Significance
Mucus propelled by cilia is key for removing particulates from lungs by mucociliary transport. The major structural components of airway mucus are two gel-forming mucins, MUC5B and MUC5AC. These mucins exhibit distinct morphologic structures. MUC5B is secreted by submucosal glands in the form of strands. MUC5AC is secreted by goblet cells as threads and thin sheets. After emerging onto the airway surface, the two mucins associate to form MUC5B strands partially covered with MUC5AC. These distinct morphologic structures likely enable efficient mucociliary transport. In cystic fibrosis, strands become entangled, MUC5B often fills submucosal gland ducts, and MUC5AC sheets are larger, are more abundant, and overlie strands. Disrupted anion secretion in cystic fibrosis alters mucin morphology, which will impair mucociliary transport.
Keywords: mucus, cystic fibrosis, lung, asthma, COPD
Abstract
Gel-forming mucins, the primary macromolecular components of airway mucus, facilitate airway clearance by mucociliary transport. In cystic fibrosis (CF) altered mucus properties impair mucociliary transport. Airways primarily secrete two closely related gel-forming mucins, MUC5B and MUC5AC. However, their morphologic structures and associations in airways that contain abundant submucosal glands and goblet cells are uncertain. Moreover, there is limited knowledge about mucins in airways not affected by inflammation, infection, or remodeling or in CF airways. Therefore, we examined airways freshly excised from newborn non-CF pigs and CF pigs before secondary manifestations develop. We found that porcine submucosal glands produce MUC5B, whereas goblet cells produce predominantly MUC5AC plus some MUC5B. We found that MUC5B emerged from submucosal gland ducts in the form of strands composed of multiple MUC5B filaments. In contrast, MUC5AC emerged from goblet cells as wispy threads and sometimes formed mucin sheets. In addition, MUC5AC often partially coated the MUC5B strands. Compared with non-CF, MUC5B more often filled CF submucosal gland ducts. MUC5AC sheets also accumulated in CF airways overlying MUC5B strands. These results reveal distinct morphology and interactions for MUC5B and MUC5AC and suggest that the two mucins make distinct contributions to mucociliary transport. Thus, they provide a framework for understanding abnormalities in disease.
Mucus propelled by ciliary activity (mucociliary transport, MCT) is an important host defense that removes particulates from airways (1–3). The predominant macromolecular components of airway mucus are two secreted mucins, MUC5B and MUC5AC (1, 4–7). These gel-forming mucins are long, heavily glycosylated proteins with similar domain organization and amino acid sequence. Previous studies described biochemical properties of these and related mucins (1, 4, 6, 8). In human airways, MUC5B is produced in submucosal glands and goblet cells, and MUC5AC is produced in goblet cells (1, 4, 5, 9). In mouse lungs, MUC5B and MUC5AC are expressed primarily in club cells (1, 10). Mice with a disrupted Muc5b gene accumulated mucus in the upper airway, whereas mice with a disrupted Muc5ac gene lacked apparent respiratory abnormalities (10). Together, these results suggest that MUC5B and MUC5AC may have different functions.
Mucin abnormalities may contribute to lung disease (1, 5). In asthma and models of airway hyperreactivity, mucus contains increased levels of MUC5B and MUC5AC, airways exhibit goblet cell hyperplasia, and MUC5AC transcripts are increased whereas MUC5B transcripts are decreased (1, 4, 5, 11–13). Chronic obstructive pulmonary disease manifests increased mucin production (9, 13). Variations in the MUC5B gene promoter/enhancer region have been associated with interstitial pulmonary fibrosis (14). In advanced cystic fibrosis (CF), airways show goblet cell hyperplasia and submucosal gland hypertrophy, and imaging of radiolabeled particles deposited in the lung indicates that MCT is reduced (1, 3). The MCT reduction is greater as the severity of the disease increases, consistent with the finding that reduced MCT has not been detected in young people with CF (15, 16).
Porcine models of CF develop airway disease that replicates that in humans (17–20). At birth CF pig lungs lack airway infection and inflammation yet display disrupted MCT, indicating a primary host defense defect (21). In vivo studies of spontaneously breathing newborn CF pigs revealed impaired movement of insufflated microdisks after cholinergic stimulation of submucosal gland secretion (21, 22). Microdisks traveled up the airways at rates that varied substantially even over the same airway region and even in the same pig. Moreover, in CF airways, some microdisks did not move at all, whereas others sped by in close proximity. The variability in microdisk behavior in both non-CF and CF suggested substantial heterogeneity in airway mucus traveling over the airway surface. In ex vivo studies, we used fluorescent nanospheres (functionalized with carboxylate, sulfate, or amine) to label mucus arising from submucosal gland ducts (21). In CF, the mucus strands sometimes failed to break, and as a result, they remained attached to ducts, halting MCT. Preventing Cl− and HCO3− secretion in non-CF pigs also partially prevented mucus from breaking free from submucosal glands, directly linking loss of CFTR and impaired MCT (21, 23, 24). These functional observations suggest that mucus forms discontinuous structures rather than a homogeneous layer on the airway surface.
Despite the importance of mucins, knowledge of the in situ structural morphology of mucins on the airway surface in health and disease is limited. First, few studies have examined mucins on the airway surface. Second, mucus has been studied on cultured cells, but they lack submucosal glands and material cannot flow onto or leave the surface. Third, mice, though valuable for many studies, have only a few submucosal glands in the proximal trachea, most mucin is secreted by club cells, and mice with a disrupted CFTR gene do not develop airway disease like that in people with CF (25). Fourth, it is difficult to obtain mucus from normal lungs, and sputum obtained from people with airway disease may contain increased proteases, accumulated DNA, and other confounders (26). Moreover, isolation, processing, and storage of mucin can alter its structure and function (4).
Whether the morphologic appearances of MUC5B and MUC5AC differ or what distinct roles they might play in airways remain uncertain. Our goal was to examine the morphology of these mucins as they are secreted onto the airway surface. We studied pigs because their airways are similar to humans (27), including the presence of submucosal glands, and they develop disease that mimics human CF (17, 19, 20). We used newborns to avoid secondary effects of the disease. In previous studies, we excised tracheas and covered the apical surface with saline to clamp pH, ionic composition, and liquid levels (21). Here, we studied airways without submersion or rinsing the apical surface.
Results
WGA Lectin Preferentially Labels MUC5B, and JAC Lectin Preferentially Labels MUC5AC.
