Abstract
Background
Nursing home residents with dementia experience increased risk for compromised eating performance due to intrapersonal, interpersonal, and environmental factors. Environmental stimulation is physical, social, and/or sensory stimulation present in the environment that can potentially trigger individuals’ emotion or motivate physical reactions. Beyond the personal factors, there is a lack of evidence on how environmental stimulation influences individuals’ eating performance at mealtimes.
Objectives
This study examined the association between environmental stimulation and eating performance among nursing home residents with dementia.
Design
This study was a secondary analysis using baseline videos selected from a communication intervention study, where videos were recorded to capture staff-resident interactions during care activities for nursing home residents with dementia. Videos were included in this study only if residents demonstrated eating activities at mealtimes.
Sample and Setting
A total of 36 videos were selected (mean length = 4 minutes). The sample included 15 residents with dementia (mean age = 86), and 19 certified nursing assistants (mean age = 36) in 8 nursing homes.
Methods
The dependent variable was eating performance as measured by the Level of Eating Independence scale (range: 15–36, with higher scores indicating better eating performance). The independent variables were characteristics of environmental stimulation measured by the Person-Environment Apathy Rating-Environment subscale (stimulation clarity, stimulation strength, stimulation specificity, interaction involvement, physical accessibility, and environmental feedback). Each characteristic was rated on a 1–4 scale with higher scores indicating more desirable environmental stimulation. Multilevel models were used to examine the association between eating performance and environmental stimulation, adjusting for resident characteristics (i.e., age, gender, dementia stage, function, comorbidity, psychoactive medication use) and nesting effects of residents and staff.
Results
Resident participants demonstrated moderate levels of eating performance (M=27.08, SD = 5.16). Eating performance was significantly lower among older residents, those with more advanced dementia, and higher comorbidity. After controlling for resident characteristics, eating performance was significantly associated with stimulation specificity (how the stimulation is delivered and tailored to the resident), and was not associated with other environmental stimulation characteristics. For each 1 point increase in stimulation specificity, eating performance increased by 8.78 points (95% CI=.59, 16.97).
Conclusions
Environmental stimulation that is personally tailored to a resident’ needs and preferences and directly offered to a resident contributed to better eating performance among residents with dementia. The findings will direct future development and implementation of person-directed mealtime care programs and dining environment arrangements for residents with dementia in nursing homes.
Keywords: eating performance, environmental stimulation, dementia, nursing homes, older adults
1. Introduction
1.1 Eating performance
Eating performance is the functional ability to get food and drinks into the mouth(1). As the most basic activity of daily living (ADL) for older adults, eating performance is usually the easiest function to restore after decline or loss(2–4). Maintaining independent eating performance at mealtimes not only promotes social engagement and enjoyment of meals, but also enhances functional autonomy and ensures adequate food intake to maintain nutritional status as a fundamental health need(5, 6).
Dementia currently affects an estimated 5.4 million older adults living in the U.S.(7). Approximately 68% of nursing home (NH) residents live with dementia(8, 9), and these residents experience high risk of compromised eating performance. NH residents with severe cognitive impairment experience the greatest deterioration in eating among all the basic ADLs within 6 months of NH admission(10). Prior work showed that 61.4% of institutionalized residents with dementia experienced eating difficulty(11). Specifically, 32% of NH residents with moderate to severe dementia had functional impairment in eating and required different levels of assistance(12).
Cognitively impaired residents with compromised eating performance are frequently confused with food during mealtimes and require close supervision and/or physical help. Residents with dementia may resist assistance by turning the head away or refusing to open the mouth when food is offered, which further compromises eating performance(13). Residents often demonstrate inability to initiate eating, locate food, use utensils or finger food appropriately, maintain attention to meals, preload food on utensils, unload food into the mouth, or bite, chew and swallow food without choking(14).
Impairment in eating performance results in various functional and nutritional consequences such as insufficient food intake, weight loss, malnutrition, infection, and loss of eating ability, which further impact residents’ health outcomes and quality of life(15–17). For example, prior research revealed that 30.7% of institutionalized residents with dementia consumed 75% or less of a meal, and this low food intake was significantly associated with eating difficulty(11). Compared to healthy controls, individuals with mild to moderate Alzheimer’s disease (AD) were more likely to experience malnutrition and those with severe AD were more likely to experience dehydration(18). All the findings point to the importance of optimal eating performance for this population in order to maintain their health and quality of life.
1.2 Dining environment in nursing homes
For nursing home residents with dementia, meals are embedded within the care environment(5). While some residents may dine in their own room or other areas in the nursing home, most meals take place in the dining room, where the resident shares the table with other residents. The dining room environment in traditional nursing homes is often busy and highly stimulating during mealtimes with a variety of food and drinks, sometimes music and/or TV programs in the background, people talking at the tables, and multiple staff members walking around serving food, setting residents up, and assisting residents with meals. The dining environments are similar in special dementia care units that have separate dining rooms for residents with dementia.
1.3 Overview of factors that influence eating performance
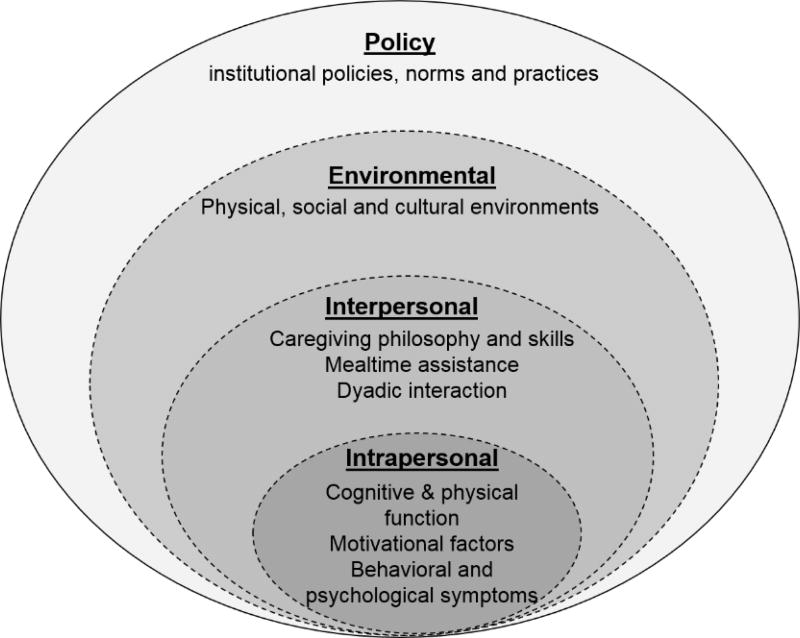
Factors that influence eating performance include intrapersonal attributes, interpersonal characteristics, environmental stimuli and policies based on the Social Ecological Model (SEM)(12, 19). Prior work used this model to describe factors that were associated with eating performance, and examined the associations of intrapersonal characteristics and institutional policy with eating performance in dementia(12). The model is briefly summarized here in relation to the context of the study (Figure 1).
Figure 1.
