Abstract
Syncytiotrophoblast extracellular vesicles (STBEVs) are placenta derived particles that are released into the maternal circulation during pregnancy. Abnormal levels of STBEVs have been proposed to affect maternal vascular function. The lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) is a multi-ligand scavenger receptor. Increased LOX-1 expression and activation has been proposed to contribute to endothelial dysfunction. As LOX-1 has various ligands, we hypothesized that, being essentially packages of lipoproteins, STBEVs are able to activate the LOX-1 receptor thereby impairing vascular function via the production of superoxide and decreased nitric oxide bioavailability. Uterine arteries were obtained in late gestation from Sprague-Dawley rats and incubated for 24h with or without human STBEVs (derived from a normal pregnant placenta) in the absence or presence of a LOX-1 blocking antibody. Vascular function was assessed using wire myography. Endothelium-dependent maximal vasodilation to methylcholine was impaired by STBEVs (MCh Emax: 57.7±5.9% in STBEV-incubated arteries vs. 77.8±2.9% in controls, p<0.05). This was prevented by co-incubation of STBEV-incubated arteries with LOX-1 blocking antibodies (MCh Emax: 78.8±4.3%, p<0.05). Pre-incubation of the vessels with a nitric oxide synthase inhibitor (L-NAME) demonstrated that the STBEV-induced impairment in vasodilation was due to decreased nitric oxide contribution (ΔAUC 12.2±11.7 in STBEV-arteries vs. 86.5±20 in controls, p<0.05), which was abolished by LOX-1 blocking antibody (ΔAUC 98.9±17, p<0.05). In STBEV-incubated vessels, LOX-1 inhibition resulted in an increased endothelial nitric oxide synthase expression (p<0.05), to a level similar to control vessels. The oxidant scavenger, superoxide dismutase, did not improve this impairment, nor were vascular superoxide levels altered. Our data support an important role for STBEVs in impairment of vascular function via activation of LOX-1 and reduced nitric oxide mediated vasodilation. Moreover, we postulate that the LOX-1 pathway could be a potential therapeutic target in pathologies associated with vascular dysfunction during pregnancy.
Introduction
STBEVs are heterogeneous membranous vesicles released into the maternal circulation by the multinucleated syncytiotrophoblast layer of the placenta. They are variable in size, ranging from smaller exosomes and ectosomes (50–150 nm) to larger extracellular vesicles (100 nm—1 μm) [1], and during pregnancy they are present from the second trimester onwards, reaching their highest levels at the end of gestation [2, 3]. While STBEVs are naturally released during pregnancy, increased concentrations of STBEVs have been suggested to contribute to potential pathological states such as systemic inflammation and endothelial dysfunction [4]. Specifically, STBEVs have been shown to activate peripheral blood monocytes and peripheral blood mononuclear cells [2, 5], disrupt the monolayer architecture and reduce proliferation of endothelial cells [6]. In addition, some ex vivo studies have shown that STBEVs affect vascular function [7] while others did not observe any changes [8]. Although it has been suggested that the proteins and the danger-associated molecular patterns (DAMPs) that STBEVs carry on their surface (such as heat shock proteins) could mediate their own activity [1], the specific receptor(s) by which STBEVs act and their exact mechanism(s) of downstream action are still unknown.
The lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) is the main receptor involved in the uptake of oxidized low-density lipoprotein (oxLDL) and it has been well-studied in cardiovascular diseases such as atherosclerosis [9] and has been shown to be increased in preeclampsia [10–12], which is characterized by systemic endothelial dysfunction. Activation of LOX-1 by oxLDL impairs vascular function [13] via increased NADPH oxidase activation, and superoxide and peroxynitrite production [12]; leading to decreased nitric oxide (NO) bioavailability [14–16]. In addition, oxLDL stimulation was shown to reduce endothelial nitric oxide synthase (eNOS) expression in endothelial cells in vitro, which was LOX-1 dependent [17, 18]. Further, previous studies from our group have shown that increased LOX-1 expression may be implicated in impaired endothelium-dependent vasodilation during pregnancy [12]. Moreover, in pregnant animals with reduced uterine perfusion pressure aortic LOX-1 expression was increased and was suggested to play a role in the observed endothelial dysfunction [11].
In addition to oxLDL, many other factors have been shown to be ligands for LOX-1 such as: other modified lipoproteins, activated platelets, apoptotic cells and even bacteria [19, 20]. As LOX-1 is a scavenger receptor and STBEVs are, in essence, packages of lipoproteins, we propose that STBEVs contribute to endothelial dysfunction during pregnancy by activating the LOX-1 receptor. In the current study, we have investigated whether syncytiotrophoblast-derived particles such as STBEVs are able to induce endothelial dysfunction in pregnant rat uterine arteries and whether this is LOX-1 dependent. We hypothesized that STBEVs impair vascular function in pregnancy via activation of LOX-1 and increased superoxide production, which leads to decreased NO bioavailability.
Methods
Ethics approval
All animal experiments were conducted at the University of Alberta, Canada, and were approved by the University of Alberta Health Sciences Animal Policy and Welfare Committee in accordance with the Canadian Council on Animal Care Guidelines (AUP #242). The study protocol for human placentae was approved by the Oxfordshire Research Ethics Committee C and STBEV isolations were conducted in Prof. Ian Sargent’s laboratory at Oxford University, U.K. STBEVs were derived from the placenta according to their standard methods described in detail in the manuscript by Dragovic et al. [21]. In short, the placenta was collected from an uncomplicated nulliparous singleton pregnant woman directly after caesarian section (elective) (age 24; 39+1 weeks of gestation; BP 120/80 mmHg; no urinary protein) and, within 20 minutes, an intact lobule was perfused for 3h. The maternal perfusate was collected and centrifuged at high speed (150,000 g) to collect the STBEVs. To confirm the placental origin and normal size distribution of the STBEVs, flow cytometry and Nanoparticle Tracking Analysis were used as described previously [21]. Pellets were diluted in PBS to 1 mg protein ml-1 and frozen until their use in experiments. Written informed consent was obtained.
