Abstract
Triple-negative breast cancers (TNBCs) lack estrogen receptor-α (ERα), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2) amplification and account for almost half of all breast cancer deaths. This breast cancer subtype largely affects women who are premenopausal, African-American, or have BRCA1/2 mutations. Women with TNBC are plagued with higher rates of distant metastasis that significantly diminish their overall survival and quality of life. Due to their poor response to chemotherapy, patients with TNBC would significantly benefit from development of new targeted therapeutics. Research suggests that the insulin-like growth factor (IGF) family and estrogen receptor beta-1 (ERβ1), due to their roles in metabolism and cellular regulation, might be attractive targets to pursue for TNBC management. Here, we review the current state of the science addressing the roles of ERβ1 and the IGF family in TNBC. Further, the potential benefit of metformin treatment in patients with TNBC as well as areas of therapeutic potential in the IGF-ERβ1 pathway are highlighted.
Keywords: insulin-like growth factors, estrogen receptors, estrogen receptor-beta, basal-like breast cancer, estrogen receptor-beta isoforms, metabolism
I. INTRODUCTION
Breast cancer (BC) is the second most common malignancy in women. Of the estimated 256,000 women diagnosed with breast cancer annually in the United States, about 10–15% of all breast cancer cases are identified as triple-negative breast cancer (TNBC).1–3 However, this BC subtype accounts for almost half of all breast cancer deaths. Triple-negative breast cancers lack estrogen receptor-alpha (ERα) and progesterone receptor (PR) expression as well as human epidermal growth factor receptor-2 (HER2) amplification. TNBC tumors are generally larger in size, are initially of higher grade, exhibit lymph node involvement at diagnosis, and are biologically more aggressive than other breast cancer subtypes. Exploring novel therapeutic approaches for TNBC is critical because the median survival for women with metastatic TNBC is less than 12 months, and virtually all women with metastatic TNBC ultimately die of their disease despite systemic therapy.4 Currently, no targeted therapies are approved for treatment of TNBC, and cytotoxic chemotherapy is the mainstay of systemic treatment.5 Although TNBC patients tend to have higher clinical response rates to chemotherapy, they also have higher rates of distant recurrence and a worse prognosis than women with other breast cancer subtypes.2,6–8 TNBC tumors occur frequently in premenopausal women, in women of African-American decent,9 and in patients with BRCA1 or BRCA2 mutations.1,8,10 Unlike breast cancers that express ERα and/or PR, patients with TNBC are not generally considered to be candidates for current targeted endocrine therapies. Further, HER-2-targeted treatments are not reported to be useful for clinical management of TNBC. This report will consider new therapeutic targets and alternative treatment strategies that may hopefully stop TNBC progression and prolong patient survival.
II. BACKGROUND
Treatment of patients with TNBC is challenging due to the heterogeneity of the disease and the absence of well-defined molecular targets.2,6,11 One of the first molecular insights into TNBCs was the report that these tumors are likely to arise in BRCA1 mutation carriers and often have gene expression patterns similar to those of BRCA1-deficient tumors.8 BRCA1 plays an important role in DNA double-strand break repair, contributing to maintenance of DNA stability. Poly ADP-ribose polymerase (PARP) enzymes are critical for the processing and repair of DNA breaks.3 Tumor cell lines lacking functional BRCA1 or BRCA2 are sensitive to PARP inhibitors in preclinical studies.12–14 Clinical trials using both PARP inhibitors and DNA-damaging agents (such as cisplatin) in TNBC are currently underway in BRCA1/2-mutant tumors.15 Independent studies identifying other molecular markers associated with TNBC, such as VEGF,16 EGFR,17 Src,18 and mTOR,19 have been important for the design of clinical trials investigating targeted treatments. Other reports indicate that a TNBC subgroup predicted to have a relatively favorable prognosis is characterized by high expression of “luminal-like” genes such as androgen-receptor (AR), whereas TNBC subgroups with worse prognosis are characterized by expression of cancer stem-cell-like markers.20 Clearly, there is a major need to better understand the molecular basis of TNBC and to develop effective treatments for this aggressive and deadly type of breast cancer. More extensive genomic, molecular, pathologic, and immunologic analyses of TNBCs are required to understand the complexity of the disease and to identify potential molecular “drivers” that can be therapeutically targeted in the clinic.
III. ESTROGEN RECEPTOR-β IN TRIPLE-NEGATIVE BREAST CANCER
Although the classical estrogen receptor form ERα is not expressed in TNBC, a number of reports have investigated expression of a more recently discovered member of the nuclear transcription factor superfamily termed ERβ.21 The two forms of the estrogen receptor are encoded by different genes, ESR1 and ESR2, on the sixth and fourteenth chromosome (6q25.1 and 14q23.2), respectively. Although ESR2 is located on a different chromosome, the DNA-binding domain of the ERβ protein product shares 96% homology with ERα and 60% homology at the ligand-binding domain, suggesting that the receptors are capable of binding similar DNA sites, with both similar and distinct ligand preferences. Five ERβ variants that are mostly generated by alternative splicing have been described and include ERβ1, ERβ2, ERβ3, ERβ4, and ERβ5.22–24 Multiple ERβ variants can occur in normal breast tissue and in breast tumors, thus presenting a challenge to understand their specific biologic functions. Importantly, only ERβ1, generally considered to be the wild-type form of ERβ, retains an intact ligand-binding domain (LBD) to interact with specific ligands, thus making ERβ1 an attractive option for potential systemic drug therapy.23,25 Notably, ERβ2 and ERβ5 are reported to form dimers and partner with ERβ1. Neither ERβ2 nor ERβ5 have known ligands, and their biologic activity is presumed to be dependent on ERβ1.23,26 The only known function of ERβ5 is to modulate or interfere with ERβ1 signaling. The potential role of other variants in malignancy remains to be clarified.
Immunohistochemical (IHC) detection of ERα in tumors is a standard assay in the clinic to plan patient management.27 In spite of the considerable structural homology shared by ERα and ERβ, ERβ is not identified in standard IHC clinical assays for ERα, nor is ERβ considered at this time in patient management decisions in the clinic. It is critical to decipher the role of ERβ in TNBC as earlier studies suggested that ERβ levels are higher in breast tumors of African-American as compared to Caucasian women, and that ERβ may play a critical role in TNBC progression.28
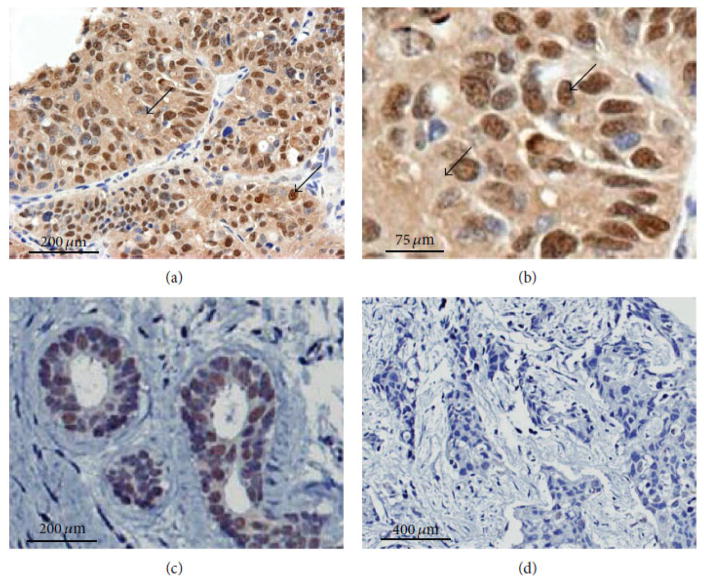
A major problem in detecting ERβ in clinical specimens using IHC is the lack of well-validated and specific antibodies. Currently, there is little to no consensus on the best laboratory protocols for ERβ detection. As a consequence, reports on ERβ expression in breast cancer tissues, particularly in TNBC and ERα-negative specimens, exhibit significant variability in findings and in correlates with clinical outcomes.23,26,29,30 Several available monoclonal and polyclonal antibodies directed to ERβ and used with different tissue preparative and staining techniques have been tested in comprehensive studies.23,31–36 From this work, two antibodies have been recommended for immunohistochemical investigation of breast and other tissue specimens—namely, PPG5/10 specific for ERβ126,35,36 and 14C8 directed to the N-terminus of ERβ that detects most isoforms of the protein.34–38 Figure 1 shows specific IHC staining of ERβ1 in TNBC specimens from the clinic using validated antibody PPG5/10 as reported before.39 Specific nuclear staining of ERβ is shown on a representative patient specimen [Figs. 1(a)–1(c)]. Extranuclear staining of ERβ1 was also present in a majority of TNBC cases examined previously (unpublished data).39 As described before, nuclear and extranuclear staining of ERβ1 and variants is often present in TNBC tumor specimens from patients23,26,34–36 and similar data are reported for non-small cell lung cancers.40–42
FIG. 1.