Factors that Influence Eating Performance using SEM Model
The intrapersonal factors that relate to resident characteristics include cognitive impairment, change of taste and appetite, comorbidities, physical capability, use of medications, motivation, mood and behavioral symptoms(8, 20–23). For example, NH residents with dementia experience greater impairment in eating function due to cognitive decline, motor apraxia, visual agnosia and behavioral symptoms compared to those without cognitive impairment(8, 22, 24). Beyond the intrapersonal factors, eating performance is also associated with multiple factors at the interpersonal, environmental, and policy levels(12). Interpersonal factors that relate to caregiver characteristics include caregivers’ perceptions and skills for engaging the resident in eating, dyadic communication and interaction, and mealtime assistance such as verbal cues and role modeling(5, 6, 25–27). Environmental factors include both physical environment (e.g., lighting, noise, tableware contrast, use of finger food, and availability of adaptive devices) (20, 23, 28), and social and cultural contexts (e.g., food delivery style, dining routines, social engagement, and cultural aspects of food choices)(29–31). Examples of policy factors include staffing and time allocated for meals, care practice (task-centered vs. person-centered), and safety considerations within institutions(32–34).
These factors interact to influence eating performance in dementia. For example, due to interpersonal barriers such as lack of dyadic interaction to engage and motivate residents in eating tasks, institutional barriers such as inadequate staffing and time to support mealtime assistance, and nursing home policies that focused excessively on preventing weight loss, nursing caregivers may provide excessive feeding assistance to maintain caloric intake and to complete feeding tasks within the dining timeframe. Excessive feeding assistance from caregivers without considering residents’ preferences and eating abilities could discourage residents’ motivation, reinforce unintended dependence, decrease residents’ autonomy, and result in resistiveness to care(5, 35).
The SEM model explains the interacting, interrelated, and interdependent dynamics of the personal and environmental factors that affect eating performance in dementia(36). Caregivers who sit in proximity and provide assistance to residents are in a strategically critical position to engage residents in eating by targeting the multilevel factors in the dining environment. Caregivers’ verbal and non-verbal behaviors toward the residents that are influenced by the institutional environment and regulations are the most direct and straightforward stimuli for residents in the dining environment. From the resident perspective, stimuli from both caregivers and the dining environment are sources of environmental stimulation during mealtimes.
Compared to intrapersonal factors that are mostly fixed, intervening on the interpersonal and environmental factors that are modifiable is more likely to support optimal eating performance in dementia. From this perspective, interventions to improve mealtime performance should include training nursing assistants and using environmental modifications or mealtime assistance. Nursing staff training programs demonstrate evidence in improving feeding difficulty(37, 38), but most programs focus on the use of feeding skills instead of engagement, interaction and other environmental factors to promote eating performance(39, 40). The strength of evidence for using environmental modifications and mealtime assistance is limited by the strength of study designs(38). However, improving dining environment in nursing homes significantly improves meal intake and nutritional status for older adults with dementia(41), and demonstrates the importance of environmental factors at mealtimes. More research is needed to identify and test innovative ways to improve the mealtime environment to maintain optimal eating performance for residents with dementia in nursing home settings(20, 37, 38).
1.4 Environmental stimulation during mealtimes for residents with dementia
The literature has increasingly recognized the importance of dining environments in dementia care(42). Environmental stimulation and opportunities for personal choices are two key factors in the physical and social environment that are associated with individuals’ eating performance(20). Environmental stimulation broadly refers to the physical, social and/or sensory stimulation that individuals are exposed to that could potentially trigger individuals’ emotional reactions or motivate physical actions(43). Common stimuli that provide environmental stimulation in nursing homes include staff caregivers, other residents, visitors, interpersonal interaction and communication, recreational activities, TV programs, food, background music, and ambient noise and odors in the room. In the nursing home environment, residents could experience few environmental stimuli (e.g., sitting in a quiet bedroom with no specific activity), one primary environment stimulus (e.g., interacting with one staff member in a quiet bedroom), or multiple stimuli (e.g., having a meal in a busy dining room). Physical environmental attributes, including the room size, furniture arrangement, environmental cues, and lighting also influence how the individuals respond to environmental stimulation(42). Because individuals with dementia have lower stress tolerance, appropriate environmental stimulation that matches individuals’ needs and preferences is important for successful mealtime experience. An overstimulating environment that exceeds individual thresholds may contribute to behavioral symptoms and negatively influence eating performance among dementia residents(44). While research identifies the importance of the dining environment and environmental stimulation, the specific characteristics of the dining environment and environmental stimulation that truly impact eating performance for residents with dementia is understudied.
The lack of evidence on how specific environmental characteristics influence individuals’ eating performance during mealtimes is partly due to limited tools for measuring environmental stimulation in dementia care. The Person-Environment Apathy Rating-Environment (PEAR-Environment) subscale is a newly developed measure designed to assess characteristics of environmental stimulation for residents with dementia in long-term care settings(43). The PEAR-Environment subscale consists of six items: stimulation clarity, stimulation strength, stimulation specificity, interaction involvement, physical accessibility, and environmental feedback. These features are identified as important aspects of environmental stimulation relevant to aging, dementia, and goal-directed behaviors. The PEAR-Environment evaluates the quality of environmental stimulation the individuals experience at the moment within the environmental context (including the physical, social and sensory stimulation). For example, stimulation specificity assesses the extent to which the simulation is delivered to the specific individual. The least specific stimulation is no stimulation delivered to the individual, either no stimulation at all or only stimulation delivered toward others (e.g., staff members talking to each other). The most specific stimulation is stimulation that is not only delivered to the individual, but also tailored to address the individual’s prior experiences, preferences, and/or needs (e.g., staff escorting the individual to his or her favorite dinning seat and serving his or her favorite food)(43). The PEAR-Environment subscale allows assessment of the quality of environmental stimulation during mealtimes, so as to examine how environmental stimulation impacts eating performance among NH residents with dementia.
1.5 Person-Centered or Person-Directed Mealtime Care
The philosophy and principles of person-centered or person-directed mealtime care is synthesized and highly recommended for long-term care residents with or without dementia. Providing choices and preferences, supporting independence, showing respect and promoting social interactions are key elements of person-centered mealtime care for nursing home residents(45). In addition to the staff-level care approaches, supportive physical environment and organizational polices in the dining context also contribute to the person-centeredness of mealtime care for long-term care residents with dementia (42, 46). With the understanding of the person-centered or person-directed mealtime care philosophy and the recommendations to develop creative staff training programs to foster person-centered mealtime care practice, the issue remains with how to effectively translate or operationalize such theoretical knowledge and principles into explicit practical techniques or strategies that could be easily applied or adapted by nursing staff who provide most of the direct care to individuals with dementia (46). This study will provide some insight to address the issue by looking at the role of environmental stimulation characteristics on eating performance among residents with dementia.
