Abstract
Purpose
Nasopharyngeal cancer (NPC) is the most common head and neck cancer in Saudi Arabia. This study reports the locoregional disease control and survival outcomes in patients with NPC treated in King Abdulaziz University Hospital.
Methods
Patients treated for NPC between June 2007 and October 2014 were retrospectively reviewed. Demographic information, clinicopathologic variables, and chemotherapy data were collected and analyzed. Cumulative survival and disease control rates were calculated by Kaplan-Meier product-limit actuarial method.
Results
Thirty-nine patients with NPC were reviewed. Thirty-five (90%) patients received definitive radiotherapy (RT) and four (10%) had palliative RT. Mean prescribed dose for definitive RT was 68 Gy (range, 60 to 70.2 Gy), delivered with mean doses per fraction of 1.9 Gy (range, 1.8 to 2.1 Gy). After a median follow-up of 15 months (range, 1 to 84 months), 22 (63%) patients who underwent definitive RT were disease free and 13 (37%) were still with disease. During this period, seven (18%) patients died of the disease; five (13%) of them received definitive RT. After 2 years’ follow-up, the actuarial estimate rates were: 85.7% for local control, 91.4% for nodal control, and 85.7% for distant control.
Conclusion
Our study showed a disease with clinical behavior similar to what has been observed in East and Southeast Asia. Further it explored the neoadjuvant chemotherapy approach in treating NPC with results that are comparable to literature. However, little is known about the molecular pathogenesis of this disease in this region, and further research integrating clinical and molecular biomarkers is required.
INTRODUCTION
Nasopharyngeal cancer (NPC) is a disease with a variable universal distribution geographically and racially. It is considered a rare human malignancy in white races in North America and Europe, with an incidence of one per 100,000 population per year,1 whereas it reaches a peak incidence rate of around 20 per 100,000 person-years in China and South Korea.2 Evidence suggests that NPC is a unique disease in terms of its risk factors and biologic association with the Epstein-Barr Virus (EBV) and its sensitivity to chemotherapy and radiotherapy (RT).2
In Saudi Arabia, NPC ranks first (35%) among all head and neck cancers and 17th (2.9%) among all cancers,3 with male predominance (2:1). Regardless of race and geography, the most common form of nasopharyngeal cancers arises from the epithelial cells lining the nasopharynx, which constitute 75% to 95% of all the cases diagnosed. Nonkeratinizing type (WHO III) is the most common type in Saudi Arabia, predominantly in the younger age population, mimicking China and South Korea in the histopathologic type and distribution.4 Despite the importance of this topic in our region, there remains a paucity of evidence on the characteristics and outcome of this disease in the Middle East.5-11 Debate continues about whether the features of this disease are comparable to those of Western or Asian studies.
The purpose of this study is to explore our experience in NPC management in King Abdulaziz University Hospital from 2008 to 2014. The observed outcomes were overall survival (OS) and locoregional control (LRC), defined from the end of treatment until death for any reason or local recurrence time, respectively.
METHODS
We retrospectively reviewed adult patients diagnosed with NPC and treated at King Abdulaziz University Hospital in Jeddah, Saudi Arabia between May 2007 and December 2014. Pediatric patients were excluded from the study. Staging of the disease was stated according to the Cancer Staging Manual by the American Joint Committee on Cancer, sixth edition.12
All the patients had a confirmed pathologic diagnosis in our center. Computed tomography (CT) and/or magnetic resonance imaging (MRI), depending on the treating physician, were used to assess radiologic staging for the primary disease and to rule out or in metastasis. Follow-up CT scans and/or MRI were performed a month after the completion of three cycles of neoadjuvant chemotherapy (NACT) and a month after the completion of concurrent chemoradiotherapy (CCRT) for response assessment. Patients were seen before each NACT cycle and at least three times while receiving CCRT. Responders were followed up with CT scans and/or MRI as per consensus guidelines, thereafter.
Chemotherapy
In our center we used the neoadjuvant or induction chemotherapy (IC) protocol, consisting of taxane, platinum, and fluorouracil for three consecutive cycles separated by 3 weeks, followed by cisplatin as radio-sensitizer, given concurrently, either 100 mg/m2 every 3 weeks or 30 mg/m2 weekly.