ERβ1 expression in archival TNBC specimens detected by immunohistochemisry using anti-ERβ1 antibody (PPG5/10, AbDSerotec). A representative example of ERβ1 immunostaining is shown using tumor and neighboring, nonmalignant tissue from the same patient. (a) Nuclear and cytoplasmic staining are shown on a representative specimen of TNBC at low magnification. (b) The same specimen from the previous panel shows nuclear and cytoplasmic staining at higher magnification as indicated by arrows. (c) Expression of nuclear ERβ1 is also present in neighboring normal tissue of the same patient tumor tissue displayed in panels (a) and (b). (d) A different TNBC tumor specimen that does not express ERβ1 is shown for comparison. Antibody binding was detected by using diaminobenzidine (DAKO). Sections were counterstained with Harris hematoxylin. (Reprinted with permission from the Hindawi Publishing Corporation, Copyright 2015).39
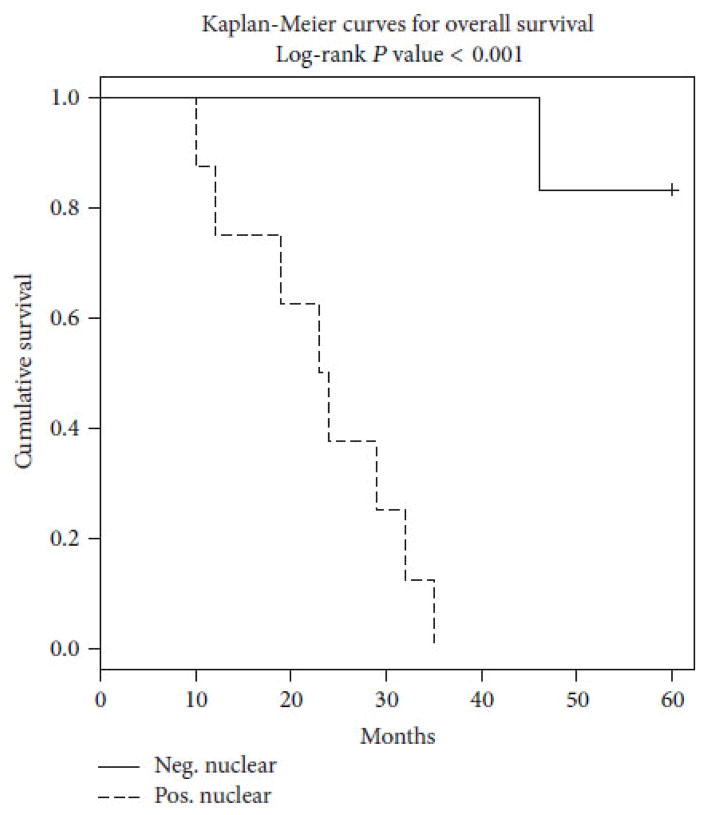
An overview of currently available evidence indicates that ERβ expression may be decreased in pre-invasive breast cancer as compared with equivalent normal tissue43 and the ERβ/ERα ratio in breast cancer subtypes other than TNBC declines significantly as disease progresses from normal epithelium to DCIS and invasive ductal carcinoma.23,43,44 Collectively, these findings led to the hypothesis that ERβ counteracts the effects of ERα when both receptors are expressed together in breast cancer cells,23,45–47 with ERβ thereby acting as a tumor suppressor. In contrast, other reports indicate a significant association between ERβ with proliferative biomarkers (such as Ki-67) in clinical specimens, and ERβ in node-negative breast cancer is reported to associate with a positive response to tamoxifen therapy and longer patient survival.23,30,33,48 In TNBC, nuclear expression of ERβ associates with significantly reduced overall patient survival (Fig. 2) in some23,25,39 but not in all studies,26 while ERβ5 expression appears to be a prognostic marker of worse prognosis in TNBC subsets in another recent investigation.26 As reported before,39 the overall survival of a small cohort of patients with advanced TNBC was worse for those who expressed high nuclear ERβ1 (positive) than for those with low ERβ1 expression (negative). Of note, a significant number of African-American patients were included in this sample and all of these patients were positive for high nuclear ERβ1 expression.39 Furthermore, high nuclear ERβ2 is an independent marker of early relapse in ERα-negative breast cancer and especially TNBC.49 Since ERβ2 can form heterodimers with ERβ1 and modulate its transactivation in a ligand-dependent manner to modulate gene expression by ERβ1,22 it will be important to assess the impact of ERβ2 on correlates with clinical outcome in future studies. It is possible that ERβ1 and ERβ2 or ERβ5 are necessary partners in promoting aggressive TNBC. Indeed, the ratio of ERβ1: ERβ2 expression in TNBC specimens may be critical in predicting clinical outcome.23,25
FIG. 2.
Tumor expression of ERβ1 associates with reduced overall survival (OS) in TNBC specimens from the clinic. TNBCs from three African-American and 11 Caucasian women were scored for nuclear (using IHC with validated antibody anti-ERβ1 antibody PPG5/10 (AbDSerotec). Allred scores >2 are denoted as positive. In this group of patients with advanced TNBC, overall survival (OS) was significantly worse for TNBC patients with high nuclear ERβ1 (positive) as compared to those with low (negative) ERβ1 (p < 0.001). We note that TNBCs from all three African-American women were high ERβ1-positive. (Reprinted with permission from the Hindawi Publishing Corporation, Copyright 2015).39
In pursuing new therapeutic approaches for TNBC, it is informative to consider results of additional studies on both TNBC and related ERα-negative disease. Hence, ERβ1 is reported to be a biomarker for improved survival in TNBC patients when treated with tamoxifen.23,25,30 Although previous work suggested that tamoxifen use only reduces the risk of ERα-positive breast cancer, Yan et al.25 report that both ERβ1 and ER co-regulator SRAP are predictive biomarkers of tamoxifen response/benefit in women with ERα-negative breast cancer. In ERα-negative tumors, ERβ1 expression correlates with Ki-67 proliferation marker, suggesting that ERβ1 may have a role in driving proliferation, and that antiestrogen treatment, by inhibiting ERβ1, could slow tumor progression.23,25 With the discovery of ERβ expression alone in TNBC, this presents the possibility that, in this ERα-negative cohort, tamoxifen or alternative antiestrogen strategies may mediate activity via ERβ1, the full-length ligand-binding receptor isoform. Further, among hereditary breast cancer cases, the majority of BCs arising in BRCA2 mutation carriers are ERα-positive, whereas most BCs arising in BRCA1 mutation carriers are ERα-negative at diagnosis including a significant proportion that are TNBC.20,23 Nevertheless, estrogen may be important in pathogenesis of breast cancers in BRCA1 mutation carriers, particularly given reports that premenopausal bilateral oophorectomy is associated with reduced breast cancer risk for BRCA1 mutation carriers. Furthermore, ERβ is commonly expressed in breast cancers of patients with BRCA1 mutation and is postulated to be a target for tamoxifen. Thus there are plausible mechanisms by which tamoxifen may prevent breast cancer for BRCA1 mutation carriers. The recent work of Phillips et al.50 provides further evidence that, for BRCA1 mutation carriers, tamoxifen use for breast cancer might reduce the risk of contralateral breast cancer. Of note, Shazer et al.51 report that the antiestrogen raloxifene, used as an ERβ-targeted therapy, inhibits growth of ERβ-positive, androgen-independent prostate cancer.
Although earlier work confirmed that ERα-positive breast cancer cells are more sensitive than ERα-negative breast cells to growth-inhibitory effects of tamoxifen, moderate anti-proliferative responses to tamoxifen and to ICI-164,384 were found in ERα-negative cells including TNBC cells.52 These effects may potentially be modulated in part by a second unique binding site identified for hydroxytamoxifen in the coactivator-binding groove of ERβ that may disrupt ERβ-coactivator interactions.53 Collectively, these reports have important implications since approved breast cancer treatments (such as tamoxifen, raloxifene) may be other alternatives for ERβ-positive TNBC patients with generally few options other than cytotoxic chemotherapy.1,25 Of note, there is an ongoing clinical trial to investigate the effectiveness of adjuvant endocrine therapy for operable ERβ-positive, TNBC patients (http://clinicaltrials.gov/show/NCT02089854). This trial includes two adjuvant endocrine therapies, either toremifene for premenopausal and perimenopausal patients or anastrozole for postmenopausal patients. The primary target of toremifene is hypothesized to be ERβ.
Although ERβ activity is generally considered antagonistic to ERα when both receptors occur together in a cell, the role of ERβ forms in isolation is not well documented. Molecular studies show that when both ERα and ERβ are present together in tumor cells, each ER restricts the binding site occupancy of the other, with ERα generally being dominant to ERβ. It is clear that ERβ binding and actions in gene regulation are different in the absence of ERα expression in BC cells.47,54 Correlation of ERβ with high proliferative biomarkers is reported in ERα-negative tumors but not in those with ERα expression.25,34 Among the first studies on stable transfection of ERβ in TNBC MDA-MB-231 cells, it was determined that the proliferation rate of the tumor cells positively correlates with the level of ERβ expression.55 The results using stable ERβ clones demonstrated that proliferation is increased as ERβ expression is increased in ERα-negative tumor cells. A confirmation of these experiments was published later when ERβ was transfected in MDA-MB-435 cells leading to significant stimulation of tumor progression as well as metastasis in vivo.56 These reports on introduction of ERβ in ERα-negative BC cells showing stimulation of tumor proliferation55,56 are consistent with more recent data using a different strategy, namely, using shRNA to silence ER-β expression, resulting in significant suppression of TNBC cell proliferation.39 In contrast, other studies on transfection of ERβ in ERα-low or -negative breast cancer cells indicate that ERβ inhibits cell proliferation.23,57 Several reasons may explain such contrasting results. For example, transfection of ERα led to the paradoxical finding that ERα was a growth inhibitor in breast cancer, a result that is clearly inconsistent with established clinical findings.58 This unexpected result and similar ERβ transfection data may be due in part to excessive levels of ERβ expression in the model systems used and other complicating factors.23 Furthermore, conflicting data may also be due to differences in expression and/or activity of ERβ2 or ERβ5 isoforms.25,26 High nuclear ERβ2 is an independent marker of early relapse in ERα-negative breast cancer and especially TNBC.23,36 Since ERβ2 can form heterodimers with ERβ1 and modulate its transactivation in a ligand-dependent manner to modulate gene expression by ERβ1,49 it will be important to assess the impact of ERβ2 in TNBC.
It is important to note that there are also conflicting reports on activity of ERβ-specifi ligands in TNBC as well as in other tumor types.59,60 ERβ1 expression correlates with tumor proliferation and progression in lung cancer,41,48,59,61 but not in colon cancer.62 A new report shows that under basal conditions ERβ agonists induce apoptosis in breast cancer cells. However, when extracellular signal-regulated kinase 1 and 2 (ERK 1/2) was activated by co-incubation with epidermal growth factor (EGF), ERβ agonist DPN induced proliferation in breast cancer cells.63 Hence, ERβ agonist DPN induces proliferation in breast cancer cells when EGFR and downstream ERK 1/2 signaling pathways are activated (see Fig. 3).63 The results indicate that cellular context such as EGFR-induced signaling activity, which is known to be frequently overexpressed and active in TNBCs, modulates ERβ growth-promoting effects.64,65
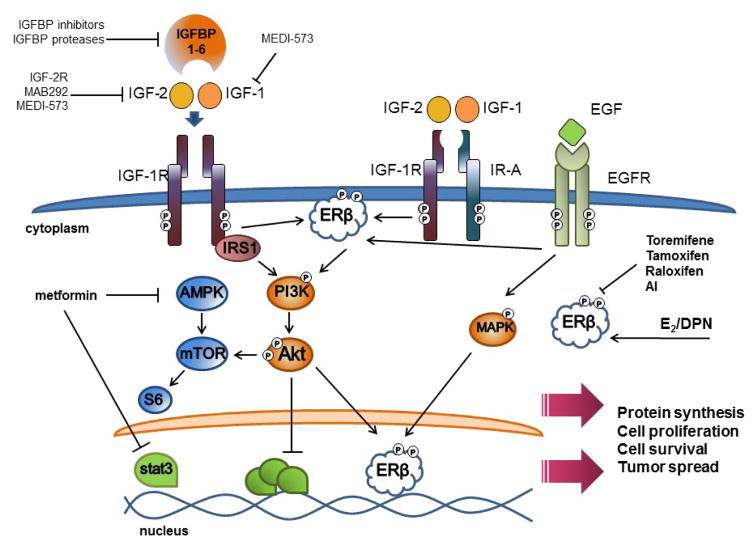
FIG. 3.
Interactions of IGF and ERβ signaling pathways in malignancy. TNBC proliferation is regulated closely by a complex network of growth factor signaling pathways. It is well known that obesity and diabetes can increase insulin and insulin-like growth factor signaling, which in turn may stimulate specific receptors to drive the activation of downstream signaling pathways in TNBC. IGF-1 and IGF-2 bind receptors activating PI3K and Akt signaling and downstream mTOR activation that promotes protein synthesis, cell proliferation, and inhibition of apoptosis. In addition, EGFR is frequently overexpressed in TNBC and is stimulated by binding with epidermal growth factor (EGF). Cross-communication of EGFR signaling with ERβ may induce downstream signaling that contributes to tumor cell survival. ERβ is activated by estrogen (E2) and by synthetic estrogen receptor-selective ligands such as diarylpropionitrile (DPN) and can reportedly be modulated in part by breast cancer therapies such as toremifene, tamoxifen, and raloxifen. IGF-2 signaling may also promote acute phosphorylation of ERβ and late induction of ERβ transcription. Metformin, a common therapeutic used to manage diabetes mellitus type 2, has been demonstrated to be effective in partial suppression of TNBC by activating AMPK (which in turn inhibits mTOR downstream signaling) and/or by suppressing systemic insulin-like growth factor levels in vivo. Arrows represent a pathway of activation and solid lines represent inhibition. See text for details.