2. Purpose
The purpose of the study was to examine the association between environmental stimulation and eating performance among NH residents with dementia. Specifically, it was hypothesized that eating performance was significantly associated with environmental stimulation characteristics (i.e. stimulation clarity, stimulation strength, stimulation specificity, interaction involvement, physical accessibility, and environmental feedback), after controlling for resident characteristics (i.e., age, gender, dementia stage, function, comorbidities and psychoactive medication use) and the clustering effects of residents and staff. This study focused on evaluating the impact of intrapersonal, interpersonal and environmental domains on eating performance in dementia based on the SEM model. The findings are fundamental for developing and implementing effective interventions to achieve optimal eating performance in NH residents with dementia.
3. Methods
3.1 Design
This study was a secondary analysis using baseline videos selected from a large archive that were collected during 2011–2014 from a randomized controlled trial of dementia communication(47). The parent study evaluated the effects of a training program to improve nursing staff communication on behavioral symptoms of NH residents with dementia(47). The parent study was approved by University Institutional Review Boards where the study was conducted.
3.2 Sample and Setting
3.2.1 Parent study
In the parent study, nursing home inclusion criteria were licensed skilled nursing facilities providing care for residents with dementia and an administrative letter of support for participation in the study. Resident inclusion criteria were a diagnosis of dementia based on medical records, long stay resident status, staff report of resistiveness to care, ability to hear staff communication, and a surrogate decision maker available to provide informed consent. Residents with Huntington’s disease, alcohol-related dementias, schizophrenia, manic-depressive disorder, deafness, or mental retardation as well as those on hospice care were excluded. Staff inclusion criteria were age 18 or greater (to legally consent), English speaking, permanent NH employee, and provision of direct care for a participating resident at least twice weekly over the past month.
The parent study enrolled 13 NHs in Kansas and randomized these NHs into intervention and control groups(47). Forty-two staff-resident dyads, consisting of 29 staff and 27 residents with dementia, were enrolled. During the week prior to the first video recording session, a practice recording session was conducted to allow the residents and staff to adjust to the novelty of being recorded, and to allow the videorecorder to become familiar with daily routines, identify least conspicuous locations for recording, evaluate adverse impact on resident subjects and establish behaviors indicating that recording should be discontinued. Morning care sessions (i.e., bathing, eating, dressing, oral care, transferring, toileting and other ADL activities) were recorded as representing the most concentrated period of communication between NH residents and staff(48). Only activities or portions of activities that did not require a curtain or door to be closed to ensure privacy were recorded. Videos were recorded during care activities before and after the intervention and at three- and six-month follow-up.
Video recordings for each dyad were selected from each timepoint following the operational inclusion criteria: 1) speech quality is adequate to understand and transcribe; 2) staff and the resident are visible during the entire interaction; and 3) only the identified staff-resident dyad is present. The duration of videos ranged from less than 1 minute up to 10 minutes, depending on the length of the dyadic interaction. The 10-minute length of interaction was used to ensure adequate and equal opportunities for observation of staff communication and subsequent resident behaviors in response to staff communication during care. The first 10 minutes of ADL care have been established as reliable representations of verbal (r = .80–.93) and nonverbal (r = .61–.92) behaviors in complete interactions(49), and have been used in dementia care research(50).
3.2.2 Present study
For this study, baseline videos from the parent study were screened. Videos were included only if there was a demonstration of residents’ eating activities. The eating activities included eating solid food and drinking liquids at mealtimes either in the dining room or the resident’s own room. Videos were excluded if there was no demonstration of any eating activity by the resident for the duration of the video. Specifically, videos were excluded if: 1) the resident was only taking medications, or 2) the resident was present in the dining room but was not demonstrating any actual eating activity, no matter whether there was food being served or not. For example, videos were excluded if they only captured the resident being transferred to or out of the dining room, waiting for the meal to be set up, or staying in the dining room for other purposes rather than for meals.
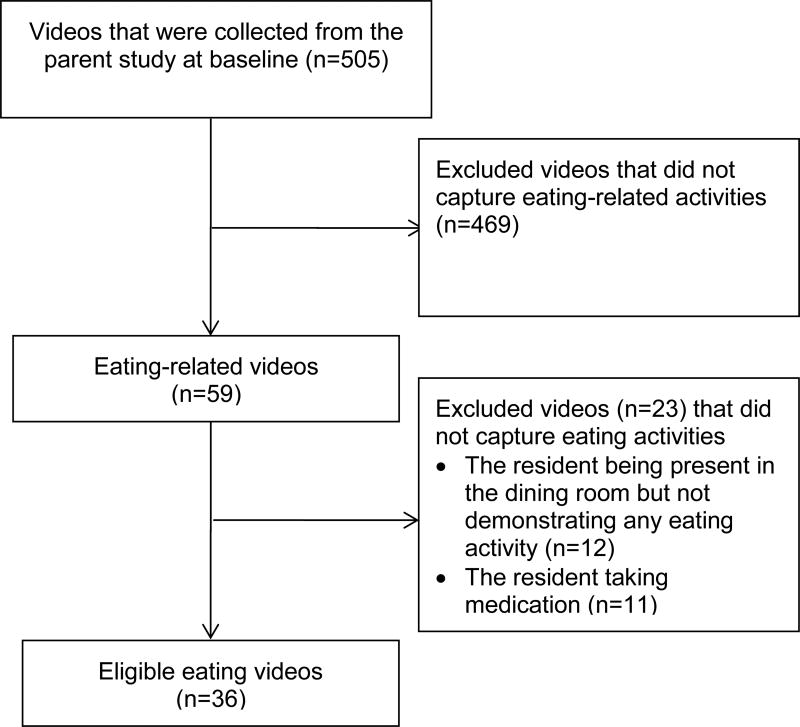
Among the 505 baseline videos collected in the parent study, 36 videos met inclusion criteria for this study (Figure 2). Of the 469 ineligible videos, 446 videos did not capture eating activities, 12 videos captured the resident being present in the dining room but not demonstrating any eating activity, and another 11 videos captured the resident taking medications. The 36 baseline videos recorded eating activities among 15 residents with dementia assisted by 19 nursing assistants at mealtimes in 8 NHs. The length of videos varied with a range from 18 seconds to 10 minutes. Based on the availability of the videos from the parent study, this study included a few short videos (7 videos ranging from 18 to 50 seconds) because these videos captured residents eating solid food and drinking liquids and provided adequate details for the coding of eating performance and environmental stimulation.
Figure 2.
Flow Diagram for Selecting Eating Videos from the Parent Study using Eligibility Criteria
3.3 Measures
In the parent study, nursing staff characteristics included age, gender, race, ethnicity, education, job title, years worked as a nursing caregiver, and years worked in the parent study nursing home. Baseline resident descriptive data included age, gender, race, ethnicity, physical comorbidities, dementia stage, functional status, and use of psychoactive medications.
Resident demographic data and functional status (ADL self-performance and support provided) were extracted from the Minimum Data Set 3.0 (MDS) section G. The MDS is mandated in the United States by the Centers for Medicare and Medicaid Services (CMS) to be completed for all NH residents to provide standardized assessment of residents to support care planning with measures established as reliable and valid(51). The MDS is completed yearly with quarterly updates (more frequently if significant changes occur). Total score for MDS section G indicates the level of self-performance and support provided for ADLs (bed mobility, transfer, locomotion, dressing, eating, toilet use, and bathing) to provide an index of functional ability. Scores can range from 0 to 160 with higher scores indicating more impairment and assistance provided.
