Abstract
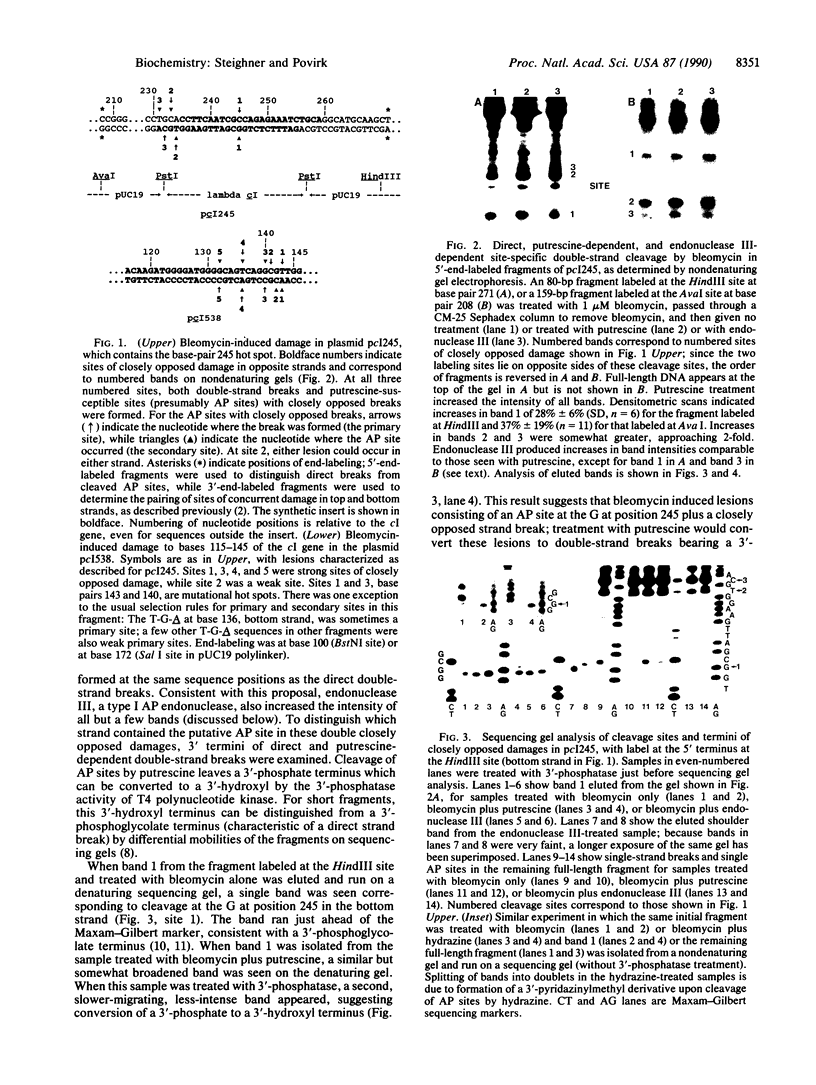
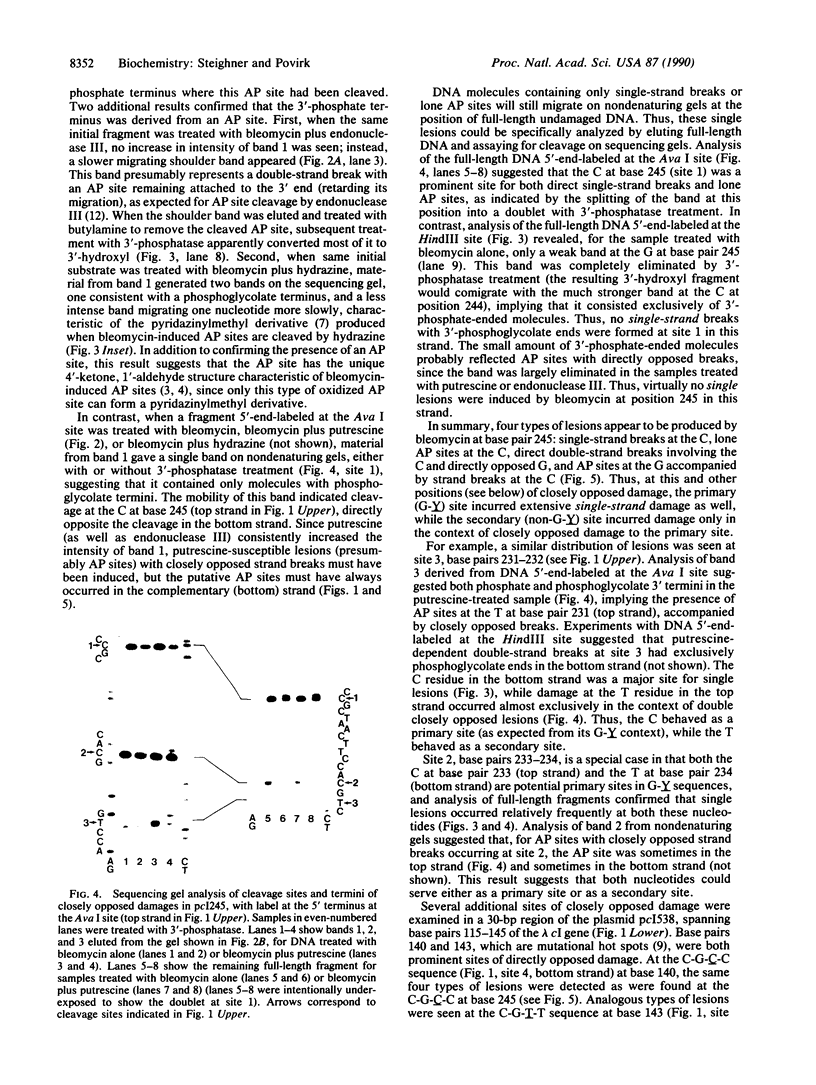
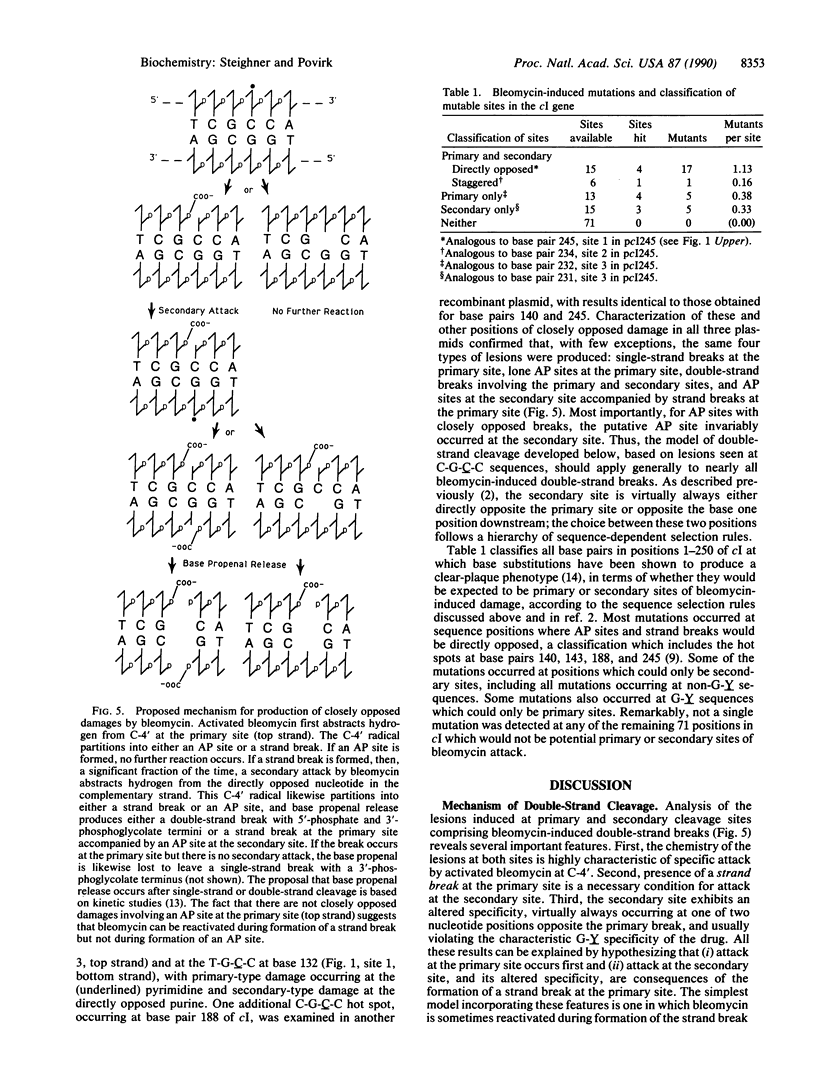
Using various end-labeled, defined-sequence DNA substrates, we examined bleomycin-induced damage at several G.C base pairs which correspond to mutational hot spots. The most frequent lesions detected were single-strand breaks and single apurinic/apyrimidinic (AP) sites at the C residue, suggesting that this was the primary site of damage. Strand breaks and AP sites also occurred, but less frequently, at a secondary damage site--i.e., the directly opposed G residue in the complementary strand. However, damage at the secondary site occurred only when a strand break was present at the primary site, and AP sites at the primary site were never accompanied by closely opposed damage in the complementary strand. Thus, formation of a strand break at the primary damage site was a necessary though not sufficient condition for attack at the secondary site. Similar patterns were seen at other sequences attacked by bleomycin, although primary and secondary sites were sometimes staggered by one nucleotide position rather than directly opposed. These and other results suggest a mechanism of double-strand cleavage in which bleomycin is reactivated during formation of the first strand break, and the reactivated drug subsequently attacks the complementary strand at a specific position which is not normally a site of bleomycin-induced cleavage. Regeneration of activated bleomycin could result from a reaction between Fe(III).bleomycin and a 4'-peroxyl derivative of deoxyribose, both produced during formation of the strand break.