Abstract
Background
In many eukaryotic cells, double-stranded RNA (dsRNA) triggers RNA interference (RNAi), the specific degradation of RNA of homologous sequence. RNAi is now a major tool for reverse-genetics projects, including large-scale high-throughput screens. Recent reports have questioned the specificity of RNAi, raising problems in interpretation of RNAi-based experiments.
Results
Using the protozoan Trypanosoma brucei as a model, we designed a functional complementation assay to ascertain that phenotypic effect(s) observed upon RNAi were due to specific silencing of the targeted gene. This was applied to a cytoskeletal gene encoding the paraflagellar rod protein 2 (TbPFR2), whose product is essential for flagellar motility. We demonstrate the complementation of TbPFR2, silenced via dsRNA targeting its UTRs, through the expression of a tagged RNAi-resistant TbPFR2 encoding a protein that could be immunolocalized in the flagellum. Next, we performed a functional complementation of TbPFR2, silenced via dsRNA targeting its coding sequence, through heterologous expression of the TbPFR2 orthologue gene from Trypanosoma cruzi: the flagellum regained its motility.
Conclusions
This work shows that functional complementation experiments can be readily performed in order to ascertain that phenotypic effects observed upon RNAi experiments are indeed due to the specific silencing of the targetted gene. Further, the results described here are of particular interest when reverse genetics studies cannot be easily achieved in organisms not amenable to RNAi. In addition, our strategy should constitute a firm basis to elaborate functional-dissection studies of genes from other organisms.
Background
RNA interference (RNAi) can be triggered by introduction of long double-stranded RNA molecules (dsRNAs) in cells [1], and proceeds in a number of sequential steps, starting with the cleavage of long dsRNAs into shorter ≈ 21–23 nucleotide-long dsRNAs called short interfering RNAs (siRNAs; these were initially discovered in plants [2]). The enzyme responsible for this chopping (DICER; [3,4]) displays RNase III activity, producing characteristic siRNAs with a phosphorylated 5' end and a two nucleotide-overhanging 3'OH end. These siRNAs enter an RNA-induced silencing complex, or RISC [5,6]. A helicase activity unwinds the two strands of the siRNA, and RISC scans the mRNAs in the cytoplasm and cleaves the molecules that are found complementary to the RISC-contained siRNA [5].
RNA-silencing processes have been described in a variety of organisms: post-transcriptional gene silencing in plants [7,8], quelling in fungi [9], homology-dependent gene silencing in ciliates [10], or RNA interference in worms [1], flies [11,12], trypanosomes [13,14] and mammals [15,16]. It is thought that this machinery has evolved to protect cells against undesirable RNAs, like RNA viruses in plants [17,18], or to limit the mobility of transposable elements in animals [19-21].
While RNAi and associated phenomena constitute exceptional recent basic science findings, they also provided a basis for the elaboration of powerful research tools. RNAi methodologies have been set up to perform reverse-genetics studies in a number of organisms. RNAi potency and flexibility have allowed to perform high-throughput genetic screens in several organisms [22-26]. In mammalian cells, the presence of long dsRNA (>50 base pairs) triggers the activation of sequence-unspecific interferon-related pathways [27-29]. To circumvent this difficulty, researchers resorted to the transfection of small interfering RNAs [16] or in vivo synthesis of small hairpin RNAs, which were demonstrated to produce gene-specific silencing [27,30,31]; reviewed in [32,33].
However, an siRNA might trigger a number of potential unspecific events such as the degradation of partially complementary mRNA due to cross-hybridization, leading to unspecific RNAi, or the translational arrest due to a micro RNA-like effect where an siRNA hybridizes to a mRNA with one or few mismatches. It is thus of paramount importance to ensure that the phenotypic effects observed as a result of siRNA presence in cells are due to silencing of the target gene only. Two large-scale studies show that siRNA-induced gene silencing of transiently- or stably-expressed mRNA is highly gene-specific and does not produce secondary effects detectable by genome-wide expression profiling [34,35]. In contrast, other works provided evidence that siRNAs can be target-unspecific, with the observation of silencing of genes that had limited sequence homology with the siRNA [36,37]. These reports should prompt scientists to assess the specificity of RNAi-silencing in any experiment. A solution to that problem, that we devised in trypanosomes and which is described in this report, is based on the rescueing of the RNAi-mediated loss-of-function phenotype by expressing an RNAi-resistant version of the target gene.
Trypanosomes are protozoan parasites belonging to the Kinetoplastida order. These unicellular flagellated organisms diverged very early in eukaryotic evolution, and exhibit a number of original features [38-40]. Trypanosomes were amongst the first organisms where RNAi was discovered [13,14], and a number of strategies have been devised to either transiently or permanently induce gene-specific RNAi-silencing in these cells [14,41-43]. Examples of successful RNAi in trypanosomes used flagellar genes as targets which yielded easily monitored phenotypes [44]. From a structural point of view, the most conserved morphological feature of eukaryotic flagella is the axoneme, which is made of nine doublets of outer microtubules plus 2 central microtubules (so-called 9+2 axonemal structure). In trypanosomes, the flagellum not only has that axone-mal structure, but it also has a lattice-like structure called the paraflagellar rod (PFR) that is positioned along the axoneme. The two main components of the PFR are TbPFR2 and TbPFR1, that share 60% primary sequence identity [45,46]. TbPFR2 silencing leads to flagellar paralysis and trypanosomes do not swim anymore [13,47]. During the cell cycle, the cell first replicates its mitochondrial DNA (kinetoplast) and starts to grow a new flagellum whilst maintaining the old flagellum in place. Hence, a trypanosome which has two kinetoplasts and two nuclei will be close to completion of its cell cycle and will possess an old and a new flagellum [48]. This aspect is an interesting feature for RNAi-based studies of flagellar morphogenesis, because bi-flagellated cells have an "internal control" flagellum (the old one), while the new one has a phenotype corresponding to the RNAi-based gene knock-down. The presence of both the old and the new flagella in the same cell gives an indication of the time course of events when RNAi is induced in trypanosomes, leading to the appearance of a visible phenotype in the new flagellum while the older one is unchanged because it is not affected by gene silencing.
We previously established the degree of identity between the gene sequences capable of leading to cross-RNAi [47,49]. However, as mentioned earlier, each time a phenotype is observed in RNAi experiments, it is necessary to ensure that it is indeed due to the specific silencing of the targeted gene(s). Inspired by the procedure with which gene knock-out is usually performed (the control experiment is done by re-introducing the knocked-out gene to ensure that the lost function gets complemented), we devised a functional complementation strategy aimed at assessing that RNAi indeed targets the intended gene. This strategy, elaborated using the TbPFR2 gene as a model system, involved the silencing of the TbPFR2 target via its UTRs and the expression of a RNAi-resistant copy of the targetted gene. The RNAi-resistant gene was either a copy of TbPFR2 with different UTRs or its Trypanosoma cruzi orthologue: TcPFR2. We found that inter-species complementation experiments were straight forward. This strategy opens a venue for functional gene dissection experiments where modified gene sequences can be tested for their ability to encode functional protein that can complement the RNAi-based loss-of-function phenotype.
Results and discussion
Multiple RNAi on trypanosomes
We wanted to establish if the co-transfection of two distinct dsRNAs, targeting two different genes, could trigger their simultaneous silencing. The genes selected were TbPFR2 and FLA1; TbPFR2 encodes one of the two major components of the paraflagellar rod and is necessary for flagellum motility [13]; FLA1 encodes a protein required for flagellum attachment to the cell body [50]. These dsRNAs were transfected simultaneously in wild-type trypanosomes. As a control experiment, we used GFP dsRNA.
