1695–1698. [Google Scholar]
- 3.Hori Y, Wakebe H, Tsukamoto T, Koga O. Electrocatalytic process of CO selectivity in electrochemical reduction of CO2 at metal electrodes in aqueous media. Electrochim Acta. 1994;39:1833–1839. [Google Scholar]
- 4.Hori Y. Electrochemical CO2 reduction on metal electrodes. In: Vayenas C, White R, Gamboa-Aldeco M, editors. Modern Aspects of Electrochemistry. Vol 42. Springer; New York: 2008. pp. 89–189. [Google Scholar]
- 5.Gattrell M, Gupta N, Co A. A review of the aqueous electrochemical reduction of CO2 to hydrocarbons at copper. J Electroanal Chem. 2006;594:1–19. [Google Scholar]
- 6.Kuhl KP, Cave ER, Abram DN, Jaramillo TF. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ Sci. 2012;5:7050–7059. [Google Scholar]
- 7.Kuhl KP, et al. Electrocatalytic conversion of carbon dioxide to methane and methanol on transition metal surfaces. J Am Chem Soc. 2014;136:14107–14113. doi: 10.1021/ja505791r. [DOI] [PubMed] [Google Scholar]
- 8.Kim YG, Javier A, Baricuatro JH, Soriaga MP. Regulating the product distribution of CO reduction by the atomic-level structural modification of the cu electrode surface. Electrocatalysis. 2016;7:391–399. [Google Scholar]
- 9.Roberts FS, Kuhl KP, Nilsson A. High selectivity for ethylene from carbon dioxide reduction over copper nanocube electrocatalysts. Angew Chem Int Ed. 2015;54:5179–5182. doi: 10.1002/anie.201412214. [DOI] [PubMed] [Google Scholar]
- 10.Loiudice A, et al. Tailoring copper nanocrystals towards C2 products in electrochemical CO2 reduction. Angew Chem Int Ed. 2016;55:5789–5792. doi: 10.1002/anie.201601582. [DOI] [PubMed] [Google Scholar]
- 11.Ma M, Djanashvili K, Smith WA. Controllable hydrocarbon formation from the electrochemical reduction of CO2 over cu nanowire arrays. Angew Chem Int Ed. 2016;55:6680–6684. doi: 10.1002/anie.201601282. [DOI] [PubMed] [Google Scholar]
- 12.Li CW, Kanan MW. CO2 reduction at low overpotential on cu electrodes resulting from the reduction of thick Cu2O films. J Am Chem Soc. 2012;134:7231–7234. doi: 10.1021/ja3010978. [DOI] [PubMed] [Google Scholar]
- 13.Kas R, et al. Electrochemical CO2 reduction on Cu2O-derived copper nanoparticles: Controlling the catalytic selectivity of hydrocarbons. Phys Chem Chem Phys. 2014;16:12194–12201. doi: 10.1039/c4cp01520g. [DOI] [PubMed] [Google Scholar]
- 14.Li CW, Ciston J, Kanan MW. Electroreduction of carbon monoxide to liquid fuel on oxide-derived nanocrystalline copper. Nature. 2014;508:504–507. doi: 10.1038/nature13249. [DOI] [PubMed] [Google Scholar]
- 15.Mistry H, et al. Highly selective plasma-activated copper catalysts for carbon dioxide reduction to ethylene. Nat Commun. 2016;7:12123. doi: 10.1038/ncomms12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dutta A, Rahaman M, Luedi NC, Mohos M, Broekmann P. Morphology matters: Tuning the product distribution of CO2 electroreduction on oxide-derived cu foam catalysts. ACS Catal. 2016;6:3804–3814. [Google Scholar]
- 17.Xiao H, Cheng T, Goddard WA, Sundararaman R. Mechanistic explanation of the pH dependence and onset potentials for hydrocarbon products from electrochemical reduction of CO on Cu(111) J Am Chem Soc. 2016;138:483–486. doi: 10.1021/jacs.5b11390. [DOI] [PubMed] [Google Scholar]
- 18.Xiao H, Cheng T, Goddard WA. Atomistic mechanisms underlying selectivities in C1 and C2 products from electrochemical reduction of CO on Cu(111) J Am Chem Soc. 2017;139:130–136. doi: 10.1021/jacs.6b06846. [DOI] [PubMed] [Google Scholar]
- 19.Sundararaman R, Goddard WA. The charge-asymmetric nonlocally determined local-electric (CANDLE) solvation model. J Chem Phys. 2015;142:064107. doi: 10.1063/1.4907731. [DOI] [PubMed] [Google Scholar]
- 20.Petrosyan SA, Rigos AA, Arias TA. Joint density-functional theory: Ab initio study of Cr2O3 surface chemistry in solution. J Phys Chem B. 2005;109:15436–15444. doi: 10.1021/jp044822k. [DOI] [PubMed] [Google Scholar]
- 21.Letchworth-Weaver K, Arias TA. Joint density functional theory of the electrode-electrolyte interface: Application to fixed electrode potentials, interfacial capacitances, and potentials of zero charge. Phys Rev B. 2012;86:075140. [Google Scholar]
- 22.Cheng T, Xiao H, Goddard WA. Reaction mechanisms for the electrochemical reduction of CO2 to CO and formate on the Cu(100) surface at 298 K from quantum mechanics free energy calculations with explicit water. J Am Chem Soc. 2016;138:13802–13805. doi: 10.1021/jacs.6b08534. [DOI] [PubMed] [Google Scholar]