Animals and experimental design
Three-month-old female Sprague Dawley rats were housed under a standard day:night cycle (10:14 hours) with ad libitum access to food and water. The presence of sperm in a vaginal smear following overnight mating with a male rat was designated as gestational day 0 of pregnancy. On gestational day 20, rats were sacrificed by exsanguination under inhaled isoflurane anesthesia. Main branch uterine arteries were isolated and cut into 2 mm pieces without side branches. Multiple 2 mm uterine artery segments were incubated for 24 hours at 4°C (as adapted from similar experiments published by others [22]) in each of the following groups: 1) physiologic salt solution (PSS) as a control, 2) PSS with LOX-1 blocking antibodies (TS20, 10 μg ml-1), 3) STBEVs (200 μg ml-1 in PSS), or 4) STBEVs (200 μg ml-1 in PSS) together with LOX-1 blocking antibodies (TS20, 10 μg ml-1). The STBEV concentration was based on previous studies [7]. There was no visible difference between the uterine artery segments; therefore each segment was randomly assigned to one of the experimental groups. Two of the incubated segments from each group were then used to assess arterial function using wire myography while the remaining segments (one to two segments per group) were snap frozen for subsequent analyses. LOX-1 blocking antibodies (TS20) were developed by Prof. Sawamura’s laboratory.
Wire myography protocols
After 24 hours of incubation, segments of uterine artery were mounted on a wire myograph (DMT, Copenhagen, Denmark). Arteries were twice exposed to phenylephrine (10 μmol L-1, Sigma-Aldrich; with washout between doses) and once to methylcholine (MCh) (3 μmol L-1, Sigma-Aldrich) following the second phenylephrine dose, to ensure intact endothelial and smooth muscle function. To assess the NO contribution to vasodilation, arteries from each experimental group were pre-incubated for 30 minutes with or without N-nitro-l-arginine methyl ester hydrochloride (n = 12; L-NAME, 100 μmol L-1, Sigma-Aldrich). To assess the influence of superoxide production on vascular function, control and STBEV incubated arteries were pre-incubated for 30 minutes with or without superoxide dismutase (n = 8; polyethylene glycol SOD, 50 U mL-1, Sigma-Aldrich). Following incubation, arteries were pre-constricted with phenylephrine (3 μmol L-1) and vasodilator responses to MCh (0.1 nmol L-1 to 100 μmol L-1) were measured. Finally, to investigate endothelium-independent vasodilator function, arteries were pre-constricted with phenylephrine (3 μmol L-1) and responses to the exogenous NO donor sodium nitroprusside (n = 8; SNP, 0.1 nmol L-1 to 10 μmol L-1, Sigma-Aldrich) were assessed.
Superoxide detection
Frozen sections of uterine artery (n = 8) were cut into 9 μm sections and stained for the presence of superoxide using dihydroethidium (DHE, Biotum, Inc. Hayward, CA, USA) staining. DHE reacts with superoxide to produce ethidium, which generates a red fluorescence that can be quantified. In short, arterial sections were thawed to room temperature for one minute and washed three times with Hanks’ Balanced Salt Solution (HBSS, Life Technologies, Burlington, ON, Canada) for 2 minutes each. Sections were then incubated with HBSS for 10 minutes at 37°C; which was then removed and diluted DHE solution (4 μmol L-1) was added for 30 minutes at 37°C. Afterwards, sections were washed thrice with HBSS (2 minutes each), covered with a coverslip, and fluorescent images were taken immediately.
Endothelial nitric oxide synthase expression, nitrotyrosine levels and LOX-1 expression
Endothelial nitric oxide synthase (eNOS) expression, nitrotyrosine levels and LOX-1 expression in frozen uterine artery sections (9 μm) were measured using immunofluorescent staining. In short, sections were fixed in ice-cold acetone (-20°C) for 10 minutes and allowed to dry for another 10 minutes. Sections were washed 3 times for 5 minutes with phosphate buffered salt solution (PBS, pH 7.4) and incubated with blocking solution (2% BSA in PBS) for 60 minutes at room temperature. Subsequently, the blocking solution was aspirated and sections were incubated with anti-eNOS antibodies (NOS3, rabbit-anti-rat, 1:200, Santa Cruz Biotechnologies), anti-nitrotyrosine antibodies (rabbit-anti-nitrotyrosine, 1:50, Life Technologies) or anti-LOX-1 antibodies (rabbit-anti-rat, 1:50, Santa Cruz Biotechnologies) in 2% BSA in PBS overnight at 4°C. The next day, sections were washed with PBS 3 times for 5 minutes and incubated with secondary goat-anti-rabbit Alexa Fluor 546 (Cy3 wavelength) labeled antibodies (1:250; Molecular Probes/Thermo Fisher Scientific) in 2% BSA in PBS for 60 minutes at room temperature in the dark. Sections were then washed with PBS 3 times for 5 minutes, mounting medium with DAPI (nuclear staining, Vector Laboratories) was added and sections were covered and allowed to dry. Images were taken on the following day.
Image analysis
Images of DHE, eNOS, nitrotyrosine and LOX-1 stained sections of uterine artery were taken using an Olympus IX81 fluorescence microscope with cellSens Dimensions software (Olympus). DHE eNOS, nitrotyrosine and LOX-1 mean staining intensity of the whole vessel was analyzed using ImageJ software. When two arterial segments were present in a sample, an average of the two mean intensities was taken.