Cells were monitored for their acquired phenotype 15 h and 22 h after transfection (Table 1). The extinction of TbPFR2 was followed by immunofluorescence microscopy using the L8C4 anti-TbPFR2 monoclonal antibody. FLA1 gene silencing was analyzed by differential interference contrast microscopy, as it results in the visible detachement of the flagellum from the cell body (Figure 2).
Table 1.
Silencing effciencies after transfection of various dsRNAs. WT trypanosomes were transfected with TbPFR2, FLA1 or GFP dsRNA.
| Time (hours) | ||||
| dsRNA | Phenotype (%) | 0 h | 15 h | 22 h |
| TbPFR2 | TbPFR2 silencing | 0 | 61.2 (n = 1069) | 74.2 (n = 1069) |
| FLA1 | FLA1 silencing | 0 | 56 (n = 586) | 54 (n = 472) |
| TbPFR2 + GFP | TbPFR2 silencing | 0 | 56.2 (n = 301) | 70.9 (n = 243) |
| TbPFR2 + FLA1 | TbPFR2 silencing | 0 | 53.6 (n = 349) | 69.7 (n = 210) |
| FLA1 silencing | 0 | 59 (n = 349) | 53 (n = 210) | |
Figure 2.
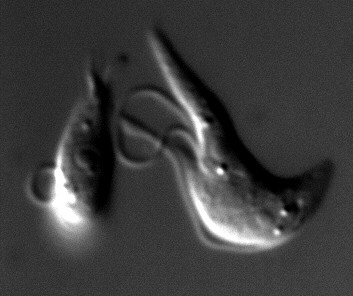
Silencing of FLA1 and TbPFR2 in Trypanosoma brucei. Wild type cells were simultaneously transfected with FLA1 and TbPFR2 dsRNA. At 22 h post-transfection, live cells were observed by differential interference contrast microscopy. Note the detached old flagellum and the dilation at the tip of the new flagellum (shorter one) of the rightmost cell.
The transfection of TbPFR2 dsRNA yielded potent silencing, as more than 60 % of the cells showed no staining for L8C4 15 h later. Since old flagella pre-exist in cells which were affected by RNAi at the beginning of cell replication, the real percentage of silenced cells is probably higher than 60 %, which is confirmed by the fact that it built up to more than 74 % at time point 22 h. The transfection of FLA1 dsRNA produced a phenotype in which the flagellum was detached from the cell body in more than 50 % of the cells. When both dsRNAs were co-transfected, both phenotypes were indeed observed, with similar frequencies to experiments where only one dsRNA was transfected. All the transfected cell populations did show a comparable growth rate (data not shown). The trypanosomes shown in Figure 2 had been transfected with both dsRNAs and the cell on the right is starting cytokinesis. The old flagellum of that cell is detached, while the new flagellum is attached along the cell body. The new flagellum exhibits a dilation of its distal tip, probably corresponding to the accumulation of TbPFR1, that is not assembled but still transported to the distal tip of the flagellum in the absence of TbPFR2 [13]. This observation demonstrates the usefulness of double-transfection experiments also for kinetics analysis. In our case, 22 h after dsRNAs transient transfection, the phenotype due to the FLA1 silencing is no longer visible in the new flagellum while that same flagellum still exhibits the phenotype due to the TbPFR2 silencing, clearly indicating different turn-over for TbPFR2 and FLA1 proteins.
The RNAi machinery could cope with two different dsRNA populations, without – in our conditions – any visible saturation effect. These results show the feasibility of experiments involving the use of multiple dsRNAs, thus allowing studies on complex processes in the cell physiology. However, such complex experiments can only be envisaged after ensuring that the phenotypes resulting from RNAi are specifically due to silencing of the target gene. In order to address that specific problem, we elaborated a method that involves RNAi experiments on trypanosomes that were engineered to possess an extra RNAi-resistant copy of the targeted gene, leading to functional complementation.
Gene silencing by dsRNA targeting UTRs
As a model for this study, we chose the TbPFR2 gene, which is present in four copies in the WT trypanosome genome (Figure 1A), all transcribed as a single long polycistronic mRNA. All these gene copies are separated by three identical intergenic UTRs (igUTR), while the first copy has a unique 5' UTR and the last copy has a unique 3'UTR. Three types of dsRNA populations were used in our experiments, and termed as follows. dsRNA homologous to the GFP sequence was labelled "GFP dsRNA"; dsRNA homologous to coding sequence of the TbPFR2 gene was labelled "CDS dsRNA"; finally, the mixture of three dsRNAs homologous to the 5' UTR, igUTR and 3'UTR of the TbPFR2 gene was termed "UTRs MIX dsRNAs" (Figure 1A). These dsRNAs were transfected into three cell lines: WT, TbPFR2tag and TbPFR2tag-ΔHLA (see Methods). For each experiment, the presence or absence of TbPFR2 in the new flagellum of bi-nucleated/bi-flagellated cells was monitored by immunofluorescence 14 h after the transfection (Table 2).
Figure 1.
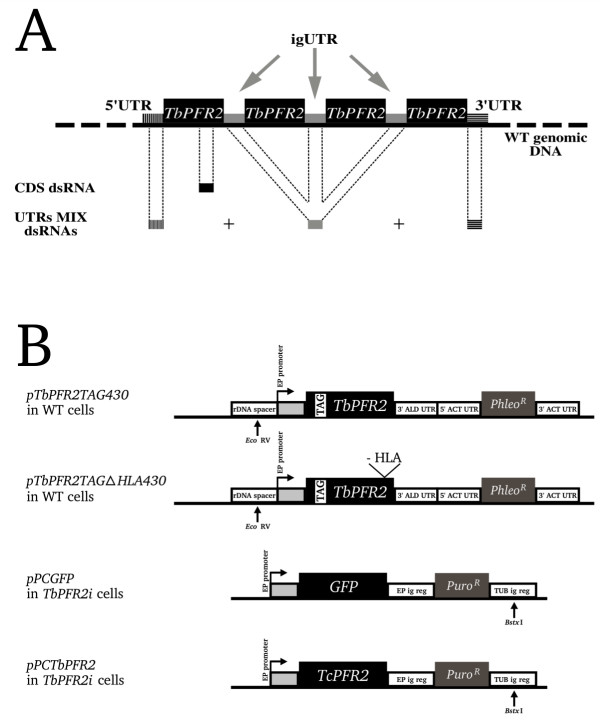
dsRNAs and plasmids used for transfections. (A) Not-to-scale schematic representation of the endogenous TbPFR2 locus, with four copies of TbPFR2 coding sequence and specific UTRs. Regions targeted by RNAi are highlighted. The TbPFR2 coding sequence was targeted using CDS dsRNA and the UTRs were targeted all together with a set of dsRNA homologous to the 3' UTR, the intergenic UTR (igUTR) and the 5' UTR (UTRs MIX dsRNAs). (B) Not-to-scale representation of the constructs used for the transfection of WT cells (pTbPFR2TAG430 and pTbPFR2TAG430-ΔHLA; integration in the rDNA spacer) or TbPFR2i cells (pPCGFP and pPCTcPFR2; integration in the tubulin intergenic region). Large boxes represent protein coding sequences (black boxes: proteins of interest; grey boxes: antibiotic-resistance activities). Each plasmid was linearized with the indicated restriction enzyme prior to transfection into the cells. 3' ALD UTR: 3' UTR of the aldolase gene; ACT UTR: 5' or 3' UTR of the actin gene; EP ig reg: EP procyclin intergenic region; TUB ig reg: tubulin intergenic region.
Table 2.
