In virology lectures, students learn the classifications: viruses have either RNA or DNA genomes; they are released either by killing the infected cell or by budding harmlessly from it; viral particles are either “enveloped” or “nonenveloped.” In cell biology classes, students learn the topology of cell membranes: cytoplasmic proteins are synthesized by cytosolic ribosomes, and proteins destined for secretion contain signal sequences that allow endoplasmic reticulum (ER)-associated ribosomes to direct them into the lumen within the ER. Then, secretion of these luminal contents proceeds via the Golgi apparatus and exocytic vesicles to the outside of the cell. Of course, such classifications beg to be violated, and in PNAS, McKnight et al. (1) consolidate a mechanism by which hepatitis A virus (HAV) does just that.
Nonenveloped or, more colorfully, “naked” viruses such as HAV and other picornaviruses (small RNA viruses) are cytoplasmic particles that contain only the genomic nucleic acid and complexed proteins. This has several consequences: for example, proteinaceous coats are often more stable to environmental insult than viral envelopes. Thus, picornaviruses such as HAV, poliovirus, and norovirus are famously stable in water, from whence they readily infect humans via oral–fecal transmission. Another consequence of this nonenveloped structure is that it should be logical that viral exit should require leakage of cytoplasm through a ruptured plasma membrane, thus killing the cell.
It has long been noted, however, that natural isolates of HAV do not appear to lyse infected cells in cultured cells or in infected people (reviewed in refs. 2 and 3). In liver biopsies of some patients, a large proportion of cells contain infectious virus, yet no cell death is evident. During acute human infection, infectious HAV particles appear in feces before any evidence of any immune-mediated hepatocyte damage occurs. Thus, it was hypothesized that the virus could be secreted nonlytically from infected hepatocytes. Given these findings, the potentially nonlytic exit of other picornaviruses was extensively discussed and investigated as well. For example, poliovirus and Simian Virus 40, a naked DNA virus, were both found to be released directionally from polarized cell monolayers (4), and persistent infections could be established in cells that nonetheless released copious amounts of virus. Nonetheless, it remained difficult to exclude the possibility that a subpopulation of lysed cells could explain the presence of extracellular virus and obviate the need for a theory of nonlytic spread.
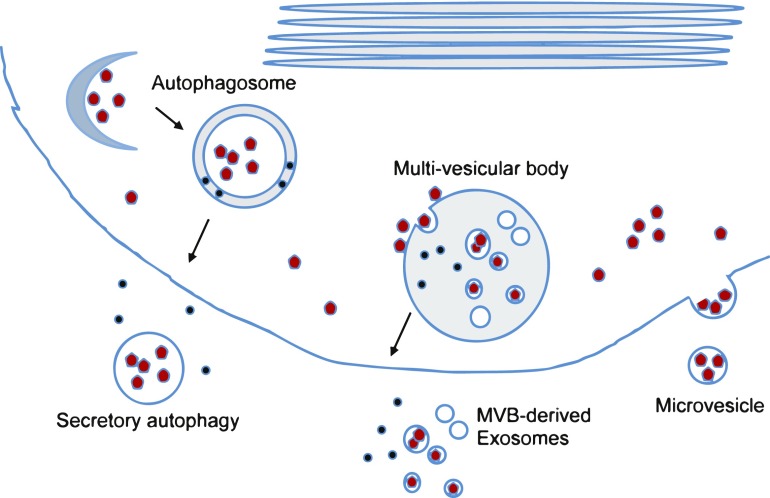
At this time, potential cell-biological mechanisms for nonlytic secretion of cytoplasmic contents were lacking. Electron-microscopic studies of cells infected with poliovirus (4) revealed membrane-bound vesicles outside poliovirus-infected cells, but their origin was not investigated. Other electron-microscopic studies demonstrated that double-membraned vesicles, which thus resemble cellular autophagosomes, are induced during poliovirus infection (5, 6). Depletion of autophagosomal protein LC3 reduced the amount of extracellular virus early in infection, leading my laboratory to the hypothesis that nonlytic exit of cytoplasmic contents could be accomplished by the fusion of the outer autophagosomal membrane with the plasma membrane, releasing the inner vesicle filled with cytosol (Fig. 1) (7). This scenario has since received experimental support from several laboratories for poliovirus, coxsackievirus, and other enteroviral picornaviruses (8–10).
Fig. 1.