We made the empirical observation that wheat germ agglutinin (WGA) lectin preferentially labeled MUC5B, and jacalin (JAC) lectin preferentially labeled MUC5AC. We found that mucous cells in the acinus of submucosal glands labeled with anti-MUC5B antibody and colocalized with WGA labeling (Fig. 1A). In contrast, neither anti-MUC5AC antibody nor JAC labeled the submucosal gland cells (Fig. 1A). The presence of MUC5B but not MUC5AC in submucosal glands is consistent with previous studies in humans and pigs (1, 4, 5, 28).
Fig. 1.
WGA and JAC lectins preferentially label MUC5B and MUC5AC. Figure shows labeling of trachea from newborn pigs by fluorophore-linked lectins and anti-mucin antibodies. (A) Images are tracheal sections showing submucosal glands labeled with anti-MUC5B antibody (white) and WGA (red) (Top) or anti-MUC5AC antibody (white) and JAC (green) (Bottom). Also shown are actin labeling (phalloidin, yellow) and nuclei labeling (DAPI, blue). (Scale bar, 10 μm.) (B) Images are airway surface epithelium showing goblet cells labeled with anti-MUC5B antibody (white) and WGA (red) (Top) or with anti-MUC5AC antibody (white) and JAC (green) (Bottom). Also shown are actin labeling (phalloidin, yellow) and nuclei labeling (DAPI, blue). (Scale bar, 10 μm.)
In cells of the surface epithelium, we found that JAC colocalized with anti-MUC5AC antibody (Fig. 1 A and B and Fig. S1A) and WGA colocalized with anti-MUC5B antibody (Fig. 1 A and B). En face images showed that ∼73% of goblet cells labeled only with JAC, about 17% labeled with WGA alone, and about 10% labeled with both WGA and JAC (Fig. 2 A and B). Despite substantial variability at different locations within individual airways, these data suggest that MUC5AC is the predominant mucin produced by goblet cells. These results are consistent with earlier studies in porcine and human airways (1, 4, 5, 28). In addition, WGA labeling did not colocalize with anti-MUC5AC antibody, and JAC labeling did not colocalize with anti-MUC5B antibody (Fig. S2 A and B). Note, however, that WGA and JAC are not specific only to these mucins; these lectins also show some staining of membranes and other structures (Fig. S2C).
Fig. S1.
Adjacent sections of the same airway were treated identically in A and B. (A) En face image of excised airway surface epithelium showing goblet cells labeled with anti-MUC5AC antibody (red), JAC (green), and merged image (yellow). Nuclei—DAPI, gray. (Scale bar, 50 μm.) Mucus in submucosal gland ducts was not labeled by anti-MUC5AC antibody or JAC. (B) En face image of excised trachea showing colocalization of mucus filling submucosal gland ducts with anti-MUC5B antibody (green) and WGA (red). Nuclei—DAPI, gray. (Scale bar, 50 μm.)
Fig. 2.
WGA and JAC lectins label surface goblet cells. (A) En face image of excised airway surface epithelium labeled with WGA (red), JAC (green), and nuclei (DAPI, gray). (Scale bar, 50 μm.) In subsequent en face images, red and green mucin staining in goblet cells, with proportions varying in individual fields, can be seen below secreted mucin. (B) Percentage of goblet cells in airway surface epithelium labeled by JAC (MUC5AC), WGA (MUC5B), or both. Each symbol represents average of experiments on epithelia from one animal, and error bars indicate SD.
Fig. S2.
(A) Images are tracheal sections showing submucosal glands and mucus in duct labeled with anti-MUC5B antibody (red). Surface goblet cells, but not submucosal glands, are detected by JAC (green). Also shown are actin labeling (phalloidin, gray) and nuclei labeling (DAPI, blue). (Scale bar, 10 μm.) (B) Images are tracheal sections showing submucosal glands detected by WGA (red) and surface goblet cells labeled by anti-Muc5AC antibody (green). Also shown are β-catenin (gray) and nuclei labeling (DAPI, blue). (Scale bar, 10 μm.) (C) WGA (red) detects glycocalyx at plasma membrane of trachea. β-catenin, gray; nuclei, DAPI, blue. JAC (green) detects glycocalyx at the plasma membrane of trachea. Actin, phalloidin, gray; nuclei, DAPI, blue. (Scale bar, 10 μm.)
In subsequent studies of secreted mucins on the airway surface, we used WGA and JAC to identify MUC5B and MUC5AC, respectively. Like in cells, we found that WGA, but not JAC, colocalized with anti-MUC5B antibody. JAC, but not WGA, colocalized with anti-MUC5AC antibody. However, we did not rely on lectin staining alone; for all key studies, some experiments were done with mucin antibodies.
Strands of MUC5B Mucus Emerge from Submucosal Glands and Associate with MUC5AC.
We found that WGA-linked fluorophore labeled mucus emerging from submucosal glands (Fig. 3A). WGA colocalized with MUC5B immunostaining (Fig. S3), as predicted based on production of MUC5B by submucosal glands. Mucins exit gland ducts not as a homogeneous tube or stream but rather as a strand of multiple MUC5B filaments (Fig. 3B). At the point of exit, strands varied from 5 to 50 μm in diameter. After that point, the diameter of strands sometimes increased. Their shape also sometimes changed, becoming less cylindrical, adopting a ribbon-like appearance, or fanning out. After leaving the duct, strands extended toward the larynx and in a ventral direction, consistent with the direction of cilia beating (22).
Fig. 3.
Mucus emerging from submucosal gland ducts labels with WGA lectin. Images in A and B are z stacks and in C are single confocal images of the excised non-CF tracheal surface. WGA is red, JAC is green, and DAPI (nuclei) is gray. (A) Airway surface with mucus strands emerging from submucosal gland (SMG) ducts. (Scale bar, 50 μm.) (See also Fig. S6.) (B) Image shows that mucin strands are comprised of WGA-labeled filaments. JAC-labeled mucus lies on the surface of the WGA-labeled strands. (Scale bar, 50 μm.) (C) Successive single-plane confocal images from the epithelial surface (Bottom) to just above the surface (Top), as indicated by blue dashed lines in Inset. (Scale bar, 50 μm.)
Fig. S3.
En face image of excised airway surface epithelium showing mucus strands labeled with anti-MUC5B antibody (green), WGA (red), and merged image (yellow). Nuclei, DAPI, gray. (Scale bar, 50 μm.)
JAC and anti-MUC5AC antibody also labeled mucus strands (Fig. 3 A and B). This seemed surprising because the submucosal glands produced MUC5B but not MUC5AC. To test if mucin strands emerging from the glands labeled with JAC, we examined strands in the ducts at the level of and just below the airway surface. Emerging strands labeled with WGA but not JAC, indicating they were composed of MUC5B (Fig. 3C).