Statistical analyses
Statistical analyses were performed using GraphPad Prism software 6.0f (GraphPad software Inc., La Jolla, CA, U.S.). All data were tested for normality using the Shapiro-Wilk normality test. Myography data were summarized as percent maximal vasodilation or area under curve (AUC) and presented as mean ± standard error of the mean. Statistical analysis was performed for comparisons between control arteries and arteries exposed to STBEVs in the absence or presence of the LOX-1 blocking antibody or pegSOD using a two-way ANOVA with Bonferroni multiple comparisons post hoc test. The contribution of NO to endothelial vasodilation was quantified by calculating the delta change in AUC between arteries exposed to L-NAME and the controls and compared between the groups using a one-way ANOVA and Dunnet’s post hoc test. Comparisons of DHE staining between control arteries and arteries exposed to STBEVs or STBEVs + LOX-1 blocking antibody were analyzed using a nonparametric Kruskal-Wallis test with Dunn’s post hoc analysis. Comparisons of vascular responses to SNP and eNOS expression between control arteries and arteries exposed to STBEVs or STBEVs + LOX-1 blocking antibody were analyzed using a one-way ANOVA with Dunnet’s post hoc test. For all statistical tests, differences were considered significant if p<0.05.
Materials
All drugs used for myography protocols were purchased from Sigma-Aldrich (St. Louis, MO, USA). The STBEVs were collected in Prof. Ian Sargent’s laboratory and were derived according to their standard methods. The LOX-1 blocking antibodies (TS20) were developed and supplied by Prof. Sawamura’s laboratory. DHE was purchased at Biotum, Inc. Hayward (CA, USA). HBSS was purchased from Life Technologies (Burlington, ON, Canada). The eNOS antibodies were obtained from Santa Cruz Biotechnologies, and the secondary goat-anti-rabbit Alexa Fluor 546-labeled antibodies from Molecular Probes/Thermo Fisher Scientific (Burlington, ON, Canada).
Results
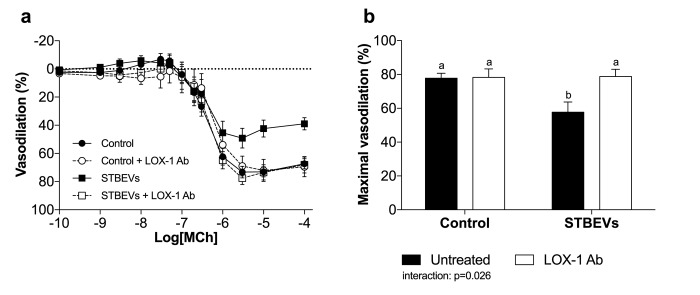
STBEVs impaired MCh-mediated vasodilation in uterine arteries
Maximal MCh-induced vasodilation was reduced in STBEV-incubated uterine arteries (Fig 1A and 1B). The addition of LOX-1 blocking antibodies to arteries exposed to STBEVs resulted in an increased responsiveness to MCh back to responses comparable with controls (Fig 1A and 1B). The LOX-1 blocking antibodies had no effect on vasodilation in control vessels (Fig 1A and 1B).
Fig 1. The effect of STBEV incubation on vascular responses to methylcholine.
(A) Arteries exposed to STBEVs (solid squares, solid line) exhibited impaired vasodilation compared with control arteries (solid circles, solid line). Inhibition of LOX-1 restored endothelial responses in STBEV-exposed arteries (open squares, dashed line) to a level comparable with controls. LOX-1 inhibition had no effect in control arteries (open circles, dashed line). (B) Summary of myography data as percent maximal vasodilation. Data are presented as means ± SEM; two-way ANOVA, ‘a’ denotes statistical difference from ‘b’, p<0.05; n = 11–18/group.
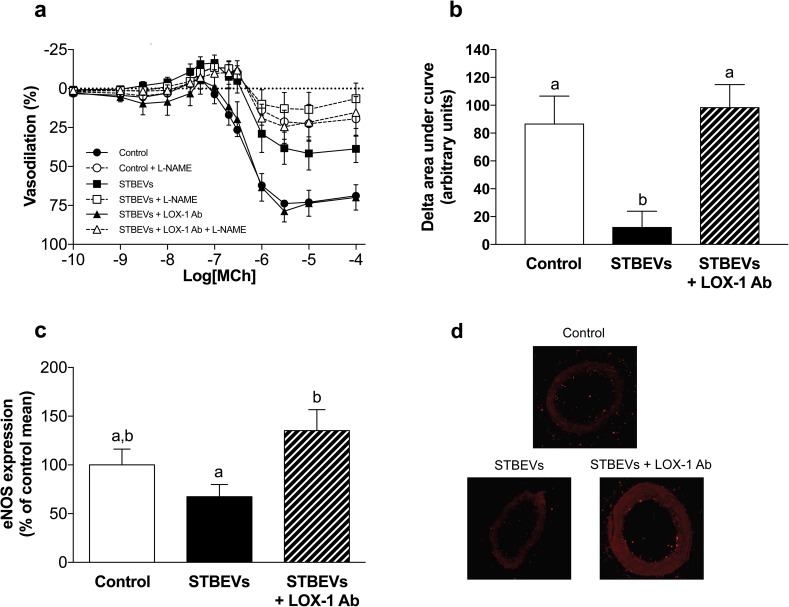
NO contribution to vasodilation was reduced in STBEV-incubated uterine arteries
Inhibition of nitric oxide synthase (NOS) by L-NAME reduced maximal vasodilation to MCh in both control and STBEV-exposed (in the absence or presence of LOX-1 blocking antibodies) vessels (Fig 2A and 2B). The contribution of NO to vasodilation, as assessed by delta AUC, was decreased in STBEV-incubated vessels compared with controls; while incubation with the LOX-1 blocking antibody increased the NO contribution to vasodilation in STBEV-incubated vessels (Fig 2B). In addition to the production of superoxide [14], LOX-1 activation has also been shown to decrease eNOS expression [17, 18]. Compared to controls, exposure to STBEVs did not significantly alter eNOS expression; however, the inhibition of LOX-1 increased eNOS expression in STBEV-exposed arteries (Fig 2C and 2D). The LOX-1 blocking antibody did not alter eNOS expression in control arteries (mean ± SEM: 28.8 ± 11.2 a.u. control vs. 28.6 ± 7.4 a.u. control + LOX-1 antibody).