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burger R. M., Projan S. J., Horwitz S. B., Peisach J. The DNA cleavage mechanism of iron-bleomycin. Kinetic resolution of strand scission from base propenal release. J Biol Chem. 1986 Dec 5;261(34):15955–15959. [PubMed] [Google Scholar]
- D'Andrea A. D., Haseltine W. A. Sequence specific cleavage of DNA by the antitumor antibiotics neocarzinostatin and bleomycin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3608–3612. doi: 10.1073/pnas.75.8.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giloni L., Takeshita M., Johnson F., Iden C., Grollman A. P. Bleomycin-induced strand-scission of DNA. Mechanism of deoxyribose cleavage. J Biol Chem. 1981 Aug 25;256(16):8608–8615. [PubMed] [Google Scholar]
- Keller T. J., Oppenheimer N. J. Enhanced bleomycin-mediated damage of DNA opposite charged nicks. A model for bleomycin-directed double strand scission of DNA. J Biol Chem. 1987 Nov 5;262(31):15144–15150. [PubMed] [Google Scholar]
- Lindahl T. DNA repair enzymes. Annu Rev Biochem. 1982;51:61–87. doi: 10.1146/annurev.bi.51.070182.000425. [DOI] [PubMed] [Google Scholar]
- Padbury G., Sligar S. G., Labeque R., Marnett L. J. Ferric bleomycin catalyzed reduction of 10-hydroperoxy-8,12-octadecadienoic acid: evidence for homolytic O-O bond scission. Biochemistry. 1988 Oct 4;27(20):7846–7852. doi: 10.1021/bi00420a039. [DOI] [PubMed] [Google Scholar]