Scanning electron microscopy also revealed strands of mucus emerging from submucosal gland ducts extending toward the larynx (Fig. S4). Mucus strands were comprised of individual filaments, consistent with the lectin and antibody labeling.
Fig. S4.
Scanning electron microscopic image of mucus strand emerging from submucosal gland duct. Trachea was stimulated with methacholine in the presence of bumetanide and the absence of HCO3−/CO2. (Scale bar, 10 μm.)
Threads and Sheets of MUC5AC Are Released from Goblet Cells onto the Airway Surface.
The JAC lectin and anti-MUC5AC antibody detected wispy threads that were distinct from the mucus strands (Figs. 3 A and B and 4A); henceforth, we refer to MUC5AC in this form as “threads.” The MUC5AC threads were ∼1–4 μm in diameter. Threads often joined MUC5B strands (Fig. 3 A and B). We also sometimes observed small thin sheets of JAC-labeled material (arrow in Fig. 4A); these were much more prominent in CF (Fig. 5B). In contrast to the frequent appearance of MUC5B strands attached to submucosal gland ducts, we seldom observed MUC5AC threads emanating from goblet cells (Fig. 4A). The lack of attachment of MUC5AC threads to surface cells suggests that MUC5AC is rapidly released from goblet cells after it is secreted. In contrast to MUC5AC, we rarely detected MUC5B threads; Fig. 4B shows a rare example.
Fig. 4.
Mucus from goblet cells forms threads and sheets. Images are z stacks of confocal images of excised trachea of non-CF pigs. (A) Left shows threads of mucin detected by MUC5AC antibody (green), a small sheet of mucus (indicated by an arrow), and position of submucosal gland ducts (indicated by white arrowheads). Right shows nuclei (DAPI, gray) to identify the position of submucosal gland ducts (indicated by white arrowheads). A small sheet of mucus is indicated by arrow. (Scale bar, 50 μm.) (B) Image of JAC-labeled mucus threads (green) from goblet cells with rare WGA (red) thread. Goblet cells are labeled by JAC and WGA beneath the threads. (Scale bar, 50 μm.)
Fig. 5.
CF airways showed entangled mucus strands and increased mucus sheets. (A) Methacholine-stimulated airways from newborn non-CF (Left) and CF pigs (Right). WGA is red, and JAC is green. (Scale bar, 50 μm.) (B) Large MUC5AC sheet (JAC, green) floating on MUC5B (WGA, red) strands in methacholine-stimulated CF trachea. (Scale bar, 50 μm.)
Compared with WGA labeling, JAC and anti-MUC5AC antibody labeled the exterior of strands (Fig. 3 A and B). The appearance of MUC5AC threads on the surface of MUC5B strands indicates that MUC5AC associates with MUC5B strands after they exit from the duct orifice onto the airway surface. Thus, mucus strands have a MUC5B core and a partial coating of MUC5AC.
The Appearance of the Mucins Differs in CF and Non-CF Airways.
Previous studies showed that abnormal mucus impaired MCT in newborn CF pigs (21). However, MUC5B and MUC5AC transcripts, Western blotting of mucin protein, goblet cell numbers, and mucus glycosylation did not differ by genotype (28, 29). In addition, controlling the solution volume and maintaining pH at 7.35 on the apical surface did not prevent the mucus abnormality or impaired MCT (21). Those observations focused attention on mucus produced below the surface in submucosal glands. However, those studies were done with airways submerged in saline, they did not identify the mucin, and they could not reveal morphologic aspects. Thus, we hypothesized that mucins would exhibit abnormal morphology in ex vivo airways not submerged in saline. We administered methacholine in vivo to stimulate mucus secretion and then removed and examined airways. Like mucins in non-CF airways, in CF airways we observed mucins in strands and threads and MUC5AC partially covering the surface of MUC5B strands (Fig. 5 A and B).
However, the morphologic appearance of the mucins differed between the two genotypes in several ways. First, in CF airways, MUC5B strands often remained attached to the ducts from which they emerged. In addition, strands emanating from different submucosal gland ducts often merged and appeared entangled (Fig. 5A). This pattern is consistent with earlier functional experiments showing that mucus strands from CF ducts sometimes failed to break and then leave submucosal gland duct openings (21). That defect produced the appearance of aggregated mucus strands on the surface of submerged CF tracheas. In contrast, in non-CF airways, MUC5B strands were less tangled (Fig. 5A).
Second, in CF, MUC5AC often appeared as thin sheets overlying MUC5B strands (Fig. 5B). In contrast, in non-CF, MUC5AC sheets were rarely observed overlying MUC5B strands.