Fig 2. Changes in nitric oxide contribution to vasodilation after STBEV incubation.
(A) Arteries exposed to STBEVs exhibited impaired vasodilation to methylcholine that was associated with reduced NO bioavailability (squares). Inhibition of LOX-1 improved NOS-mediated vascular responses in STBEV-exposed arteries (triangles) to a level comparable with controls (circles). (B) Quantitative summary of the NO contribution to endothelial vasodilation assessed as delta area under curve (arbitrary units). (C) Endothelial nitric oxide synthase (eNOS) expression in uterine arteries after 24-hour exposure in the absence (control) or presence of STBEVs, with or without a LOX-1 blocking antibody. The expression of eNOS was not significantly altered by exposure to STBEVs (solid bar) as compared with controls (open bars). Inhibition of LOX-1 in STBEV-treated arteries significantly increased eNOS expression (dashed bar). (D) Representative images of eNOS staining. Data are presented as means ± SEM; one-way ANOVA, ‘a’ denotes statistical difference from ‘b’, p<0.05; n = 6–12/group.
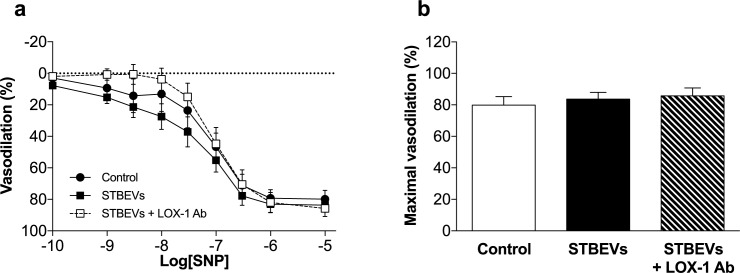
To distinguish whether the effects of STBEVs on NO-mediated vasodilation was the result of effects on altered endothelial or vascular smooth muscle function, we analyzed vascular responses to SNP, an exogenous NO donor. We found that vascular (smooth muscle) responses to SNP were not significantly different between control arteries and those exposed to STBEVs, or STBEVs in the presence of LOX-1 inhibition (Fig 3A and 3B).
Fig 3. Intrinsic vascular smooth muscle cell function is not affected by STBEV incubation.
(A) Vascular smooth muscle cell responses to the exogenous NO donor sodium nitroprusside (SNP) were not significantly different between controls (circles) or arteries exposed to STBEVs, with (open squares) or without (solid squares) a LOX-1 blocking antibody. (B) Summary of myography data as percent maximal vasodilation (Emax). Data are presented as means ± SEM; one-way ANOVA; n = 6–8/group.
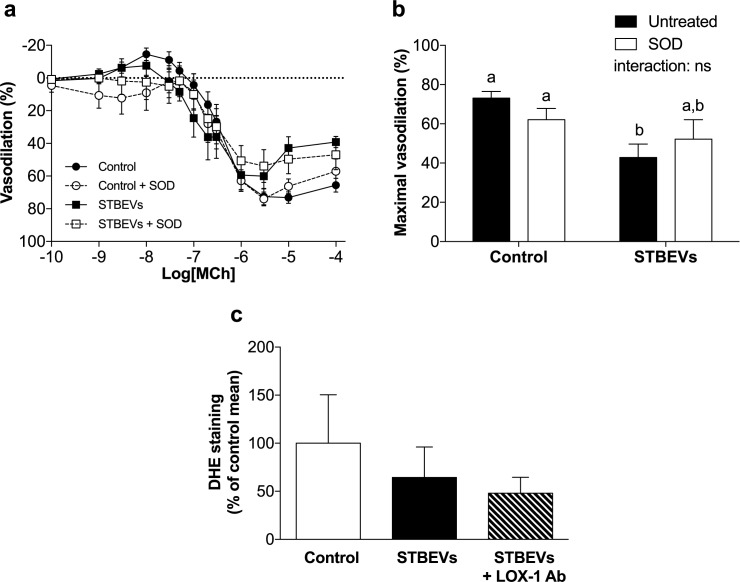
STBEV-induced impaired vasodilation is not mediated by superoxide anions
LOX-1 receptor activation has been shown to increase superoxide production in diseased vasculature [12, 14]. Contrary to our hypothesis that STBEVs would activate LOX-1 and this would induce superoxide production, incubation with pegSOD did not improve MCh-induced vasodilation in STBEV-exposed arteries (Fig 4A and 4B). In addition, sections of uterine arteries were assessed for superoxide production using DHE staining and no differences were found between the groups (Fig 4C). This suggests that the STBEV-induced impairment in endothelial vasodilation was mediated via LOX-1 activation but not associated with increased superoxide production after 24 hours of exposure. Peroxynitrite is an oxidant formed by the reaction of superoxide with nitric oxide that affects vascular function [23]. Nitrotyrosine is one of the reaction products of peroxynitrite. Nitrotyrosine levels in uterine artery sections were not affected by STBEV stimulation (see Online Data Supplement, S1 Fig).
Fig 4. The effect of STBEVs on superoxide production in uterine arteries.
(A) Vascular responses to methylcholine, an endothelium-dependent vasodilator, were impaired after overnight incubation with STBEVs (solid squares, solid line) as compared with controls (solid circles, solid line). However, scavenging of superoxide (pegSOD) did not significantly alter endothelial vasodilation in either control (open circles, dashed line) or STBEV exposed arteries (open squares, dashed line). (B) Summary of myography data as percent maximal vasodilation (Emax). Data in A and B are presented as means ± SEM; two-way ANOVA, ‘a’ denotes statistical difference from ‘b’, ns = not significant; p<0.05. (C) Dihydroethidium (DHE) staining was not significantly different between control, STBEV, and STBEV + anti-LOX-1 antibody exposed arteries. Data in C are presented as median (range); Kruskal-Wallis test. n = 6–8/group.
