Abstract
Background
Various noninvasive liver reserve markers were proposed to indicate the severity of liver damage. However, the role and feasibility of these markers to predict the prognosis of patients with hepatocellular carcinoma (HCC) are unknown. We aimed to identify the prognostic role of the 8 currently used hepatic reserve markers in patients with HCC undergoing transarterial chemoembolization (TACE).
Methods
Between 2002 and 2013, a total of 881 patients with HCC undergoing TACE were prospectively identified and retrospectively analyzed. The baseline characteristics, tumor status and noninvasive markers were collected. Homogeneity and corrected Akaike information criteria (AICc) were compared between these markers. The Cox proportional hazards model was used to identify independent predictors of survival.
Results
Significant differences in survival distribution were found for albumin-bilirubin (ALBI) grade, Child-Turcotte-Pugh (CTP) class, Lok index, fibrosis index based on 4 factors (FIB-4), Göteborg University cirrhosis index (GUCI), cirrhosis discriminant index (CDI) and model for end-stage liver disease (MELD) score (all p values <0.05). Among these markers, the ALBI grade showed the highest homogeneity and lowest AICc value, indicating a better prognostic performance. Cox multivariate analysis confirmed that ALBI grade 2, ascites, serum alkaline phosphatase and α-fetoprotein level, tumor diameter, vascular invasion and performance status were significant independent prognostic predictors. The distribution of the ALBI score well correlated with baseline CTP and MLED scores.
Conclusions
Our data suggest that among the currently used liver reserve markers, ALBI grade may serve as an objective and feasible surrogate to predict the prognosis of HCC patients undergoing TACE.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignancy and the third leading cause of cancer-related mortality worldwide [1]. The incidence of HCC is highest in Southeast Asia and sub-Saharan Africa where hepatitis B virus (HBV) infection is endemic. Hepatitis C virus (HCV)-associated HCC increased rapidly in United State [2, 3]. HCC typically develops on a background of chronic liver disease or cirrhosis in 70–90% of all cases [4, 5]. As a result, various degrees of liver functional insufficiency are usually present at the time of cancer diagnosis. Surgical resection is generally recommended for HCC [6], but is indicated only for patients with early stage and well preserved liver function. For patients not suitable for curative treatment, transarterial chemoembolization (TACE) may provide better loco-regional tumor control and increase patient survival [7].
In comparison with other solid cancers, management and prognosis HCC highly depend on tumor extent and underlying liver functional reserve [8]. The Child-Turcotte-Pugh (CTP) classification is widely used to evaluate liver reserve in patients with HCC. However, it has some limitations because the cut-off values of some variables (serum albumin, bilirubin and international normalized ratio of prothrombin time) are arbitrarily defined; in addition, ascites and encephalopathy are highly subjective [9]. Although liver biopsy is usually the gold standard to assess hepatic fibrosis or cirrhosis, it is an invasive procedure that is associated with potential risks and prone to sampling errors as well as intra- and inter-observer variation [10].
Up to now, at least 8 noninvasive liver reserve markers have been proposed to define the degree of functional liver reserve in patients with chronic liver diseases [11]. The model for end-stage liver disease (MELD) has been adopted for end-stage cirrhotic patients waiting for liver transplantation and has been used to assess liver dysfunction in HCC [12]. Alternative tools to evaluate liver dysfunction are Lok index, cirrhosis discriminant index (CDS) and Göteborg University Cirrhosis Index (GUCI) [13–15]. These markers incorporate clinical parameters such as serum aspartate aminotransferase, alanine aminotransferase, international normalized ratio of prothrombin time (INR) and platelet count. Other liver reserve markers, fibrosis index based on 4 factors (FIB-4) and aspartate aminotransferase-to-platelet ratio (APRI), have also been proposed to assess liver dysfunction [16, 17]. Both FIB-4 index and APRI can be readily calculated by using clinical variables including age and serum biochemistry. Lastly, the albumin-bilirubin (ALBI) grade is a more recently introduced prognostic marker based solely on serum albumin and bilirubin level [18].
Given all these choices, their role and accuracy in predicting the outcome of HCC patients are largely unclear. Selection of an optimal surrogate marker for these patients is highly controversial. This study aimed to assess the feasibility and compare the prognostic role of these markers in patients with HCC undergoing TACE.
Methods
Patients
Between 2002 and 2013, patients with HCC undergoing TACE in Taipei Veterans General Hospitals were prospectively collected and retrospectively analyzed. The baseline characteristics, including demographic data, etiology of chronic liver diseases, performance status, diabetes mellitus, laboratory parameters, tumor status (tumor nodules, tumor diameter and vascular invasion) and various noninvasive markers for liver reserve, were comprehensively recorded at the time when the diagnosis was established. Patients were followed every 3–6 months until death or dropout from follow-up. This study was approved by the Institutional Review Broad of Taipei Veterans General Hospital (IRB protocol number 2014-03-007AC), and complies with the standards of the Declaration of Helsinki and current ethical guidelines. Waiver of consent was obtained, and patient records/information was anonymized and de-identified prior to analysis.