Resident physical comorbidities were evaluated by reviewing the MDS 3.0 and clinical records using the Modified Cumulative Illness Rating Scale(52). This scale rates impairment of 14 systems, each on a 1–5 scale ranging from no impairment to severe impairment. Dementia stage was measured using Functional Assessment Staging in Alzheimer’s Disease (FAST), which is a 16-item scale used to identify functional disabilities that correspond to stage of dementia(53). A final FAST score ranges from 1–7, with 1 indicating normal cognition and functioning, 2 very mild memory loss, 3 early dementia, 4 mild dementia, 5 moderate dementia, 6 moderately severe dementia, and 7 severe dementia.
Eating performance was assessed by the adapted Level of Eating Independence (LEI) scale, which is a 9-item scale measuring the level of independence with eating and drinking activities(54). The first five items focus on eating solid food and include grasping and loading the utensil, moving the utensil to the mouth, unloading the utensil, and swallowing. The other four items focus on drinking liquids and include grasping the cup/glass, moving to the mouth, unloading, and swallowing. All the items are scored from 1 (total dependence) to 4 (total independence), except for the two swallowing items, which are consistently scored as 4. The total scores range from 15 to 36, with higher scores indicating better eating performance. The inter-rater reliability was 0.96(54). Resident eating performance in each video was reviewed and coded using the LEI scale based on the resident’s overall performance, and a total rating was assigned for each video by summing up all the item scores. The internal consistency (Cronbach’s α = .834) was good in this study.
Environmental Stimulation was measured by the Person-Environment Apathy Rating Environment subscale (PEAR-Environment)(43), which was designed to assess the immediate physical and social environment that potentially impacted individuals’ motivation and goal-directed behaviors for individuals with dementia. The subscale consists of six items: 1) stimulation clarity (how discernible and straightforward the stimulation is), 2) stimulation strength (how strong and unique the stimulation is), 3) stimulation specificity (how the stimulation is delivered and tailored to the resident), 4) interaction involvement (whether the stimulation includes interaction with the resident), 5) physical accessibility (whether the stimulation is present at an accessible distance), and 6) environmental feedback (whether the stimulation prompts resident response). Each item is rated on a 1–4 scale with a higher score indicating more desirable environmental stimulation. The subscale had good internal consistency (Cronbach’s α = 0.84), inter-rater reliability (weighted kappa = 0.49–0.94) and intra-rater reliability (weighted kappa = 0.63–0.94) among long-term care residents with dementia(43). The scale had moderate internal consistency (Cronbach’s α = .651) in this study.
All videos were coded second-by-second using Noldus Observer® XT10.5 software (Noldus Information Technology Inc, Leesburg, VA, USA). The raters first identified the environmental stimulation and rated the stimulation quality for each item. If there were multiple stimuli captured in the video, the rater rated the primary one. In this study, primary stimuli were most often the food and sometimes the staff feeding or assisting the resident. The rater did not have prior knowledge about the participants’ background or preferences, so the rating was fully based on the environmental context captured within each video. For example, if a staff member addressed the resident by name, served the food in front of the resident, and said “here is your favorite burger”, the rater would give the highest rating on stimulation specificity (directed and tailored to the resident)(43). Based on the second-by-second coding, a weighted average rating was calculated for each item in each video. For example, for a 3-minute video, if the stimulation clarity was rated as 1 for 1 minute and 4 for 2 minutes, the weighted average rating of stimulation clarity for that video would be 3 (1*1/3 + 4*2/3=3). Therefore, the weighted rating for each item ranged from 1 to 4.
3.4 Data Analysis
Descriptive statistics were used to summarize sample characteristics. Multi-level linear modeling using maximum likelihood estimation was applied using STATA software version 13.0 (StataCorp, College Station, TX, USA)(55, 56). Specifically, Random Intercept (RI) models using independent covariance structure was used to estimate the impact of environmental stimulation on eating performance, accounting for the clustering effect of observations within the same resident and the clustering effect of residents assisted by the same staff.
Three models were calibrated to examine change of variance in eating performance that was explained by the clustering effect of resident and staff as well as by personal and environmental covariates using the Intraclass Correlation Coefficient (ICC). The 3-level RI null model (model 1) captures the resident- and staff -level variations in eating performance before adding any covariates. In equation 1 below, “Eating performance” is the resident-level dependent variable for resident j (level 1) assisted by staff k (level 2), β0 is the “fixed” intercept, µjk and µk are the resident- and staff -level random intercepts, respectively, and εijk is the random error component.
| (1) |
The 3-level null model had significant intercept and variance components, suggesting significant resident and staff variations in eating performance. The null model shows that 76% of variance in eating performance is accounted for by staff (28%) and resident (48%) variations. It indicates a significant clustering effect on eating performance both within resident and within staff. Therefore, multilevel linear modeling is appropriate for examining the independent effects of personal and environmental characteristics on eating performance.
The 3-level RI models were computed by adding personal and environmental covariates including resident demographic, cognitive and functional indicators (model 2), and characteristics of environmental stimulation (model 3). In equation 2 below, R is a set of resident characteristics (i.e., age, gender, dementia stage, function, comorbidity, and psychoactive medication use), and E is six dimensions of environmental stimulation (i.e., stimulation clarity, strength and specificity involvement, physical accessibility, and environmental feedback), βs are the fixed intercepts, and µs are the resident- and staff-specific random slopes.
| (2) |
Coefficients with 95% C.I. for fixed effects of all covariates and the intercepts are reported for the three models. The ICC in the null model estimates the proportion of variance in eating performance that is accounted for at resident- and staff- levels. The ICCs in model 2 and 3 represent the percent of variance in eating performance that remains at resident- and staff-levels after adding covariates. The log likelihood ratio is reported for each model, and the likelihood ratio difference is reported as appropriate (when two models used the same sample) to compare the fit of the model to the data. Assumption of normal distribution is examined by the distribution of level-1 residuals (i.e., histogram and Q-Q plot). The level of significance was .05 for all the analyses.
4. Results
4.1 Sample characteristics
A total of 36 videos were included in this study. The average length of the videos was 4 minutes and 7 seconds (SD= 203.87 seconds) with a range from 18 seconds to 10 minutes. A total of 15 residents and 19 staff were observed in the 36 videos. The characteristics of the participants are shown in Table 1 and 2. The resident participants were 86 years old (SD=8.29) with a range from 71 to 104 years, and had moderate comorbidity level (M=26.73, SD=5.21), severe dementia (M=6.82, SD=.24) and moderate functional level (M=22.82, SD=5.17). All residents were white. The majority were non-Hispanic (n=13, 86.7%). Seven residents were male and eight female. Six residents were taking one or more psychoactive medications and five were not taking any psychoactive medication.
Table 1.