LOX-1 expression after STBEV incubation in uterine arteries
LOX-1 staining was performed on uterine artery sections to check whether STBEV stimulation had any effect on LOX-1 expression. No changes were observed in LOX-1 expression between the experimental groups (see Online Data Supplement, S2 Fig).
All raw data is provided in S1 File.
Discussion
In the current study, we have shown that STBEVs impaired endothelium-dependent vasodilation in uterine arteries, which appeared to be LOX-1 receptor mediated. We also demonstrated that arteries exposed to STBEVs exhibited an increased LOX-1 contribution to impaired NO-mediated vasodilation. However, vascular superoxide production was unaltered by exposure to STBEVs. Inhibition of LOX-1 in STBEV-incubated vessels increased eNOS expression. These data support our hypothesis that STBEVs can play a role in vascular dysfunction through the activation of LOX-1.
Our data have shown that STBEVs can increase LOX-1 mediated vascular activation and dysfunction. Endothelium-dependent vasodilation in uterine arteries was significantly inhibited by incubation with STBEVs and restored by the presence of LOX-1 blocking antibodies. To our knowledge, we are the first to show that this STBEV-induced endothelial impairment could potentially be mediated via the LOX-1 receptor. LOX-1 activation has previously been suggested to play a role in the endothelial dysfunction commonly seen in cardiovascular diseases, for example, atherosclerosis [19] and during pregnancy [10, 12]. Previous literature has examined the effects of STBEVs on vascular function, but has resulted in conflicting reports. In line with our findings, Cockell et al. have shown that STBEVs impair endothelial-dependent vasodilation in human omental fat arteries [7]. In contrast, while using a similar vascular bed as the current study, Van Wijk et al. did not find any effect of STBEVs in human myometrial arteries [8]. However, this difference could be attributed either to their use of lower STBEV concentrations (i.e. not sufficient to activate LOX-1) or the use of vessels from a different species. By utilizing a pregnant rat model, we obtained uterine arteries for our experiments because they constitute the most important vascular bed supplying the growth and development of the uterus, placenta and fetus during pregnancy.
LOX-1 associated impairment of vasodilation after STBEV stimulation may have several intracellular causes. We have shown that the overall endothelial NO contribution to vasodilation was lower in STBEV incubated arteries as compared with controls. In addition, no change in SNP-induced vasodilation was observed after STBEV stimulation; therefore, smooth muscle cell responses to NO appear unchanged and the STBEV-induced effects appear to be endothelium specific. Activation of LOX-1 by oxLDL has been shown to induce activation of Rho kinase A which may liberate arginase from the mitochondria and increase catabolism of L-arginine, the primary substrate for NOS [24]. In addition, oxLDL has also been shown to decrease eNOS activity in bovine aortic endothelial cells by dephosphorylation, a process that was LOX-1 mediated [25]. Hence, the impaired NO contribution to vasodilation by STBEVs could be mediated via 1) a reduction in NO production by NOS as a result of a LOX-1-induced increase in arginase production and decreased L-arginine availability; or 2) a lack of NO production due to changes in eNOS phosphorylation/activation [26] and/or intracellular localization [27], aspects which could be investigated in further studies.
While previous studies have shown that LOX-1 activation can decrease eNOS expression [17, 18, 28], and our functional data demonstrated that LOX-1 activation by STBEVs reduced NO-mediated relaxation, no changes in vascular eNOS expression were observed after STBEV exposure. Our eNOS measurements included both endothelial and smooth muscle cells. It has been previously demonstrated that all three NOS isoforms are strongly expressed in smooth muscle cells of various types of blood vessels, and specifically in small arterioles [29]. In addition, eNOS has been shown to be expressed in uterine artery vascular smooth muscle cells [30]. Interestingly, there appears to be a regulatory relationship between LOX-1 and eNOS expression as we found that blocking the LOX-1 receptor increased vascular eNOS expression in STBEV-stimulated vessels, while LOX-1 blocking on its own had no effect. In line with these findings, other investigators have shown that eNOS expression can be decreased by LOX-1 activation and returned to control levels after blocking the LOX-1 receptor [17, 18, 28].
LOX-1 activation induces NADPH oxidase dependent superoxide production in bovine aortic and human umbilical vein endothelial cells [14, 31, 32]. Superoxide is then able to scavenge NO, thus reducing NO bioavailability and leading to peroxynitrite formation [14, 31, 32], both of which result in impaired vascular function. In an ex vivo setting, oxLDL-induced impairment of NO mediated vasodilation of mice coronary arterioles was also shown to be LOX-1 and NAPDH oxidase dependent [33]. Interestingly, even though LOX-1 activation was apparent, we did not observe increased superoxide or peroxynitrite (nitrotyrosine) production in sections of STBEV-incubated rat uterine arteries; nor did we observe an improvement of vascular function after adding pegSOD to STBEV-exposed vessels. However, as reactive oxygen species, including superoxide, are short-lived [34], this finding might not have captured an STBEV-induced production of superoxide at an earlier phase of stimulation. Hence, it is possible that superoxide or peroxynitrite may be produced at earlier stages, which activates other signaling pathways that continue to influence vasodilation after 24 hours.