Diagnosis and definition
The diagnosis of HCC was histologically confirmed by needle biopsy or based on the findings of typical radiological features in at least two imaging examinations including sonography, contrast-enhanced dynamic computed tomography (CT), magnetic resonance imaging (MRI), and hepatic arterial angiography [19]. Patients who were seropositive for hepatitis B antigen (HBsAg), seronegative for anti-HCV antibody, and without history of alcoholism were classified as HBV-related HCC. HCV-related HCC was defined as seropositive for anti-HCV antibody, seronegative for HBsAg and no history of alcoholism. Dual HBV- and HCV-related HCC was defined as seropositive for HBsAg and anti-HCV antibody [20]. The 8 liver reserve markers were calculated according to their original formula, and grading of severity was classified at the time of diagnosis according to the scores [15, 21, 22].
Treatment
When the diagnosis of HCC was confirmed, patients’ medical data were reviewed at the multidisciplinary HCC broad of Taipei Veterans General Hospital. The management of unresectable HCC and indication of TACE were according to the American Association for the Study of Liver Disease or European Association for the Study of Liver guidelines [23]. TACE was performed in patients who had unresectable lesions and were not eligible or unwilling to receive other therapies. The HCC nodule(s) was considered unresectable if there were multifocal lesions which made extended resection necessary to eradicate all tumors, or hepatic reserve was insufficient with an indocyanine green 15-min retention rate > 30%. The criteria for patients undergoing TACE were: (1) no main portal vein trunk involvement or extrahepatic metastasis, (2) CTP functional class A or B, (3) normal renal function with a serum creatinine concentration < 1.5 mg/dL, and (4) no gross ascites by ultrasound or CT [24, 25]. Therapeutic information including benefits and risks was provided to individual patient based on shared decision making. Written informed consent was obtained prior to initiation of treatment.
The Seldinger’s technique of arterial embolization was administered as standard TACE procedure [23–25]. In brief, the four-French catheter (Terumo, Tokyo, Japan) were used for femoral artery puncture. HCC nodules were localized with hepatic arteriography and superior mesenteric arterial portovenography. Tumor stain (vascularity of HCC tumor) was investigated with 50 to 100 mL radiocontrast agent (Telebrix; Laboratoire Guerbet, Aulnay-Sous-Bois, France) injected by a power injector (CT9000 ADV; Liebel-Flarsheim, St. Louis, MO, USA). Infusion of a mixture of 20–30 mg adriamycin (Carlo Erba, Milan, Italy) and 5–10 mL Lipiodol (Laboratoire Guerbet) was performed after the arteries supplying the tumor were catheterized with three-French catheter superselectively. Sufficient amount of emulsion to the tumoral area and 2–3 mm strips of Gelfoam (Upjohn, Kalamazoo, MI, USA) were delivered till complete flow stagnation was achieved.
Statistics
The two-tailed chi-squared or Fisher exact test was employed to compare categorical data. The Mann-Whitney rank sum test was used for continuous variables. Missing values were handled by multiple imputation while a complete case was used as benchmark analysis. Logistic regression on missing data indicators using completely observed variables as covariates was implemented. The statistical output obtained from multiple imputation was similar to statistical output from a complete case analysis in which patients with missing data were omitted. Pooled results by multiple imputation were reported in this study [26, 27]. The survival distributions for liver reserve markers were examined by the Kaplan-Meier methods and compared by log-rank test. Comparison of prognostic performance of these markers was calculated by corrected Akaike information criteria (AICc) and homogeneity. Prognostic factors that were possibly linked to survival, including sex, etiology of chronic liver disease, performance status, laboratory parameters and tumor status were comprehensively included in survival analysis. Factors that were significant in the univariate analysis were introduced into the multivariate Cox proportional hazards model to determine the adjusted risk ratio. Box plot was used to describe correlation between ALBI grade and CTP and MELD grades. All statistical analyses were conducted using the SPSS for Windows version 21 release (SPSS Inc., Chicago, IL, USA). Statistical significance was set at p value < 0.05 in two-tailed tests.
Results
A total of 881 patients who received TACE as primary anti-cancer treatment were enrolled between 2002 and 2013. The median age was 68 years and 673 (76%) patients were male. The median overall survival of the study patients was 24 months. The etiology of HCC were hepatitis B in 311 (35%), hepatitis C in 241 (27%), both hepatitis B and C in 35 (4%), and alcoholism in 162 (19%) patients. There were 166 (18%) patients who had ascites at the time of diagnosis and 226 (26%) patients had history of diabetes mellitus. Four hundred and forty-two (50%) patients had a single tumor at initial presentation and 450 (51%) patients had maximum tumor diameter < 5 cm (Table 1). The calculation formula and severity grading of the 8 liver reserve markers are described in Table 2.
Table 1. Baseline characteristics of patients of hepatocellular carcinoma undergoing TACE.