Resident and Staff Characteristics (Continuous Variables)
| Resident characteristics | Mean | SD | Range |
|---|---|---|---|
| Resident age, year | 86. 29 | 8.29 | 71–104 |
| Comorbidity score | 26.73 | 5.21 | 19–36 |
| Dementia stage | 6.82 | 0.24 | 6.6–7.4 |
| Function-ADL score | 22.82 | 5.17 | 12–31 |
|
|
|||
| Staff characteristics | |||
|
|
|||
| Staff age | 36.42 | 13.92 | 24 −79 |
| Years worked as Professional Caregiver | 11.32 | 9.38 | 1.5–31 |
| Years worked in current facility | 5.87 | 4.47 | 0.2–13 |
Notes. The study included 15 residents and 19 staff.
Table 2.
Resident and Staff Characteristics (Categorical Variables)
| Resident characteristics | n | % |
|---|---|---|
| Gender | ||
| Male | 7 | 46.7 |
| Female | 8 | 53.3 |
| Race | ||
| White | 15 | 100.0 |
| Ethnicity | ||
| Non-Hispanic | 13 | 86.7 |
| Hispanic | 2 | 13.3 |
| Psychoactive Medications use | ||
| No | 5 | 33.3 |
| Yes (Takes >=1 psychoactive medications) | 6 | 40.0 |
|
|
||
| Staff characteristics | ||
|
|
||
| Gender | ||
| Male | 2 | 10.5 |
| Female | 17 | 89.5 |
| Race | ||
| White | 12 | 63.2 |
| African American | 7 | 36.8 |
| Ethnicity | ||
| Non-Hispanic | 16 | 84.2 |
| Hispanic | 3 | 15.8 |
| Education | ||
| High School | 2 | 10.5 |
| College | 13 | 68.4 |
Notes. The study included 15 residents and 19 staff.
On average, the staff aged 36 years (SD = 13.92), worked for 11 years as nursing caregivers, and worked for almost 6 years in the study nursing homes. The majority of staff participants were female (n=17, 89.5%), non-Hispanic (n=16%, 84.2%), and college educated (n = 13, 68.4%). Twelve participants were white (63.2) and seven were African American (36.8%). All the staff were Certified Nursing Assistants (CNAs), and some CNAs had additional training for roles including activity assistants (n=1, 5.3%), or medication or rehabilitation aides (n=3, 15.8%).
The 15 resident participants in the 36 videos demonstrated moderate levels of eating performance (M=27.08, SD = 5.16, range: 19–36). Environmental stimulation was slightly higher with moderate variability on stimulation specificity (M=3.03, SD=0.19, range: 2.36–3.80), interaction involvement (M=2.73, SD=0.67, range: 1.40–4), and environmental feedback (M=3.04, SD=0.45, range: 2.18–3.90). However, ratings were identical with no variability among all the 36 videos on stimulation clarity (M=4.00, SD=0), stimulation strength (M=3.00, SD=0), and physical accessibility (M=4.00, SD=0).
4.2 Multilevel models
The percentage of variance in eating performance that remained at the resident and staff levels due to clustering effects changed across models when personal and environmental characteristics were added (Table 3). The percentage of variance that was accounted for by the resident and staff clustering effects was more than 76% in the null model (model 1). The percentage decreased from 76% to 40% after adding resident demographic, cognitive and functional indicators (age, gender, dementia stage, function, comorbidity and psychoactive medication use, model 2), and further decreased from 40% to 14% after adding the six dimensions of environmental stimulation (model 3). The percent change of variance indicated that the personal and environmental covariates accounted for almost 62% of variance (62% = 76% – 14%) in eating performance, of which, 36% was accounted for by resident characteristics and 26% by stimulation specificity, interaction involvement, and environmental feedback. Model 3 did not fit significantly better than model 2 based on the likelihood ratio difference test [x2(df) = 4.71 (3), p = 0.19]. In model 3, the histogram and the Q-Q plot showed that the level-1 residuals were normally distributed.
Table 3.
The Association of Eating Performance with Resident Characteristics and Environmental Stimulation
| Variables (measure or reference) |
Model 1 | Model 2 | Model 3 |
|---|---|---|---|
|
| |||
| Coefficient (95% CI) | |||
| Resident age (years) | −.61**(−.97, −.24) | −.57**(−.92, −.22) | |
| Resident gender (male) | −1.92(−6.27, 2.43) | −3.12(−6.86, .62) | |
| Dementia Stage | −13.25**(−22.39, −4.10) | −12.38**(−20.69, −4.07) | |
| Function | .04 (−.48, .57) | −.12 (−.66, .41) | |
| Comorbidity | −.83*** (−1.29, −.36) | −.82*** (−1.22, −.41) | |
| Psychoactive Medication use (no) | 2.91 (−1.37, 7.19) | 2.95 (−.89, 6.79) | |
| Stimulation specificity | 8.78*(.59, 16.97) | ||
| Interaction involvement | .32 (−3.57, 4.21) | ||
| Environmental feedback | .65 (−4.16, 5.48) | ||
| Constant | 26.84***(24.27, 29.40) | 149.47***(69.23, 229.72) | 116.63***(37.72, 195.54) |
| ICC at resident & staff levels a | .7638 | .4012 | .1405 |
| Log Likelihood ratio | −104.84 (no p) | −70.95*** | −68.59 *** |
| Likelihood ratio difference, x2(df) | 4.71 (3) b | ||
Notes.
p<.001,
p<.01,
p<.05.
ICC= Intraclass Correlation Coefficient. RI=Random Intercept. 95% CI=95% Confidence Interval.
variance in eating performance accounted for by resident ID and staff ID.
comparison of model 2 and model 3. No comparison available between model 1, 2 and 3 due to different sample size in the analysis.
Model 1: The null model that only adjusted for both resident ID and staff ID due to clustering effect.
Model 2: Controlling for resident characteristics.
Model 3: Controlling for stimulation specificity, interaction involvement, and environmental feedback. Stimulation clarity and strength, and physical accessibility were omitted from the model due to collinearity and no variability.
Model 3 shows the association of eating performance with resident characteristics and environmental stimulation. Eating performance as measured by the LEI scale was lower among residents with older age, more advanced dementia, and higher comorbidity. For each 1 year increase in resident age, eating performance decreased by .57 points (95%CI= −.92, −.22). As residents’ stage of dementia determined by FAST scoring (ranged from 1 to 7) increased by 1 stage, eating performance decreased by 12.38 points (95%CI= −20.69, −4.07). With each point increase in comorbidity scoring, eating performance decreased by .82 points (95%CI=−1.22, −.41). Eating performance was not significantly associated with resident gender, functional status, or use of psychoactive medications.
After adjusting for resident characteristics, eating performance was significantly associated with stimulation specificity (how the stimulation is delivered and tailored to residents). An environment with more specific stimulation was associated with better eating performance. For each 1 point increase in stimulation specificity, eating performance increased by 8.78 points (95% CI= .59, 16.97). For example, the LEI total score (ranged from 19 to 36) increased by 8.78 points, when the stimulation specificity score went up from 1 (stimulus not toward the resident) to 2 (stimulus partially toward the resident), from 2 to 3 (stimulus directly toward the resident), or from 3 to 4 (stimulus tailored to the resident and directly offered to the resident). Eating performance was not significantly associated with interaction involvement or environmental feedback. The other three dimensions of environmental stimulation, including stimulation clarity and strength, and physical accessibility, were omitted from the model due to collinearity and lack of variability.