A diverse range of LOX-1 receptor ligands have been described [35] and, because STBEVs are essentially packages of lipoproteins, we proposed that the LOX-1 scavenger receptor might also be activated by STBEVs. In the current study we are, to our knowledge, the first to show that STBEVs could indeed be LOX-1 ligands. Since this study was conducted as an initial proof-of- principle investigation to determine whether syncytiotrophoblast derived particles per se could activate LOX-1, we used a single STBEV-sample that contained a heterogeneous population of extracellular vesicles. The biological relevance of the presented data remains to be further investigated, however, our data suggest that STBEVs are indeed able to activate LOX-1. Interestingly, women with the preeclampsia present with vascular endothelial dysfunction while at the same time this pregnancy disorder has been associated with increased STBEV concentrations [3, 36] in combination with higher LOX-1 expression [12]. Together with our findings, this could suggest that STBEV-induced LOX-1 mediated vascular dysfunction could potentially play a role in this syndrome. This would be of interest in future studies using STBEVs derived from pregnancies complicated by preeclampsia.
During pregnancy, the maternal vasculature is constantly exposed to circulating STBEVs over several months of gestation. The concentrations of STBEVs measured in plasma from pregnant (and preeclamptic) women (20–100 ng ml-1) [36–38] are lower than the STBEV concentration of 200 μg ml-1 that we utilized in our current experiments. This higher concentration of STBEVs for a shorter (24 hours) duration of exposure was used to enable us to assess the possible role of LOX-1 in an ex vivo bioassay and is in-line with other previous studies [7]. We used overnight incubation (similar to the study by van Wijk et al. [22]) to ensure there was enough time for potential interaction, and we have not observed any obvious differences in basal vascular function between overnight-incubated and freshly isolated arteries.
As STBEVs are heterogeneous [1], it may be speculated that mainly vesicles that contain oxidized lipids are able to bind LOX-1. In addition, it has been shown that phosphatidylserine can bind and activate the LOX-1 receptor [39], and these lipoproteins have also been shown to be present on STBEVs [40]. Whether STBEVs are internalized by endocytosis upon LOX-1 activation and have subsequent intracellular effects, which is a general feature of scavenger receptors [35], remains to be studied. Scavenger receptors play an important role in distinguishing self from non-self and in the inflammatory response [35]. Thus it is tempting to speculate from our results that activation of monocytes and peripheral blood mononuclear cells by STBEVs [2, 5] could potentially also be LOX-1 receptor (or other scavenger receptor) dependent. This remains to be investigated further but would greatly enhance the therapeutic potential of LOX-1 blocking.
From a clinical perspective, endothelial dysfunction is a key point of convergence underlying many pathologies; however, the exact mechanism of how placental circulating factors affect the maternal vasculature is still under investigation. In this study, we have provided evidence that STBEVs play a role in the vascular dysfunction. STBEVs, containing vesicles and exosomes derived from placental syncytiotrophoblasts, impaired endothelial vasodilation and were associated with reduced NO bioavailability via the LOX-1 receptor. Not only does this increase our collective understanding of the vascular pathophysiology, but it also provides insight into potential therapeutic strategies by targeting the LOX-1 pathway.
Supporting information
No differences in nitrotyrosine levels were observed between all of the experimental groups. Bars represent means ± SEM; two-way ANOVA. ns = not significant. n = 6–7/group.
(PDF)
No differences in uterine artery LOX-1 expression were found between the experimental groups. Bars represent means ± SEM; two-way ANOVA. ns = not significant. n = 6–7/group.
(PDF)
(ZIP)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by grants from Canadian Institutes of Health Research (CIHR; MOP142320), by the Women and Children’s Health Research Institute (WCHRI) through the generosity of the Stollery Children's Hospital Foundation and supporters of the Lois Hole Hospital for Women, and by the Medical Research Council Programme grant MR/J003360/1. Dr. Spaans was funded by a Postgraduate Fellowship award from Alberta Innovates - Health Solutions (AIHS; PF 201400457), and Dr. Kao by a Clinical Investigator Program of Alberta Health Services (AHS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Redman CW, Tannetta DS, Dragovic RA, Gardiner C, Southcombe JH, Collett GP, et al. Review: Does size matter? Placental debris and the pathophysiology of pre-eclampsia. Placenta. 2012;33 Suppl:S48–54. Epub 2012/01/06. doi: 10.1016/j.placenta.2011.12.006 . [DOI] [PubMed] [Google Scholar]
- 2.Germain SJ, Sacks GP, Sooranna SR, Sargent IL, Redman CW. Systemic inflammatory priming in normal pregnancy and preeclampsia: the role of circulating syncytiotrophoblast microparticles. J Immunol. 2007;178(9):5949–56. . [DOI] [PubMed] [Google Scholar]