| Variables | Patients (n = 881) |
|---|---|
| Age (years, median [interquartile range]) | 68 (55–75) |
| Male, n (%) | 673 (76) |
| Etiologies of liver disease | |
| HBV, n (%) | 311 (35) |
| HCV, n (%) | 241 (27) |
| HBV+HCV, n (%) | 35 (4) |
| Alcohol, n (%) | 162 (19) |
| Diabetes mellitus, n (%) | 226 (26) |
| Performance status (0/1/2/3/4), n (%) | 521/207/106/39/8 (59/24/12/4/1) |
| Ascites, n (%) | 166 (18) |
| Laboratory values | |
| α-fetoprotein (ng/mL), mean ± SD | 13515.0 ± 98741.32 |
| Alanine aminotransferase (IU/L), mean± SD | 67.2±70.1 |
| Aspartate aminotransferase (IU/L), mean± SD | 94.2± 184.1 |
| Alkaline phosphatase (IU/L), mean± SD | 138.62±103.77 |
| Albumin (g/L), mean ± SD | 36± 6 |
| Total bilirubin (μmol/L), mean ± SD | 18.8 ± 16.6 |
| Creatinine (mg/dl), mean ± SD | 1.151±0.956 |
| Platelets (1,000/μL), mean ± SD | 160± 95 |
| INR of prothrombin time (mean ± SD) | 1.1 ± 0.2 |
| Non-invasive liver reserve markers | |
| ALBI grade (1/2/3), n (%) | 297/540/44 (34/61/5) |
| APRI grade (1/2/3), n (%) | 195/368/318 (22/42/36) |
| CTP classification (A/B/C), n (%) | 698/263/20 (79/19/2) |
| CDS grade (1/2/3), n (%) | 209/500/172 (24/56/20) |
| FIB-4 grade (1/2/3), n (%) | 401/234/246 (45/27/28) |
| GUCI grade (1/2/3), n (%) | 154/362/365 (18/41/41) |
| Lok index grade (1/2/3), n (%) | 365/298/218 (41/34/25) |
| MELD score (<10/10-14/>14), n (%) | 613/202/66 (70/23/7) |
| Tumor nodules (1/2/≥3), n (%) | 442/177/262 (50/20/30) |
| Maximal tumor diameter (≤2/ 2-5/ >5cm), n (%) | 121/329/431 (14/37/49) |
| Vascular invasion, n (%) | 157 (18%) |
Table 2. Formula and grading of the 8 noninvasive hepatic reserve markers.
| Noninvasive blood tests as liver reserve markers | Formula |
|---|---|
| ALBI, Grade 1/2/3(<-2.6/-2.6- ≤-1.39 / >-1.39) | (log(bilirubin[μmol/L]) x 0.66)—(albumin[g/L] x 0.085) |
| APRI, Grade 1/2/3(<0.5/0.5–1.5/ >1.5) | AST (/UNL)/platelet (109/L) × 100 |
| CTP, A/B/C, grade 1/2/3/(5-6/7-9/10-15) | encephalopathy: none = 1, grade 1 or 2 = 2, grade 3 or 4 = 3 ascites: none = 1, mild to moderate = 2, severe = 3 bilirubin (mg/dl): <2 = 1, 2–3 = 2, >3 = 3albumin (g/dl): >3.5 = 1, 2.8–3.5 = 2, < 2.8 = 3PT sec (INR): < 4 (1.7) = 1, 4–6 (1.7–2.3) = 2, > 6 (>2.3) = 3 |
| CDS, Grade 1/2/3(<4/4-7/>7) | platelet count (× 109/L): >340 = 0; 280–339 = 1; 220–279 = 2; 160–219 = 3; 100–159 = 4; 40–99 = 5; <40 = 6 |
| ALT/AST ratio: >1.7 = 0; 1.2–1.7 = 1; 0.6–1.19 = 2; <0.6 = 3 | |
| INR: <1.1 = 0; 1.1–1.4 = 1; >1.4 = 2; CDS is the sum of the above (possible value 0–11) | |
| FIB-4 index, Grade 1/2/3(<1.45/1.45–3.25/>3.25) | Age × AST/[Platelet × (ALT)1/2] |
| GUCI, Grade 1/2/3(<0.5/0.5–1.56/>1.56) | AST/TOP NORMAL AST x INR x100/(Platelets x109) |
| Lok index, Grade 1/2/3(<0.5/0.5–0.8/>0.8) | Lok Index = e(LogOddsLok) / (1 + e(LogOddsLok))Log Odds Lok = (1.26x AST / ALT) + (5.27 x INR)—(0.0089 x Platelets x109)—5.56 |
| MELD, Grade 1/2/3(<10/10-14/>14) | 10 x ((0.957 x ln(Creatinine)) + (0.378 x ln(Bilirubin)) + (1.12 x ln(INR))) + 6.43 |
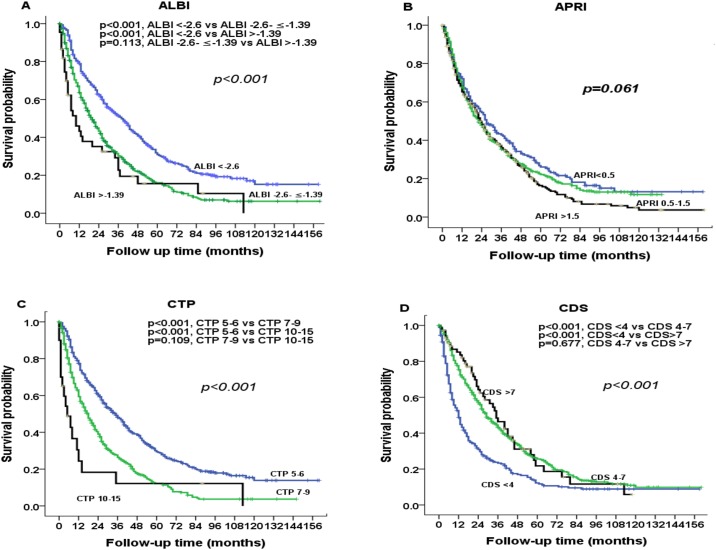
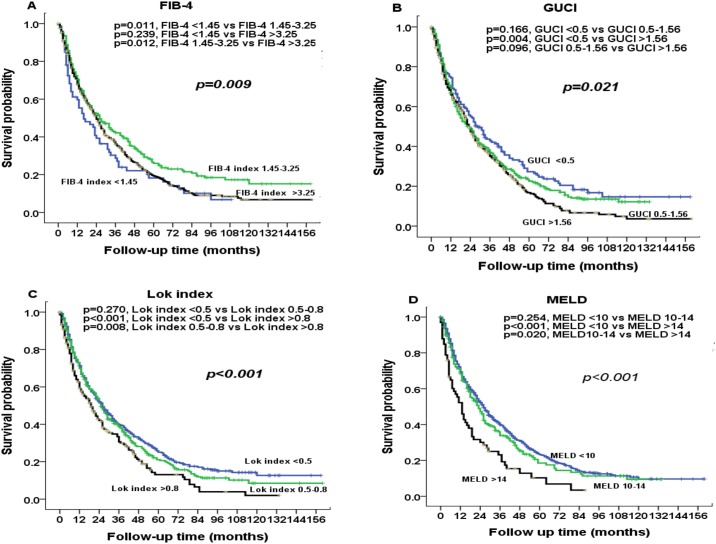
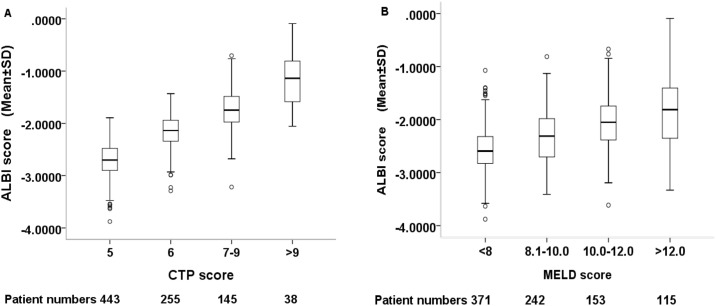