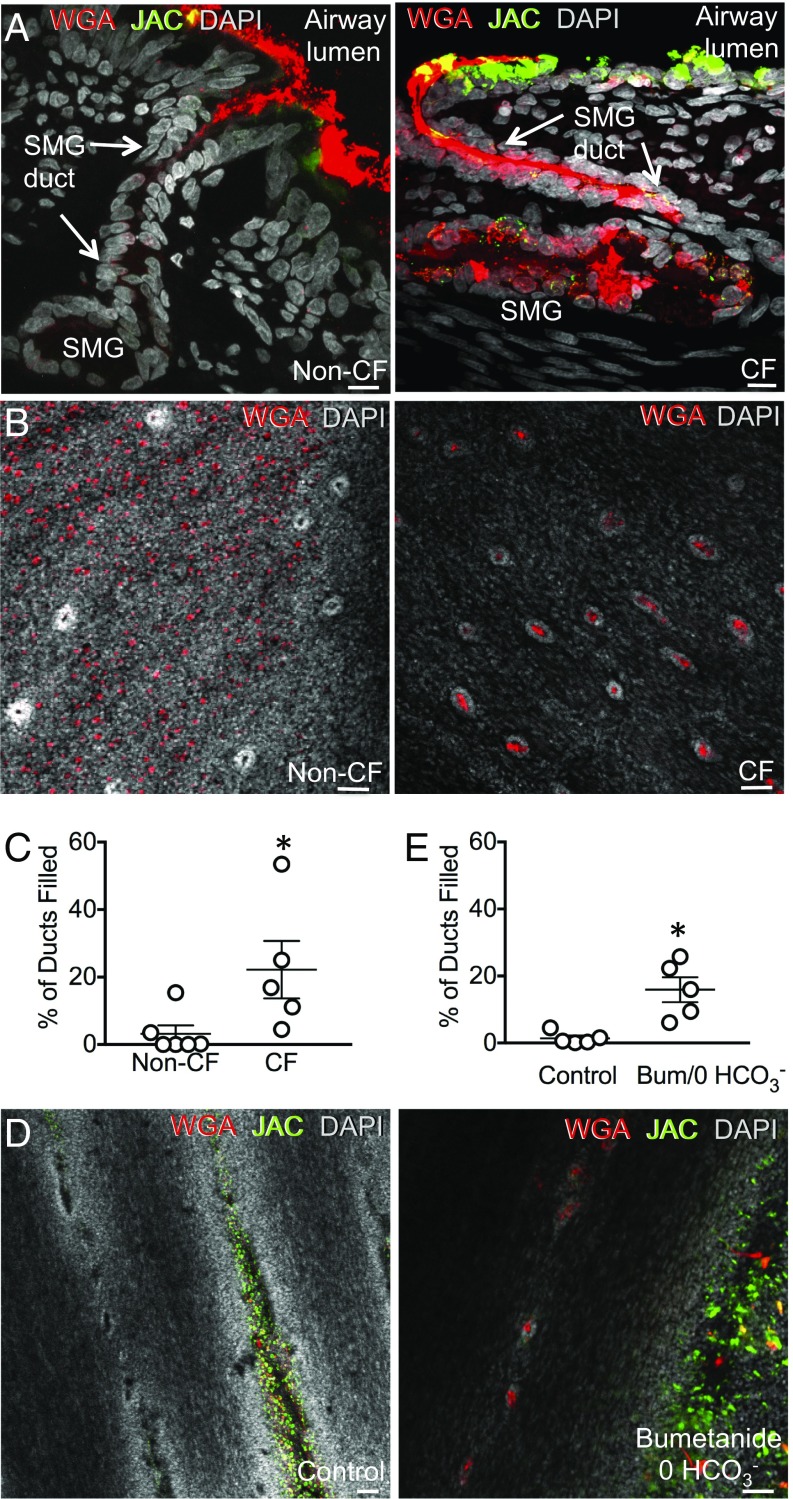
Third, compared with non-CF, CF submucosal glands were more often distended with mucin from the acinus up through the duct to the airway surface (Fig. 6 A and B). These findings suggested that ducts of CF submucosal glands would be filled with mucus more frequently than non-CF ducts. To test this prediction, we counted ducts filled with WGA-labeled mucin. Compared with non-CF, a greater fraction of CF ducts were filled with MUC5B (Fig. 6 B and C). Finding more mucus-filled ducts in CF is in seeming contrast with previous studies showing that CF submucosal glands are smaller and secrete less liquid than non-CF (28, 30, 31). However, less liquid secretion together with more mucus filling of the ducts suggests that the mucus has abnormal biophysical properties, a conclusion consistent with earlier studies (21).
Fig. 6.
CF submucosal gland ducts are filled with mucus. (A) Images are from pigs treated in vivo with methacholine. WGA (MUC5B) is red, JAC (MUC5AC) is green, and DAPI (nuclei) is gray. Shown are vertical sections of airway excised from non-CF (Left) and CF (Right) pigs. (Scale bar, 10 μm.) (B) En face image of excised trachea from methacholine-stimulated newborn non-CF (Left) and CF (Right) pigs. (Scale bar, 50 μm.) (C) Percentage of submucosal gland ducts filled with mucin in excised trachea from non-CF and CF pigs treated in vivo with methacholine. Each data point is from a different pig. Bars and whiskers indicate mean ± SEM. *P < 0.05. (D) Data are z stacks of confocal images at the level of the apical membrane. Excised tracheas from non-CF pigs incubated with methacholine in HCO3−/CO2-buffered saline (control) or Hepes-buffered saline containing bumetanide. WGA, red; JAC, green; DAPI, gray. (Scale bar, 50 μm.) (E) Percentage of submucosal gland ducts filled with mucus. Pigs received methacholine in vivo. Each data point is from a different pig. n = 5 pigs for each condition. Average number of ducts counted per condition = 340 ± 53. Bars and whiskers indicate mean ± SEM. *P < 0.05. (See also Fig. S7.)
HCO3−/CO2-Free Saline Plus Bumetanide Produced Mucus Abnormalities in Non-CF Airways.
In non-CF airways, inhibiting Cl− secretion with bumetanide and blocking HCO3− secretion with HCO3−/CO2-free solution reproduced some features of CF with impaired breakage and release of mucus from submucosal glands (21, 23, 24). These studies were performed with excised airways submerged in saline. We hypothesized that inhibiting anion secretion in non-CF airways would reproduce morphologic features of mucins in excised, nonsubmerged airways. We found that nominally HCO3−/CO2-free solution plus bumetanide increased the fraction of submucosal gland ducts filled with mucus (Fig. 6 D and E) without changing pH on the airway surface (Fig. S5). The mucus in ducts was positive for WGA and anti-MUC5B antibody labeling, but it did not label with JAC or anti-MUC5AC antibody (Fig. S1 A and B). These studies are consistent with earlier work (21, 23, 24) and suggest that loss of anion secretion in submucosal glands alters the properties of mucus.
Fig. S5.
ASL pH 30 min after basolateral incubation of tracheal rings. Control, Krebs bicarbonate buffer, pH 7.4, with methacholine incubated at 37 °C in a humidified incubator with 5% CO2. Bumet, 0 HCO3−, 10 mM Hepes, pH 7.4, no HCO3−, with bumetanide and methacholine incubated at 37 °C in a humidified incubator without CO2. Each symbol represents a single pH measurement. Results are from 3–4 measurements per condition in two pigs.
Discussion
MUC5B Forms Mucus Strands and MUC5AC Forms Mucus Threads and Sheets.
Our findings indicate that MUC5B and MUC5AC have distinct morphologic and structural appearances. MUC5B emerges from submucosal gland ducts as a strand-like structure composed of MUC5B filaments (Fig. 7). The MUC5B filaments probably emanate from individual secretory granules or individual mucus-producing cells within the submucosal glands. Multiple filaments passing through the long, thin submucosal gland duct then facilitate formation of the mucin into a strand. The individual filaments are reminiscent of the histopathological appearance of individual mucin filaments produced by the gallbladder and intestine of newborn CF pigs (32).
Fig. 7.
Model of mucin secretion in pig airway.
In contrast to MUC5B, MUC5AC forms thin, wispy threads. In CF, and less commonly in non-CF, MUC5AC appears as thin mucin sheets. Although goblet cells also produce MUC5B, we rarely detected it as threads or sheets. The explanation is uncertain, but perhaps goblet cells secrete less MUC5B.