Based on current data, estrogen receptors are known to regulate gene expression by both genomic and non-genomic inputs.66,67 Genomic signals involve direct action of nuclear-localized estrogen receptors as an estradiol-regulated transcription factor or co-regulator. By contrast, non-genomic signaling involves extranuclear events mediated by extranuclear estrogen receptors often in cooperation with co-activator or adaptor proteins;25 these then impact gene expression indirectly by stimulating signaling cascades such as MAPK and PI3K/AKT to activate co-regulators or other transcription factors.66–68 In target cells, extranuclear ERα forms derive from the same transcript as nuclear ERα, but minor extranuclear ERα splice variants occur.66,67 Notably, several reports note that ERβ may localize in tumor cell nuclei as well as in extranuclear sites.39,69 Work in our laboratory indicates that ERβ expression is reduced in the nuclei of metastatic TNBCs from the clinic, but levels of ERβ localized in extranuclear sites of the metastatic TNBC specimens are either maintained or increased relative to that found in paired primary TNBC specimens (unpublished observations).39
Recent studies indicate that the tumor microenvironment also has a significant impact on tumor progression, particularly on tumor metastasis, which is a critical factor in TNBC. The tumor microenvironment includes surrounding supporting cells and fibroblasts, blood endothelial vessels, immune cells, secreted molecules, and extracellular matrix. Notably, tumor infiltrating leucocytes (TILs) that play a role in immune recognition of tumor cells express estrogen receptors that are known to be involved in the regulation of immune functions and inflammation.70–72 A recent report shows that about 61% of TILs express ERβ, but ERα is not expressed,44 thus raising questions on the role of ERβ in immune modulation in breast cancer. Further, growth-stimulating effects of ERβ in TNBC may be due, in part, to downstream actions to promote production and secretion of other growth factors such as vascular endothelial growth factor (VEGF), amphiregulin, and Wnt-10b, which can act in turn to activate specific receptor signaling pathways known to be associated with TNBC progression.39
IV. IGF AXIS AND TRIPLE-NEGATIVE BREAST CANCER
In the twenty-first century, the obesity rate among men (≥20 years) increased from 20.2% to 31.1% while the rate among women increased from 25.4% to 33.2% (Healthy People 2010; www.healthypeople.gov). Obesity is an established risk factor for breast cancer. In fact, obese patients with a high waist-to-hip ratio (WHR) have more frequent tumor recurrence, metastasis, and worse outcomes than nonobese patients.73,74 Obesity and lack of physical activity have been associated with hyperinsulinemia and insulin resistance, and obesity often influences the amount of free insulin-like growth factor (IGF) available to cells.75 The insulin family of proteins have critical pleiotropic effects on metabolism and growth. A large body of evidence indicates the insulin/insulin-like growth factor (IGF-1, IGF-2) pathway plays a role in breast cancer progression.31,75–78 The insulin-like growth factor (IGF) pathways mediate metabolism, cell proliferation, differentiation, migration, invasion, and survival.79–81 The tyrosine kinase receptors, insulin-like growth factor-1 receptor (IGF-1R), and the insulin receptor isoform A (IR-A) are able to form homo- or heterodimers when bound by their ligands insulin, insulin-like growth factor-1 (IGF-1), or insulin-like growth factor-2 (IGF-2). Ligand binding then activates the downstream mitogen-activated protein kinase (MAPK) and the phosphatidylinositol 3-kinase (PI3K)-AKT/protein kinase B (PKB) pathways.82 Although early phase clinical trials raised hope for the use of IGF1R-specific antibodies for cancer therapy, initial phase III results in unselected patients have not been effective. Further clinical studies may benefit from use of predictive biomarkers to identify probable responders, the use of rational combination therapies, and consideration of alternative targeting.83
Studies in ERα-positive breast tumor cell lines confirm a relationship between ERα and IGF-1R.84,85 Since increased expression of IGF-1 and IGF-2 are associated with aggressive tumor types as well as metastatic phenotypes,86–89 the IGF axis is now being explored in TNBC. The gene for IGF-2 (Igf2) is transcribed from the paternal allele and lies tangential to H19.90,91 The expression of Igf2 and H19 are regulated by a differentially methylated region (DMR) that contains estrogen response elements (ERE).92,93 In murine and rat testis, Pathak et al. demonstrate a direct association between ERβ and Igf2 methylation.93,94 Preclinical studies show that ERβ activation and localization in TNBC cell lines may be initiated by IGF-2, acting via IGF-1R and IR (Fig. 3).77 Expression of ERβ in TNBC cell lines is due, in part, to the ability of IGF-2 to modulate ERβ transcription.39,77 Furthermore, assessment of archival breast tissue obtained from patients with TNBC reveal high levels of both IGF-2 and ERβ1 as compared to controls.39 Further investigation of the cross talk between IGF-2 and ERβ may improve our understanding of TNBC progression as well as present potentially unique opportunities for treatment and screening.
A. IGF Binding Proteins
Even though IGF-239,79 is implicated as a potential biomarker for TNBC development, it is important to consider that this growth hormone is a single component of a superfamily. The bioavailability of both IGF-1 and IGF-2 are modulated by IGF binding proteins (IGFBPs). Six IGFBPs have been identified and studied.82,95,96 Circulating IGFBPs 1–5 have equal binding affinity for IGF-1 and IGF-2, whereas IGFBP-6 has a significant preference for IGF-2.96,97 Complexes formed between IGFBPs and IGFs, accompanied with an acid-labile subunit (ALS), increase the half-life of unbound IGFs and prevent their interaction with IGF-1R. Their sequestration of IGFs suggests IGFBPs act as tumor suppressors. Investigating the viability and activity of IGFBPs in the absence of ALS and/or IGFs, as well as their ability to form associations with other growth hormones, may help to improve our understanding of these binding proteins and clarify their potential therapeutic roles.
1. IGFBP-1
TNBCs are highly proliferative, and an IGFBP significantly associated with cell proliferation and motility is IGFBP-1. This ~30 kDa non-glycosylated protein contains an integrin recognition sequence, Arg-Gly-Asp, allowing IGFBP-1 to bind the α5β1 integrin (fibronectin) receptor.98 As shown by Zhang and Yee, inhibition of IGF-1R and integrin motif of IGFBP-1 prevented migration of bone-seeking metastatic TNBC MDA-MB-231 (MDA-231BO) cells.99 In TNBC/basal-like murine tumors lacking matrix metalloproteinase-9 (MMP9), increased levels of IGFBP-1 were detected resulting in slower tumor growth and reduced IGF-1R phosphorylation.100 Consequently, increasing IGFBP-1 levels, whether endogenously or exogenously, could conceivably lead in part to improved TNBC patient outcomes.
2. IGFBP-2
IGFBP-2, the second most abundant IGFBP in the circulation, is a non-glycosylated ~36 kDa protein whose proliferative effects are well documented.96,101,102 Like IGFBP-1, IGFBP-2 contains an integrin motif and plays a role in cell mobility.95,103 This RGD sequence enables IGFBP-2 to enhance cell adhesion and facilitate neovascularization.104 It is also an adipocyte-secreted factor that increases migration of tumors cells.105 In fact, secreted IGFBP-2 enables migration and invasion of MCF-7 cells, an ERα-positive cancer cell line with low metastatic potential.105 Furthermore, adipocytes cocultured with tumor cells have a greater than tenfold increase of IGFBP-2 mRNA expression.105 These data underscore the effect of stroma on tumor invasiveness. Given that obesity is risk factor for breast cancer, Catsburg et al. assessed the relationship between atypical hyperplasia and IGFBP-2.106 Their study suggested that in postmenopausal, nondiabetic women, an inverse relationship exists between serum IGFBP-2 and patient BMI.106 In this population, high circulating IGFBP-2 might protect against the development of atypical hyperplasia, but clearly further studies are needed. Of note, IGFBP-2 contains a nuclear localization signal and via importin-α is transported to the nucleus.107 Target genes of IGFBP-2 include those involved in focal adhesion, MAPK and Wnt signaling.108 Tumor metastasis is also reported to be regulated in part by IGFBP-2. Knockdown of IGFBP-2 decreases levels of β-catenin, a marker of metastasis, through the IGF-1R or integrin signaling pathways.108 Assessment of lymph node metastasis reveals a positive correlation between IGFBP-2 and β-catenin.108 Although little data currently exists on the role of IGFBP-2 in TNBC, assessment of IGFBP-2 expression may be helpful in predicting the progression of this cancer subtype since TNBC exhibits a high proliferative index and a propensity to widely metastasize.
3. IGFBP-3
IGFBP-3, the most abundant of all the circulating IGFBPs, forms a 150 kDa ternary complex with IGF-1 and an acid-labile subunit (ALS).95 Through processes not completely defined, nuclear IGFBP-3 in combination with other regulatory complexes may either stimulate or inhibit cellular processes and tends to support genomic integrity.96,109 In invasive tumors, expression of IGFBP-3 tends to associate with tumor proliferation, ERα negativity, and HER-2 overexpression, while in ductal carcinomas in situ, a significant association exists between IGFBP-3 and ERα negativity.110 In early stages of breast cancer, IGFBP-3 behaves as a tumor suppressor while in a more advanced stages, IGFBP-3 may interact with the tumor microenvironment to promote tumor progression.111–113 Again, little data is currently available in understanding the role of IGFBP-3 specifically in TNBC.
4. IGFBP-4 and IGFBP-5
In the circulation IGFBP-4 exists as a 24 kDa doublet,95 and as other members of this family, IGFBP-4 limits the bioavailability of IGF-1 and IGF-2. When IGFBP-4 is cleaved by pregnancy-associated plasma protein-a (PAPP-A), or prostatic kallikreins, hK2 and hK3, IGF-1 is released.114,115 In a murine model, PAPP-A-resistant IGFBP-4 reduced mammary tumor growth and increased cell apoptosis.116 Another binding protein, IGFBP-5, affects cell proliferation and migration via IGF-dependent and -independent modes.117–123 Based on in vitro experiments, IGFBP-5 confers resistance to BMS-536924, an IGF-1/IR inhibitor and it is significantly expressed in invasive tumors and the adjacent normal tissue.124 Independently, the prognostic potential of IGFBP-5 or IGFBP-4 in breast malignancy appears limited.