Radiotherapy
The Radiation Therapy Oncology Group atlas was followed for target volume contouring.13 Gross primary and nodal tumors were contoured as gross tumor volume (GTV) on the basis of clinical findings and CT/MRI imaging that was performed before the neoadjuvant chemotherapy. Clinical target volume consisted of computer-generated 1-cm isotropic expansion around each gross tumor volume, respecting anatomic barriers, and included all nodal groups with a greater than 10% to 15% risk of containing subclinical disease (all the neck nodes bilaterally).14 Planning target volume was constructed by an automated 0.3- to 0.5-cm expansion of the clinical target volume surfaces, to account for setup error and daily uncertainty. Patients were positioned for simulation using customized thermoplastic mask. CT scans with intravenous contrast were used for treatment planning. Both intensity-modulated RT and three-dimensional conformal RT were used for treatment technique. Dose limits for the critical tissue structures and plan evaluation were followed as defined by the Radiation Therapy Oncology Group 0225.15
Statistical Analysis
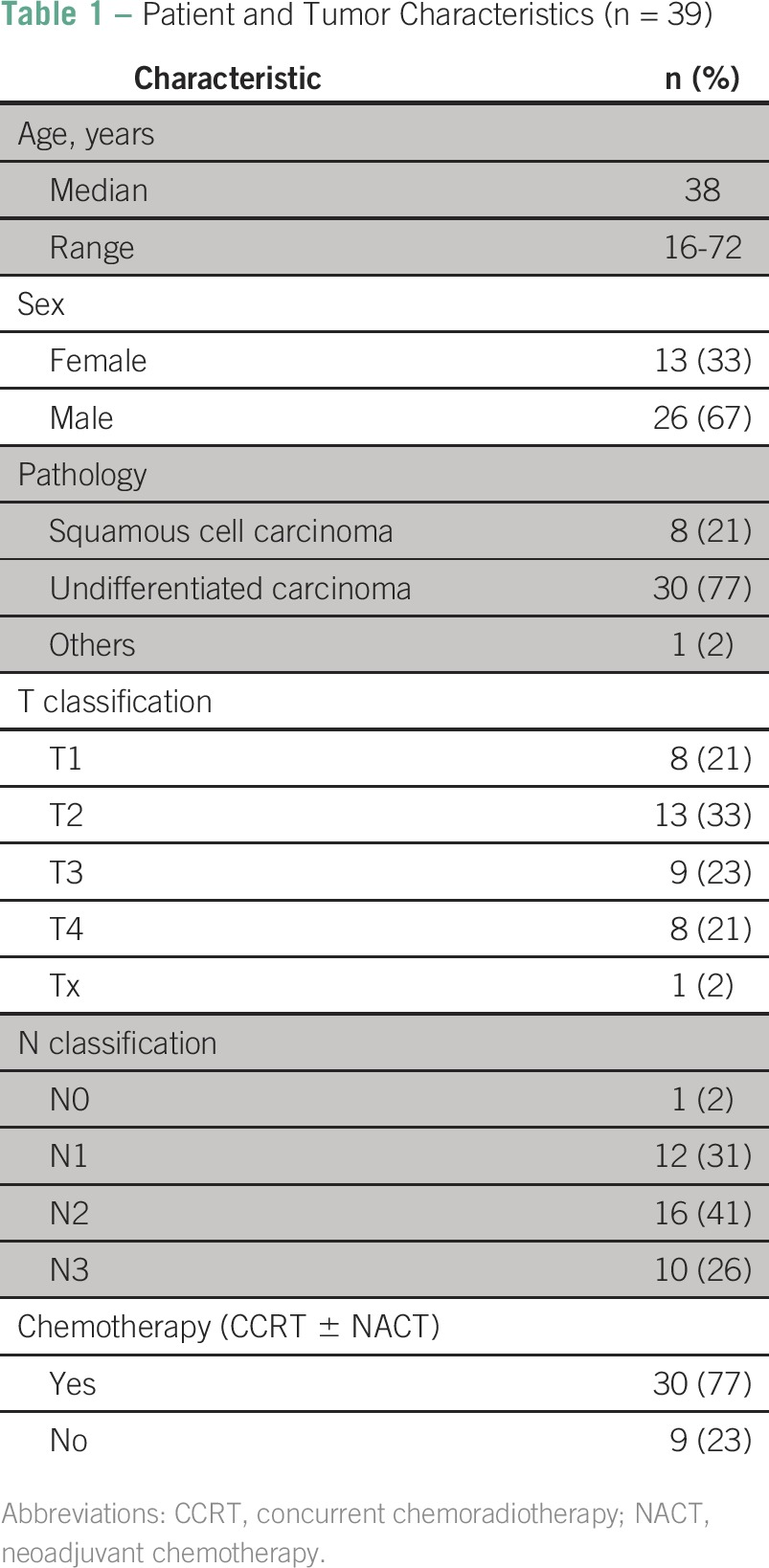
Patients’ demographic and clinical characteristics, such as age at the time of diagnosis, sex, stage, and treatment data, were collected and analyzed (Table 1).