Production of MUC5B strands coated with MUC5AC may be facilitated by the airway anatomy. When mucus emerges from submucosal gland ducts, it is not released piecemeal. Instead, for a time, it remains anchored at the duct, growing in length as a strand. We propose that wispy threads and sheets of MUC5AC move across the surface, collide with elongating MUC5B strands, and associate with them. The MUC5B strand with associated MUC5AC then eventually breaks and moves up the airway (Fig. 7). The chemical or physical basis of the association between MUC5AC and MUC5B remains uncertain.
It is estimated that the volume of mucus in submucosal glands is ∼50 times that in goblet cells (1), suggesting that the main function of glands is to produce large amounts of mucus driven by neuronal stimulation. Our findings suggest that an additional role for mucus production by submucosal glands may be to produce mucus in a specific structural form—that is, strands (Fig. 7). Those strands with associated MUC5AC sheets and threads may have properties that are optimal for trapping and sweeping material from the lung.
CF Alters the Appearance of Airway Mucus.
We previously showed that in CF, strands of mucus sometimes fail to break and thus remain attached to submucosal gland ducts (21). Our current results in nonsubmerged airways are consistent with that finding. In addition, we found more MUC5AC sheets in CF than in non-CF. There are several potential explanations. CF airways might have secreted more MUC5AC. MUC5AC might more readily form sheets in CF airways. MUC5AC sheets may be produced similarly in CF and non-CF, but their attachment to stationary MUC5B strands may prevent their movement. A combination of these or other factors is also possible.
Loss of CFTR reduces Cl− and HCO3− secretion (33, 34). These defects decrease the rate of liquid secretion by submucosal glands and reduce the pH of the secreted liquid (30, 31, 35). We found that blocking Cl− secretion and eliminating HCO3− secretion in non-CF airways reproduced some of the abnormalities of CF airways. Whether the abnormalities in CF mucus result from reduced liquid volume, decreased HCO3− concentration, abnormally acidic pH, or some combination of these is uncertain (21). A reduced HCO3− concentration was reported to contribute to intestinal mucin abnormalities in CF mice (36–38). In CF pigs, a decreased pH, rather than a decreased HCO3− concentration, increased airway surface liquid viscosity (29). Earlier studies in CF pigs showed that inhibiting both Cl− and HCO3− secretion in non-CF airways was required to produce mucus abnormalities that resemble those in CF (21, 23, 24). These observations suggest that both liquid volume and the pH or HCO3− concentration are important.
This Work Has Advantages and Limitations.
We studied an animal model with anatomical and physiological similarities to humans (27). CF pigs develop lung disease that mimics that in humans with CF (19, 20). Because we studied newborn pigs, the properties of mucus were not altered by airway infection and inflammation. We studied mucus rather than sputum, which introduces confounding variables. Because we examined mucus on the surface of freshly excised airways, we avoided alterations that occur with collecting, processing, and storing mucus (4). We studied freshly excised airways at the air–liquid interface without rinsing the surface. The results are similar to those in airways submerged in saline, thus excluding the possibility that decreased airway liquid was responsible for the findings.
These studies also have some limitations. We studied a large airway with submucosal glands, yet small airways lacking glands may also contribute to CF pathogenesis (17, 39). In addition to mucins, other proteins, sugars, and lipids contribute to mucus and may influence MCT (1). We fixed the trachea, which could introduce artifacts; however, the results are consistent with earlier functional studies in living airways and in vivo studies (21). As CF disease progresses, airway remodeling and the mix of proteolytic enzymes, inflammatory cells, and infection may also alter mucus properties and MCT.
For both cells and secreted mucins, the data showed preferential binding of WGA to MUC5B and JAC to MUC5AC. Although preferential lectin labeling provided convenient reagents, caution prevents conclusions about the causes of differential labeling. There are several possibilities. The two mucins might display different glycans. WGA binds to sialic acid and N-acetyl-d-glucosamine (40), and JAC has been reported to bind galactose and galactosyl (β-1,3) N-acetylgalactosamine on O-glycoproteins (41). However, such determinations are based largely on competition with monosaccharides (42). Binding of lectins also depends on protein hydrophobic interactions, electrostatic interactions, and complex glycan structures that could differ between MUC5B and MUC5AC. Access of the lectins to the two mucins could also differ, just as access of antibodies to mucins may be limited by their glycans. However, the abundance of glycans on mucins (70–80% of mass) can make labeling with lectins more prominent than for other glycoproteins.
These Results Raise Questions for Future Studies.
Here we highlight three questions:
First, why do airways have strands, threads, and sheets? Presumably airways evolved these structures to produce the most effective MCT. It could be that strands are required to remove large particulates, whereas threads and sheets are sufficient to remove smaller particulates. That would explain why large airways contain submucosal glands whereas small airways lack submucosal glands and thus presumably lack strands. In this regard, it is interesting that smaller mammals, such as mice and rats, have few submucosal glands in intrapulmonary airways (1). Only relatively small particulates gain access to their airways, and thus, perhaps mucus strands are not required to remove them.
Second, why does MUC5B form strands and MUC5AC form threads and sheets? Is it a function of the mucin—that is, its primary structure or glycan composition—or a function of its site of origin—that is, submucosal glands or goblet cells?
Third, how do the physical structures of MUC5B and MUC5AC relate to abnormalities in disease? In CF, loss of Cl− and HCO3− secretion alters the morphology of mucin strands and sheets and disrupts MCT in large airways (21). Loss of CFTR might also disrupt the structure of mucin threads and sheets in small airways. Mucins may also contribute to other lung diseases, including asthma and chronic obstructive pulmonary disease. Thus, knowledge of mucin structure and biophysical properties may aid understanding of the origins of lung disease and suggest new therapeutic strategies.
Materials and Methods
Animals.
We studied non-CF (CFTR+/+, CFTR+/−) and CF (CFTR−/−, CFTR∆F508/∆F508) pigs 8–15 h after birth, as reported previously (19–21). Tracheal segments were obtained between the opening of the right cranial lobe and the larynx. For studies that examined the effect of inhibiting Cl− and HCO3− secretion, segments of trachea were removed from non-CF pigs, wrapped in gauze soaked in either HCO3− containing solutions or HCO3−-free Hepes solution plus bumetanide, and incubated at 37 °C. After incubation, tracheas were cut ventrally, pinned out, and treated for immunocytochemistry. The University of Iowa Animal Care and Use Committee approved all animal studies.
Immunocytochemistry and Scanning Electron Microscopy.