The prognostic value of these markers was evaluated according to their grading (Figs 1 and 2). Significant differences in survival distribution were found in all markers with the exception of APRI score. Pairwise comparison showed that there were no significance survival differences between ALBI grade 2 vs 3 (p = 0.113), CTP grade 2 vs 3 (p = 0.109), CDS grade 2 vs 3 (p = 0.677), FIB-4 grade 1 vs 3 (p = 0.239), GUCI grade 1 vs 2 (p = 0.166), GUCI grade 2 vs 3 (p = 0.096), Lok index grade 1 vs 2 (p = 0.270) and MELD grade 1 vs 2 (p = 0.254). Fig 3 shows the correlation between the ALBI score and baseline CTP and MLED scores. The ALBI score increased with increasing CTP and MELD scores, indicating a worsened liver functional reserve.
Fig 1. Comparison of survival distributions according to (A) ALBI, (B) APRI, (C) CTP, and (D) CDS grading.
Significant survival differences are found for ALBI grade, CTP class and CDS grading.
Fig 2. Comparison of survival distributions according to (A) FIB-4 index, (B) GUCI, (C) Lok index, and (D) MELD grading.
Significant survival differences are found in all 4 markers.
Fig 3. Correlation between ALBI score with CTP score and MELD score.
The ALBI score increases with increasing CTP and MELD scores. The dark line in the middle of the boxes is the median of ALBI score. The bottom of the box indicates the 25th percentile and the top of the box represents the 75th percentile. T-bar at the top and bottom of the box is maximum and minimum values, respectively. ○ indicates extreme values. SD; standard deviation.
Comparison of the prognostic performance of the 8 markers is shown in Table 3. Of these, the ALBI grade had the highest homogeneity and lowest AICc value, followed by the CDS and CTP class. The predictive role of the ALBI score was analyzed along with other clinically important prognostic predictors. In univariate survival analysis, alcoholism, presence of ascites, alkaline phosphatase level, serum platelet count and AFP level, maximum tumor diameter, vascular invasion, performance status and ALBI grade were the factors associated with a poor prognosis (Table 4; all p values <0.05). Cox multivariate analysis revealed that ascites [hazard ratio (HR): 1.536, 95% confidence interval (CI): 1.227–1.924, p< 0.001], alkaline phosphatase ≧100 IU/L [HR:1.362, 95% CI: 1.155–1.607, p< 0.001], AFP ≧20 ng/dL [HR: 2.006, 95% CI: 1.694–2.372, p< 0.001], maximum tumor diameter > 5 cm [HR:1.791, 95% CI: 1.510–1.2.124, p< 0.001], vascular invasion [HR: 1.999, 95% CI: 1.622–2.646, p< 0.001], poor performance status [HR: 1.463, 95% CI: 1.221–1.751, p< 0.001], and ALBI grade 2 [HR: 1.531, 95% CI: 1.285–2.823, p< 0.001] were independent predictors associated with a decreased survival; ALBI grade 3 was associated with a decreased survival at marginal significance [HR: 1.525, 95% CI: 0.967–2.38, p = 0.064] in the Cox model.
Table 3. Comparison of prognostic performance of noninvasive liver reserve markers.
| Homogeneity (Wald χ2) | Corrected Akaike information criteria (AICc) | |
|---|---|---|
| ALBI | 43.655 | 8094.296 |
| APRI | 2.050 | 8135.901 |
| CTP | 26.861 | 8111.090 |
| CDS | 35.635 | 8102.143 |
| FIB-4 | 0.173 | 8137.571 |
| GUCI | 7.613 | 8130.338 |
| Lok index | 11.512 | 8126.439 |
| MELD | 6.700 | 8131.251 |
ALBI, albumin-bilirubin; APRI, aspartate transaminase-to-platelet ratio; CDS, cirrhosis discriminant index; CTP, Child-Turcotte-Pugh score; FIB-4, fibrosis index based on the four factors (FIB-4); MELD, model for end-stage liver disease; GUCI, Göteborg University Cirrhosis Index.