Table 1.
Patient and Tumor Characteristics (n = 39)
Local control of the disease was determined from the day of treatment completion to the last documented clinic visit without recurrence. The same starting point was used for OS and disease-free survival (DFS).
For patients receiving definitive treatment, the correlation between disease control and patient characteristics and between disease control and treatment data (RT dose, time from diagnosis to treatment initiation) was assessed by logistic regression, using IBM SPSS statistic software version 20. A P < .05 was considered significant.
Cumulative survival and disease control rates were calculated by Kaplan-Meier product-limit actuarial method. Data censoring occurred in February 2015.
RESULTS
Thirty-nine patients with NPC were reviewed. Thirty-five (90%) patients received definitive treatment, and only four (10%) received palliative treatment. Among the patients who received definitive treatments, 31 patients (86%) were treated with chemotherapy and radiation subdivided by the chemotherapy setting as follow: 15 patients received NACT followed by CCRT, 14 patients received only CCRT, and two patients had NACT followed by RT alone.
Mean prescribed dose for definitive treatments was 68 Gy (range, 60 to 70.2 Gy), delivered with mean doses per fraction of 1.9 Gy (range, 1.8 to 2.1 Gy). The median time between diagnosis and RT initiation was 4 months (range, 0 to 7 months).
Disease Control and Survival
After a median follow-up of 15 months (range, 1 to 84 months), 22 (63%) patients who underwent definitive RT with or without chemotherapy were free of disease, and 13 (37%) were with disease. During this period, seven (18%) patients died of the disease; out of these, five (13%) received definitive RT (Table 2).
Table 2.
Clinical Outcome of Patients Receiving Definitive Radiotherapy (n = 35)

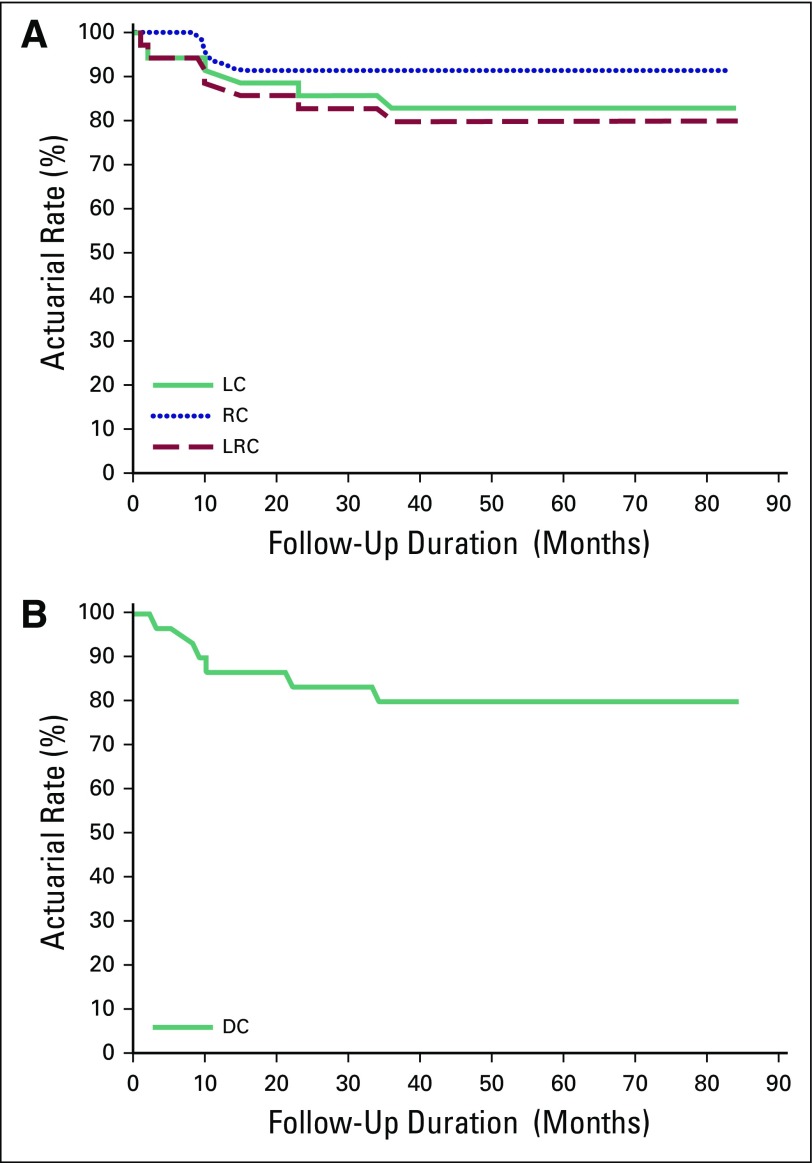
The 2-year actuarial rates of local control, regional nodal control, and distant control were: 85.7%, 91.4%, and 85.7%, respectively (Fig 1).