Silencing effciencies after transfection of various dsRNAs.
| Cell line | TbPFR2 silencing in the new flagellum (% bi-flagellated cells) | |
| dsRNA transfected | ||
| TbPFR2 CDS | TbPFR2 UTRs MIX | |
| WT | 88 % (n = 53) | 62 % (n = 147) |
| TbPFR2tag | 68 % (n = 113) | 2 % (n = 51) |
| TbPFR2tag-ΔHLA | 78 % (n = 100) | 72 % (n = 100) |
WT, TbPFR2tag and TbPFR2tag-ΔHLA trypanosomes were transfected with GFP, TbPFR2 CDS or TbPFR2 UTRs MIX dsRNAs and bi-nucleated/bi-flagellated cells were counted for the status of their new flagellum. The data presented in this table correspond to the immunofluorescence experiment described in Figure 3 (see text for details).
Reports in [14,24] showed that RNAi silencing of a gene can be accomplished by targeting transcribed non-coding sequences. Here, we wanted to make sure that this kind of experiment was still feasible with a more complex system such as the TbPFR2 multigene locus, where multiple and distinct UTRs regulate the expression of four TbPFR2 isogenes. We first transfected WT trypanosomes with GFP dsRNA as a negative control and did not detect any TbPFR2 silencing (Figure 3A). Second, WT trypanosomes were transfected with the CDS dsRNA: 88 % of cells showed typical TbPFR2 silencing with an anti-TbPFR2 immunofluorescence showing that the protein was missing from the new flagellum (Figure 3B). Finally, the WT trypanosomes were transfected with the UTRs MIX dsRNAs, yielding the same phenotype as for the CDS dsRNA, although the silencing appeared less pronounced (Figure 3C and Table 2). Overall, these results demonstrate that RNAi could efficiently silence all of the TbPFR2 gene copies by targeting non-coding sequences present at the mRNA level.
Figure 3.
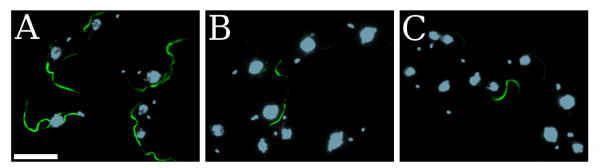
TbPFR2 silencing by targeting its UTRs all together. WT cells were transfected with GFP dsRNA (A), CDS dsRNA (B) or UTRs MIX dsRNAs (C). At 14 h post-transfection, cells were treated for immunofluorescence using the L8C4 anti-TbPFR2 antibody (green) and counterstained with DAPI (blue). Cells of interest are bi-nucleated/bi-flagellated. Transfection of GFP dsRNA did not produce any specific phenotype. Both the CDS dsRNA and the UTRs MIX dsRNAs successfully silenced TbPFR2. Scale bar: 10 μm. See text for details.
When WT trypanosomes were transfected with TbPFR2 dsRNA complementary to only one UTR, the cells did not display any specific phenotype (data not shown). This observation is probably explained by the organization of the TbPFR2 locus: the polycistronic transcript is rapidly spliced into three different types of mRNA, each encoding one of the four copies of TbPFR2 [51-53]. Thus, even if one type of TbPFR2 RNA is destroyed, the three remaining ones would likely provide enough RNA to synthesize TbPFR2 levels compatible with normal PFR formation.
To demonstrate that the silencing observed upon transfection of WT trypanosomes with the UTRs MIX dsRNAs was due to the actual targeting of TbPFR2, we used two cell lines expressing a supplementary tagged TbPFR2 gene copy. The TbPFR2tag cell line expresses the TbPFR2-TAG protein which correctly localizes to the flagellum.
The TbPFR2tag-ΔHLA cell line expresses TbPFR2-TAG-ΔHLA, lacking the HLA tripeptide, which prevents its localization to the flagellum. To determine both the cellular localization of the tagged TbPFR2 proteins (TbPFR2-TAG and TbPFR2-TAG-ΔHLA) and the completeness of the PFR assembly, immunofluorescence experiments were carried out with the BB2 and ROD-1 antibodies; the former recognizes the Ty-1 epitope tag present on the two tagged TbPFR2 proteins [54], while the latter is a marker for full PFR assembly [55,56].
GFP dsRNA was transfected as a negative control in each cell line. As expected, this did not yield any TbPFR2 silencing: TbPFR2 was decorated in both the old and new flagella by the anti-TbPFR2 antibody, and the PFR could be assembled fully, as evidenced by its staining with the ROD-1 antibody (data not shown, Table 2). In TbPFR2tag cells, TbPFR2-TAG was able to localize to the PFR, as evidenced by the PFR decoration with BB2 (red color, Figure 4A). In contrast, TbPFR2-TAG-ΔHLA failed to do so in TbPFR2tag-ΔHLA cells, and the BB2 signal was detected in the cytoplasm, as expected (red color, Figure 4D).
Figure 4.
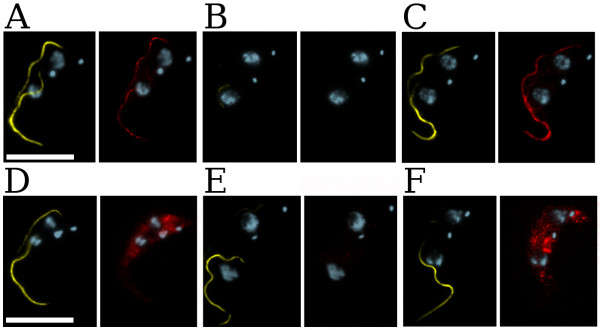
Structural complementation of RNAi mutants. TbPFR2tag (panels A–C) or TbPFR2tag-ΔHLA (panels D–F) cells were transfected with GFP dsRNA (first column), CDS dsRNA (second column) or UTRs MIX dsRNAs (last column). At 14 h post-transfection, cells were treated for immunofluorescence with both the ROD-1 antibody (marker for full PFR assembly, yellow) and the BB2 anti-TAG antibody (red). All cells were counterstained with DAPI (blue). Cells of interest are bi-nucleated/bi-flagellated. Transfection of GFP dsRNA (A, D) did not produce any specific phenotype, whatever the recipient cells: the paraflagellar rod could assemble normally (yellow). As expected, TbPFR2-TAG localized to the flagellum (A, red), while TbPFR2-TAG-ΔHLA accumulated in the cytosol (D, red). The CDS dsRNA (B, E) successfully silenced the WT and the tagged TbPFR2 genes on both cell lines: no tagged TbPFR2 was stained with BB2; the paraflagellar rod could not assemble (no yellow signal is visible in the new flagellum). When using the UTRs MIX dsRNAs in TbPFR2tag and TbPFR2tag-ΔHLA cells, the wild-type TbPFR2 gene is silenced (see Figure 3). However, only in the TbPFR2tag cells does TbPFR2-TAG complement the missing TbPFR2 protein: TbPFR2-TAG was stained in the flagellum in (C, red). TbPFR2-TAG-ΔHLA failed to complement in the TbPFR2tag-ΔHLA cells: TbPFR2-TAG-ΔHLA was stained in the cytoplasm in (F, red). Thus, the paraflagellar rod could assemble fully in (C, yellow) but failed to do so in (F, yellow). Scale bar: 10 μm.
We next compared TbPFR2tag trypanosomes after transfection with either CDS dsRNA or UTRs MIX dsRNAs. TbPFR2tag cells transfected with CDS dsRNA had a flagellum not (or faintly) decorated with the anti-TbPFR2 antibody, demonstrating that both the WT and the recombinant TbPFR2 gene copies were effciently silenced (Table 2). That result was confirmed with anti-TAG immunofluorescence that showed no staining of the flagellum, demonstrating that TbPFR2-TAG was absent (no red color, Figure 4B). This lack of both TbPFR2 and TbPFR2-TAG led to an incomplete assembly of the PFR, which was therefore not decorated with the ROD-1 antibody (no yellow color, Figure 4B). In contrast, cells transfected with the UTRs MIX dsRNAs exhibited a WT phenotype, with only 2 % of the cells displaying TbPFR2 silencing in the flagellum (Table 2). In this case, the tagged protein was expressed, leading to complete assembly of the PFR (yellow color, Figure 4C) because the protein is functional and localized to the flagellum (red color, Figure 4C; [56]). This remarkable result indicates a complementation phenomenon that is explained by the fact that the tagged TbPFR2 gene was not silenced, as it was expressed from a coding sequence flanked by UTRs from the expression vector: from the 5' UTR of the procyclin gene and from the 3'UTR of the aldolase gene (Figure 1B; [57]).