Vesicular pathways of unconventional secretion and models for their hijack by nonenveloped viruses. (From Left) Nascent autophagosomes surround cytoplasmic cargo to form a double-membraned autophagosome, whose fusion with the plasma membrane can release both luminal contents and cytoplasmic cargo bounded by a single membrane to the extracellular milieu. Multivesicular bodies (MVBs) can receive cytoplasmic cargo, which can bud into their lumen in single-membraned vesicles using constituents of the ESCRT pathways. Fusion of the MVB with the plasma membranes can then release both luminal contents and small exosomes that contain material of cytoplasmic origin. Microvesicles can bud directly from the plasma membrane with cytoplasmic cargo. In the diagram, luminal compartments are shown in gray, naked viral particles are shown in red, and luminal proteins, like IL1-β during its unconventional secretion, are shown in blue.
However, the mechanism by which HAV can spread through one’s liver without cell destruction remains the most convincing biological example of why nonlytic secretion of naked viruses is important. In a groundbreaking paper, Feng et al. (11) showed that HAV, consistent with being sheltered by a membrane, could spread between cultured cells in the presence of excess neutralizing antibodies. Extracellular membranes could be isolated by gradient centrifugation that contained HAV particles; these vesicle/virus complexes proved to be just as infectious as membrane-free particles. The visualized complexes were surprisingly small, with each usually containing only one virion. This observation blurred the distinction between enveloped and nonenveloped viruses; describing HAV as a “quasi-enveloped” virus was both appropriate and heretical.
During these years, cell biologists were far from idle. Many examples of secreted proteins that lacked signal sequences were identified, giving birth to the new field of “unconventional secretion” (reviewed in refs. 12 and 13). Defined as the externalization of cellular proteins with no dependence on the ER or Golgi apparatus, examples of unconventional secretion are now plentiful and their mechanisms myriad. Fibroblast growth factor 2, for example, directly translocates across the plasma membrane by docking on the cytoplasmic surface and undergoing elaborate conformational changes that take it to the other side. Unconventional secretion mechanisms that are mediated by vesicular structures include the autophagosome-mediated release of cytoplasm bounded by a single membrane, the release of small “exosomes” derived from multivesicular bodies (MVBs), and the shedding of vesicles directly from the cell membrane (Fig. 1). Distinctions between these apparently separate pathways are not always clear, however. For example, the unconventional secretion of fungal morphogen Acb1 depends on components from the autophagy, MVB, and endosomal pathways (14). Spectacularly, the secretion of inflammatory cytokine IL1-β involves both direct translocation and vesicular transport. Cytoplasmic IL1-β, bound to the outer surface of an autophagosome, first translocates directly into the lumen between the two membranes (Fig. 1). Then, when the outer membrane of the autophagosome fuses to the cell membrane, IL1-β in its luminal location is directly secreted into the extracellular milieu (15). Finally, the acquisition of its envelope by HIV requires most of the components of the MVB pathway. This line of investigation began in the laboratory of Carol Carter (16), who found that HIV capsid proteins bound specifically to Tsg101, a component of the ESCRT-I machinery needed for cargo selection.
It is in this context that the manuscript by McKnight et al. reports the use of mass spectrometry to determine the protein composition of the quasi-envelope of HAV. Extracellular, membrane-bound, infectious HAV particles were recovered and their constituent proteins quantitatively compared with those of similar vesicle preparations from uninfected cells. The experiments were expertly performed and carefully interpreted. Quasi-enveloped HAV particles from the medium of HAV-infected cells, and from the
The manuscript by McKnight reports the use of mass spectrometry to determine the protein composition of the quasi-envelope of HAV.
serum of HAV-infected patients, were found to be greatly enriched in proteins of the late MVB pathway and of MVB-derived exosomes. Although it is perilous in a mass spectrometry experiment to draw conclusions from proteins that are not present, it is interesting that LC3, the only specific marker for the autophagosome, was not found in the quasi-enveloped HAV preparations. Also not found were any of the plentiful components of the early MVB pathway, such as Tsg101. The authors suggest an alternative strategy for the specific packaging of HAV in the absence of the early MVB pathway. They had previously characterized a direct interaction between the HAV capsid and ALIX, a protein required in the late stages of MVB formation, and suggest that this recognition event could allow the early MVB steps to be bypassed. This attractive idea would be an excellent example of how two different viruses, HIV and HAV, use the same important cellular pathway via independently evolved mechanisms.