Fig 1.
(A) Disease control for all patients (n = 39), locoregional control (LRC). (B) Disease control for all patients (n = 39), distant control (DC). LC, local control; RC, regional nodal control.
A statistically significant correlation was demonstrated between local control and radiation dose (R2 = 0.12; P = .041) but not for regional (P = .486) and distant control (P = .151). The use of chemotherapy was significantly associated with distant (P = .023) and regional (P = .043) control but not with local control (P = .097).
The median time from diagnosis to treatment initiation for disease-free patients was 2 months (range, 0 to 6 months), and for those who relapsed it was 4 months (range, 1 to 7 months). The time between diagnosis and treatment initiation showed a statistically significant relation with local control (R2 = 0.16; P = .039) but not with regional (P = .075) and distant control (P = .369).
Pattern of Failure
From a total of 35 patients receiving definitive RT, 13 (37%) developed disease recurrence during follow-up: six (17%) had local recurrence, three (8%) had regional recurrence, two (5%) had both local and regional recurrences, and six (17%) had distant relapse.
No statistically significant correlation was found between patients’ demographic and clinical characteristics and disease control. Patients with stage T1 to T2 disease had lower rates of local failure than those with stage T3 to T4 (20% v 23%; P = .207) but were not statistically significant. Freedom from distant metastasis was only significantly affected by patient age (P = .016).
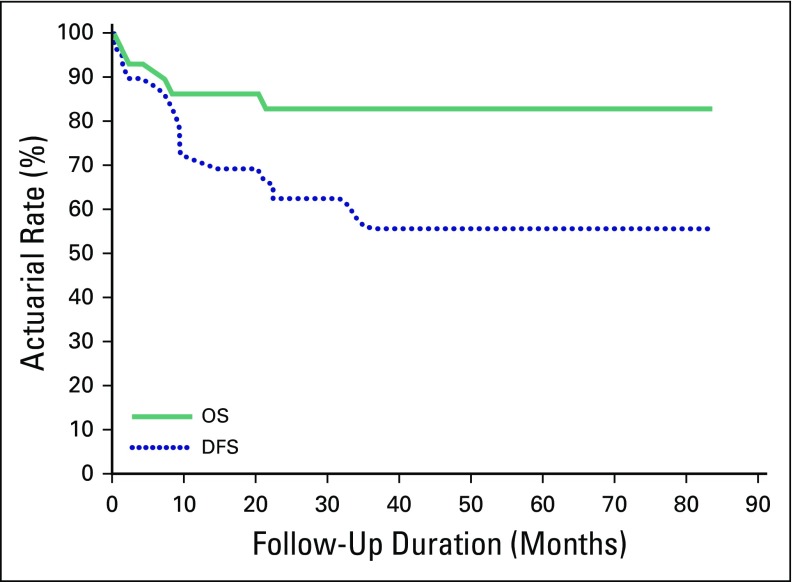
The 2-year OS and DFS actuarial rates were 85.7% and 68.6%, respectively (Fig 2). The use of chemotherapy did not show statistical significant influence on either OS (P = .286) or DFS (P = .211).
Fig 2.
Survival for all patients (n = 39). DFS, disease-free survival; OS, overall survival.
DISCUSSION
This study reports the outcomes of patients with cancer of the nasopharynx who were treated in King Abdulaziz University Hospital, Jeddah, Saudi Arabia between the years 2007 and 2014. Patients in our center were treated with definitive RT with or without chemotherapy. Although adjuvant chemotherapy is considered the standard of care after the treatment with CCRT,16 the administration of adjuvant chemotherapy in our center was limited because of poor patient compliance. Alternatively, we used NACT with taxane, platinum, and fluorouracil followed by CCRT,10 which in our experience offered the patients a rapid clinical improvement and allowed a more conservative RT planning that spares critical structures such as chiasm, optic nerves, and brain stem. Selecting patients for NACT was based on the treating medical and radiation oncologists’ preference, but overall patients with nodal disease received NACT regardless of the tumor extent.