To definitely demonstrate that the complementation described above is indeed due to the expression of functional TbPFR2-TAG, we transfected the same dsRNA into TbPFR2tag-ΔHLA trypanosomes expressing a modified TbPFR2 protein missing three amino acids (that is nine nucleotides out of 1800). TbPFR2tag-ΔHLA does not access the flagellar compartment and thus cannot be functional [58]. Transfecting either CDS dsRNA or UTRs MIX dsRNAs produced cells in which the new flagellum was not (or faintly) decorated by the anti-TbPFR2 antibody (Table 2). TbPFR2-TAG-ΔHLA was not decorated by the anti-TAG antibody when cells were transfected with CDS dsRNA (red color, Figure 4E), indicating that both the WT and the tagged TbPFR2 copies were silenced, thus leading to an incomplete PFR edification (yellow color, Figure 4E). In contrast, transfection of UTRs MIX dsRNAs did not prevent the expression of the recombinant TbPFR2-TAG-ΔHLA protein, as it appeared stained by the anti-TAG antibody (red color, Figure 4F). However, that non-functional protein could not participate in the construction of the PFR, as shown by the absence of ROD-1 signal in the new flagellum, since it cannot access the flagellum (yellow color, Figure 4F).
RNA-directed RNA polymerase activity (RdRP) has been implicated as one possible step in the formation of siRNA in fungi [59], plants [17,18], and worms [60]. The fact that we could specifically silence WT TbPFR2 by targeting its UTRs, without interfering with the tagged TbPFR2 genes, suggests that spreading of silencing beyond the initial targeted sequence does not occur in trypanosomes [61-64].
Functional complementation with orthologue genes
We next asked if an RNAi-mediated loss of function could be complemented by the expression of a gene orthologue to the silenced one. The system used to answer that question involved the TbPFR2i cell line – that expresses TbPFR2 dsRNA under the control of a tetracycline-inducible promoter [47] – into which constitutive expression of Trypanosoma cruzi TbPFR2 orthologue (TcPFR2) was established using stable transfection procedures. TbPFR2 and TcPFR2 proteins share 90 % identity (both of them are recognized by the anti-TbPFR2 L8C4 antibody), but their gene sequences have diverged enough for us to envisage that the RNAi-silencing of TbPFR2 would not affect significantly the introduced TcPFR2 gene (83 % overall nucleotide identity). We thus created two new cell lines based on the previously described TbPFR2i cells [47] (see Methods). TbPFR2 expression and cell motility were analyzed.
Our first experiment showed that the PCGFP cells constitutively expressed GFP, as detected by microscope observation of living cells (data not shown). Both the PCGFP and PCTcPFR2 cell lines were induced to express TbPFR2 dsRNA for 48 hours. Immunofluorescence revealed that non-induced PCGFP cells exhibit a WT-like phenotype (Figure 5A, -TET). When these cells were induced with tetracycline, expected TbPFR2 silencing occurred (Figure 5A, +TET). Non-induced PCTcPFR2 cells displayed an intense anti-TbPFR2 antibody decoration with bright dots in the cytoplasm, indicative of TcPFR2 overexpression (such overexpression by the EP procyclin promoter is frequent; Figure 5B, -TET). When these same cells were tetracycline-induced, the flagellar staining was still perfectly visible, at a level comparable to the one previously observed in the non-induced PCGFP control cells (Figure 5B, +TET). Bright dots previously observed had disappeared, probably as a result of TbPFR2 silencing. The fact that the paraflagellar rod was still neatly decorated by the anti-TbPFR2 antibody demonstrated that the structural inter-species complementation had indeed taken place in these cells, with TcPFR2 being effectively located at the flagellum.
Figure 5.
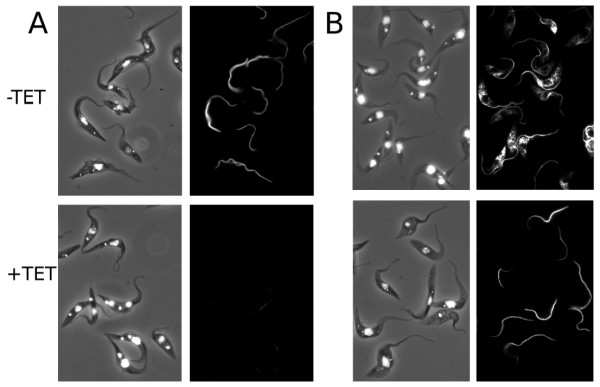
Interspecies structural complementation of RNAi mutants. TbPFR2i-derived trypanosomes, constitutively expressing GFP (panel A; PCGFP cells) or TcPFR2 (panel B; PCTcPFR2 cells) were cultured for 48 h in the absence (-TET) or in the presence (+TET) of tetracycline. In these TbPFR2i-derived cells, tetracyline-induction triggers RNAi-silencing of the endogenous TbPFR2 gene. The cells were then treated for immunofluorescence using L8C4 as an anti-TbPFR2 (right panel, black background) and counterstained with DAPI (left panel, merged with phase contrast image). Non-induced PCGFP cells show normal flagellar staining (A, -TET) while induced cells show an almost total loss of flagellar signal (A, +TET) due to silencing of TbPFR2. Non-induced PCTcPFR2 cells show an intense L8C4 signal in the flagellum and sometimes in the cytoplasm, due to overexpression (B, -TET). Upon tetracycline-induction of these cells, the flagellar L8C4 decoration did not disappear, indicating that TcPFR2 was not subject to RNAi and that TcPFR2 could successfully localize to the flagellum (B, +TET).
Did these structurally-complemented cells show a functional complementation, i.e. a normal flagellum motility (hence a normal cellular mobility)? To address this question, we performed a sedimentation assay [56] on non-induced and tetracycline-induced PCGFP and PCTcPFR2 trypanosomes (Figure 6). Non-induced PCGFP cells showed a little tendency to sediment due to the fact that expression of TbPFR2 dsRNA in TbPFR2i cells is partially leaky, producing low amounts of TbPFR2 dsRNA even in the absence of tetracycline ([43]; Durand-Dubief and Bastin, unpublished data). When expression of TbPFR2 dsRNA was fully induced, motility stopped leading to increased sedimentation (Figure 6, left panel). In contrast, expression of TbPFR2 dsRNA in PCTcPFR2 cells did not reduce motility (Figure 6, right panel). That result definitely demonstrates that the ortholog protein TcPFR2 fully complemented the loss of function resulting from TbPFR2 silencing.
Figure 6.
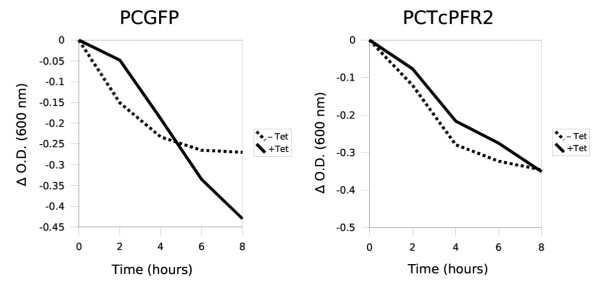
Phenotype analysis by sedimentation assay. PCGFP and PCTcPFR2 trypanosomes were induced (+Tet) with tetracycline for 48 h or non-induced (-Tet) and grown in cuvettes. Non-motile cells do not swim and sediment to the bottom of the cuvette, leading to a reduction in optical density that can be monitored over time. The PCTcPFR2 cells show an almost perfect functional complementation phenotype, as induced and non-induced cells had virtually identical sedimentation curves.