Table 4. Univariate and multivariate survival analysis in patients with hepatocellular carcinoma undergoing TACE.
| Overall survival | Number | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| HR | CI | p | HR | CI | p | ||
| §All patients (n = 881) | |||||||
| Age (<65/≥65 years) | 373/508 | 0.933 | 0.801–1.088 | 0.377 | |||
| Sex (male/female) | 673/208 | 0.936 | 0.855–1.025 | 0.153 | |||
| HBsAg (negative/positive) | 466/415 | 1.1271 | 0.969–1.311 | 0.120 | |||
| Anti-HCV (negative/positive) | 572/309 | 0.894 | 0.764–1.048 | 0.167 | |||
| Alcoholism (no/yes) | 719/162 | 1.240 | 1.018–1.509 | 0.032 | |||
| aDiabetes mellitus (no/yes) | 651/226 | 1.041 | 0.873–1.240 | 0.656 | |||
| Ascites (absent/present) | 715/166 | 1.796.522 | 1.488–2.169 | <0.001 | 1.536 | 1.227–1.924 | <0.001 |
| Creatinine (<1/≥1 mg/dL) | 442/439 | 0.897 | 0.772–1.043 | 0.159 | |||
| Alanine transaminase (≤40/>40 IU/L) | 337/544 | 1.151 | 0.985–1.346 | 0.077 | |||
| aAlkaline phosphatase (<100/≥100 IU/L) 1.06 | 370/492 | 1.678 | 1.435–1.963 | <0.001 | 1.362 | 1.155–1.607 | <0.001 |
| INR of PT (<1/≥1) | 236/645 | 1.145 | 0.967–1.354 | 0.116 | |||
| Platelet (≥150,000/<150,000/μL) | 408/473 | 0.794 | 0.683–0.924 | 0.003 | |||
| Alpha-fetoprotein (<20/≥20 ng/mL) | 349/532 | 1.927 | 1.642–2.261 | <0.001 | 2.006 | 1.694–2.372 | <0.001 |
| Tumor nodules (single/multiple) | 442/439 | 1.152 | 0.990–1.339 | 0.067 | |||
| Maximal tumor diameter (≤5/>5 cm) | 450/431 | 2.031 | 1.743–2.366 | <0.001 | 1.791 | 1.510–2.124 | <0.001 |
| Vascular invasion (no/yes) | 724/157 | 2.511 | 2.075–3.039 | <0.001 | 1.999 | 1.622–2.464 | <0.001 |
| Performance status (0/1-4) | 521/360 | 1.779 | 1.521–2.081 | <0.001 | 1.463 | 1.221–1.751 | <0.001 |
| Albumin-Bilirubin grade | |||||||
| Grade 1 | 297 | 1 | |||||
| Grade 2 | 540 | 1.678 | 1.421–1.981 | <0.001 | 1.531 | 1.285–2.823 | <0.001 |
| Grade 3 | 44 | 1.501 | 1.251–1.801 | <0.001 | 1.525 | 0.976–2.382 | 0.064 |
HR, hazards ratio; CI, confidence interval; HCV, hepatitis C virus, INR, international normalized ratio; PT, prothrombin time
a: Missing data of DM and alkaline phosphatase in 4 (0.5%) and 19 (2.2%) patients, respectively.
Discussion
Liver functional reserve is a critical concern in determining the prognosis of HCC. Efforts were undertaken to identify accurate surrogate markers to indicate the severity of liver dysfunction in HCC patients during the past decades [6, 28]. In this study, we enrolled a large, well-documented, and adequately followed-up HCC cohort. Up to 8 noninvasive liver reserve markers and possible prognostic predictors were comprehensively evaluated. We confirm that the key prognostic predictors of HCC are the severity of liver reserve, tumor burden and performance status of patients. We also show that among the 8 noninvasive markers, the ALBI grade is the best predictive model to assess the degree of liver damage in HCC patients undergoing TACE. These data, which are consistent with previous cohort studies [18, 22, 29], can discriminate patient survival and indicate the predictive accuracy of the ALBI grade for HCC patients.
With Kaplan-Meier survival analyses, we systematically investigated 8 noninvasive liver reserve markers in HCC patients undergoing TACE. Our results show that ALBI, CDS and CTP are the three most accurate prognostic markers to distinguish hepatic functional reserve according to the AICc analysis. Of these markers, we found that the ALBI model is the best in discriminating survival for different severity grades. In addition, the ALBI has the greatest homogeneity of survival among patients within the same stage, suggesting ALBI is a more feasible tool for outcome prediction among the 8 markers. In multivariate Cox analysis, patients with ALBI grade 2 and grade 3 had 52–53% increased risk of mortality as compared with patients with ALBI grade 1. These findings indicate that ALBI grade is superior in representing liver reserve and providing prognostic information.
In this study, we aim to determine the independent prognostic predictors for HCC. In additional to ALBI grade, we found that the number and size of tumor nodule were closely related to overall survival of HCC patients. Additionally, consistent with published data [29–33], vascular invasion was identified as an important predictor for patient survival. The performance status of patient, as determined according to the Eastern Cooperative Oncology Group scale, may also independently influence the survival in HCC patients [34, 35]. Moreover, in accordance with previous studies [26, 32, 35–37], our study found that presence of ascites, high AFP and serum alkaline phosphatase level were strongly linked with patient outcomes. Taken together, the extent of tumor involvement, performance status and severity of liver reserve are the hallmarks of survival predictors.