5. IGFBP-6
Unlike other IGFBPs, the expression of IGFBP-6 is low in the liver.103,125 A unique characteristic of this IGFBP is its preferential binding affinity for IGF-2. IGFBP-6, along with IGF-2R, has the principle role in limiting IGF-2 bioavailability.97 Oncogenic action of IGFBP-6 has been described126 as well as an ability to inhibit tumor cell migration and tumor-associated angiogenesis.127,128 Conversely, IGFBP-6 promotes cell migration in rhabdomyosarcoma and colon cancer cells independent of IGF-2, an action mediated by p38, pERK, JNK1 signaling pathways.129–131 Evaluation of the role of IGFBP-6 in ovarian cancer decreased migration of HEY cells, an aggressive ovarian cancer cell line, whereas in less aggressive cultures such as SKOV3, IGFBP-6 stimulated cell migration.132 Although IGFBP-6 induced phosphorylation of ERK and JNK in both cell lines, exposure to inhibitors of ERK and JNK only reduced the migration of HEY cells.132 This suggests that IGFBP-6 has the potential to reduce metastasis independent of IGF-2.128–131 Under hypoxic conditions, IGFBP-6 also inhibits angiogenesis independent of IGF-2 in vitro and in vivo.128 Concurrently, IGFBP-6 has the ability to induce cell migration by binding directly to prohibin-2 (PHB2), a single-span membrane protein.129–131 What makes IGFBP-6 an attractive therapeutic target is its preference for partnering with IGF-2, which is highly expressed in invasive ductal carcinoma and TNBC,39,133 as well as its IGF-independent activity.
The IGF superfamily offers a plethora of potential therapeutic targets against TNBC. The ability of family members to impact cell proliferation, migration, angiogenesis, and adhesion, critical factors that affect patient outcome, underscores the need for further research in the role of the IGF family in TNBC development.
B. Metformin and TNBC
As noted above, obesity and the metabolic syndrome are associated with multiple factors that may cause an increased risk for breast cancer and breast cancer-related mortality.31 A known link between obesity and breast cancer risk is insulin resistance, a condition commonly treated with metformin, an orally bioavailable biguanide for diabetes mellitus type 2. Metformin decreases hepatic glucose production and intestinal glucose absorption. While increasing insulin sensitivity, metformin decreases circulating levels of insulin and insulin-like growth factors. Investigations of the therapeutic potential of metformin reveals an ability to inhibit TNBC proliferation and reduce TNBC tumor growth in vitro and in vivo.134,135 Moreover, survival outcomes among women with TNBC taking metformin demonstrate reduced distant metastasis even among postmenopausal, black, and obese women.136
Metformin is an attractive potential therapeutic for TNBC due to is availability and impact on tumor growth and metastasis. This oral, antidiabetic mediation reduces circulating glucose creating a beneficial environment for TNBC treatment. At physiologic or low levels of glucose, the ability of metformin to inhibit cell proliferation and tumor formation, and induce apoptosis is optimized.137,138 Metformin induces apoptosis of TNBC. Although not completely understood, metformin mediates this process by activating miR-193b, an inhibitor of fatty acid synthase, and AMP-activated protein kinase (AMPK), which inhibits signaling by mammalian target of rapamycin (mTOR) and signal transducer and activator of transcription-3 (STAT-3).138–141 In vitro metformin reduces mRNA levels of vimentin and snail, markers of epithelial-mesenchymal transition (EMT), while increasing E-cadherin highlighting a preventive action against TNBC metastasis.141 In endometrial cells, metformin inhibits cell proliferation stimulated by IGF-2 and mTOR signaling while increasing the expression of the progesterone receptor (PR) and sensitivity to medroxyprogesterone acetate (MPA).142 In these same cells, metformin upregulated IGFBP-1 mRNA and protein, reduced IGF-1R mRNA, and decreased proliferation, an anti-proliferative effect enhanced with PPP, an IGF-1R inhibitor.143 In addition to the IGF-1R promoter, metformin significantly reduces promoter activity of IR,144 accentuating its putative effect on the IGF/IR receptor axis. In lung tumorgenesis, metformin reduces circulating levels of insulin and IGF-1, and inhibits AMPK-independent receptor tyrosine kinase (RTK) signaling pathways (e.g., EGFR, cMET, VEGFR, FGFR;).145 However, Capp et al. did not detect a significant change in IGF-1R or IGF-2R in the presence of metformin using primary cultures.146 Another study evaluating patients >50 years with endometrial cancer and type 2 diabetes mellitus (DM2) did not detect a significant difference in IGF-1R among patients receiving metformin, insulin, or sulfonylurea derivatives.147 To develop a better understanding of the impact of metformin on the IGF axis, further studies are clearly needed.
V. FUTURE DIRECTIONS FOR TNBC TREATMENT
Unfortunately, patients afflicted with TNBC often respond poorly to chemotherapy, and currently targeted therapies for TNBC are not available. However, preclinical in vitro and in vivo studies utilizing multiple therapies provide hope for improved outcomes for those with TNBC. Lau et al. found that basal breast cancer responds well to metformin and erlotinib.137 A combination of gefitinib, an epidermal growth factor receptor (EGFR) inhibitor, and inhibition of activation of sphingosine kinase-1 (SphK1)/sphingosine 1-phosphate (S1P) system by IGFBP-3 prevented TNBC tumor growth.148 To prevent metastasis, Mancini et al.79 investigated the efficacy of co-targeting IGF1 and IGF-2, and their receptors. In this study NVP-AEW541, an antagonist IGF-1R, was sufficient to inhibit IGF-1 initiated migration of MDA-MB-231 cells, whereas NVP-AEW541 complemented with MAB292, an IGF-2 monoclonal antibody, were necessary to inhibit IGF-2 mediated migration.79 This highlights the benefit in determining levels of IGF-1 and IGF-2 in TNBC tumors in investigations to reduce metastasis. Another study assessed the response of TNBC cultures to BMS-754807, an anti-IGF-1R/IR inhibitor, and found significant reduction in cell proliferation. Furthermore, xenografts underwent complete regression when treated with BMS-754807 and docetaxel.149 In addition, development of novel delivery methods to introduce IGFBP-1, IGFBP-2, IGFBP-6, or protease-resistant IGFBP-4 could promote improved methods to elicit tumor regression including TNBC (Fig. 3). Finally, as detailed in recent reports, cooperative interactions between IGF and ERβ signaling pathways in TNBC and potential racial/ethnic differences in the biology of TNBC may lead to additional therapeutic strategies in this deadly disease.28,39
VI. CONCLUSION
Targeted and more effective therapies for TNBC are urgently needed. It is clear that TNBC is a molecularly, pathologically, and clinically diverse disease that will likely require more targeted therapeutic approaches.1–4,6 Standard chemotherapy has some efficacy in a subset of patients with TNBC, and better strategies to identify these patients, as well as those less likely to respond prior to treatment, could have a significant impact on clinical management and patient outcomes. The success of clinical trials going forward in patients with TNBC may require stratification of tumors by molecular subtypes, specific genomic alterations, and/or pathologic prescreening for “driver” signal transduction pathways in TNBC specimens. For example, some novel therapeutic strategies are currently being tested in phase I–III trials, including antiestrogens and aromatase inhibitors as adjuvant therapies in TNBC, antiandrogens (such as enzalutamide) for androgen receptor-positive, and potentially other TNBC subtypes that have androgen receptor dependence,150,151 and poly(ADP-ribose) polymerase inhibitors for BRCA-mutated TNBC[142]. Treatment of TNBC based on other actionable targets such as IGF-2 also deserve consideration for future clinical translation. The discovery of ERβ and its expression in TNBC initially raised hope that targeting ERβ might offer new treatment options for TNBC patients, but several caveats, as discussed above, still remain. To accomplish the goal of discovering new targeted treatments for TNBC, further preclinical investigation utilizing a wide array of established TNBC models will help to develop more effective therapeutics and guide strategies for clinical intervention.
Acknowledgments
This work is dedicated to the memory of our distinguished colleague and friend Dr. Lee Goodglick (1960–2014) who made major contributions in cancer research during his professional career.
Funding support was from the National Institutes of Health (NIH)/National Cancer Institute Grant No. U54 CA14393 Partnership to Eliminate Cancer Health Disparities, Grant No. NIH CA-86366 Early Detection Research Network, NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant No. UL1TR000124, California Breast Cancer Research Program IDEA Awards No. 16 IB-0042 and No. 18IB-0034, UCLA Jonsson Cancer Center Foundation Transdisciplinary Research Grant, Stiles Program in Integrative Oncology, Tower Cancer Research Foundation-Jessica M. Berman Senior Investigator Award, Hickey Family Foundation, and the Robert Wood Johnson Foundation Nurse Faculty Scholar Award No. 69352.