The complementation described above shows the robustness of our strategy, because TbPFR2 and TcPFR2 are highly similar (they share 82 % identity at the nucleotide level [65]) and are nonetheless correctly differenciated by the RNAi machinery. However, our complementation strategy might be more diffcult to implement when the gene studied is too similar to the T. brucei counterpart. While this unfavorable case might happen with extremely evolutionarily-related organisms, studies have shown that the overall genetic sequence identity between Trypanosoma brucei and Trypanosoma cruzi, for example (the closest evolutionarily-related organisms envisaged for these studies), is roughly 80 % ([65] and [66]). [47] showed that this identity percentage is still compatible with an RNAi-based complementation strategy. It goes without saying that when the organisms are evolutionarily-distant, gene sequences diverge more rapidly than the protein sequences, thus laying off a field where our strategy can be implemented with good confidence that complementation will occur.
Conclusions
In this report, we demonstrated that RNAi-mediated silencing of a gene by targeting its UTRs is useful in studies where the loss of function resulting from this silencing must be complemented with the expression of an RNAi-resistant copy of the silenced gene, in order to demonstrate that the phenotype is indeed due to silencing of that gene, and not to inactivation of another one. The results obtained in this work are of particular interest when reverse-genetics studies cannot be easily achieved in organisms not amenable to RNAi, like Leishmania [67] or Trypanosoma cruzi [68], or where genetics experiments are hardly set up, like mammals. When genes from these organisms are to be studied, a complementation experiment can be set up as a three-step procedure whereby: 1) the ortholog gene in Trypanosoma brucei is RNAi-silenced and the loss-of-function phenotype is established; 2) T. brucei cells are engineered to ensure constitutive heterologous expression of the gene of interest, still allowing RNAi-mediated silencing of the T. brucei gene; 3) function of the investigated gene is assessed by checking if the loss-of-function phenotype observed in the first place gets complemented. Additionally, one application of the strategy described herein is genetic functional dissection, which is of interest when protein domains are to be characterized with respect to their function (e.g. the HLA tripeptide sequence in TbPFR2 that localizes the protein to the flagellum).
Complementation had previously been demonstrated following transformation of mammalian cells with EGFP siRNA and expression of a codon-modified, but functional, EGFP version [69]. Our strategies are increasing flexibility for complementation studies after RNAi as unmodified genes can be used for rescue.
Methods
Trypanosomes
The procyclic T. brucei brucei strain 427 (or its derivatives) was used throughout this work. Cells were cultured at 27°C in semi-defined medium 79 (SDM 79) containing 10% foetal calf serum. PFRAi cells were described in [47]. The TbPFR2i trypanosomes can be tetracycline-induced to express TbPFR2 dsRNA, thus eliciting an RNAi response against that gene. Note that this cell line is referred to as TbPFR2i in this article because of a change in the gene nomenclature [70].
RNAi assays by transient transfection
RNA was synthesized in vitro with T3 and Sp6 polymerases using PCR products as templates [71]. The following primers (incorporating T3 or Sp6 promoters) were used:
for GFP (from the nucleotide coding sequence 476–691 of the EGFPN2 gene; Clontech), AATTAACCCTCACTAAAGGGAGAAG AACGGCATCAAGGTGAAC (T3 promoter italicized) and ATTTAGGTGACACTATAGAAG AGTGATCCCGGCGGCGGTCACG (Sp6 promoter italicized);
for FLA1, AATTAACCCTCACTAAAGGGAGA CCAAACCGTGGGCACCAAGG (T3 promoter italicized) and ATTTAGGTGAACTATAGAAGAG GTGGGATGATTAAAACGAGC (Sp6 promoter italicized);
for the TbPFR2 5' untranslated region (5' UTR; nucleotide sequence [-545→-1] upstream of TbPFR2 ATG start codon), AATTAACCCTCACTAAAGGGAGA (T3 promoter) and ATTTAGGTGACACT-ATAGAAGAG (Sp6 promoter);
for the TbPFR2 intergenic untranslated region (igUTR), AATTAACCCTCACTAAAGGGAGA CGCTGCGCTTAAATGTCTT (T3 promoter italicized) and ATTTAGGTGACACTATAGAAGA GTGATGCTTTATTGCTTTCT (Sp6 promoter italicized);
for the TbPFR2 3'untranslated region (3'UTR; nucleotide sequence [1→533] downstream of the TbPFR2 TAG stop codon), AATTAACCCTCACTAAAGGGAGA (universal T3 promoter) and ATTTAGGTGACACTATAGAAGAG (universal Sp6 promoter);
for the TbPFR2 coding sequence (CDS; nucleotide coding sequence [1084→1358]), ATTTAGGTGACACTATAGA GAGGTGAAGCGCCGTATTGAGGA (Sp6 promoter italicized) and AATTAACCCTCACTAAAGGGAGA GTTTTGTACAGGCGACGGAA (T3 promoter italicized);
Figure 1A shows the TbPFR2 locus and the position of the two dsRNA populations that were used, and their homology to either the coding sequence (labelled "CDS dsRNA") or the different 5'UTR, igUTR and 3'UTR all together (labelled "UTRs MIX dsRNAs"). A third dsRNA, homologous to the GFP gene is labelled "GFP dsRNA" throughout this work and was used as a control dsRNA. dsRNA was introduced into trypanosomes by electroporation, as described [14].
Plasmids
Plasmid pPC was generated from plasmid pSk1-GFP [50] as follows: pSk1-GFP was digested with Hind III and Eco RI to remove the GFP gene. Oligonucleotides AGCT GTCTAGCGATATCGGATCCG (forward) and AATT CGGATCCGATATCGCTAGCA (reverse) were annealed (protruding ends italicized) and the resulting double-strand oligonucleotide was ligated into the pSk1-GFP plasmid, resulting in the insertion of a poly-linker containing restriction sites Cla I, Hind III, Nhe I, Eco RV, Bam HI and Eco RI (Branche and Bastin; unpublished data). Plas-mid pPCTcPFR2 was generated as follows: amplification of the TcPFR2 gene was performed using Trypanosoma cruzi genomic DNA (kind gift of Cécile Gallet and Philippe Grellier, MNHN) and the two primers TcPFR2H (GAGTCTAAGCTTATGAGCTACAAGGAGGCATC) and TcPFR2ER (GCGTGGAATTCTTACTGTGTGATCTGCTGCAC). Both the amplified DNA fragment and the pPC plasmid were digested with Eco RI and Hind III. The fragment was ligated into pPC so as to yield the plasmid pPCTcPFR2 (Figure 1B).
Cell lines
The different constructs used to transform trypanosomes are shown on Figure 1B. The cell lines were established as follows.
WT-derived trypanosomes constitutively expressing TbPFR2-TAG proteins
The TbPFR2tag cell line was derived from the WT cell line into which the pTbPFR2TAG430 plasmid [72] was transfected. The recombinant cells constitutively expressed the TbPFR2-TAG protein, that is localized in the flagellum (Fig 4D). Tagged TbPFR2 is known to be functional [56,72]. In contrast, transformation of WT cells with the pTbPFR2TAGΔHLA430 plasmid lead to the expression of slightly modified TbPFR2 protein, missing only three amino acids, that failed to enter the flagellum compartment and hence was found in the cell body cytoplasm [58] (Fig 4G). This cell line was called TbPFR2tag-ΔHLA. After electroporation [73], cells were grown overnight and then distributed in 24-well plates in the presence of phleomycin (2 μg/mL) for selection.