Immunocytochemistry of frozen sections of trachea, treated excised tracheal segments, and vertical sections of excised tracheal segments is described in SI Materials and Methods and ref. 29. Both sections and segments were subsequently imaged by confocal microscopy. Scanning electron microscopy is described in SI Materials and Methods.
Quantitation of Confocal Images.
We assessed the percentage of filled submucosal gland ducts using single planes at the membrane level from original confocal images of excised trachea. Ducts were counted by blinded readers on images of airways of paired trachea from individual pigs. We quantified the number of goblet cells stained by JAC or WGA from confocal images of excised trachea.
SI Materials and Methods
Animals.
We studied non-CF (CFTR+/+, CFTR+/−) and CF (CFTR−/−, CFTR∆F508/∆F508) pigs that were produced by mating (19–21). Results were the same for CFTR−/− and CFTR∆F508/∆F508 and are combined. Newborn littermates were obtained from Exemplar Genetics.
Animals were studied 8–15 h after birth. We anesthetized the animals with ketamine (I.M., Phoenix Pharmaceutical, Inc., or Akorn Animal Science) and xylazine (I.M., Bimeda, or Akorn Animal Science). Sedation was maintained with i.v. propofol (Diprivan). We administered acetyl-β-methacholine chloride (1.28 × 10−7 mol/kg, i.v.) to stimulate mucus secretion. Thirty minutes later, animals were euthanized with i.v. Euthasol (Diprivan). Tracheal segments ∼0.5–1 cm long between the opening of the right cranial lobe and the larynx were cut with a surgical blade between cartilage rings and removed. We also used sections of main stem bronchi with similar results. The University of Iowa Animal Care and Use Committee approved all animal studies.
Immunocytochemistry.
For studies examining cross-sections of airways, rings (0.5 cm) of trachea were immersed in 4% paraformaldehyde (Electron Microscopy Sciences) for 1 h at room temperature. The fixed rings were then quickly frozen in optimal cutting temperature compound (O.C.T.) (Sakura) using an ethanol/dry ice bath and kept at –80 °C. In addition, following 1 h of paraformaldehyde fixation, some rings were immersed overnight in 30% sucrose at 4 °C and then frozen in O.C.T. Mucin staining was similar with or without sucrose immersion. Sections (5–7 μm) were cryo-sectioned onto SuperfrostPlus slides (Fisher) and stored at –80 °C. At the time of immunostaining, slides were warmed to room temperature, rinsed briefly in Ca2+ and Mg2+-free PBS (Sigma), permeabilized with 0.3% TX-100 (Thermo Fisher) in PBS, and then blocked in Super-Block (Thermo Fisher) with 10% normal goat serum (Vector Laboratories) before labeling with antibodies. We used the following anti-mucin antibodies: rabbit anti-MUC5B (1:1,000; Santa Cruz) and mouse anti-Muc5AC (clone 45M1) (1:5,000, Abcam or Novus Biologicals), followed by goat anti-rabbit and goat anti-mouse secondary antibodies conjugated to Alexa-Fluor 488 or 568 at 1:1,000 dilution. In some studies, anti–β-catenin antibodies (Zymed) were added. The lectins were added with the secondary antibodies: WGA-rhodamine (Vector Laboratories) or WGA-AlexaFluor 594 (Molecular Probes) and Jacalin-FITC (Vector Laboratories) at 1:3,500 dilution as well as phalloidin 633 (1:300 dilution). Slides were mounted in Vectashield plus DAPI (Vector Labs), coverslipped, and visualized using an Olympus Fluoview FV1000 confocal microscope.
For en face studies of untreated trachea, excised tracheal rings (1 cm) were immediately opened with a longitudinal cut on the ventral aspect and pinned out on six-well plates coated with Silgard 184 (Dow Corning) and covered with 4% paraformaldehyde (EMS) to fix. For studies that examined the effect of inhibiting Cl− and HCO3− secretion, tracheas were removed from non-CF pigs; wrapped in gauze soaked in a solution that contained (in mΜ) 118.9 NaCl, 25 NaHCO3, 2.4 K2HPO4, 0.6 KH2PO4, 1.2 CaCl2, 1.2 MgCl2, 10 dextrose, pH 7.35, with 5% CO2; and placed in a 37 °C humidified chamber with 5% CO2. An adjacent tracheal ring was wrapped in gauze soaked in 135 NaCl, 2.4 K2HPO4, 0.6 KH2PO4, 1.2 CaCl2, 1.2 MgCl2, 10 dextrose, 5 Hepes, pH 7.3, and 10 μM bumetanide, which inhibits NKCC-mediated entry of Cl− across the basolateral membrane, and placed in a 37 °C humidified chamber in the absence of CO2. Methacholine (1.28 × 10−5 mol/L) was added to both solutions. After 30 min, gauze was removed, and tracheas were cut on the ventral aspect, pinned out, and fixed as above.
Following overnight fixation in paraformaldehyde, tracheas were permeabilized with 0.3% TX-100 (Thermo Fisher) in PBS overnight and then blocked overnight in Super-Block (Thermo Fisher) with 10% normal goat serum if antibodies were added subsequently. Tracheas were then incubated with the lectins, WGA-rhodamine (Vector Laboratories), or WGA-AlexaFluor 594 (Molecular Probes) and Jacalin-FITC (Vector Laboratories) at 1:1,000 dilution, again overnight. All overnight incubations were at 4 °C. Some excised tracheas were incubated with anti-MUC5B (1:1,000; Santa Cruz) or anti-MUC5AC (1:5,000; Abcam or Novus Biologicals) followed by a subsequent overnight incubation with goat anti-mouse and goat anti-rabbit Alexa Fluor 488 or 568 (Molecular Probes). Tracheas were mounted in Vectashield plus DAPI (Vector Labs), coverslipped, and visualized using an Olympus Fluoview FV1000 confocal microscope.
For vertical sections of fixed, permeabilized, and lectin-stained excised trachea, tracheas were removed from the slides, briefly rinsed, and rapidly frozen in O.C.T. using an ethanol-dry ice bath as described above. Vertical 10–20-μm cross-sections perpendicular to the line of submucosal gland ducts were cryo-sectioned onto slides, covered with DAPI, and visualized as described above.
Confocal images were processed using the Olympus Fluoview program.
Quantitation of Confocal Images.
To assess the percentage of submucosal gland ducts that were filled with mucus, we formatted TIF files from single planes at the membrane level from original confocal images of excised trachea labeled with DAPI and WGA. Two blinded observers calculated the number of ducts (outlined by DAPI staining) in each image as well as the number of ducts that were filled with WGA staining. We did not quantitate the abundance of mucus. The counts were done at the membrane level of the trachea and do not include secreted mucus lying above the membrane itself or ducts with strands still attached. In Fig. S6, we present the data from both readers separately. To quantify the number of goblet cells, a blinded reader examined TIF files of original confocal images and determined staining by JAC and WGA.