ABBREVIATIONS
- AR
androgen receptor
- BC
breast cancer
- EGFR
epidermal growth factor receptor
- ER
estrogen receptor
- ERα
estrogen receptor-alpha
- ERβ
estrogen receptor-beta
- IGF-2
insulin-like growth factor-2
- IGFBP
IGF binding protein
- IHC
immunohistochemistry
- PR
progesterone receptor
- TNBC
triple-negative breast cancer
- VEGF
vascular endothelial growth factor
References
- 1.Foulkes WD, Stefansson IM, Chappuis PO, Begin LR, Goffin JR, Wong N, Trudel M, Akslen LA. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst. 2003;95:1482–5. doi: 10.1093/jnci/djg050. [DOI] [PubMed] [Google Scholar]
- 2.Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML, Perou CM. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–34. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 3.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 4.Abramson VG, Lehmann BD, Ballinger TJ, Pietenpol JA. Subtyping of triple-negative breast cancer: implications for therapy. Cancer. 2015;121:8–16. doi: 10.1002/cncr.28914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minami CA, Chung DU, Chang HR. Management options in triple-negative breast cancer. Breast Cancer (Auckl) 2011;5:175–99. doi: 10.4137/BCBCR.S6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–67. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaklamani VG, Siziopikou K, Scholtens D, Lacouture M, Gordon J, Uthe R, Meservey C, Hansen N, Khan SA, Jeruss JS, Bethke K, Cianfrocca M, Rosen S, Von Roenn J, Wayne J, Parimi V, Jovanovic B, Gradishar W. Pilot neoadjuvant trial in HER2 positive breast cancer with combination of nab-paclitaxel and lapatinib. Breast Cancer Res Treat. 2012;132:833–42. doi: 10.1007/s10549-011-1411-8. [DOI] [PubMed] [Google Scholar]
- 8.Haffty BG, Yang Q, Reiss M, Kearney T, Higgins SA, Weidhaas J, Harris L, Hait W, Toppmeyer D. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;24:5652–7. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 9.O’Toole SA, Beith JM, Millar EK, West R, McLean A, Cazet A, Swarbrick A, Oakes SR. Therapeutic targets in triple negative breast cancer. J Clin Pathol. 2013;66:530–42. doi: 10.1136/jclinpath-2012-201361. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Angulo AM, Timms KM, Liu S, Chen H, Litton JK, Potter J, Lanchbury JS, Stemke-Hale K, Hennessy BT, Arun BK, Hortobagyi GN, Do KA, Mills GB, Meric-Bernstam F. Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clin Cancer Res. 2011;17:1082–9. doi: 10.1158/1078-0432.CCR-10-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiggans RG, Woolley PV, Smythe T, Hoth D, Macdonald JS, Green L, Schein PS. Phase-II trial of tamoxifen in advanced breat cancer. Cancer Chemother Pharmacol. 1979;3:45–8. doi: 10.1007/BF00254419. [DOI] [PubMed] [Google Scholar]
- 12.D’Andrea AD, Grompe M. The Fanconi anaemia/BRCA pathway. Nat ReviewsCancer. 2003;3:23–34. doi: 10.1038/nrc970. [DOI] [PubMed] [Google Scholar]
- 13.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, Martin NM, Jackson SP, Smith GC, Ashworth A. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–21. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 14.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–74. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 15.Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O’Connor MJ, Ashworth A, Carmichael J, Kaye SB, Schellens JH, de Bono JS. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. New Engl J Med. 2009;361:123–34. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 16.Burstein HJ, Elias AD, Rugo HS, Cobleigh MA, Wolff AC, Eisenberg PD, Lehman M, Adams BJ, Bello CL, DePrimo SE, Baum CM, Miller KD. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2008;26:1810–6. doi: 10.1200/JCO.2007.14.5375. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler L, Akslen LA, Ragaz J, Gown AM, Gilks CB, van de Rijn M, Perou CM. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–74. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 18.Finn RS, Dering J, Ginther C, Wilson CA, Glaspy P, Tchekmedyian N, Slamon DJ. Dasatinib, an orally active small molecule inhibitor of both the src and abl kinases, selectively inhibits growth of basal-type/“triple-negative” breast cancer cell lines growing in vitro. Breast Cancer Res Treat. 2007;105:319–26. doi: 10.1007/s10549-006-9463-x. [DOI] [PubMed] [Google Scholar]
- 19.Ellard SL, Clemons M, Gelmon KA, Norris B, Kennecke H, Chia S, Pritchard K, Eisen A, Vandenberg T, Taylor M, Sauerbrei E, Mishaeli M, Huntsman D, Walsh W, Olivo M, McIntosh L, Seymour L. Randomized phase II study comparing two schedules of everolimus in patients with recurrent/metastatic breast cancer: NCIC Clinical Trials Group IND.163. J Clin Oncol. 2009;27:4536–41. doi: 10.1200/JCO.2008.21.3033. [DOI] [PubMed] [Google Scholar]
- 20.Yu KD, Zhu R, Zhan M, Rodriguez AA, Yang W, Wong S, Makris A, Lehmann BD, Chen X, Mayer I, Pietenpol JA, Shao ZM, Symmans WF, Chang JC. Identification of prognosis-relevant subgroups in patients with chemoresistant triple-negative breast cancer. Clin Cancer Res. 2013;19:2723–33. doi: 10.1158/1078-0432.CCR-12-2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–70. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 22.Leung YK, Mak P, Hassan S, Ho SM. Estrogen receptor (ER)-beta isoforms: a key to understanding ER-beta signaling. Proc Natl Acad Sci U S A. 2006;103:13162–7. doi: 10.1073/pnas.0605676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy LC, Leygue E. The role of estrogen receptor-beta in breast cancer. Semin Reprod Med. 2012;30:5–13. doi: 10.1055/s-0031-1299592. [DOI] [PubMed] [Google Scholar]
- 24.Smart E, Hughes T, Smith L, Speirs V. Estrogen receptor beta: putting a positive into triple negative breast cancer? Hormone Mol Biol Clin Invest. 2013;16:117–23. doi: 10.1515/hmbci-2013-0042. [DOI] [PubMed] [Google Scholar]
- 25.Yan Y, Li X, Blanchard A, Bramwell VH, Pritchard KI, Tu D, Shepherd L, Myal Y, Penner C, Watson PH, Leygue E, Murphy LC. Expression of both estrogen receptor-beta 1 (ER-beta1) and its co-regulator steroid receptor RNA activator protein (SRAP) are predictive for benefit from tamoxifen therapy in patients with estrogen receptor-alpha (ER-alpha)-negative early breast cancer (EBC) Ann Oncol. 2013;24:1986–93. doi: 10.1093/annonc/mdt132. [DOI] [PubMed] [Google Scholar]
- 26.Wimberly H, Han G, Pinnaduwage D, Murphy LC, Yang XR, Andrulis IL, Sherman M, Figueroa J, Rimm DL. ERbeta splice variant expression in four large cohorts of human breast cancer patient tumors. Breast Cancer Res Treat. 2014;146:657–67. doi: 10.1007/s10549-014-3050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen EV, Greene GL, DeSombre ER. Immunochemical studies of estrogen receptors. Prog Clin Biol Res. 1987;249:283–305. [PubMed] [Google Scholar]
- 28.Poola I, Fuqua SA, De Witty RL, Abraham J, Marshallack JJ, Liu A. Estrogen receptor alpha-negative breast cancer tissues express significant levels of estrogen-independent transcription factors, ERbeta1 and ERbeta5: potential molecular targets for chemoprevention. Clin Cancer Res. 2005;11:7579–85. doi: 10.1158/1078-0432.CCR-05-0728. [DOI] [PubMed] [Google Scholar]
- 29.Chen JQ, Russo J. ERalpha-negative and triple negative breast cancer: molecular features and potential therapeutic approaches. Biochim Biophys Acta. 2009;1796:162–75. doi: 10.1016/j.bbcan.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Honma N, Horii R, Iwase T, Saji S, Younes M, Takubo K, Matsuura M, Ito Y, Akiyama F, Sakamoto G. Clinical importance of estrogen receptor-beta evaluation in breast cancer patients treated with adjuvant tamoxifen therapy. J Clin Oncol. 2008;26:3727–34. doi: 10.1200/JCO.2007.14.2968. [DOI] [PubMed] [Google Scholar]
- 31.Belardi V, Gallagher EJ, Novosyadlyy R, LeRoith D. Insulin and IGFs in obesity-related breast cancer. J Mammary Gland Biol Neoplasia. 2013;18:277–89. doi: 10.1007/s10911-013-9303-7. [DOI] [PubMed] [Google Scholar]
- 32.Gruvberger-Saal SK, Bendahl PO, Saal LH, Laakso M, Hegardt C, Eden P, Peterson C, Malmstrom P, Isola J, Borg A, Ferno M. Estrogen receptor beta expression is associated with tamoxifen response in ERalpha-negative breast carcinoma. Clin Cancer Res. 2007;13:1987–94. doi: 10.1158/1078-0432.CCR-06-1823. [DOI] [PubMed] [Google Scholar]
- 33.Mann S, Laucirica R, Carlson N, Younes PS, Ali N, Younes A, Li Y, Younes M. Estrogen receptor beta expression in invasive breast cancer. Human Pathol. 2001;32:113–8. doi: 10.1053/hupa.2001.21506. [DOI] [PubMed] [Google Scholar]
- 34.Skliris GP, Leygue E, Curtis-Snell L, Watson PH, Murphy LC. Expression of oestrogen receptor-beta in oestrogen receptor-alpha negative human breast tumours. Br J Cancer. 2006;95:616–26. doi: 10.1038/sj.bjc.6603295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skliris GP, Parkes AT, Limer JL, Burdall SE, Carder PJ, Speirs V. Evaluation of seven oestrogen receptor beta antibodies for immunohistochemistry, western blotting, and flow cytometry in human breast tissue. J Pathol. 2002;197:155–62. doi: 10.1002/path.1077. [DOI] [PubMed] [Google Scholar]
- 36.Speirs V, Green CA, Shaaban AM. Oestrogen receptor beta immunohistochemistry: time to get it right? J Clin Pathol. 2008;61:1150–1. Author reply 1151. [PubMed] [Google Scholar]
- 37.Weitsman GE, Skliris G, Ung K, Peng B, Younes M, Watson PH, Murphy LC. Assessment of multiple different estrogen receptor-beta antibodies for their ability to immunoprecipitate under chromatin immunoprecipitation conditions. Breast Cancer Res Treat. 2006;100:23–31. doi: 10.1007/s10549-006-9229-5. [DOI] [PubMed] [Google Scholar]
- 38.Fuqua SA, Schiff R, Parra I, Moore JT, Mohsin SK, Osborne CK, Clark GM, Allred DC. Estrogen receptor beta protein in human breast cancer: correlation with clinical tumor parameters. Cancer Res. 2003;63:2434–9. [PMC free article] [PubMed] [Google Scholar]
- 39.Hamilton N, Marquez-Garban D, Mah V, Fernando G, Elshimali Y, Garban H, Elashoff D, Vadgama J, Goodglick L, Pietras R. Biologic roles of estrogen receptor-beta and insulin-like growth factor-2 in triple-negative breast cancer. BioMed Res Int. 2015;2015:925703. doi: 10.1155/2015/925703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinberg OK, Marquez-Garban DC, Fishbein MC, Goodglick L, Garban HJ, Dubinett SM, Pietras RJ. Aromatase inhibitors in human lung cancer therapy. Cancer Res. 2005;65:11287–91. doi: 10.1158/0008-5472.CAN-05-2737. [DOI] [PubMed] [Google Scholar]