TbPFR2i-derived trypanosomes constitutively expressing GFP and TcPFR2
TbPFR2i cells [47] constituted the genetic background into which we established the PCGFP and PCTcPFR2 new cell lines. The PCGFP cell line was established by transfecting TbPFR2i cells with plasmid pPCGFP after linearization with BstX I. For establishing the PCTcPFR2 cell line, the pPCTcPFR2 plasmid was linearized with BstX I and transfected into TbPFR2i cells. Recombinant cells were selected by addition of puromycin (1 μg/mL), phleomycin (2 μg/mL), G418 (15 μg/mL) and hygromycin (20 μg/mL) to the culture medium.
Immunofluorescence and microscopy
Three different monoclonal antibodies were used as hybridoma supernatants: L8C4, IgG recognizing T. brucei TbPFR2 and cross-reacting with T. cruzi orthologue TcPFR2 [74]; BB2, IgG recognizing the Ty-1 tag of the TbPFR2-TAG and TbPFR2-TAG-ΔHLA recombinant proteins [54]; and ROD-1, IgM recognizing a doublet of minor PFR proteins [55]. For immunofluorescence, trypanosomes were spread onto poly-L-lysine-coated slides, fixed in cold methanol and processed as described [75]. Experiments involving the use of L8C4 only were performed with an FITC-conjugated anti-mouse IgG secondary antibody. Double-staining experiments using BB2 and ROD-1 were performed with a TRITC-conjugated specific anti-mouse IgG secondary antibody and an FITC-conjugated specific anti-mouse IgM secondary antibody. DNA was systematically stained with 4',6-diamidino-2-phenylindole (DAPI). Slides were examined with a Leica DMR microscope, images were captured using a cooled CCD camera (Cool Snap HQ, Roper Scientific) and processed with the GNU image manipulation program version 2 [76].
Cell sedimentation assay
The trypanosome sedimentation assay was performed as described in [56]. Briefly: trypanosomes were grown at ≈ 5.106 cells/mL in normal culture medium, with or without 48 hour tetracycline induction. 1 mL of these cultures was dispensed to 5 plastic spectrophotometry cuvettes, for time points 0, 2, 4, 6, 8 hours, and left still. At each time point, the optical density at 600 nm was measured twice: first without mixing (O.D.no mix) and second after mixing the cuvette (O.D.mix). Data were plotted as a function of time.
Authors' contributions
F.R. carried out most of the experiments reported and wrote the manuscript, M.D.-D. performed the double transfection reported at Table 1 & Figure 2 and P.B. conceived the study and participated in its design and coordination.
Acknowledgments
Acknowledgements
We wish to thank Linda Kohl for live image acquisition, Carole Branche for providing pPC, Cécile Gal-let and Philippe Grellier for providing T. cruzi genomic DNA and Sabrina Benghanem for helpful discussions. M.D.-D. is supported by a "Bourse de formation recherche du Gouvernement luxembourgeois". This work was financed with the following grants: "ACI dynamique et réactivité des assemblages biologiques", (CNRS and Ministère de la recherche), "ACI biologie du développement et physiologie intégrative" (Ministère de la recherche), "GIS recherche sur les maladies rares" (INSERM and Institut des Maladies rares).
Contributor Information
Filippo Rusconi, Email: rusconi@mnhn.fr.
Mickaël Durand-Dubief, Email: duranddu@mnhn.fr.
Philippe Bastin, Email: pbastin@mnhn.fr.
References
- Fire A, Xu S, Montgomery M, Kostas S, Driver S, Mello C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–11. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Hamilton A, Baulcombe D. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science. 1999;286:950–2. doi: 10.1126/science.286.5441.950. [DOI] [PubMed] [Google Scholar]
- Bernstein E, Caudy A, Hammond S, Hannon G. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–6. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- Zamore P, Tuschl T, Sharp P, Bartel D. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell. 2000;101:25–33. doi: 10.1016/S0092-8674(00)80620-0. [DOI] [PubMed] [Google Scholar]
- Nykanen A, Haley B, Zamore P. ATP requirements and small interfering RNA structure in the RNA interference pathway. Cell. 2001;107:309–21. doi: 10.1016/S0092-8674(01)00547-5. [DOI] [PubMed] [Google Scholar]
- Schwarz D, Hutvagner G, Haley B, Zamore P. Evidence that siRNAs function as guides, not primers, in the Drosophila and human RNAi pathways. Mol Cell. 2002;10:537–48. doi: 10.1016/S1097-2765(02)00651-2. [DOI] [PubMed] [Google Scholar]
- Napoli C, Lemieux C, Jorgensen R. Introduction of a Chimeric Chalcone Synthase Gene into Petunia Results in Reversible Co-Suppression of Homologous Genes in trans. Plant Cell. 1990;2:279–289. doi: 10.1105/tpc.2.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Krol A, Mur L, Beld M, Mol J, Stuitje A. Flavonoid genes in petunia: addition of a limited number of gene copies may lead to a suppression of gene expression. Plant Cell. 1990;2:291–9. doi: 10.1105/tpc.2.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogoni C, Irelan J, Schumacher M, Schmidhauser T, Selker E, Macino G. Transgene silencing of the al-1 gene in vegetative cells of Neurospora is mediated by a cytoplasmic effector and does not depend on DNA-DNA interactions or DNA methylation. EMBO J. 1996;15:3153–63. [PMC free article] [PubMed] [Google Scholar]
- Ruiz F, Vayssie L, Klotz C, Sperling L, Madeddu L. Homology-dependent gene silencing in Paramecium. Mol Biol Cell. 1998;9:931–43. doi: 10.1091/mbc.9.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerdell J, Carthew R. Use of dsRNA-mediated genetic interference to demonstrate that frizzled and frizzled 2 act in the wingless pathway. Cell. 1998;95:1017–26. doi: 10.1016/S0092-8674(00)81725-0. [DOI] [PubMed] [Google Scholar]
- Misquitta L, Paterson B. Targeted disruption of gene function in Drosophila by RNA interference (RNA-i): a role for nautilus in embryonic somatic muscle formation. Proc Natl Acad Sci U S A. 1999;96:1451–6. doi: 10.1073/pnas.96.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastin P, Sherwin T, Gull K. Paraflagellar rod is vital for trypanosome motility. Nature. 1998;391:548. doi: 10.1038/35300. [DOI] [PubMed] [Google Scholar]
- Ngô H, Tschudi C, Gull K, Ullu E. Double-stranded RNA induces mRNA degradation in Trypanosoma brucei. Proc Natl Acad Sci U S A. 1998;95:14687–92. doi: 10.1073/pnas.95.25.14687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wianny F, Zernicka-Goetz M. Specific interference with gene function by double-stranded RNA in early mouse development. Nat Cell Biol. 2000;2:70–5. doi: 10.1038/35000016. [DOI] [PubMed] [Google Scholar]
- Elbashir S, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–8. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Mourrain P, Beclin C, Elmayan T, Feuerbach F, Godon C, Morel J, Jouette D, Lacombe A, Nikic S, Picault N, Remoue K, Sanial M, Vo T, Vaucheret H. Arabidopsis SGS2 and SGS3 genes are required for posttranscriptional gene silencing and natural virus resistance. Cell. 2000;101:533–42. doi: 10.1016/S0092-8674(00)80863-6. [DOI] [PubMed] [Google Scholar]