Fig. S6.
En face image of unfixed, nonpermeabilized, freshly excised trachea labeled with WGA (red) and JAC (green). DAPI is gray. (Scale bar, 50 μm.) Arrows point to submucosal gland ducts.
Lectin Staining of Nonfixed Excised Trachea.
We routinely fixed excised tracheas for several reasons. Fixation prevented the strands and sheets from being washed away and/or altering the mucus pattern from in situ condition. Fixation allowed subsequent antibody staining of the airway. We also permeabilized the trachea to allow access of antibodies and lectins to mucins in goblet cells and submucosal gland cells; these provided a positive control for detection of extracellular mucins. We reasoned that permeabilization might also increase access of lectins and antibodies to sites on mucus strands and sheets (42, 43).
As a control, we lectin-stained some tracheas without fixation or permeabilization. For en face studies, excised tracheal rings (1 cm) were immediately opened with a longitudinal cut on the ventral aspect and pinned out on six-well plates coated with Silgard 184 (Dow Corning) and covered with WGA (1:1,000) and JAC (1:1,000) in PBS++ (plus 1 mM MgCl2 and 0.1 mM CaCl2) overnight at 4 °C. Trachea were rinsed and incubated with Hoescht (10 μg/mL) (Sigma) in PBS++ for 15 min, rinsed, and placed on a slide with Vectashield Hardset without DAPI (Vector Labs). Confocal images were processed using Olympus Fluoview program. Fig. S7 shows an example.
Fig. S7.
Percentage of submucosal gland ducts that were filled with mucus. Control, Krebs bicarbonate buffer, pH 7.4, with methacholine incubated at 37 °C in a humidified incubator with 5% CO2. Bumet & 0 HCO3−, 10 mM Hepes, pH 7.4, no HCO3−, with bumetanide and methacholine incubated at 37 °C in a humidified incubator without CO2. n = 5 paired trachea samples each from a different pig. Each symbol represents data from one pig. Each panel shows percent filled ducts from a different reader. Bars and whiskers indicate mean ± SEM.
Scanning Electron Microscopy.
Trachea samples were processed for scanning electron microscopy using a nonaqueous method. Tissues were fixed for 2 h in FC-72 perfluorocarbon (3M) containing 2% osmium tetroxide and then dehydrated in three changes of 100% ethanol over 3 h. Samples were then transitioned to hexamethyldisilizane and air-dried overnight. Tissues were mounted on aluminum stubs, sputter coated with 80:20 gold:palladium, and imaged in a Hitachi S-4800 FE-SEM (Hitachi High Technologies America, Inc.).
Quantitation of airway surface liquid pH.
Adjacent tracheal rings were excised from methacholine-stimulated newborn piglets and wrapped in gauze soaked in either the Hepes buffer or the HCO3−/CO2 as described above. Tracheal segments soaked in Hepes buffer were incubated in 37 °C humidified incubator in the absence of CO2. Tracheal segments soaked in HCO3− buffer were incubated in a 37 °C humidified incubator in the presence of 5% CO2. Rings were opened, and the pH of the airway surface liquid was measured using an optode (a pH-sensitive foil, PreSens GmbH). We measured 1–2 sites per tracheal ring, using two rings for each of two non-CF pigs, for a total of 3–4 measurements per pig per condition (Fig. S5).
Analysis.
Images of airways represent examples from studies performed on paired trachea from five different animals for the filled ducts data. Graphs show data points from individual pigs. Statistical significance was tested with an unpaired Student’s t test. P ≤ 0.05 was considered statistically significant.
Acknowledgments
We thank Sarah Horgen for help in preparing the manuscript and Patrick Allen and Rachel Hedinger for image analysis. This work was in part supported by the National Institutes of Health Grants HL091842 and HL51670 (to M.J.W.) and HL117744 (to D.A.S.), a Cystic Fibrosis Foundation Research Development Program, and the Roy J. Carver Charitable Trust. M.J.W. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Conflict of interest statement: The University of Iowa has licensed CF pigs to Exemplar Genetics, and M.J.W. receives royalties from the license.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1703228114/-/DCSupplemental.
References
- 1.Widdicombe JH, Wine JJ. Airway gland structure and function. Physiol Rev. 2015;95:1241–1319. doi: 10.1152/physrev.00039.2014. [DOI] [PubMed] [Google Scholar]
- 2.Wanner A, Salathé M, O’Riordan TG. Mucociliary clearance in the airways. Am J Respir Crit Care Med. 1996;154:1868–1902. doi: 10.1164/ajrccm.154.6.8970383. [DOI] [PubMed] [Google Scholar]
- 3.Robinson M, Bye PT. Mucociliary clearance in cystic fibrosis. Pediatr Pulmonol. 2002;33:293–306. doi: 10.1002/ppul.10079. [DOI] [PubMed] [Google Scholar]
- 4.Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol. 2008;70:459–486. doi: 10.1146/annurev.physiol.70.113006.100702. [DOI] [PubMed] [Google Scholar]
- 5.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363:2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ambort D, Johansson ME, Gustafsson JK, Ermund A, Hansson GC. Perspectives on mucus properties and formation–Lessons from the biochemical world. Cold Spring Harb Perspect Med. 2012;2:a014159. doi: 10.1101/cshperspect.a014159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verdugo P. Supramolecular dynamics of mucus. Cold Spring Harb Perspect Med. 2012;2:a009597. doi: 10.1101/cshperspect.a009597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lillehoj EP, Kato K, Lu W, Kim KC. Cellular and molecular biology of airway mucins. Int Rev Cell Mol Biol. 2013;303:139–202. doi: 10.1016/B978-0-12-407697-6.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turner J, Jones CE. Regulation of mucin expression in respiratory diseases. Biochem Soc Trans. 2009;37:877–881. doi: 10.1042/BST0370877. [DOI] [PubMed] [Google Scholar]
- 10.Roy MG, et al. Muc5b is required for airway defence. Nature. 2014;505:412–416. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans CM, Kim K, Tuvim MJ, Dickey BF. Mucus hypersecretion in asthma: Causes and effects. Curr Opin Pulm Med. 2009;15:4–11. doi: 10.1097/MCP.0b013e32831da8d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lachowicz-Scroggins ME, et al. Abnormalities in MUC5AC and MUC5B protein in airway mucus in asthma. Am J Respir Crit Care Med. 2016;194:1296–1299. doi: 10.1164/rccm.201603-0526LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voynow JA, Gendler SJ, Rose MC. Regulation of mucin genes in chronic inflammatory airway diseases. Am J Respir Cell Mol Biol. 2006;34:661–665. doi: 10.1165/rcmb.2006-0035SF. [DOI] [PubMed] [Google Scholar]