- 41.Siegfried JM, Stabile LP. Estrongenic steroid hormones in lung cancer. Semin Oncol. 2014;41:5–16. doi: 10.1053/j.seminoncol.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Z, Li Z, Ding X, Shen Z, Liu Z, An T, Duan J, Zhong J, Wu M, Zhao J, Zhuo M, Wang Y, Wang S, Sun Y, Bai H, Wang J. ERbeta localization influenced outcomes of EGFR-TKI treatment in NSCLC patients with EGFR mutations. Sci Rep. 2015;5:11392–11404. doi: 10.1038/srep11392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roger P, Sahla ME, Makela S, Gustafsson JA, Baldet P, Rochefort H. Decreased expression of estrogen receptor beta protein in proliferative preinvasive mammary tumors. Cancer Res. 2001;61:2537–41. [PubMed] [Google Scholar]
- 44.Huang B, Warner M, Gustafsson JA. Estrogen receptors in breast carcinogenesis and endocrine therapy. Mol Cell Endocrinol. 2014;418(3):240–244. doi: 10.1016/j.mce.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 45.Lindberg MK, Moverare S, Skrtic S, Gao H, Dahlman-Wright K, Gustafsson JA, Ohlsson C. Estrogen receptor (ER)-beta reduces ERalpha-regulated gene transcription, supporting a “ying yang” relationship between ERalpha and ERbeta in mice. Mol Endocrinol. 2003;17:203–8. doi: 10.1210/me.2002-0206. [DOI] [PubMed] [Google Scholar]
- 46.Powell E, Shanle E, Brinkman A, Li J, Keles S, Wisinski KB, Huang W, Xu W. Identification of estrogen receptor dimer selective ligands reveals growth-inhibitory effects on cells that co-express ERalpha and ERbeta. PloS One. 2012;7:e30993. doi: 10.1371/journal.pone.0030993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sotoca AM, van den Berg H, Vervoort J, van der Saag P, Strom A, Gustafsson JA, Rietjens I, Murk AJ. Influence of cellular ERalpha/ERbeta ratio on the ERalpha-agonist induced proliferation of human T47D breast cancer cells. Toxicol Sci. 2008;105:303–11. doi: 10.1093/toxsci/kfn141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Novelli F, Milella M, Melucci E, Di Benedetto A, Sperduti I, Perrone-Donnorso R, Perracchio L, Venturo I, Nistico C, Fabi A, Buglioni S, Natali PG, Mottolese MA. divergent role for estrogen receptor-beta in node-positive and node-negative breast cancer classified according to molecular subtypes: an observational prospective study. Breast Cancer Res. 2008;10:R74–86. doi: 10.1186/bcr2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chantzi NI, Tiniakos DG, Palaiologou M, Goutas N, Filippidis T, Vassilaros SD, Dhimolea E, Mitsiou DJ, Alexis MN. Estrogen receptor beta 2 is associated with poor prognosis in estrogen receptor alpha-negative breast carcinoma. J Cancer Res Clin Oncol. 2013;139:1489–98. doi: 10.1007/s00432-013-1467-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Phillips KA, Milne RL, Rookus MA, Daly MB, Antoniou AC, Peock S, Frost D, Easton DF, Ellis S, Friedlander ML, Buys SS, Andrieu N, Nogues C, Stoppa-Lyonnet D, Bonadona V, Pujol P, McLachlan SA, John EM, Hooning MJ, Seynaeve C, Tollenaar RA, Goldgar DE, Terry MB, Caldes T, Weideman PC, Andrulis IL, Singer CF, Birch K, Simard J, Southey MC, Olsson HL, Jakubowska A, Olah E, Gerdes AM, Foretova L, Hopper JL. Tamoxifen and risk of contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2013;31:3091–9. doi: 10.1200/JCO.2012.47.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shazer RL, Jain A, Galkin AV, Cinman N, Nguyen KN, Natale RB, Gross M, Green L, Bender LI, Holden S, Kaplan L, Agus DB. Raloxifene, an oestrogen-receptor-beta-targeted therapy, inhibits androgen-independent prostate cancer growth: results from preclinical studies and a pilot phase II clinical trial. BJU Int. 2006;97:691–7. doi: 10.1111/j.1464-410X.2006.05974.x. [DOI] [PubMed] [Google Scholar]
- 52.Stewart J, King R, Hayward J, Rubens R. Estrogen and progesterone receptors: correlation of response rates, site and timing of receptor analysis. Breast Cancer Res Treatment. 1982;2:243–50. doi: 10.1007/BF01806937. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, Chirgadze NY, Briggs SL, Khan S, Jensen EV, Burris TP. A second binding site for hydroxytamoxifen within the coactivator-binding groove of estrogen receptor beta. Proc Natl Acad Sci U S A. 2006;103:908–11. doi: 10.1073/pnas.0510596103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Charn TH, Liu ET, Chang EC, Lee YK, Katzenellenbogen JA, Katzenellenbogen BS. Genome-wide dynamics of chromatin binding of estrogen receptors alpha and beta: mutual restriction and competitive site selection. Mol Endocrinol. 2010;24:47–59. doi: 10.1210/me.2009-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tonetti DA, Rubenstein R, DeLeon M, Zhao H, Pappas SG, Bentrem DJ, Chen B, Constantinou A, Craig Jordan V. Stable transfection of an estrogen receptor beta cDNA isoform into MDA-MB-231 breast cancer cells. J Steroid Biochem Mol Biol. 2003;87:47–55. doi: 10.1016/j.jsbmb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 56.Hou YF, Yuan ST, Li HC, Wu J, Lu JS, Liu G, Lu LJ, Shen ZZ, Ding J, Shao ZM. ERbeta exerts multiple stimulative effects on human breast carcinoma cells. Oncogene. 2004;23:5799–806. doi: 10.1038/sj.onc.1207765. [DOI] [PubMed] [Google Scholar]
- 57.Thomas C, Rajapaksa G, Nikolos F, Hao R, Katchy A, McCollum CW, Bondesson M, Quinlan P, Thompson A, Krishnamurthy S, Esteva FJ, Gustafsson JA. ERbeta1 represses basal breast cancer epithelial to mesenchymal transition by destabilizing EGFR. Breast Cancer Res. 2012;14:R148–163. doi: 10.1186/bcr3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang SY, Jordan VC. Growth regulation of estrogen receptor-negative breast cancer cells transfected with complementary DNAs for estrogen receptor. J Natl Cancer Inst. 1992;84:580–91. doi: 10.1093/jnci/84.8.580. [DOI] [PubMed] [Google Scholar]
- 59.Hershberger PA, Stabile LP, Kanterewicz B, Rothstein ME, Gubish CT, Land S, Shuai Y, Siegfried JM, Nichols M. Estrogen receptor beta (ERbeta) subtype-specific ligands increase transcription, p44/p42 mitogen activated protein kinase (MAPK) activation and growth in human non-small cell lung cancer cells. J Steroid Biochem Mol Biol. 2009;116:102–9. doi: 10.1016/j.jsbmb.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nair HB, Kirma NB, Ganapathy M, Vadlamudi RK, Tekmal RR. Estrogen receptor-beta activation in combination with letrozole blocks the growth of breast cancer tumors resistant to letrozole therapy. Steroids. 2011;76:792–6. doi: 10.1016/j.steroids.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 61.Stabile LP, Davis AL, Gubish CT, Hopkins TM, Luketich JD, Christie N, Finkelstein S, Siegfried JM. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res. 2002;62:2141–50. [PubMed] [Google Scholar]
- 62.Rudolph A, Toth C, Hoffmeister M, Roth W, Herpel E, Jansen L, Marx A, Brenner H, Chang-Claude J. Expression of oestrogen receptor beta and prognosis of colorectal cancer. Br J Cancer. 2012;107:831–9. doi: 10.1038/bjc.2012.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cotrim CZ, Fabris V, Doria ML, Lindberg K, Gustafsson JA, Amado F, Lanari C, Helguero LA. Estrogen receptor beta growth-inhibitory effects are repressed through activation of MAPK and PI3K signalling in mammary epithelial and breast cancer cells. Oncogene. 2013;32:2390–402. doi: 10.1038/onc.2012.261. [DOI] [PubMed] [Google Scholar]
- 64.Tremblay A, Tremblay GB, Labrie F, Giguere V. Ligand-independent recruitment of SRC-1 to estrogen receptor beta through phosphorylation of activation function AF-1. Mol Cell. 1999;3:513–9. doi: 10.1016/s1097-2765(00)80479-7. [DOI] [PubMed] [Google Scholar]
- 65.Corkery B, Crown J, Clynes M, O’Donovan N. Epidermal growth factor receptor as a potential therapeutic target in triple-negative breast cancer. Ann Oncol. 2009;20:862–7. doi: 10.1093/annonc/mdn710. [DOI] [PubMed] [Google Scholar]
- 66.Pietras RJ, Marquez-Garban DC. Membrane-associated estrogen receptor signaling pathways in human cancers. Clin Cancer Res. 2007;13:4672–6. doi: 10.1158/1078-0432.CCR-07-1373. [DOI] [PubMed] [Google Scholar]
- 67.Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Endocrine Rev. 2007;28:726–41. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- 68.Madak-Erdogan Z, Kieser KJ, Kim SH, Komm B, Katzenellenbogen JA, Katzenellenbogen BS. Nuclear and extranuclear pathway inputs in the regulation of global gene expression by estrogen receptors. Mol Endocrinol. 2008;22:2116–27. doi: 10.1210/me.2008-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shaaban AM, Green AR, Karthik S, Alizadeh Y, Hughes TA, Harkins L, Ellis IO, Robertson JF, Paish EC, Saunders PT, Groome NP, Speirs V. Nuclear and cytoplasmic expression of ERbeta1, ERbeta2, and ERbeta5 identifies distinct prognostic outcome for breast cancer patients. Clin Cancer Res. 2008;14:5228–35. doi: 10.1158/1078-0432.CCR-07-4528. [DOI] [PubMed] [Google Scholar]
- 70.DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9:212–222. doi: 10.1186/bcr1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 72.Cunningham M, Gilkeson G. Estrogen receptors in immunity and autoimmunity. Clinical Rev Allergy Immunol. 2011;40:66–73. doi: 10.1007/s12016-010-8203-5. [DOI] [PubMed] [Google Scholar]
- 73.Hauner D, Hauner H. Metabolic syndrome and breast cancer: is there a link? Breast Care. 2014;9:277–81. doi: 10.1159/000365951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Davis AA, Kaklamani VG. Metabolic syndrome and triple-negative breast cancer: a new paradigm. Int J Breast Cancer. 2012;2012:809291. doi: 10.1155/2012/809291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Coughlin SS, Smith SA. The insulin-like growth factor axis, adipokines, physical activity, and obesity in relation to breast cancer incidence and recurrence. Cancer Clin Oncol. 2015;4:24–31. doi: 10.5539/cco.v4n2p24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Osborne CK, Clemmons DR, Arteaga CL. Regulation of breast cancer growth by insulin-like growth factors. J Steroid Biochem Mol Biol. 1990;37:805–9. doi: 10.1016/0960-0760(90)90423-i. [DOI] [PubMed] [Google Scholar]