The CTP, MELD and ALBI grades are three major composite models to assess the severity of liver functional reserve. A major shortcoming of the CTP score is its arbitrary cut-off values and subjective variables such as encephalopathy [9]. MELD score is an alternative commonly used marker to indicate liver dysfunction in HCC, and may perform better than the CTP score in different clinical settings. However, serum creatinine level, which is one of the parameters in the MELD, may be less reliable in patients with HCC because cancer-related cachexia might not be fully reflected. Notably, the prognostic role of the other 5 models (Lok index, FIB-4, APRI, GUCI and CDS) has never been validated in patients with HCC undergoing different therapies. In comparison with the other 7 models, the ALBI grade, which incorporates only serum albumin and bilirubin level, is more objective and can be rapidly computed without the need for other special tests [18, 22, 29, 38, 39]. Our study suggests that the ALBI grade is a more feasible and readily available liver reserve marker for risk stratification in HCC patients undergoing TACE.
Among the 8 markers, APRI and FIB-4 index were principally designed as liver fibrosis markers. Therefore, it should be noted that the prognostic impact of these markers could be through the high hepato-carcinogenic potential in the background liver which is associated with increased risk of tumor recurrence, and may not be necessarily through the deterioration of liver functional reserve.
The ALBI score well correlated with baseline CTP and MELD score in HCC patients, suggesting these three models are intrinsically similar tools in assessing liver functional reserve. However, the latter two models performed less well in the cohort of HCC patients undergoing TACE. These results imply that the evaluation based on albumin and bilirubin is clinically more robust and accurate, and the possibility that the ALBI grade may be integrated into the cancer staging system to further refine prognostic information should be considered. However, a potential weakness of the ALBI score is that it could be influenced by albumin replacement therapy or the presence of obstructive jaundice when in some cases HCC may present with obstructive jaundice, and thus might not accurately reflect true liver functional reserve at all times.
This study has a few limitations. First, this is a single center, retrospective study in a predominantly HBV endemic area. Our results require external validation from independent research groups. Second, this study is limited to HCC patients undergoing TACE to specifically address the prognostic role of different liver reserve markers. The accuracy of ALBI grade in patients receiving other therapies needs further studies to establish. Third, some patients did not strictly adhere to the Barcelona Clinic Liver Cancer (BCLC) recommendations. Rather, treatment decisions were decided by the patients and the multidisciplinary HCC team based on shared decision making.
In conclusion, our results indicate that the ALBI grade is the most accurate prognostic model among the 8 noninvasive liver reserve markers. The ALBI grade may serve as an objective, discriminatory and evidence-based method in assessing liver functional reserve. The ALBI grade is clinically more useful due to is superior prognostic power in HCC patients undergoing TACE. Further studies are urgently needed to validate the feasibility of ALBI grade in different clinical scenarios.
Abbreviations
- AFP
α-fetoprotein
- ALBI
albumin-bilirubin
- APRI
aspartate aminotransferase-to-platelet ratio
- BCLC
Barcelona Clinic Liver Cancer
- CDS
cirrhosis discriminant index
- CI
confidence interval
- CT
computed tomography
- CTP
Child-Turcotte-Pugh
- FIB-4
fibrosis index based on 4 factors
- GUCI
Göteborg University cirrhosis index
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- INR
international normalized ratio
- MELD
model for end-stage liver disease
- MRI
magnetic resonance imaging
- SD
standard deviation
- TACE
transarterial chemoembolization
Data Availability
The restrictions set by the Institutional Review Board of Taipei Veterans General Hospital prohibit the authors from making the minimal data set publicly available. For data access, please contact the Director of the IRB of Taipei Veterans General Hospital at d-mre@vghtpe.gov.tw.