- 23.Bendavid LI, Carter EA. CO2 adsorption on Cu2O(111): A DFT+U and DFT-D study. J Phys Chem C. 2013;117:26048–26059. [Google Scholar]
- 24.Soon A, Todorova M, Delley B, Stampfl C. Thermodynamic stability and structure of copper oxide surfaces: A first-principles investigation. Phys Rev B. 2007;75:125420. [Google Scholar]
- 25.Önsten A, Göthelid M, Karlsson UO. Atomic structure of Cu2O(111) Surf Sci. 2009;603:257–264. [Google Scholar]
- 26.Nie X, Griffin GL, Janik MJ, Asthagiri A. Surface phases of Cu2O(111) under CO2 electrochemical reduction conditions. Catal Commun. 2014;52:88–91. [Google Scholar]
- 27.Bendavid LI, Carter EA. First-principles predictions of the structure, stability, and photocatalytic potential of Cu2O surfaces. J Phys Chem B. 2013;117:15750–15760. doi: 10.1021/jp406454c. [DOI] [PubMed] [Google Scholar]
- 28.Lian X, Xiao P, Yang SC, Liu R, Henkelman G. Calculations of oxide formation on low-index Cu surfaces. J Chem Phys. 2016;145:044711. doi: 10.1063/1.4959903. [DOI] [PubMed] [Google Scholar]
- 29.Schouten KJP, Qin Z, Gallent EP, Koper MTM. Two pathways for the formation of ethylene in CO reduction on single-crystal copper electrodes. J Am Chem Soc. 2012;134:9864–9867. doi: 10.1021/ja302668n. [DOI] [PubMed] [Google Scholar]
- 30.Schouten KJP, Pérez Gallent E, Koper MTM. The influence of pH on the reduction of CO and to hydrocarbons on copper electrodes. J Electroanal Chem. 2014;716:53–57. [Google Scholar]
- 31.Gao S, et al. Partially oxidized atomic cobalt layers for carbon dioxide electroreduction to liquid fuel. Nature. 2016;529:68–71. doi: 10.1038/nature16455. [DOI] [PubMed] [Google Scholar]
- 32.Montoya JH, Shi C, Chan K, Nørskov JK. Theoretical insights into a CO dimerization mechanism in CO2 electroreduction. J Phys Chem Lett. 2015;6:2032–2037. doi: 10.1021/acs.jpclett.5b00722. [DOI] [PubMed] [Google Scholar]
- 33.Nie X, Esopi MR, Janik MJ, Asthagiri A. Selectivity of CO2 reduction on copper electrodes: The role of the kinetics of elementary steps. Angew Chem Int Ed. 2013;52:2459–2462. doi: 10.1002/anie.201208320. [DOI] [PubMed] [Google Scholar]
- 34.Kresse G, Hafner J. Ab initio molecular dynamics for liquid metals. Phys Rev B. 1993;47:558–561. doi: 10.1103/physrevb.47.558. [DOI] [PubMed] [Google Scholar]
- 35.Kresse G, Furthmuller J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput Mater Sci. 1996;6:15–50. doi: 10.1103/physrevb.54.11169. [DOI] [PubMed] [Google Scholar]
- 36.Kresse G, Furthmuller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B. 1996;54:11169–11186. doi: 10.1103/physrevb.54.11169. [DOI] [PubMed] [Google Scholar]
- 37.Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys Rev Lett. 1996;77:3865–3868. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 38.Kresse G, Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev B. 1999;59:1758–1775. [Google Scholar]
- 39.Henkelman G, Uberuaga BP, Jónsson H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J Chem Phys. 2000;113:9901–9904. [Google Scholar]
- 40.Henkelman G, Jónsson H. A dimer method for finding saddle points on high dimensional potential surfaces using only first derivatives. J Chem Phys. 1999;111:7010–7022. [Google Scholar]
- 41.Bertie JE, Whalley E. Optical spectra of orientationally disordered crystals. II. Infrared spectrum of ice Ih and ice Ic from 360 to 50 cm−1. J Chem Phys. 1967;46:1271–1284. [Google Scholar]
- 42.Liu H, Wang Y, Bowman JM. Vibrational analysis of an ice Ih model from 0 to 4000 cm−1 using the ab initio WHBB potential energy surface. J Phys Chem B. 2013;117:10046–10052. doi: 10.1021/jp405865c. [DOI] [PubMed] [Google Scholar]
- 43.Sundararaman R, Gunceler D, Letchworth-Weaver K, Schwarz KA, Arias TA. 2012. JDFTx. Available at jdftx.org/
- 44.Garrity KF, Bennett JW, Rabe KM, Vanderbilt D. Pseudopotentials for high-throughput DFT calculations. Comput Mater Sci. 2014;81:446–452. [Google Scholar]
- 45.Arias TA, Payne MC, Joannopoulos JD. Ab initio molecular dynamics: Analytically continued energy functionals and insights into iterative solutions. Phys Rev Lett. 1992;69:1077–1080. doi: 10.1103/PhysRevLett.69.1077. [DOI] [PubMed] [Google Scholar]
- 46.Freysoldt C, Boeck S, Neugebauer J. Direct minimization technique for metals in density functional theory. Phys Rev B. 2009;79:241103. [Google Scholar]