5. Discussion
This study examined the associations of resident characteristics and environmental stimulation with eating performance among nursing home residents with dementia. The study showed that multiple resident characteristics were associated with eating performance, which is consistent with prior research(12, 20). Specifically, individuals who are older and those who have advanced dementia or higher comorbidity exhibit lower eating performance. In addition, the study found that a higher level of stimulation specificity is associated with better eating performance. The hypotheses of the study based on the SEM model were partially supported by the findings. Overall, 76% of variance in eating performance was attributable to the resident-and staff-level variations, of which, 36% was explained by the resident characteristics and 26% by environmental stimulation. The findings indicate that environmental stimulation, specifically stimulation specificity, contributes significantly to differences in eating performance after adjusting for resident characteristics. Prior research indicated that superior quality of dementia care environment contributed to a delay in the onset of eating disability(20). Building upon that study, this study was the first that examined the influence of specific characteristics of the dining environment on eating performance in dementia.
5.1 Stimulation specificity
This study indicated that highly specific environmental stimulation was better received by persons with dementia as evidenced by better eating performance. Study findings were consistent with nursing assistants’ perspectives in engaging residents at mealtimes in a qualitative study(23). Stimulation specificity refers to the extent to which the environmental stimulation is individualized and directly delivered to the resident, and is an important indicator of the quality of environmental stimulation. The findings demonstrated that individuals performed better in eating tasks when they ate in an environment that provided stimuli that was specifically directed toward and personally tailored to them.
From this perspective, strategies to enhance stimulation specificity at mealtimes can be applied at interpersonal and environmental levels. At the interpersonal level, it is important that the staff get to know residents’ capabilities, meal preferences, and dining routines for mealtimes by observing residents’ performance and talking to families, residents and other staff, and prepare the meals and dining environment in a manner that match their preferences and routines. Other techniques at the staff level include maintaining appropriate interactions with residents (e.g., sitting in proximity, establishing eye contact, addressing the resident by name in communication, using the resident’s language for simple communication), and providing specific verbal or nonverbal cues as needed (e.g., verbal and nonverbal reminds, prompts and encouragement) to engage residents in eating activities throughout the mealtime.
At the environmental level, residents should be provided with appropriate assistive devices and adaptive utensils that matched their vision and/or hearing impairment and functional abilities in eating, preferred music if feasible, and minimal environmental distractions in the dining environment (e.g., minimizing noisy background, limiting conversations with other residents or staff). Tailored environmental stimulation should be based on the individual’s status and environmental contexts. Future work is needed to incorporate the techniques into staff training programs and to modify the dining environment in NH settings.
5.2 Other dimensions of environmental stimulation
Surprisingly, neither interaction involvement (whether there is interpersonal interaction with the resident) nor environmental feedback (whether the stimulation prompts resident response) was significantly associated with eating performance in this study. One possible reason might be that food and drinks were considered the primary and most important stimuli during mealtimes, while social interaction and environmental feedback from others were deemed as secondary stimuli. The other possible reason was that the resident participants in this study had a relatively higher level of eating performance (an averaged eating performance rating of 27 on the possible rating scale of 15–36). Interaction involvement and environmental feedback could have greater impact on eating performance for individuals with a lower functional level who rely more on caregivers’ assistance. Future research is needed to further examine the associations between interaction involvement, environmental feedback, and eating performance using heterogeneous samples.
The impact of stimulation clarity, stimulation strength, and physical accessibility on eating performance was not examined in this study due to lack of variation in the video sample. This study used baseline videos capturing eating activities from a communication intervention study, in which the communication between staff and residents was primarily one-on-one interaction. Recordings would therefore not include examples of staff-resident interactions that were inaccessible or unclear. Additionally, given the nature of this study focusing on eating performance, it is logical to expect that all the recordings would involve food and drinks, which were the most straightforward and accessible stimuli at mealtimes. It may be premature to exclude these environmental factors for correlates of eating performance in future studies. Rather, these environmental factors should be examined using different samples with diverse dining environment characteristics and staff-resident interactions.
5.3 Implications for research
The study pointed out important directions for future research in examining factors that influence eating performance in dementia. Video-recorded data are ideal for this research because recordings can be reviewed repeatedly to specifically assess different aspects of the dining environment and eating performance. Future research should collect eating videos that capture longer durations or whole mealtimes to accurately represent eating performance, and recordings that capture varied dining environment and staff-resident interactions to maximize the variations on environmental stimulation. Also, future work may need to adjust for variables representing motivation, goal-directed behaviors, behavioral symptoms and/or individuals’ preferences and routines at mealtimes to help identify person-centered needs and provide specific and individualized environmental support. This study focused on the quality of environmental stimulation, and future research could assess the types of environmental stimulation (e.g., food or social interaction) or other environmental features (e.g., dining room settings) during mealtimes and examine the associations with eating performance. To examine the causal relationships between environmental stimulation and eating performance, further work could conduct sequential analysis to precisely examine the relationship, examine within-individual differences in eating performance in relation to changing environmental stimulation, and apply experimental designs to examine the impact of environmental stimulation on eating performance. In addition, the scope of the study considered the intrapersonal, interpersonal and environmental domains in examining the association with eating performance. Future research should consider the policy level factors and test other components of the SEM model to achieve a better understanding of the multilevel factors that influence eating performance.
5.4 Implications for clinical practice
The findings of the study have important implications for clinical practice. These findings fall within the scope of operationalizing the person-centered or person-directed mealtime care philosophy and principles at both environmental and caregiver levels for nursing home care practice. Specifically, the findings add a clearer understanding of the role of environmental stimulation specificity in improving eating performance, along with examples of how stimulation specificity can be operationalized by nursing staff during daily mealtime care to improve eating performance. Providing highly specific environmental stimulation is consistent with the current focus on person-directed dementia care and quality of life, which is a major initiative within Advancing Excellence and the Centers for Medicare & Medicaid Services National Partnership to Improve Dementia Care in Nursing Homes(32). Stimuli from the dining environment or staff caregivers that are individually tailored and personally directed to residents by targeting the different t modifiable interpersonal and environmental factors are needed to actively engage residents with dementia in their highest level of functional performance in eating(39, 57).