- 3.Lok CA, Van Der Post JA, Sargent IL, Hau CM, Sturk A, Boer K, et al. Changes in microparticle numbers and cellular origin during pregnancy and preeclampsia. Hypertens Pregnancy. 2008;27(4):344–60. doi: 10.1080/10641950801955733 . [DOI] [PubMed] [Google Scholar]
- 4.van der Post JA, Lok CA, Boer K, Sturk A, Sargent IL, Nieuwland R. The functions of microparticles in pre-eclampsia. Semin Thromb Hemost. 2011;37(2):146–52. doi: 10.1055/s-0030-1270342 . [DOI] [PubMed] [Google Scholar]
- 5.Messerli M, May K, Hansson SR, Schneider H, Holzgreve W, Hahn S, et al. Feto-maternal interactions in pregnancies: placental microparticles activate peripheral blood monocytes. Placenta. 2010;31(2):106–12. doi: 10.1016/j.placenta.2009.11.011 . [DOI] [PubMed] [Google Scholar]
- 6.Kertesz Z, Linton EA, Redman CW. Adhesion molecules of syncytiotrophoblast microvillous membranes inhibit proliferation of human umbilical vein endothelial cells. Placenta. 2000;21(2–3):150–9. doi: 10.1053/plac.1999.0476 . [DOI] [PubMed] [Google Scholar]
- 7.Cockell AP, Learmont JG, Smarason AK, Redman CW, Sargent IL, Poston L. Human placental syncytiotrophoblast microvillous membranes impair maternal vascular endothelial function. Br J Obstet Gynaecol. 1997;104(2):235–40. . [DOI] [PubMed] [Google Scholar]
- 8.Van Wijk MJ, Boer K, Nisell H, Smarason AK, Van Bavel E, Kublickiene KR. Endothelial function in myometrial resistance arteries of normal pregnant women perfused with syncytiotrophoblast microvillous membranes. BJOG. 2001;108(9):967–72. . [DOI] [PubMed] [Google Scholar]
- 9.Pirillo A, Norata GD, Catapano AL. LOX-1, OxLDL, and atherosclerosis. Mediators Inflamm. 2013;2013:152786 doi: 10.1155/2013/152786 ; PubMed Central PMCID: PMCPMC3723318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.English FA, McCarthy FP, McSweeney CL, Quon AL, Morton JS, Sawamura T, et al. Inhibition of lectin-like oxidized low-density lipoprotein-1 receptor protects against plasma-mediated vascular dysfunction associated with pre-eclampsia. Am J Hypertens. 2013;26(2):279–86. doi: 10.1093/ajh/hps035 . [DOI] [PubMed] [Google Scholar]
- 11.Morton JS, Abdalvand A, Jiang Y, Sawamura T, Uwiera RR, Davidge ST. Lectin-like oxidized low-density lipoprotein 1 receptor in a reduced uteroplacental perfusion pressure rat model of preeclampsia. Hypertension. 2012;59(5):1014–20. doi: 10.1161/HYPERTENSIONAHA.112.191825 . [DOI] [PubMed] [Google Scholar]
- 12.Sankaralingam S, Xu Y, Sawamura T, Davidge ST. Increased lectin-like oxidized low-density lipoprotein receptor-1 expression in the maternal vasculature of women with preeclampsia: role for peroxynitrite. Hypertension. 2009;53(2):270–7. doi: 10.1161/HYPERTENSIONAHA.108.122630 . [DOI] [PubMed] [Google Scholar]
- 13.Mehta JL, Sanada N, Hu CP, Chen J, Dandapat A, Sugawara F, et al. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ Res. 2007;100(11):1634–42. doi: 10.1161/CIRCRESAHA.107.149724 . [DOI] [PubMed] [Google Scholar]
- 14.Cominacini L, Rigoni A, Pasini AF, Garbin U, Davoli A, Campagnola M, et al. The binding of oxidized low density lipoprotein (ox-LDL) to ox-LDL receptor-1 reduces the intracellular concentration of nitric oxide in endothelial cells through an increased production of superoxide. J Biol Chem. 2001;276(17):13750–5. doi: 10.1074/jbc.M010612200 . [DOI] [PubMed] [Google Scholar]
- 15.Eichhorn B, Muller G, Leuner A, Sawamura T, Ravens U, Morawietz H. Impaired vascular function in small resistance arteries of LOX-1 overexpressing mice on high-fat diet. Cardiovasc Res. 2009;82(3):493–502. doi: 10.1093/cvr/cvp089 . [DOI] [PubMed] [Google Scholar]
- 16.Goulopoulou S, Davidge ST. Molecular mechanisms of maternal vascular dysfunction in preeclampsia. Trends Mol Med. 2015;21(2):88–97. doi: 10.1016/j.molmed.2014.11.009 . [DOI] [PubMed] [Google Scholar]
- 17.Bao MH, Zhang YW, Lou XY, Cheng Y, Zhou HH. Protective effects of let-7a and let-7b on oxidized low-density lipoprotein induced endothelial cell injuries. PLoS One. 2014;9(9):e106540 doi: 10.1371/journal.pone.0106540 ; PubMed Central PMCID: PMCPMC4172475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu L, Wang S, Li B, Sun A, Zou Y, Ge J. A protective role of ciglitazone in ox-LDL-induced rat microvascular endothelial cells via modulating PPARgamma-dependent AMPK/eNOS pathway. J Cell Mol Med. 2015;19(1):92–102. doi: 10.1111/jcmm.12463 ; PubMed Central PMCID: PMCPMC4288353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen M, Masaki T, Sawamura T. LOX-1, the receptor for oxidized low-density lipoprotein identified from endothelial cells: implications in endothelial dysfunction and atherosclerosis. Pharmacol Ther. 2002;95(1):89–100. . [DOI] [PubMed] [Google Scholar]
- 20.Sawamura T, Kakino A, Fujita Y. LOX-1: a multiligand receptor at the crossroads of response to danger signals. Curr Opin Lipidol. 2012;23(5):439–45. doi: 10.1097/MOL.0b013e32835688e4 . [DOI] [PubMed] [Google Scholar]
- 21.Dragovic RA, Collett GP, Hole P, Ferguson DJ, Redman CW, Sargent IL, et al. Isolation of syncytiotrophoblast microvesicles and exosomes and their characterisation by multicolour flow cytometry and fluorescence Nanoparticle Tracking Analysis. Methods. 2015;87:64–74. doi: 10.1016/j.ymeth.2015.03.028 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vanwijk MJ, Svedas E, Boer K, Nieuwland R, Vanbavel E, Kublickiene KR. Isolated microparticles, but not whole plasma, from women with preeclampsia impair endothelium-dependent relaxation in isolated myometrial arteries from healthy pregnant women. Am J Obstet Gynecol. 2002;187(6):1686–93. . [DOI] [PubMed] [Google Scholar]