The actuarial rates of locoregional control (80%) and OS (85.7%) were comparable to those reported in the literature, and these results further support the neoadjuvant treatment method for NPC.10 The addition of chemotherapy did not influence the rates of local control but decreased distant recurrence. However, the improved distant control rates with the addition of chemotherapy did not translate into OS or DSF difference. In a phase III trial from China, CCRT proved its advantage on local and distant control,17 whereas the benefit of adding adjuvant and neoadjuvant chemotherapy to the CCRT is still not well established.18,19 The majority of patients in both trials had undifferentiated NPC, which is similar to what is reported in our cohort.
We observed a trend toward improved overall survival with chemotherapy, but statistical significance was not attained. This could be attributed to several confounding factors, which include the small number of patients, the short follow-up period, and the underutilization of chemotherapy during the early years of this study. This is in addition to the insufficient understanding of the natural history of this cancer in this region of the world. We acknowledge that our study represents a single institute experience with limited generalizability, but it certainly adds to the evidence that supports the neoadjuvant approach for treating NPC in countries where disparities in cancer treatments are a recognizable issue.
The molecular basis of undifferentiated NPC is still an active area of ongoing investigation.8 Recently, a local study was done to investigate the molecular evidence of this disease in which biopsies were obtained from 25 patients in Saudi Arabia20 with NPC and were examined for the presence of EBV DNA and for the frequency of p53 mutations. Results showed that despite a high association of EBV infection in the Saudi patients with NPC, the frequency of p53 mutations was low, which makes the results consistent with the worldwide observation of infrequent p53 mutations in NPC. Despite the promising results in such a chemotherapy- and radiotherapy-sensitive disease that is routinely treated with high-dose radiation, questions remain as to why there are still local control failures. A recent study with similar NPC pathology from China showed that the local failure remains the most common site of failure despite high-dose RT coupled with conformal and precise techniques.21 Further studies, which take these variables into account, will need to be undertaken to answer this question to help recognize and select more effective treatment modalities.
A future study is in process involving retrieving pathologic material that will be used for specific virologic and genetic molecular biomarkers to investigate their contribution to the initiation and progression of NPC in this region.
In Saudi Arabia, NPC is the most common head and neck malignancy. Undifferentiated, nonkeratinizing squamous cell carcinoma subtype accounts for the majority of cases. The current study showed a disease with clinical behavior similar to what has been observed in East and Southeast Asia. Our study further supports the neoadjuvant chemotherapy methodology in treating NPC with results that are comparable to the literature. However, little is known about the molecular pathogenesis of this disease in this region, and further research integrating clinical and molecular biomarkers is required.
Footnotes
Authors’ disclosures of potential conflicts of interest and contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Rolina Al-Wassia
Administrative support: Nesreen Awad, Camelia Constantinescu
Collection and assembly of data: Rolina Al-Wassia, Nesreen Awad, Camelia Constantinescu
Data analysis and interpretation: Rolina Al-Wassia, Atlal Abusanad, Hani Marzouki, Shadi Alkhayyat, Talal Al-Khatib
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Rolina Al-Wassia
No relationship to disclose
Atlal Abusanad
No relationship to disclose
Nesreen Awad
No relationship to disclose
Hani Marzouki
No relationship to disclose
Shadi Alkhayyat
No relationship to disclose
Talal Al-Khatib
No relationship to disclose
Camelia Constantinescu
No relationship to disclose
REFERENCES
- 1.Ferlay J. Cancer incidence in five continents. Processing of data. IARC Sci Publ 120:39-44, 1992. [PubMed] [Google Scholar]
- 2.Ma BB, Hui EP, Chan AT. Systemic approach to improving treatment outcome in nasopharyngeal carcinoma: Current and future directions. Cancer Sci. 2008;99:1311–1318. doi: 10.1111/j.1349-7006.2008.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Al-Eid HS, Arteh SO: Cancer incidence report: Saudi Arabia 1999–2000. Riyadh, Saudi Arabia, National Cancer Registry Authority, 2010.