5.5 Limitations
This study had some limitations. A small sample size with multiple short videos were used in the study. The number of videos capturing residents eating solid food and drinking liquids was limited in the parent study sample. Possible reasons are that residents who needed assistance with other ADLs might be independent at mealtimes, or that other residents and/or staff were captured in the recordings in the dining room. These recordings would not meet selection criteria of the parent study if dyadic communication was not present or if residents and staff in the recordings were not all consented. Also, some videos that might be relevant to mealtimes were excluded, because it only captured the resident sitting at the dining table with food in front but not performing any eating activity and it was unclear whether the resident had already finished the meal or was unable or unwilling to eat the meal. This may have resulted in selection bias that could influence the variations of the rating for eating performance and environmental stimulation, and influence the generalizability of findings. In addition, the raters of environmental stimulation did not have prior knowledge of the residents’ preferences and routines, so ratings were based on the information and environmental context captured in the videos. Future research could address this limitation by collecting data of residents’ dining preferences, needs, and routines. The study examined cross-sectional associations between eating performance and environmental stimulation, and a causal relationship could not be demonstrated. Despite the limitations, the video sample in the study provided detailed data on dining experiences of residents with dementia allowing the assessment of environmental stimulation and eating performance.
6. Conclusion
This study supports the association of eating performance with resident characteristics and stimulation specificity in the dining environment. Environmental stimulation specificity is modifiable and can be included in nursing home staff educational programs and mealtime environmental modifications. Mealtime interventions should be individualized and tailored to residents’ needs and preferences, and stimuli from the physical environment and social interaction with staff should be directed to residents to optimize eating performance. Future work should continue to explore the influence of other dimensions of environmental stimulation on eating performance. Findings can be used to guide environmental design and intervention development in nursing home settings to promote eating performance, and consequently improve nutritional status and quality of care for residents with dementia.
Acknowledgments
The authors acknowledge that the parent study was supported by NIH grant NR011455-04, Changing Talk to Reduce Resistiveness in Dementia Care (CHAT), K. Williams, PI. ClinicalTrials.gov Identifier: NCT01324219. The sponsor was not involved in study design, data collection and analysis, interpretation of findings, and manuscript preparation. The authors also acknowledge the assistance from Megan Lough and Alicia Freiburg for screening for baseline eating videos, and the assistance from Margaret Turk for coding environmental stimulation.
References
- 1.Liu W, Galik E, Nahm E-S, Boltz M, Resnick B. Optimizing eating performance for long-term care residents with dementia: testing the impact of function-focused care for cognitively impaired. Journal of the American Medical Directors Association. 2015;16(12):1062–8. doi: 10.1016/j.jamda.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 2.Liu W, Unick J, Galik E, Resnick B. Barthel Index of Activities of Daily Living: Item Response Theory Analysis of Ratings for Long-Term Care Residents. Nursing Research. 2015;64(2):88–99. doi: 10.1097/NNR.0000000000000072. [DOI] [PubMed] [Google Scholar]
- 3.Morris JN, Fiatarone M, Kiely DK, Belleville-Taylor P, Murphy KJ, Littlehale S, et al. Nursing rehabilitation and exercise strategies in the nursing home. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 1999;54(10):M494–M500. doi: 10.1093/gerona/54.10.m494. [DOI] [PubMed] [Google Scholar]
- 4.Kovar M, Lawton M. Functional disability. Activities and instrumental activities of daily living. In: Lawton M, Teresi J, editors. Focus on Assessment Techniques The Annual Review of Gerontology and Geriatrics. Vol. 14. 1994. pp. 57–75. [Google Scholar]
- 5.Gibbs-Ward AJ, Keller HH. Mealtimes as active processes in long-term care facilities. Canadian journal of dietetic practice and research. 2005;66(1):5–11. doi: 10.3148/66.1.2005.5. [DOI] [PubMed] [Google Scholar]
- 6.Palacios-Ceña D, Losa-Iglesias ME, Cachón-Pérez JM, Gómez-Pérez D, Gómez-Calero C, Fernández-de-las-Peñas C. Is the mealtime experience in nursing homes understood? A qualitative study. Geriatrics & gerontology international. 2013;13(2):482–9. doi: 10.1111/j.1447-0594.2012.00925.x. [DOI] [PubMed] [Google Scholar]
- 7.Alzhermer’s Association. 2016 Alzheimer’s Disease Facts and Figures. 2016 doi: 10.1016/j.jalz.2016.03.001. Retrieved from http://wwwalzorg/documents_custom/2016-facts-and-figurespdf. [DOI] [PubMed]
- 8.Zimmerman S, Sloane PD, Reed D. Dementia Prevalence And Care In Assisted Living. Health Affairs. 2014;33(4):658–66. doi: 10.1377/hlthaff.2013.1255. [DOI] [PubMed] [Google Scholar]
- 9.Thies W, Bleiler L. Alzheimer’s disease facts and figures. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2013;9(2):208–45. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter GI, Hastie CL, Morris JN, Fries BE, Ankri J. Measuring change in activities of daily living in nursing home residents with moderate to severe cognitive impairment. BMC geriatrics. 2006;6(1):7. doi: 10.1186/1471-2318-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin LC, Watson R, Wu SC. What is associated with low food intake in older people with dementia? Journal of Clinical Nursing. 2010;19(1–2):53–9. doi: 10.1111/j.1365-2702.2009.02962.x. [DOI] [PubMed] [Google Scholar]
- 12.Liu W, Galik E, Boltz M, Nahm ES, Lerner N, Resnick B. Factors associated with eating performance for long-term care residents with moderate-to-severe cognitive impairment. Journal of advanced nursing. 2016;72(2):348–60. doi: 10.1111/jan.12846. [DOI] [PubMed] [Google Scholar]
- 13.Watson R. Measuring feeding difficulty in patients with dementia: perspectives and problems. Journal of advanced nursing. 1993;18(1):25–31. doi: 10.1046/j.1365-2648.1993.18010025.x. [DOI] [PubMed] [Google Scholar]
- 14.Tully MW. The Eating behavior Scale. A simple method of assessing functional ability in patients with Alzheimer’s disease. Journal of gerontological nursing. 1997;23(7):9–15. doi: 10.3928/0098-9134-19970701-08. quiz 54–5. [DOI] [PubMed] [Google Scholar]
- 15.Hanson LC, Ersek M, Lin FC, Carey TS. Outcomes of feeding problems in advanced dementia in a nursing home population. Journal of the American Geriatrics Society. 2013;61(10):1692–7. doi: 10.1111/jgs.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slaughter SE, Eliasziw M, Morgan D, Drummond N. Incidence and predictors of eating disability among nursing home residents with middle-stage dementia. Clinical Nutrition. 2011;30(2):172–7. doi: 10.1016/j.clnu.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Chang CC, Roberts BL. Malnutrition and feeding difficulty in Taiwanese older with dementia. Journal of Clinical nursing. 2011;20(15–16):2153–61. doi: 10.1111/j.1365-2702.2010.03686.x. [DOI] [PubMed] [Google Scholar]