- 23.Forstermann U. Nitric oxide and oxidative stress in vascular disease. Pflugers Arch. 2010;459(6):923–39. doi: 10.1007/s00424-010-0808-2 . [DOI] [PubMed] [Google Scholar]
- 24.Ryoo S, Bhunia A, Chang F, Shoukas A, Berkowitz DE, Romer LH. OxLDL-dependent activation of arginase II is dependent on the LOX-1 receptor and downstream RhoA signaling. Atherosclerosis. 2011;214(2):279–87. doi: 10.1016/j.atherosclerosis.2010.10.044 ; PubMed Central PMCID: PMCPMC3031764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou J, Abid MD, Xiong Y, Chen Q, Chen J. ox-LDL downregulates eNOS activity via LOX-1-mediated endoplasmic reticulum stress. Int J Mol Med. 2013;32(6):1442–50. doi: 10.3892/ijmm.2013.1513 . [DOI] [PubMed] [Google Scholar]
- 26.Michel T, Li GK, Busconi L. Phosphorylation and subcellular translocation of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1993;90(13):6252–6. ; PubMed Central PMCID: PMCPMC46906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michel T, Feron O. Nitric oxide synthases: which, where, how, and why? J Clin Invest. 1997;100(9):2146–52. doi: 10.1172/JCI119750 ; PubMed Central PMCID: PMCPMC508408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakamoto N, Ishibashi T, Sugimoto K, Sawamura T, Sakamoto T, Inoue N, et al. Role of LOX-1 in monocyte adhesion-triggered redox, Akt/eNOS and Ca2+ signaling pathways in endothelial cells. J Cell Physiol. 2009;220(3):706–15. doi: 10.1002/jcp.21818 . [DOI] [PubMed] [Google Scholar]
- 29.Buchwalow IB, Podzuweit T, Bocker W, Samoilova VE, Thomas S, Wellner M, et al. Vascular smooth muscle and nitric oxide synthase. FASEB J. 2002;16(6):500–8. . [DOI] [PubMed] [Google Scholar]
- 30.Welter H, Bollwein H, Weber F, Rohr S, Einspanier R. Expression of endothelial and inducible nitric oxide synthases is modulated in the endometrium of cyclic and early pregnant mares. Reprod Fertil Dev. 2004;16(7):689–98. . [DOI] [PubMed] [Google Scholar]
- 31.Shin HK, Kim YK, Kim KY, Lee JH, Hong KW. Remnant lipoprotein particles induce apoptosis in endothelial cells by NAD(P)H oxidase-mediated production of superoxide and cytokines via lectin-like oxidized low-density lipoprotein receptor-1 activation: prevention by cilostazol. Circulation. 2004;109(8):1022–8. doi: 10.1161/01.CIR.0000117403.64398.53 . [DOI] [PubMed] [Google Scholar]
- 32.Chen XP, Xun KL, Wu Q, Zhang TT, Shi JS, Du GH. Oxidized low density lipoprotein receptor-1 mediates oxidized low density lipoprotein-induced apoptosis in human umbilical vein endothelial cells: role of reactive oxygen species. Vascul Pharmacol. 2007;47(1):1–9. doi: 10.1016/j.vph.2007.01.004 . [DOI] [PubMed] [Google Scholar]
- 33.Xu X, Gao X, Potter BJ, Cao JM, Zhang C. Anti-LOX-1 rescues endothelial function in coronary arterioles in atherosclerotic ApoE knockout mice. Arterioscler Thromb Vasc Biol. 2007;27(4):871–7. doi: 10.1161/01.ATV.0000259358.31234.37 . [DOI] [PubMed] [Google Scholar]
- 34.Ho E, Karimi Galougahi K, Liu CC, Bhindi R, Figtree GA. Biological markers of oxidative stress: Applications to cardiovascular research and practice. Redox Biol. 2013;1:483–91. doi: 10.1016/j.redox.2013.07.006 ; PubMed Central PMCID: PMCPMC3830063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nat Rev Immunol. 2013;13(9):621–34. doi: 10.1038/nri3515 . [DOI] [PubMed] [Google Scholar]
- 36.Knight M, Redman CW, Linton EA, Sargent IL. Shedding of syncytiotrophoblast microvilli into the maternal circulation in pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1998;105(6):632–40. . [DOI] [PubMed] [Google Scholar]
- 37.Dragovic RA, Southcombe JH, Tannetta DS, Redman CW, Sargent IL. Multicolor flow cytometry and nanoparticle tracking analysis of extracellular vesicles in the plasma of normal pregnant and pre-eclamptic women. Biol Reprod. 2013;89(6):151 doi: 10.1095/biolreprod.113.113266 . [DOI] [PubMed] [Google Scholar]
- 38.Chen Y, Huang Y, Jiang R, Teng Y. Syncytiotrophoblast-derived microparticle shedding in early-onset and late-onset severe pre-eclampsia. Int J Gynaecol Obstet. 2012;119(3):234–8. doi: 10.1016/j.ijgo.2012.07.010 . [DOI] [PubMed] [Google Scholar]
- 39.Oka K, Sawamura T, Kikuta K, Itokawa S, Kume N, Kita T, et al. Lectin-like oxidized low-density lipoprotein receptor 1 mediates phagocytosis of aged/apoptotic cells in endothelial cells. Proc Natl Acad Sci U S A. 1998;95(16):9535–40. ; PubMed Central PMCID: PMCPMC21373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gohner C, Weber M, Tannetta DS, Groten T, Plosch T, Faas MM, et al. A New Enzyme-linked Sorbent Assay (ELSA) to Quantify Syncytiotrophoblast Extracellular Vesicles in Biological Fluids. Am J Reprod Immunol. 2015;73(6):582–8. doi: 10.1111/aji.12367 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
No differences in nitrotyrosine levels were observed between all of the experimental groups. Bars represent means ± SEM; two-way ANOVA. ns = not significant. n = 6–7/group.
(PDF)
No differences in uterine artery LOX-1 expression were found between the experimental groups. Bars represent means ± SEM; two-way ANOVA. ns = not significant. n = 6–7/group.
(PDF)
(ZIP)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.