Funding Statement
This study was supported by grants from the Center of Excellence for Cancer Research at Taipei Veterans General Hospital (MOHW104-TDU-B-211-124-001), Taiwan, from Taipei Veterans General Hospital (V105C-009, V105A-011, VN106-11), Taiwan. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: a cancer journal for clinicians. 2015;65(2):87–108. Epub 2015/02/06. doi: 10.3322/caac.21262 . [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118–27. Epub 2011/10/14. doi: 10.1056/NEJMra1001683 . [DOI] [PubMed] [Google Scholar]
- 3.Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS. Future of Hepatocellular Carcinoma Incidence in the United States Forecast Through 2030. J Clin Oncol. 2016;34(15):1787–94. doi: 10.1200/JCO.2015.64.7412 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee MH, Yang HI, Liu J, Batrla-Utermann R, Jen CL, Iloeje UH, Lu SN, You SL, Wang LY, Chen CJ. Prediction models of long-term cirrhosis and hepatocellular carcinoma risk in chronic hepatitis B patients: risk scores integrating host and virus profiles. Hepatology. 2013. August;58(2):546–54. doi: 10.1002/hep.26385 . [DOI] [PubMed] [Google Scholar]
- 5.Schutte K, Bornschein J, Malfertheiner P. Hepatocellular carcinoma—epidemiological trends and risk factors. Dig Dis. 2009;27(2):80–92. Epub 2009/06/24. doi: 10.1159/000218339 . [DOI] [PubMed] [Google Scholar]
- 6.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–36. Epub 2005/10/27. doi: 10.1002/hep.20933 . [DOI] [PubMed] [Google Scholar]
- 7.Lee IC, Huo TI, Huang YH, Chao Y, Li CP, Lee PC, et al. Transarterial chemoembolization can prolong survival for patients with metastatic hepatocellular carcinoma: a propensity score matching analysis. Hepatol Int. 2012;6(4):753–62. Epub 2012/10/01. doi: 10.1007/s12072-011-9322-7 . [DOI] [PubMed] [Google Scholar]
- 8.Marrero JA, Fontana RJ, Barrat A, Askari F, Conjeevaram HS, Su GL, et al. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology. 2005;41(4):707–16. Epub 2005/03/30. doi: 10.1002/hep.20636 . [DOI] [PubMed] [Google Scholar]
- 9.Durand F, Valla D. Assessment of prognosis of cirrhosis. Semin Liver Dis. 2008;28(1):110–22. Epub 2008/02/23. doi: 10.1055/s-2008-1040325 . [DOI] [PubMed] [Google Scholar]
- 10.Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97(10):2614–8. Epub 2002/10/19. doi: 10.1111/j.1572-0241.2002.06038.x . [DOI] [PubMed] [Google Scholar]
- 11.Castera L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology. 2012;142(6):1293–302.e4. Epub 2012/04/28. doi: 10.1053/j.gastro.2012.02.017 . [DOI] [PubMed] [Google Scholar]
- 12.Huo TI, Lee PC, Huang YH, Wu JC, Lin HC, Chiang JH, et al. The sequential changes of the model for end-stage liver disease score correlate with the severity of liver cirrhosis in patients with hepatocellular carcinoma undergoing locoregional therapy. J Clin Gastroenterol. 2006;40(6):543–50. Epub 2006/07/11. . [DOI] [PubMed] [Google Scholar]
- 13.Lok AS, Ghany MG, Goodman ZD, Wright EC, Everson GT, Sterling RK, et al. Predicting cirrhosis in patients with hepatitis C based on standard laboratory tests: results of the HALT-C cohort. Hepatology. 2005;42(2):282–92. Epub 2005/06/30. doi: 10.1002/hep.20772 . [DOI] [PubMed] [Google Scholar]
- 14.Bonacini M, Hadi G, Govindarajan S, Lindsay KL. Utility of a discriminant score for diagnosing advanced fibrosis or cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1997;92(8):1302–4. Epub 1997/08/01. . [PubMed] [Google Scholar]
- 15.Udell JA, Wang CS, Tinmouth J, FitzGerald JM, Ayas NT, Simel DL, et al. Does this patient with liver disease have cirrhosis? JAMA. 2012;307(8):832–42. Epub 2012/02/24. doi: 10.1001/jama.2012.186 . [DOI] [PubMed] [Google Scholar]
- 16.Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32–6. Epub 2007/06/15. doi: 10.1002/hep.21669 . [DOI] [PubMed] [Google Scholar]
- 17.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518–26. Epub 2003/07/29. doi: 10.1053/jhep.2003.50346 . [DOI] [PubMed] [Google Scholar]
- 18.Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33(6):550–8. Epub 2014/12/17. doi: 10.1200/JCO.2014.57.9151 ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35(3):421–30. Epub 2001/10/11. . [DOI] [PubMed] [Google Scholar]
- 20.Kao WY, Su CW, Chau GY, Lui WY, Wu CW, Wu JC. A comparison of prognosis between patients with hepatitis B and C virus-related hepatocellular carcinoma undergoing resection surgery. World J Surg. 2011;35(4):858–67. Epub 2011/01/06. doi: 10.1007/s00268-010-0928-z . [DOI] [PubMed] [Google Scholar]
- 21.Choi WM, Lee JH, Ahn H, Cho H, Cho YY, Lee M, et al. Forns index predicts recurrence and death in patients with hepatitis B-related hepatocellular carcinoma after curative resection. Liver Int. 2015;35(8):1992–2000. Epub 2015/01/06. doi: 10.1111/liv.12776 . [DOI] [PubMed] [Google Scholar]
- 22.Liu PH, Hsu CY, Hsia CY, Lee YH, Chiou YY, Huang YH, et al. ALBI and PALBI Grade Predict Survival for HCC across Treatment Modalities and BCLC Stages in the MELD Era. J Gastroenterol Hepatol. 2016. Epub 2016/10/04. doi: 10.1111/jgh.13608 . [DOI] [PubMed] [Google Scholar]