- 77.Richardson AE, Hamilton N, Davis W, Brito C, De Leon D. Insulin-like growth factor-2 (IGF-2) activates estrogen receptor-alpha and -beta via the IGF-1 and the insulin receptors in breast cancer cells. Growth Factors. 2011;29:82–93. doi: 10.3109/08977194.2011.565003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Westley BR, May FE. Role of insulin-like growth factors in steroid modulated proliferation. J Steroid Biochem Mol Biol. 1994;51:1–9. doi: 10.1016/0960-0760(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 79.Mancini M, Gariboldi MB, Taiana E, Bonzi MC, Craparotta I, Pagin M, Monti E. Co-targeting the IGF system and HIF-1 inhibits migration and invasion by (triple-negative) breast cancer cells. Br J Cancer. 2014;110:2865–73. doi: 10.1038/bjc.2014.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khajah MA, Al Saleh S, Mathew PM, Luqmani YA. Differential effect of growth factors on invasion and proliferation of endocrine resistant breast cancer cells. PloS One. 2012;7:e41847. doi: 10.1371/journal.pone.0041847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Singh SK, Moretta D, Almaguel F, Wall NR, De Leon M, De Leon D. Differential effect of proIGF-II and IGF-II on resveratrol induced cell death by regulating survivin cellular localization and mitochondrial depolarization in breast cancer cells. Growth Factors. 2007;25:363–72. doi: 10.1080/08977190801886905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lodhia KA, Tienchaiananda P, Haluska P. Understanding the key to targeting the IGF axis in cancer: a biomarker assessment. Front Oncol. 2015;5:142–156. doi: 10.3389/fonc.2015.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12:159–69. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 84.Klotz DM, Hewitt SC, Ciana P, Raviscioni M, Lindzey JK, Foley J, Maggi A, DiAugustine RP, Korach KS. Requirement of estrogen receptor-alpha in insulin-like growth factor-1 (IGF-1)-induced uterine responses and in vivo evidence for IGF-1/estrogen receptor cross-talk. J Biol Chem. 2002;277:8531–7. doi: 10.1074/jbc.M109592200. [DOI] [PubMed] [Google Scholar]
- 85.Kahlert S, Nuedling S, van Eickels M, Vetter H, Meyer R, Grohe C. Estrogen receptor alpha rapidly activates the IGF-1 receptor pathway. J Biol Chem. 2000;275:18447–53. doi: 10.1074/jbc.M910345199. [DOI] [PubMed] [Google Scholar]
- 86.Hirano H, Lopes MB, Laws ER, Jr, Asakura T, Goto M, Carpenter JE, Karns LR, VandenBerg SR. Insulin-like growth factor-1 content and pattern of expression correlates with histopathologic grade in diffusely infiltrating astrocytomas. Neuro-Oncology. 1999;1:109–19. doi: 10.1093/neuonc/1.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sohda T, Oka Y, Iwata K, Gunn J, Kamimura S, Shijo H, Okumura M, Yun K. Co-localisation of insulin-like growth factor II and the proliferation marker MIB1 in hepatocellular carcinoma cells. J Clin Pathol. 1997;50:135–7. doi: 10.1136/jcp.50.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kawamoto K, Onodera H, Kondo S, Kan S, Ikeuchi D, Maetani S, Imamura M. Expression of insulin-like growth factor-2 can predict the prognosis of human colorectal cancer patients: correlation with tumor progression, proliferative activity and survival. Oncology. 1998;55:242–8. doi: 10.1159/000011858. [DOI] [PubMed] [Google Scholar]
- 89.Livingstone C. IGF2 and cancer. Endocrine-Relat Cancer. 2013;20:R321–39. doi: 10.1530/ERC-13-0231. [DOI] [PubMed] [Google Scholar]
- 90.Bartolomei MS, Zemel S, Tilghman SM. Parental imprinting of the mouse H19 gene. Nature. 1991;351:153–5. doi: 10.1038/351153a0. [DOI] [PubMed] [Google Scholar]
- 91.DeChiara TM, Robertson EJ, Efstratiadis A. Parental imprinting of the mouse insulin-like growth factor II gene. Cell. 1991;64:849–59. doi: 10.1016/0092-8674(91)90513-x. [DOI] [PubMed] [Google Scholar]
- 92.Thorvaldsen JL, Duran KL, Bartolomei MS. Deletion of the H19 differentially methylated domain results in loss of imprinted expression of H19 and Igf2. Genes Dev. 1998;12:3693–702. doi: 10.1101/gad.12.23.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pathak S, D’Souza R, Ankolkar M, Gaonkar R, Balasinor NH. Potential role of estrogen in regulation of the insulin-like growth factor2-H19 locus in the rat testis. Mol Cell Endocrinol. 2010;314:110–7. doi: 10.1016/j.mce.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 94.Pathak S, Kedia-Mokashi N, Saxena M, D’Souza R, Maitra A, Parte P, Gill-Sharma M, Balasinor N. Effect of tamoxifen treatment on global and insulin-like growth factor 2-H19 locus-specific DNA methylation in rat spermatozoa and its association with embryo loss. Fertil Steril. 2009;91:2253–63. doi: 10.1016/j.fertnstert.2008.07.1709. [DOI] [PubMed] [Google Scholar]
- 95.Kelley KM, Oh Y, Gargosky SE, Gucev Z, Matsumoto T, Hwa V, Ng L, Simpson DM, Rosenfeld RG. Insulin-like growth factor-binding proteins (IGFBPs) and their regulatory dynamics. Int J Biochem Cell Biol. 1996;28:619–37. doi: 10.1016/1357-2725(96)00005-2. [DOI] [PubMed] [Google Scholar]
- 96.Baxter RC. IGF binding proteins in cancer: mechanistic and clinical insights. Nat Rev Cancer. 2014;14:329–41. doi: 10.1038/nrc3720. [DOI] [PubMed] [Google Scholar]
- 97.Bach LA. IGFBP-6 five years on; not so ‘forgotten’? Growth Hormone IGF Res. 2005;15:185–92. doi: 10.1016/j.ghir.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 98.Jones JI, Gockerman A, Busby WH, Jr, Wright G, Clemmons DR. Insulin-like growth factor binding protein 1 stimulates cell migration and binds to the alpha 5 beta 1 integrin by means of its Arg-Gly-Asp sequence. Proc Natl Acad Sci U S A. 1993;90:10553–7. doi: 10.1073/pnas.90.22.10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang X, Yee D. Insulin-like growth factor binding protein-1 (IGFBP-1) inhibits breast cancer cell motility. Cancer Res. 2002;62:4369–75. [PubMed] [Google Scholar]
- 100.Park JH, Rasch MG, Qiu J, Lund IK, Egeblad M. Presence of insulin-like growth factor binding proteins correlates with tumor-promoting effects of matrix metalloproteinase 9 in breast cancer. Neoplasia. 2015;17:421–33. doi: 10.1016/j.neo.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Foulstone EJ, Zeng L, Perks CM, Holly JM. Insulin-like growth factor binding protein 2 (IGFBP-2) promotes growth and survival of breast epithelial cells: novel regulation of the estrogen receptor. Endocrinology. 2013;154:1780–93. doi: 10.1210/en.2012-1970. [DOI] [PubMed] [Google Scholar]
- 102.Frommer KW, Reichenmiller K, Schutt BS, Hoeflich A, Ranke MB, Dodt G, Elmlinger MW. IGF-independent effects of IGFBP-2 on the human breast cancer cell line Hs578T. J Mol Endocrinol. 2006;37:13–23. doi: 10.1677/jme.1.01955. [DOI] [PubMed] [Google Scholar]
- 103.Shimasaki S, Ling N. Identification and molecular characterization of insulin-like growth factor binding proteins (IGFBP-1, -2, -3, -4, -5 and -6) Prog Growth Factor Res. 1991;3:243–66. doi: 10.1016/0955-2235(91)90003-m. [DOI] [PubMed] [Google Scholar]
- 104.Feng N, Zhang Z, Wang Z, Zheng H, Qu F, He X, Wang C. Insulin-like growth factor binding protein-2 promotes adhesion of endothelial progenitor cells to endothelial cells via integrin alpha5beta1. J Mol Neurosci. 2015;57(3):426–34. doi: 10.1007/s12031-015-0589-3. [DOI] [PubMed] [Google Scholar]
- 105.Wang C, Gao C, Meng K, Qiao H, Wang Y. Human adipocytes stimulate invasion of breast cancer MCF-7 cells by secreting IGFBP-2. PloS One. 2015;10:e0119348. doi: 10.1371/journal.pone.0119348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Catsburg C, Gunter MJ, Tinker L, Chlebowski RT, Pollak M, Strickler HD, Cote ML, Page DL, Rohan TE. Serum IGFBP-2 and risk of atypical hyperplasia of the breast. J Cancer Epidemiol. 2015;2015:203284. doi: 10.1155/2015/203284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Azar WJ, Zivkovic S, Werther GA, Russo VC. IGFBP-2 nuclear translocation is mediated by a functional NLS sequence and is essential for its pro-tumorigenic actions in cancer cells. Oncogene. 2014;33:578–88. doi: 10.1038/onc.2012.630. [DOI] [PubMed] [Google Scholar]
- 108.Sehgal P, Kumar N, Praveen Kumar VR, Patil S, Bhattacharya A, Vijaya Kumar M, Mukherjee G, Kondaiah P. Regulation of protumorigenic pathways by insulin like growth factor binding protein2 and its association along with beta-catenin in breast cancer lymph node metastasis. Mol Cancer. 2013;12:63–78. doi: 10.1186/1476-4598-12-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lin MZ, Marzec KA, Martin JL, Baxter RC. The role of insulin-like growth factor binding protein-3 in the breast cancer cell response to DNA-damaging agents. Oncogene. 2014;33:85–96. doi: 10.1038/onc.2012.538. [DOI] [PubMed] [Google Scholar]
- 110.Vestey SB, Perks CM, Sen C, Calder CJ, Holly JM, Winters ZE. Immunohistochemical expression of insulin-like growth factor binding protein-3 in invasive breast cancers and ductal carcinoma in situ: implications for clinicopathology and patient outcome. Breast Cancer Res. 2005;7:R119–29. doi: 10.1186/bcr963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McIntosh J, Dennison G, Holly JM, Jarrett C, Frankow A, Foulstone EJ, Winters ZE, Perks CM. IGFBP-3 can either inhibit or enhance EGF-mediated growth of breast epithelial cells dependent upon the presence of fibronectin. J Biol Chem. 2010;285:38788–800. doi: 10.1074/jbc.M110.177311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ioachim E, Charchanti A, Briasoulis E, Karavasilis V, Tsanou H, Arvanitis DL, Agnantis NJ, Pavlidis N. Immunohistochemical expression of extracellular matrix components tenascin, fibronectin, collagen type IV and laminin in breast cancer: their prognostic value and role in tumour invasion and progression. Eur J Cancer. 2002;38:2362–70. doi: 10.1016/s0959-8049(02)00210-1. [DOI] [PubMed] [Google Scholar]
- 113.Burrows C, Holly JM, Laurence NJ, Vernon EG, Carter JV, Clark MA, McIntosh J, McCaig C, Winters ZE, Perks CM. Insulin-like growth factor binding protein 3 has opposing actions on malignant and nonmalignant breast epithelial cells that are each reversible and dependent upon cholesterol-stabilized integrin receptor complexes. Endocrinology. 2006;147:3484–500. doi: 10.1210/en.2006-0005. [DOI] [PubMed] [Google Scholar]
- 114.Lawrence JB, Oxvig C, Overgaard MT, Sottrup-Jensen L, Gleich GJ, Hays LG, Yates JR, 3rd, Conover CA. The insulin-like growth factor (IGF)-dependent IGF binding protein-4 protease secreted by human fibroblasts is pregnancy-associated plasma protein-A. Proc of Natl Acad Sci U S A. 1999;96:3149–53. doi: 10.1073/pnas.96.6.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rehault S, Monget P, Mazerbourg S, Tremblay R, Gutman N, Gauthier F, Moreau T. Insulin-like growth factor binding proteins (IGFBPs) as potential physiological substrates for human kallikreins hK2 and hK3. Eur J Biochem/FEBS. 2001;268:2960–8. doi: 10.1046/j.1432-1327.2001.02185.x. [DOI] [PubMed] [Google Scholar]