- Dalmay T, Hamilton A, Rudd S, Angell S, Baulcombe D. An RNA-dependent RNA polymerase gene in Arabidopsis is required for posttranscriptional gene silencing mediated by a transgene but not by a virus. Cell. 2000;101:543–53. doi: 10.1016/S0092-8674(00)80864-8. [DOI] [PubMed] [Google Scholar]
- Tabara H, Sarkissian M, Kelly W, Fleenor J, Grishok A, Timmons L, Fire A, Mello C. The rde-1 gene, RNA interference, and transposon silencing in C. elegans. Cell. 1999;99:123–32. doi: 10.1016/S0092-8674(00)81644-X. [DOI] [PubMed] [Google Scholar]
- Ketting R, Haverkamp T, van Luenen H, Plasterk R. Mut-7 of C. elegans, required for transposon silencing and RNA interference, is a homolog of Werner syndrome helicase and RNaseD. Cell. 1999;99:133–41. doi: 10.1016/S0092-8674(00)81645-1. [DOI] [PubMed] [Google Scholar]
- Sijen T, Plasterk R. Transposon silencing in the Caenorhabditis elegans germ line by natural RNAi. Nature. 2003;426:310–4. doi: 10.1038/nature02107. [DOI] [PubMed] [Google Scholar]
- Fraser A, Kamath R, Zipperlen P, Martinez-Campos M, Sohrmann M, Ahringer J. Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature. 2000;408:325–30. doi: 10.1038/35042517. [DOI] [PubMed] [Google Scholar]
- Gonczy P, Echeverri C, Oegema K, Coulson A, Jones S, Copley R, Duperon J, Oegema J, Brehm M, Cassin E, Hannak E, Kirkham M, Pichler S, Flohrs K, Goessen A, Leidel S, Alleaume A, Martin C, Ozlu N, Bork P, Hyman A. Functional genomic analysis of cell division in C. elegans using RNAi of genes on chromosome III. Nature. 2000;408:331–6. doi: 10.1038/35042526. [DOI] [PubMed] [Google Scholar]
- Morris J, Wang Z, Drew M, Englund P. Glycolysis modulates trypanosome glycoprotein expression as revealed by an RNAi library. EMBO J. 2002;21:4429–38. doi: 10.1093/emboj/cdf474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiger A, Baum B, Jones S, Jones M, Coulson A, Echeverri C, Perrimon N. A functional genomic analysis of cell morphology using RNA interference. J Biol. 2003;2:27. doi: 10.1186/1475-4924-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath R, Fraser A, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, Welchman D, Zipperlen P, Ahringer J. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–7. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- Paddison P, Caudy A, Bernstein E, Hannon G, Conklin D. Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev. 2002;16:948–58. doi: 10.1101/gad.981002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda P, Stein P, Schultz R. RNAi in mouse oocytes and preimplantation embryos: effectiveness of hairpin dsRNA. Biochem Biophys Res Commun. 2001;287:1099–104. doi: 10.1006/bbrc.2001.5707. [DOI] [PubMed] [Google Scholar]
- Stark G, Kerr I, Williams B, Silverman R, Schreiber R. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–64. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- Yu J, DeRuiter S, Turner D. RNA interference by expression of short-interfering RNAs and hairpin RNAs in mammalian cells. Proc Natl Acad Sci U S A. 2002;99:6047–52. doi: 10.1073/pnas.092143499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui G, Soohoo C, Affar el B, Gay F, Shi Y, Forrester W, Shi Y. A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc Natl Acad Sci U S A. 2002;99:5515–20. doi: 10.1073/pnas.082117599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus M, Sharp P. Gene silencing in mammals by small interfering RNAs. Nat Rev Genet. 2002;3:737–47. doi: 10.1038/nrg908. [DOI] [PubMed] [Google Scholar]
- Dykxhoorn D, Novina C, Sharp P. Killing the messenger: short RNAs that silence gene expression. Nat Rev Mol Cell Biol. 2003;4:457–67. doi: 10.1038/nrm1129. [DOI] [PubMed] [Google Scholar]
- Chi J, Chang H, Wang N, Chang D, Dunphy N, Brown P. Genomewide view of gene silencing by small interfering RNAs. Proc Natl Acad Sci U S A. 2003;100:6343–6. doi: 10.1073/pnas.1037853100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semizarov D, Frost L, Sarthy A, Kroeger P, Halbert D, Fesik S. Specificity of short interfering RNA determined through gene expression signatures. Proc Natl Acad Sci U S A. 2003;100:6347–52. doi: 10.1073/pnas.1131959100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A, Bartz S, Schelter J, Kobayashi S, Burchard J, Mao M, Li B, Cavet G, Linsley P. Expression profiling reveals off-target gene regulation by RNAi. Nat Biotechnol. 2003;21:635–7. doi: 10.1038/nbt831. [DOI] [PubMed] [Google Scholar]
- Scacheri P, Rozenblatt-Rosen O, Caplen N, Wolfsberg T, Umayam L, Lee J, Hughes C, Shanmugam K, Bhattacharjee A, Meyerson M, Collins F. Short interfering RNAs can induce unexpected and divergent changes in the levels of untargeted proteins in mammalian cells. Proc Natl Acad Sci U S A. 2004;101:1892–7. doi: 10.1073/pnas.0308698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton C, Hausler T, Blattner J. Protein trafficking in kinetoplastid protozoa. Microbiol Rev. 1995;59:325–44. doi: 10.1128/mr.59.3.325-344.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gull K. The cytoskeleton of trypanosomatid parasites. Annu Rev Microbiol. 1999;53:629–55. doi: 10.1146/annurev.micro.53.1.629. [DOI] [PubMed] [Google Scholar]
- Vanhamme L, Pays E. Control of gene expression in trypanosomes. Microbiol Rev. 1995;59:223–40. doi: 10.1128/mr.59.2.223-240.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastin P, Ellis K, Kohl L, Gull K. Flagellum ontogeny in trypanosomes studied via an inherited and regulated RNA interference system. J Cell Sci. 2000;113:3321–8. doi: 10.1242/jcs.113.18.3321. [DOI] [PubMed] [Google Scholar]
- LaCount D, Bruse S, Hill K, Donelson J. Double-stranded RNA interference in Trypanosoma brucei using head-to-head promoters. Mol Biochem Parasitol. 2000;111:67–76. doi: 10.1016/S0166-6851(00)00300-5. [DOI] [PubMed] [Google Scholar]
- Wang Z, Morris J, Drew M, Englund P. Inhibition of Trypanosoma brucei gene expression by RNA interference using an integratable vector with opposing T7 promoters. J Biol Chem. 2000;275:40174–9. doi: 10.1074/jbc.M008405200. [DOI] [PubMed] [Google Scholar]
- Kohl L, Robinson D, Bastin P. Novel roles for the flagellum in cell morphogenesis and cytokinesis of trypanosomes. EMBO J. 2003;22:5336–46. doi: 10.1093/emboj/cdg518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastin P, Matthews K, Gull K. The paraflagellar rod of Kinetoplastida: solved and unsolved questions. Parasitol Today. 1996;12:302–307. doi: 10.1016/0169-4758(96)10031-4. [DOI] [PubMed] [Google Scholar]
- Maga J, LeBowitz J. Unravelling the kinetoplastid paraflagellar rod. Trends Cell Biol. 1999;9:409–13. doi: 10.1016/S0962-8924(99)01635-9. [DOI] [PubMed] [Google Scholar]
- Durand-Dubief M, Kohl L, Bastin P. Efficiency and specificity of RNA interference generated by intra- and intermolecular double stranded RNA in Trypanosoma brucei. Mol Biochem Parasitol. 2003;129:11–21. doi: 10.1016/S0166-6851(03)00071-9. [DOI] [PubMed] [Google Scholar]