- Povirk L. F. Bleomycin-induced mutagenesis in repackaged lambda phage: base substitution hotspots at the sequence C-G-C-C. Mutat Res. 1987 Sep;180(1):1–9. doi: 10.1016/0027-5107(87)90061-3. [DOI] [PubMed] [Google Scholar]
- Povirk L. F., Houlgrave C. W. Effect of apurinic/apyrimidinic endonucleases and polyamines on DNA treated with bleomycin and neocarzinostatin: specific formation and cleavage of closely opposed lesions in complementary strands. Biochemistry. 1988 May 17;27(10):3850–3857. doi: 10.1021/bi00410a049. [DOI] [PubMed] [Google Scholar]
- Royer-Pokora B., Gordon L. K., Haseltine W. A. Use of exonuclease III to determine the site of stable lesions in defined sequences of DNA: the cyclobutane pyrimidine dimer and cis and trans dichlorodiammine platinum II examples. Nucleic Acids Res. 1981 Sep 25;9(18):4595–4609. doi: 10.1093/nar/9.18.4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steighner R. J., Povirk L. F. Effect of in vitro cleavage of apurinic/apyrimidinic sites on bleomycin-induced mutagenesis of repackaged lambda phage. Mutat Res. 1990 Feb;240(2):93–100. doi: 10.1016/0165-1218(90)90012-q. [DOI] [PubMed] [Google Scholar]
- Sugiyama H., Xu C., Murugesan N., Hecht S. M., van der Marel G. A., van Boom J. H. Chemistry of the alkali-labile lesion formed from iron(II) bleomycin and d(CGCTTTAAAGCG). Biochemistry. 1988 Jan 12;27(1):58–67. doi: 10.1021/bi00401a011. [DOI] [PubMed] [Google Scholar]
- Takeshita M., Grollman A. P., Ohtsubo E., Ohtsubo H. Interaction of bleomycin with DNA. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5983–5987. doi: 10.1073/pnas.75.12.5983. [DOI] [PMC free article] [PubMed] [Google Scholar]