- 23.Hsu CY, Huang YH, Su CW, Lin HC, Chiang JH, Lee PC, et al. Renal failure in patients with hepatocellular carcinoma and ascites undergoing transarterial chemoembolization. Liver Int. 2010;30(1):77–84. Epub 2009/10/13. doi: 10.1111/j.1478-3231.2009.02128.x . [DOI] [PubMed] [Google Scholar]
- 24.Huo TI, Wu JC, Huang YH, Chiang JH, Lee PC, Chang FY, et al. Acute renal failure after transarterial chemoembolization for hepatocellular carcinoma: a retrospective study of the incidence, risk factors, clinical course and long-term outcome. Aliment Pharmacol Ther. 2004;19(9):999–1007. Epub 2004/04/29. doi: 10.1111/j.1365-2036.2004.01936.x . [DOI] [PubMed] [Google Scholar]
- 25.Huang YH, Chen CH, Chang TT, Chen SC, Chiang JH, Lee HS, et al. The role of transcatheter arterial embolization for patients with unresectable hepatocellular carcinoma: a nationwide, multicentre study evaluated by cancer stage. Aliment Pharmacol Ther. 2005;21(6):687–94. Epub 2005/03/18. doi: 10.1111/j.1365-2036.2005.02404.x . [DOI] [PubMed] [Google Scholar]
- 26.Liu PH, Hsu CY, Hsia CY, Lee YH, Su CW, Huang YH, et al. Prognosis of hepatocellular carcinoma: Assessment of eleven staging systems. J Hepatol. 2016;64(3):601–8. Epub 2015/11/10. doi: 10.1016/j.jhep.2015.10.029 . [DOI] [PubMed] [Google Scholar]
- 27.Ibrahim JG, Chu H, Chen MH. Missing data in clinical studies: issues and methods. J Clin Oncol. 2012;30(26):3297–303. Epub 2012/06/01. doi: 10.1200/JCO.2011.38.7589 ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–43. Epub 2012/03/20. doi: 10.1016/j.jhep.2011.12.001 . [DOI] [PubMed] [Google Scholar]
- 29.Waked I, Berhane S, Toyoda H, Chan SL, Stern N, Palmer D, et al. Transarterial chemo-embolisation of hepatocellular carcinoma: impact of liver function and vascular invasion. Br J Cancer. 2017;116(4):448–54. Epub 2017/01/27. doi: 10.1038/bjc.2016.423 ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu PH, Hsia CY, Lee YH, Hsu CY, Huang YH, Su CW, et al. Surgical resection versus transarterial chemoembolization for BCLC stage C hepatocellular carcinoma. J Surg Oncol. 2015;111(4):404–9. Epub 2015/02/04. doi: 10.1002/jso.23854 . [DOI] [PubMed] [Google Scholar]
- 31.Lee YH, Hsu CY, Huang YH, Hsia CY, Chiou YY, Su CW, et al. Vascular invasion in hepatocellular carcinoma: prevalence, determinants and prognostic impact. J Clin Gastroenterol. 2014;48(8):734–41. Epub 2013/10/09. doi: 10.1097/MCG.0b013e3182a8a254 . [DOI] [PubMed] [Google Scholar]
- 32.Huo TI, Hsu CY, Huang YH, Su CW, Lin HC, Lee RC, et al. Prognostic prediction across a gradient of total tumor volume in patients with hepatocellular carcinoma undergoing locoregional therapy. BMC Gastroenterology. 2010;10:146 Epub 2011/01/05. doi: 10.1186/1471-230X-10-146 ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su YW, Liu PH, Hsu CY, Lee YH, Hsia CY, Ho SY, et al. Prognostic impact of diabetes mellitus on hepatocellular carcinoma: Special emphasis from the BCLC perspective. PloS One. 2017;12(3):e0174333 Epub 2017/03/24. doi: 10.1371/journal.pone.0174333 ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu CY, Lee YH, Hsia CY, Huang YH, Su CW, Lin HC, et al. Performance status in patients with hepatocellular carcinoma: determinants, prognostic impact, and ability to improve the Barcelona Clinic Liver Cancer system. Hepatology. 2013;57(1):112–9. Epub 2012/07/19. doi: 10.1002/hep.25950 . [DOI] [PubMed] [Google Scholar]
- 35.Liu PH, Hsu CY, Hsia CY, Lee YH, Huang YH, Su CW, et al. Proposal and validation of a new model to estimate survival for hepatocellular carcinoma patients. Eur J Cancer. 2016;63:25–33. Epub 2016/06/04. doi: 10.1016/j.ejca.2016.04.023 . [DOI] [PubMed] [Google Scholar]
- 36.Tournoux-Facon C, Paoletti X, Barbare JC, Bouche O, Rougier P, Dahan L, et al. Development and validation of a new prognostic score of death for patients with hepatocellular carcinoma in palliative setting. J Hepatol. 2011;54(1):108–14. Epub 2010/11/05. doi: 10.1016/j.jhep.2010.06.015 . [DOI] [PubMed] [Google Scholar]
- 37.Hsu CY, Liu PH, Lee YH, Hsia CY, Huang YH, Lin HC, et al. Using serum alpha-fetoprotein for prognostic prediction in patients with hepatocellular carcinoma: what is the most optimal cutoff? PloS One. 2015;10(3):e0118825 Epub 2015/03/05. doi: 10.1371/journal.pone.0118825 ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hiraoka A, Kumada T, Michitaka K, Toyoda H, Tada T, Ueki H, et al. Usefulness of albumin-bilirubin grade for evaluation of prognosis of 2584 Japanese patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2016;31(5):1031–6. Epub 2015/12/10. doi: 10.1111/jgh.13250 . [DOI] [PubMed] [Google Scholar]
- 39.Chan AW, Kumada T, Toyoda H, Tada T, Chong CC, Mo FK, et al. Integration of albumin-bilirubin (ALBI) score into Barcelona Clinic Liver Cancer (BCLC) system for hepatocellular carcinoma. J Gastroenterol Hepatol. 2016;31(7):1300–6. Epub 2016/01/12. doi: 10.1111/jgh.13291 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The restrictions set by the Institutional Review Board of Taipei Veterans General Hospital prohibit the authors from making the minimal data set publicly available. For data access, please contact the Director of the IRB of Taipei Veterans General Hospital at d-mre@vghtpe.gov.tw.