- Sherwin T, Gull K. The cell division cycle of Trypanosoma brucei brucei: timing of event markers and cytoskeletal modulations. Philos Trans R Soc Lond B Biol Sci. 1989;323:573–88. doi: 10.1098/rstb.1989.0037. [DOI] [PubMed] [Google Scholar]
- Redmond S, Vadivelu J, Field M. RNAit: an automated web-based tool for the selection of RNAi targets in Trypanosoma brucei. Mol Biochem Parasitol. 2003;128:115–8. doi: 10.1016/S0166-6851(03)00045-8. [DOI] [PubMed] [Google Scholar]
- LaCount D, Barrett B, Donelson J. Trypanosoma brucei FLA1 is required for flagellum attachment and cytokinesis. J Biol Chem. 2002;277:17580–8. doi: 10.1074/jbc.M200873200. [DOI] [PubMed] [Google Scholar]
- Schlaeppi K, Deflorin J, Seebeck T. The major component of the paraflagellar rod of Trypanosoma brucei is a helical protein that is encoded by two identical, tandemly linked genes. J Cell Biol. 1989;109:1695–709. doi: 10.1083/jcb.109.4.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deflorin J, Rudolf M, Seebeck T. The major components of the paraflagellar rod of Trypanosoma brucei are two similar, but distinct proteins which are encoded by two different gene loci. J Biol Chem. 1994;269:28745–51. [PubMed] [Google Scholar]
- Birkett C, Parma A, Gerke-Bonet R, Woodward R, Gull K. Isolation of cDNA clones encoding proteins of complex structures: analysis of the Trypanosoma brucei cytoskeleton. Gene. 1992;110:65–70. doi: 10.1016/0378-1119(92)90445-U. [DOI] [PubMed] [Google Scholar]
- Bastin P, Bagherzadeh Z, Matthews K, Gull K. A novel epitope tag system to study protein targeting and organelle biogenesis in Trypanosoma brucei. Mol Biochem Parasitol. 1996;77:235–9. doi: 10.1016/0166-6851(96)02598-4. [DOI] [PubMed] [Google Scholar]
- Woods A, Sherwin T, Sasse R, MacRae T, Baines A, Gull K. Definition of individual components within the cytoskeleton of Trypanosoma brucei by a library of monoclonal antibodies. J Cell Sci. 1989;93:491–500. doi: 10.1242/jcs.93.3.491. [DOI] [PubMed] [Google Scholar]
- Bastin P, Pullen T, Sherwin T, Gull K. Protein transport and flagellum assembly dynamics revealed by analysis of the paralysed trypanosome mutant snl-1. J Cell Sci. 1999;112:3769–77. doi: 10.1242/jcs.112.21.3769. [DOI] [PubMed] [Google Scholar]
- Wirtz E, Clayton C. Inducible gene expression in trypanosomes mediated by a prokaryotic repressor. Science. 1995;268:1179–83. doi: 10.1126/science.7761835. [DOI] [PubMed] [Google Scholar]
- Ersfeld K, Gull K. Targeting of cytoskeletal proteins to the flagellum of Trypanosoma brucei. J Cell Sci. 2001;114:141–148. doi: 10.1242/jcs.114.1.141. [DOI] [PubMed] [Google Scholar]
- Cogoni C, Macino G. Gene silencing in Neurospora crassa requires a protein homologous to RNA-dependent RNA polymerase. Nature. 1999;399:166–9. doi: 10.1038/20215. [DOI] [PubMed] [Google Scholar]
- Smardon A, Spoerke J, Stacey S, Klein M, Mackin N, Maine E. EGO-1 is related to RNA-directed RNA polymerase and functions in germ-line development and RNA interference in C. elegans. Curr Biol. 2000;10:169–78. doi: 10.1016/S0960-9822(00)00323-7. [DOI] [PubMed] [Google Scholar]
- Sijen T, Fleenor J, Simmer F, Thijssen K, Parrish S, Timmons L, Plasterk R, Fire A. On the role of RNA amplification in dsRNA-triggered gene silencing. Cell. 2001;107:465–76. doi: 10.1016/S0092-8674(01)00576-1. [DOI] [PubMed] [Google Scholar]
- Elbashir S, Martinez J, Patkaniowska A, Lendeckel W, Tuschl T. Functional anatomy of siRNAs for mediating efficient RNAi in Drosophila melanogaster embryo lysate. EMBO J. 2001;20:6877–88. doi: 10.1093/emboj/20.23.6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J, Patkaniowska A, Urlaub H, Luhrmann R, Tuschl T. Single-stranded antisense siRNAs guide target RNA cleavage in RNAi. Cell. 2002;110:563–74. doi: 10.1016/S0092-8674(02)00908-X. [DOI] [PubMed] [Google Scholar]
- Roignant J, Carre C, Mugat B, Szymczak D, Lepesant J, Antoniewski C. Absence of transitive and systemic pathways allows cell-specific and isoform-specific RNAi in Drosophila. RNA. 2003;9:299–308. doi: 10.1261/rna.2154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard C, Saborio J, Tewari D, Krieglstein K, Henschen A, Manning J. Evidence for two distinct major protein components, PAR 1 and PAR 2, in the paraflagellar rod of Trypanosoma cruzi. Complete nucleotide sequence of PAR. J Biol Chem. 1992;267:21656–62. [PubMed] [Google Scholar]
- Worthey E, Schnaufer A, Mian I, Stuart K, Salavati R. Comparative analysis of editosome proteins in trypanosomatids. Nucleic Acids Res. 2003;31:6392–408. doi: 10.1093/nar/gkg870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson K, Beverley S. Improvements in transfection efficiency and tests of RNA interference (RNAi) approaches in the protozoan parasite Leishmania. Mol Biochem Parasitol. 2003;128:217–28. doi: 10.1016/S0166-6851(03)00079-3. [DOI] [PubMed] [Google Scholar]
- DaRocha W, Otsu K, Teixeira S, Donelson J. Tests of cytoplasmic RNA interference (RNAi) and construction of a tetracycline-inducible T7 promoter system in Trypanosoma cruzi. Mol Biochem Parasitol. 2004;133:175–86. doi: 10.1016/j.molbiopara.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Kim D, Rossi J. Coupling of RNAi-mediated target downregulation with gene replacement. Antisense Nucleic Acid Drug Dev. 2003;13:151–5. doi: 10.1089/108729003768247619. [DOI] [PubMed] [Google Scholar]
- Gadelha C, LeBowitz J, Manning J, Seebeck T, Gull K. Relationships between the major kinetoplastid paraflagellar rod proteins: a consolidating nomenclature. Mol Biochem Parasitol. 2004;136:113–5. doi: 10.1016/j.molbiopara.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Durand-Dubief M, Bastin P. TbAGO1, an Argonaute protein required for RNA interference, is involved in mitosis and chromosome segregation in Trypanosoma brucei. BMC Biol. 2003;1:2. doi: 10.1186/1741-7007-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastin P, MacRae T, Francis S, Matthews K, Gull K. Flagellar morphogenesis: protein targeting and assembly in the paraflagellar rod of trypanosomes. Mol Cell Biol. 1999;19:8191–200. doi: 10.1128/mcb.19.12.8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley S, Clayton C. Transfection of Leishmania and Trypanosoma brucei by electroporation. Methods Mol Biol. 1993;21:333–48. doi: 10.1385/0-89603-239-6:333. [DOI] [PubMed] [Google Scholar]
- Kohl L, Sherwin T, Gull K. Assembly of the paraflagellar rod and the flagellum attachment zone complex during the Trypanosoma brucei cell cycle. J Eukaryot Microbiol. 1999;46:105–9. doi: 10.1111/j.1550-7408.1999.tb04592.x. [DOI] [PubMed] [Google Scholar]
- Sherwin T, Schneider A, Sasse R, Seebeck T, Gull K. Distinct localization and cell cycle dependence of COOH terminally tyrosinolated alpha-tubulin in the microtubules of Trypanosoma brucei brucei. J Cell Biol. 1987;104:439–46. doi: 10.1083/jcb.104.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball S, Mattis P, Others The GIMP, the GNU image manipulation program http://www.gimp.org