The authors complement their proteomics platform with biochemical analysis to determine the disposition of MVB and exosomal proteins on the quasi-enveloped HAV particles. Gratifyingly, antibodies against the proteins predicted to be on the outside surface of MVB-derived exosomes could successfully immunoprecipitate the HAV particles. Antibodies to LC3, the autophagosomal marker, did not immunoprecipitate HAV virions, but would not be predicted to do so given the cytosolic location of LC3, inside of the vesicles released by autophagy-mediated unconventional secretion. Nonetheless, the preponderance of MVB and exosomal proteins and the small size of the quasi-enveloped particles support an exciting argument that HAV uses the MVB pathway to acquire its membranous coat. Perhaps a quasi-envelope is, in some ways, superior to a “real” envelope. A quasi-enveloped virus is infectious with or without its surrounding membrane and, even better, contains no viral antigens on its surface. It is likely that other naked viruses showing links to the MVB pathway (17) also benefit from wearing a versatile, and optional, invisibility cloak.
Footnotes
The author declares no conflict of interest.
See companion article on page 6587 in issue 25 of volume 114.
References
- 1.McKnight KL, et al. Protein composition of the hepatitis A virus quasi-envelope. Proc Natl Acad Sci USA. 2017;114:6587–6592. doi: 10.1073/pnas.1619519114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lemon SM. Type A viral hepatitis. New developments in an old disease. N Engl J Med. 1985;313:1059–1067. doi: 10.1056/NEJM198510243131706. [DOI] [PubMed] [Google Scholar]
- 3.Hollinger FB, Ticehurst J. Hepatitis A virus. In: Fields BN, Knipe DM, editors. Virology. 2nd Ed. Raven Press; New York: 1990. pp. 631–667. [Google Scholar]
- 4.Tucker SP, Thornton CL, Wimmer E, Compans RW. Vectorial release of poliovirus from polarized human intestinal epithelial cells. J Virol. 1993;67:4274–4282. doi: 10.1128/jvi.67.7.4274-4282.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dales S, Eggers HJ, Tamm I, Palade GE. Electron microscopic study of the formation of poliovirus. Virology. 1965;26:379–389. doi: 10.1016/0042-6822(65)90001-2. [DOI] [PubMed] [Google Scholar]
- 6.Schlegel A, Giddings TH, Jr, Ladinsky MS, Kirkegaard K. Cellular origin and ultrastructure of membranes induced during poliovirus infection. J Virol. 1996;70:6576–6588. doi: 10.1128/jvi.70.10.6576-6588.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson WT, et al. Subversion of cellular autophagosomal machinery by RNA viruses. PLoS Biol. 2005;3:e156. doi: 10.1371/journal.pbio.0030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bird SW, Maynard ND, Covert MW, Kirkegaard K. Nonlytic viral spread enhanced by autophagy components. Proc Natl Acad Sci USA. 2014;111:13081–13086. doi: 10.1073/pnas.1401437111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson SM, et al. Coxsackievirus B exits the host cell in shed microvesicles displaying autophagosomal markers. PLoS Pathog. 2014;10:e1004045. doi: 10.1371/journal.ppat.1004045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen YH, et al. Phosphatidylserine vesicles enable efficient en bloc transmission of enteroviruses. Cell. 2015;160:619–630. doi: 10.1016/j.cell.2015.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng Z, et al. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature. 2013;496:367–371. doi: 10.1038/nature12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rabouille C, Malhotra V, Nickel W. Diversity in unconventional protein secretion. J Cell Sci. 2012;125:5251–5255. doi: 10.1242/jcs.103630. [DOI] [PubMed] [Google Scholar]
- 13.Steringer JP, Müller HM, Nickel W. Unconventional secretion of fibroblast growth factor 2—a novel type of protein translocation across membranes? J Mol Biol. 2015;427:1202–1210. doi: 10.1016/j.jmb.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Bruns C, McCaffery JM, Curwin AJ, Duran JM, Malhotra V. Biogenesis of a novel compartment for autophagosome-mediated unconventional protein secretion. J Cell Biol. 2011;195:979–992. doi: 10.1083/jcb.201106098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang M, Kenny SJ, Ge L, Xu K, Schekman R. Translocation of interleukin-1β into a vesicle intermediate in autophagy-mediated secretion. eLife. 2015;4:e11205. doi: 10.7554/eLife.11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.VerPlank L, et al. Tsg101, a homologue of ubiquitin-conjugating (E2) enzymes, binds the L domain in HIV type 1 Pr55(Gag) Proc Natl Acad Sci USA. 2001;98:7724–7729. doi: 10.1073/pnas.131059198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng Z, Hirai-Yuki A, McKnight KL, Lemon SM. Naked viruses that aren’t always naked: Quasi-enveloped agents of acute hepatitis. Annu Rev Virol. 2014;1:539–560. doi: 10.1146/annurev-virology-031413-085359. [DOI] [PMC free article] [PubMed] [Google Scholar]