- 116.Ryan AJ, Napoletano S, Fitzpatrick PA, Currid CA, O’Sullivan NC, Harmey JH. Expression of a protease-resistant insulin-like growth factor-binding protein-4 inhibits tumour growth in a murine model of breast cancer. Br J Cancer. 2009;101:278–86. doi: 10.1038/sj.bjc.6605141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jones JI, Gockerman A, Busby WH, Jr, Camacho-Hubner C, Clemmons DR. Extracellular matrix contains insulin-like growth factor binding protein-5: potentiation of the effects of IGF-I. J Cell Biol. 1993;121:679–87. doi: 10.1083/jcb.121.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Butt AJ, Dickson KA, McDougall F, Baxter RC. Insulin-like growth factor-binding protein-5 inhibits the growth of human breast cancer cells in vitro and in vivo. J Biol Chem. 2003;278:29676–85. doi: 10.1074/jbc.M301965200. [DOI] [PubMed] [Google Scholar]
- 119.Hsieh T, Gordon RE, Clemmons DR, Busby WH, Jr, Duan C. Regulation of vascular smooth muscle cell responses to insulin-like growth factor (IGF)-I by local IGF-binding proteins. J Biol Chem. 2003;278:42886–92. doi: 10.1074/jbc.M303835200. [DOI] [PubMed] [Google Scholar]
- 120.Perks CM, McCaig C, Clarke JB, Clemmons DR, Holly JM. Effects of a non-IGF binding mutant of IGFBP-5 on cell death in human breast cancer cells. Biochem Biophys Res Commun. 2002;294:995–1000. doi: 10.1016/S0006-291X(02)00570-3. [DOI] [PubMed] [Google Scholar]
- 121.Xu Q, Yan B, Li S, Duan C. Fibronectin binds insulin-like growth factor-binding protein 5 and abolishes Its ligand-dependent action on cell migration. J Biol Chem. 2004;279:4269–77. doi: 10.1074/jbc.M311586200. [DOI] [PubMed] [Google Scholar]
- 122.McCaig C, Perks CM, Holly JM. Intrinsic actions of IGFBP-3 and IGFBP-5 on Hs578T breast cancer epithelial cells: inhibition or accentuation of attachment and survival is dependent upon the presence of fibronectin. J Cell Sci. 2002;115:4293–303. doi: 10.1242/jcs.00097. [DOI] [PubMed] [Google Scholar]
- 123.Kricker JA, Towne CL, Firth SM, Herington AC, Upton Z. Structural and functional evidence for the interaction of insulin-like growth factors (IGFs) and IGF binding proteins with vitronectin. Endocrinology. 2003;144:2807–15. doi: 10.1210/en.2002-221086. [DOI] [PubMed] [Google Scholar]
- 124.Becker MA, Hou X, Harrington SC, Weroha SJ, Gonzalez SE, Jacob KA, Carboni JM, Gottardis MM, Haluska P. IGFBP ratio confers resistance to IGF targeting and correlates with increased invasion and poor outcome in breast tumors. Clin Cancer Res. 2012;18:1808–17. doi: 10.1158/1078-0432.CCR-11-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Delhanty PJ, Hill DJ, Shimasaki S, Han VK. Insulin-like growth factor binding protein-4, -5 and -6 mRNAs in the human fetus: localization to sites of growth and differentiation? Growth Regul. 1993;3:8–11. [PubMed] [Google Scholar]
- 126.Kuo YS, Tang YB, Lu TY, Wu HC, Lin CT. IGFBP-6 plays a role as an oncosuppressor gene in NPC pathogenesis through regulating EGR-1 expression. J Pathol. 2010;222:299–309. doi: 10.1002/path.2735. [DOI] [PubMed] [Google Scholar]
- 127.Zhao HM, Sheng MJ, Yu J. Expression of IGFBP-6 in a proliferative vitreoretinopathy rat model and its effects on retinal pigment epithelial cell proliferation and migration. Int J Ophthalmol. 2014;7:27–33. doi: 10.3980/j.issn.2222-3959.2014.01.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang C, Lu L, Li Y, Wang X, Zhou J, Liu Y, Fu P, Gallicchio MA, Bach LA, Duan C. IGF binding protein-6 expression in vascular endothelial cells is induced by hypoxia and plays a negative role in tumor angiogenesis. Int J Cancer. 2012;130:2003–12. doi: 10.1002/ijc.26201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fu P, Liang GJ, Khot SS, Phan R, Bach LA. Cross-talk between MAP kinase pathways is involved in IGF-independent, IGFBP-6-induced Rh30 rhabdomyosarcoma cell migration. J Cell Physiol. 2010;224:636–43. doi: 10.1002/jcp.22156. [DOI] [PubMed] [Google Scholar]
- 130.Fu P, Thompson JA, Bach LA. Promotion of cancer cell migration: an insulin-like growth factor (IGF)-independent action of IGF-binding protein-6. J Biol Chem. 2007;282:22298–306. doi: 10.1074/jbc.M703066200. [DOI] [PubMed] [Google Scholar]
- 131.Fu P, Yang Z, Bach LA. Prohibitin-2 binding modulates insulin-like growth factor-binding protein-6 (IGFBP-6)-induced rhabdomyosarcoma cell migration. J Biol Chem. 2013;288:29890–900. doi: 10.1074/jbc.M113.510826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang Z, Bach LA. Differential effects of insulin-like growth factor binding protein-6 (IGFBP-6) on migration of two ovarian cancer cell lines. Front Endocrinol. 2014;5:231. doi: 10.3389/fendo.2014.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kalla Singh S, Tan QW, Brito C, De Leon M, Garberoglio C, De Leon D. Differential insulin-like growth factor II (IGF-II) expression: A potential role for breast cancer survival disparity. Growth Hormone IGF Res. 2010;20:162–70. doi: 10.1016/j.ghir.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu B, Fan Z, Edgerton SM, Deng XS, Alimova IN, Lind SE, Thor AD. Metformin induces unique biological and molecular responses in triple negative breast cancer cells. Cell Cycle. 2009;8:2031–40. doi: 10.4161/cc.8.13.8814. [DOI] [PubMed] [Google Scholar]
- 135.Alimova IN, Liu B, Fan Z, Edgerton SM, Dillon T, Lind SE, Thor AD. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle. 2009;8:909–15. doi: 10.4161/cc.8.6.7933. [DOI] [PubMed] [Google Scholar]
- 136.Bayraktar S, Hernadez-Aya LF, Lei X, Meric-Bernstam F, Litton JK, Hsu L, Hortobagyi GN, Gonzalez-Angulo AM. Effect of metformin on survival outcomes in diabetic patients with triple receptor-negative breast cancer. Cancer. 2012;118:1202–11. doi: 10.1002/cncr.26439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lau YK, Du X, Rayannavar V, Hopkins B, Shaw J, Bessler E, Thomas T, Pires MM, Keniry M, Parsons RE, Cremers S, Szabolcs M, Maurer MA. Metformin and erlotinib synergize to inhibit basal breast cancer. Oncotarget. 2014;5:10503–17. doi: 10.18632/oncotarget.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wahdan-Alaswad R, Fan Z, Edgerton SM, Liu B, Deng XS, Arnadottir SS, Richer JK, Anderson SM, Thor AD. Glucose promotes breast cancer aggression and reduces metformin efficacy. Cell Cycle. 2013;12:3759–69. doi: 10.4161/cc.26641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66:10269–73. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 140.Deng XS, Wang S, Deng A, Liu B, Edgerton SM, Lind SE, Wahdan-Alaswad R, Thor AD. Metformin targets Stat3 to inhibit cell growth and induce apoptosis in triple-negative breast cancers. Cell Cycle. 2012;11:367–76. doi: 10.4161/cc.11.2.18813. [DOI] [PubMed] [Google Scholar]
- 141.Zhao Z, Cheng X, Wang Y, Han R, Li L, Xiang T, He L, Long H, Zhu B, He Y. Metformin inhibits the IL-6-induced epithelial-mesenchymal transition and lung adenocarcinoma growth and metastasis. PloS One. 2014;9:e95884. doi: 10.1371/journal.pone.0095884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xie Y, Wang YL, Yu L, Hu Q, Ji L, Zhang Y, Liao QP. Metformin promotes progesterone receptor expression via inhibition of mammalian target of rapamycin (mTOR) in endometrial cancer cells. J Steroid Biochem Mol Biol. 2011;126:113–20. doi: 10.1016/j.jsbmb.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 143.Xie Y, Wang JL, Ji M, Yuan ZF, Peng Z, Zhang Y, Wen JG, Shi HR. Regulation of insulin-like growth factor signaling by metformin in endometrial cancer cells. Oncol Lett. 2014;8:1993–9. doi: 10.3892/ol.2014.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sarfstein R, Friedman Y, Attias-Geva Z, Fishman A, Bruchim I, Werner H. Metformin downregulates the insulin/IGF-I signaling pathway and inhibits different uterine serous carcinoma (USC) cells proliferation and migration in p53-dependent or -independent manners. PloS One. 2013;8:e61537. doi: 10.1371/journal.pone.0061537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Quinn BJ, Dallos M, Kitagawa H, Kunnumakkara AB, Memmott RM, Hollander MC, Gills JJ, Dennis PA. Inhibition of lung tumorigenesis by metformin is associated with decreased plasma IGF-I and diminished receptor tyrosine kinase signaling. Cancer Prevent Res. 2013;6:801–10. doi: 10.1158/1940-6207.CAPR-13-0058-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Capp E, Jauckus J, von Eye Corleta H, Toth B, Strowitzki T, Germeyer A. Does metformin influence the insulin-, IGF I- and IGF II-receptor gene expression and Akt phosphorylation in human decidualized endometrial stromal cells? Eur J Obstet Gynecol Reprod Biol. 2011;158:248–53. doi: 10.1016/j.ejogrb.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 147.Markowska A, Pawalowska M, Filas V, Korski K, Grybos M, Sajdak S, Olejek A, Bednarek W, Spiewankiewicz B, Lubin J, Markowska J. Does Metformin affect ER, PR, IGF-1R, beta-catenin and PAX-2 expression in women with diabetes mellitus and endometrial cancer? Diabetol Metabol Synd. 2013;5:76–87. doi: 10.1186/1758-5996-5-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Martin JL, de Silva HC, Lin MZ, Scott CD, Baxter RC. Inhibition of insulin-like growth factor-binding protein-3 signaling through sphingosine kinase-1 sensitizes triple-negative breast cancer cells to EGF receptor blockade. Mol Cancer Ther. 2014;13:316–28. doi: 10.1158/1535-7163.MCT-13-0367. [DOI] [PubMed] [Google Scholar]
- 149.Litzenburger BC, Creighton CJ, Tsimelzon A, Chan BT, Hilsenbeck SG, Wang T, Carboni JM, Gottardis MM, Huang F, Chang JC, Lewis MT, Rimawi MF, Lee AV. High IGF-IR activity in triple-negative breast cancer cell lines and tumorgrafts correlates with sensitivity to anti-IGF-IR therapy. Clin Cancer Res. 2011;17:2314–27. doi: 10.1158/1078-0432.CCR-10-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Barton VN, D’Amato NC, Gordon MA, Christenson JL, Elias A, Richer JK. Androgen receptor biology in triple negative breast cancer: a case for classification as AR+ or quadruple negative disease. Hormones Cancer. 2015;6(5–6):206–213. doi: 10.1007/s12672-015-0232-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Barton VN, D’Amato NC, Gordon MA, Lind HT, Spoelstra NS, Babbs BL, Heinz RE, Elias A, Jedlicka P, Jacobsen BM, Richer JK. Multiple molecular subtypes of triple-negative breast cancer critically rely on androgen receptor and respond to enzalutamide in vivo. Mol Cancer Ther. 2015;14:769–78. doi: 10.1158/1535-7163.MCT-14-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]