- 4.Yu MC, Yuan JM. Epidemiology of nasopharyngeal carcinoma. Semin Cancer Biol. 2002;12:421–429. doi: 10.1016/s1044579x02000858. [DOI] [PubMed] [Google Scholar]
- 5.Laramore GE, Clubb B, Quick C, et al. Nasopharyngeal carcinoma in Saudi Arabia: A retrospective study of 166 cases treated with curative intent. Int J Radiat Oncol Biol Phys. 1988;15:1119–1127. doi: 10.1016/0360-3016(88)90193-9. [DOI] [PubMed] [Google Scholar]
- 6.Al-Rajhi N, El-Sebaie M, Khafaga Y, et al. Nasopharyngeal carcinoma in Saudi Arabia: Clinical presentation and diagnostic delay. East Mediterr Health J. 2009;15:1301–1307. [PubMed] [Google Scholar]
- 7.Geara FB, Nasr E, Tucker SL, et al. Nasopharyngeal cancer in the Middle East: Experience of the American University of Beirut Medical Center. Int J Radiat Oncol Biol Phys. 2005;61:1408–1415. doi: 10.1016/j.ijrobp.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 8.Andejani AA, Kundapur V, Malaker K. Age distribution of nasopharyngeal cancer in Saudi Arabia. Saudi Med J. 2004;25:1579–1582. [PubMed] [Google Scholar]
- 9.el-Akkad SM, Amer MH, Lin GS, et al. Pattern of cancer in Saudi Arabs referred to King Faisal Specialist Hospital. Cancer. 1986;58:1172–1178. doi: 10.1002/1097-0142(19860901)58:5<1172::aid-cncr2820580533>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 10.Al-Amro A, Al-Rajhi N, Khafaga Y, et al. Neoadjuvant chemotherapy followed by concurrent chemo-radiation therapy in locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2005;62:508–513. doi: 10.1016/j.ijrobp.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 11. Alqadah FD, Alsafadi N, Shukla V, et al: Nasopharyngeal carcinoma (NPC) in the Kingdom of Saudi Arabia. The experience of Princess Nourah Oncology Center (POC), Jeddah. J Clin Oncol 23, 2005 (suppl; abstr 5597)
- 12. Frederick LG, Page DL, Fleming ID, et al: AJCC Cancer Staging Manual. Berlin, Germany, Springer Science and Business Media, 2013. [Google Scholar]
- 13.Grégoire V, Levendag P, Ang KK, et al. CT-based delineation of lymph node levels and related CTVs in the node-negative neck: DAHANCA, EORTC, GORTEC, NCIC, RTOG consensus guidelines. Radiother Oncol. 2003;69:227–236. doi: 10.1016/j.radonc.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Ho FC, Tham IW, Earnest A, et al. Patterns of regional lymph node metastasis of nasopharyngeal carcinoma: A meta-analysis of clinical evidence. BMC Cancer. 2012;12:98. doi: 10.1186/1471-2407-12-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee AW, Tung SY, Chua DT, et al. Randomized trial of radiotherapy plus concurrent-adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst. 2010;102:1188–1198. doi: 10.1093/jnci/djq258. [DOI] [PubMed] [Google Scholar]
- 16.Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: Phase III randomized intergroup study 0099. J Clin Oncol. 1998;16:1310–1317. doi: 10.1200/JCO.1998.16.4.1310. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Liu MZ, Liang SB, et al. Preliminary results of a prospective randomized trial comparing concurrent chemoradiotherapy plus adjuvant chemotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma in endemic regions of China. Int J Radiat Oncol Biol Phys. 2008;71:1356–1364. doi: 10.1016/j.ijrobp.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 18.Chen L, Hu CS, Chen XZ, et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: A phase 3 multicentre randomised controlled trial. Lancet Oncol. 2012;13:163–171. doi: 10.1016/S1470-2045(11)70320-5. [DOI] [PubMed] [Google Scholar]
- 19.Kong L, Zhang YW, Hu CS, et al. Neoadjuvant chemotherapy followed by concurrent chemoradiation for locally advanced nasopharyngeal carcinoma. Chin J Cancer 29:551-555, 2010. [DOI] [PubMed]
- 20.Nasrin N, Taiba K, Hannan N, et al. A molecular study of EBV DNA and p53 mutations in nasopharyngeal carcinoma of Saudi Arab patients. Cancer Lett. 1994;82:189–198. doi: 10.1016/0304-3835(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 21.Li JX, Huang SM, Jiang XH, et al. Local failure patterns for patients with nasopharyngeal carcinoma after intensity-modulated radiotherapy. Radiat Oncol. 2014;9:87. doi: 10.1186/1748-717X-9-87. [DOI] [PMC free article] [PubMed] [Google Scholar]