- 14.Evans CM, et al. Idiopathic pulmonary fibrosis: A genetic disease that involves mucociliary dysfunction of the peripheral airways. Physiol Rev. 2016;96:1567–1591. doi: 10.1152/physrev.00004.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McShane D, et al. Normal nasal mucociliary clearance in CF children: Evidence against a CFTR-related defect. Eur Respir J. 2004;24:95–100. doi: 10.1183/09031936.04.00097503. [DOI] [PubMed] [Google Scholar]
- 16.Regnis JA, et al. Mucociliary clearance in patients with cystic fibrosis and in normal subjects. Am J Respir Crit Care Med. 1994;150:66–71. doi: 10.1164/ajrccm.150.1.8025774. [DOI] [PubMed] [Google Scholar]
- 17.Stoltz DA, Meyerholz DK, Welsh MJ. Origins of cystic fibrosis lung disease. N Engl J Med. 2015;372:351–362. doi: 10.1056/NEJMra1300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogers CS, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoltz DA, et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med. 2010;2:29ra31. doi: 10.1126/scitranslmed.3000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ostedgaard LS, et al. The ΔF508 mutation causes CFTR misprocessing and cystic fibrosis-like disease in pigs. Sci Transl Med. 2011;3:74ra24. doi: 10.1126/scitranslmed.3001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoegger MJ, et al. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science. 2014;345:818–822. doi: 10.1126/science.1255825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoegger MJ, et al. Assessing mucociliary transport of single particles in vivo shows variable speed and preference for the ventral trachea in newborn pigs. Proc Natl Acad Sci USA. 2014;111:2355–2360. doi: 10.1073/pnas.1323633111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trout L, Gatzy JT, Ballard ST. Acetylcholine-induced liquid secretion by bronchial epithelium: Role of Cl- and HCO3- transport. Am J Physiol. 1998;275:L1095–L1099. doi: 10.1152/ajplung.1998.275.6.L1095. [DOI] [PubMed] [Google Scholar]
- 24.Ballard ST, Spadafora D. Fluid secretion by submucosal glands of the tracheobronchial airways. Respir Physiol Neurobiol. 2007;159:271–277. doi: 10.1016/j.resp.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grubb BR, Boucher RC. Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol Rev. 1999;79(1) Suppl:S193–S214. doi: 10.1152/physrev.1999.79.1.S193. [DOI] [PubMed] [Google Scholar]
- 26.Voynow JA, Rubin BK. Mucins, mucus, and sputum. Chest. 2009;135:505–512. doi: 10.1378/chest.08-0412. [DOI] [PubMed] [Google Scholar]
- 27.Rogers CS, et al. The porcine lung as a potential model for cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;295:L240–L263. doi: 10.1152/ajplung.90203.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyerholz DK, et al. Loss of cystic fibrosis transmembrane conductance regulator function produces abnormalities in tracheal development in neonatal pigs and young children. Am J Respir Crit Care Med. 2010;182:1251–1261. doi: 10.1164/rccm.201004-0643OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang XX, et al. Acidic pH increases airway surface liquid viscosity in cystic fibrosis. J Clin Invest. 2016;126:879–891. doi: 10.1172/JCI83922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joo NS, Cho HJ, Khansaheb M, Wine JJ. Hyposecretion of fluid from tracheal submucosal glands of CFTR-deficient pigs. J Clin Invest. 2010;120:3161–3166. doi: 10.1172/JCI43466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee RJ, Foskett JK. cAMP-activated Ca2+ signaling is required for CFTR-mediated serous cell fluid secretion in porcine and human airways. J Clin Invest. 2010;120:3137–3148. doi: 10.1172/JCI42992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyerholz DK, Stoltz DA, Pezzulo AA, Welsh MJ. Pathology of gastrointestinal organs in a porcine model of cystic fibrosis. Am J Pathol. 2010;176:1377–1389. doi: 10.2353/ajpath.2010.090849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith JJ, Welsh MJ. cAMP stimulates bicarbonate secretion across normal, but not cystic fibrosis airway epithelia. J Clin Invest. 1992;89:1148–1153. doi: 10.1172/JCI115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poulsen JH, Fischer H, Illek B, Machen TE. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci USA. 1994;91:5340–5344. doi: 10.1073/pnas.91.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song Y, Salinas D, Nielson DW, Verkman AS. Hyperacidity of secreted fluid from submucosal glands in early cystic fibrosis. Am J Physiol Cell Physiol. 2006;290:C741–C749. doi: 10.1152/ajpcell.00379.2005. [DOI] [PubMed] [Google Scholar]
- 36.Gustafsson JK, et al. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J Exp Med. 2012;209:1263–1272. doi: 10.1084/jem.20120562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia MA, Yang N, Quinton PM. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J Clin Invest. 2009;119:2613–2622. doi: 10.1172/JCI38662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang N, Garcia MA, Quinton PM. Normal mucus formation requires cAMP-dependent HCO3- secretion and Ca2+-mediated mucin exocytosis. J Physiol. 2013;591:4581–4593. doi: 10.1113/jphysiol.2013.257436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flores-Delgado G, Lytle C, Quinton PM. Site of fluid secretion in small airways. Am J Respir Cell Mol Biol. 2016;54:312–318. doi: 10.1165/rcmb.2015-0238RC. [DOI] [PubMed] [Google Scholar]
- 40.Mandal C, Mandal C. Sialic acid binding lectins. Experientia. 1990;46:433–441. doi: 10.1007/BF01954221. [DOI] [PubMed] [Google Scholar]
- 41.Tachibana K, et al. Elucidation of binding specificity of Jacalin toward O-glycosylated peptides: Quantitative analysis by frontal affinity chromatography. Glycobiology. 2006;16:46–53. doi: 10.1093/glycob/cwj038. [DOI] [PubMed] [Google Scholar]
- 42.Roth J. Lectins for histochemical demonstration of glycans. Histochem Cell Biol. 2011;136:117–130. doi: 10.1007/s00418-011-0848-5. [DOI] [PubMed] [Google Scholar]
- 43.Howat WJ, Wilson BA. Tissue fixation and the effect of molecular fixatives on downstream staining procedures. Methods. 2014;70:12–19. doi: 10.1016/j.ymeth.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]