- 18.Buffa R, Mereu R, Putzu P, Floris G, Marini E. Bioelectrical impedance vector analysis detects low body cell mass and dehydration in patients with Alzheimer’s disease. The journal of nutrition, health & aging. 2010;14(10):823–7. doi: 10.1007/s12603-010-0115-9. [DOI] [PubMed] [Google Scholar]
- 19.Bronfenbrenner U. Ecological systems theory. In: Vasta Ross., editor. Six theories of child development: Revised formulations and current issues. London, England: Jessica Kingsley Publishers; 1992. pp. 187–249. [Google Scholar]
- 20.Slaughter SE, Hayduk LA. Contributions of Environment, Comorbidity, and Stage of Dementia to the Onset of Walking and Eating Disability in Long-Term Care Residents. Journal of the American Geriatrics Society. 2012;60(9):1624–31. doi: 10.1111/j.1532-5415.2012.04116.x. [DOI] [PubMed] [Google Scholar]
- 21.den Ouden ME, Schuurmans MJ, Mueller-Schotte S, Brand JS, van der Schouw YT. Domains contributing to disability in activities of daily living. Journal of the American Medical Directors Association. 2013;14(1):18–24. doi: 10.1016/j.jamda.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Desai AK, Schwartz L, Grossberg GT. Behavioral disturbance in dementia. Current Psychiatry Reports. 2012;14(4):298–309. doi: 10.1007/s11920-012-0288-5. [DOI] [PubMed] [Google Scholar]
- 23.Liu W. Facilitators and Barriers in Optimizing Eating Performance among Cognitively Impaired Older Adults; Submitted abstract at the 21st IAGG World Congress of Gerontology and Geriatrics; San Francisco, CA, USA. 2017. [Google Scholar]
- 24.Cortes F, Nourhashémi F, Guérin O, Cantet C, Gillette-Guyonnet S, Andrieu S, et al. Prognosis of Alzheimer’s disease today: a two-year prospective study in 686 patients from the REAL-FR Study. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2008;4(1):22–9. doi: 10.1016/j.jalz.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Amella EJ, Aselage MB. Facilitating ADLs by Caregivers of Persons with Dementia: The C3P Model. Occupational therapy in health care. 2014;28(1):51–61. doi: 10.3109/07380577.2013.867388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu W, Freiburg A, Williams KN. Facilitators and Barriers to Engaging Cognitively Impaired Residents in Self-Eating; Paper presentation at the Gerontological Society of America’s 69th Annual Scientific Meeting; New Orleans, LA, USA. 2016. [Google Scholar]
- 27.Pelletier CA. Feeding beliefs of certified nurse assistants in the nursing home: a factor influencing practice. Journal of gerontological nursing. 2005;31(7):5–10. doi: 10.3928/0098-9134-20050701-04. [DOI] [PubMed] [Google Scholar]
- 28.Brush JA, Meehan RA, Calkins MP. Using the environment to improve intake for people with dementia. Alzheimer’s Care Quarterly. 2002;3(4):330–8. [Google Scholar]
- 29.Evans BC, Crogan NL, Shultz JA. The meaning of mealtimes: connection to the social world of the nursing home. Journal of gerontological nursing. 2005;31(2):11–7. doi: 10.3928/0098-9134-20050201-05. [DOI] [PubMed] [Google Scholar]
- 30.Andreoli NA, Breuer L, Marbury D, Williams S, Rosenblut MN. Serving Culture Change at Mealtimes. 2007 Retrieved from http://wwwltlmagazinecom/article/serving-culture-change-mealtimes.
- 31.Chang CC, Roberts BL. Cultural perspectives in feeding difficulty in Taiwanese elderly with dementia. Journal of nursing scholarship. 2008;40(3):235–40. doi: 10.1111/j.1547-5069.2008.00231.x. [DOI] [PubMed] [Google Scholar]
- 32.Centers for Medicare & Medicaid Services C. Dementia Care in Nursing Homes. 2013 retrieved from http://wwwcmsgov/Outreach-and-Education/Outreach/NPC/National-Provider-Calls-and-Events-Items/2013-01-31-Dementia-Care-html.
- 33.Bowers BJ, Esmond S, Jacobson N. The relationship between staffing and quality in long-term care facilities: Exploring the views of nurse aides. Journal of nursing care quality. 2000;14(4):55–64. doi: 10.1097/00001786-200007000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Schnelle JF, Simmons SF, Harrington C, Cadogan M, Garcia E, Bates-Jensen BM. Relationship of nursing home staffing to quality of care. Health services research. 2004;39(2):225–50. doi: 10.1111/j.1475-6773.2004.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amella EJ. Resistance at mealtimes for persons with dementia. The Journal of nutrition, health aging. 2002;6(2):117–22. [PubMed] [Google Scholar]
- 36.Bronfenbrenner U. Toward an experimental ecology of human development. American psychologist. 1977;32(7):513–31. [Google Scholar]
- 37.Liu W, Cheon J, Thomas SA. Interventions on mealtime difficulties in older adults with dementia: A systematic review. International journal of nursing studies. 2014;51(1):14–27. doi: 10.1016/j.ijnurstu.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 38.Liu W, Galik E, Boltz M, Nahm ES, Resnick B. Optimizing Eating Performance for Older Adults With Dementia Living in Long-term Care: A Systematic Review. Worldviews on Evidence-Based nursing. 2015;12(4):228–35. doi: 10.1111/wvn.12100. [DOI] [PubMed] [Google Scholar]
- 39.Aselage MB, Amella EJ, Rose SB, Bales CW. Handbook of Clinical Nutrition and Aging. New York: Springer; 2015. Dementia-Related Mealtime Difficulties: Assessment and Management in the Long-Term Care Setting; pp. 287–301. [Google Scholar]
- 40.Chang C, Lin L. Effects of a feeding skills training programme on nursing assistants and dementia patients. Journal of Clinical nursing. 2005;14(10):1185–92. doi: 10.1111/j.1365-2702.2005.01240.x. [DOI] [PubMed] [Google Scholar]
- 41.Douglas JW, Lawrence JC. Environmental considerations for improving nutritional status in older adults with dementia: a narrative review. Journal of the Academy of Nutrition and Dietetics. 2015;115(11):1815–31. doi: 10.1016/j.jand.2015.06.376. [DOI] [PubMed] [Google Scholar]
- 42.Chaudhury H, Hung L, Badger M. The Role of Physical Environment in Supporting Person-centered Dining in Long-Term Care A Review of the Literature. American journal of alzheimer’s disease and other dementias. 2013;28(5):491–500. doi: 10.1177/1533317513488923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jao Y-L, Algase DL, Specht JK, Williams K. Developing the Person–Environment Apathy Rating for persons with dementia. Aging & mental health. 2015:1–10. doi: 10.1080/13607863.2015.1043618. [DOI] [PubMed] [Google Scholar]
- 44.Smith M, Gerdner LA, Hall GR, Buckwalter KC. History, development, and future of the progressively lowered stress threshold: a conceptual model for dementia care. Journal of the American Geriatrics Society. 2004;52(10):1755–60. doi: 10.1111/j.1532-5415.2004.52473.x. [DOI] [PubMed] [Google Scholar]
- 45.Reimer HD, Keller HH. Mealtimes in nursing homes: Striving for person-centered care. Journal of Nutrition for the Elderly. 2009;28(4):327–47. doi: 10.1080/01639360903417066. [DOI] [PubMed] [Google Scholar]
- 46.Hung L, Chaudhury H. Exploring personhood in dining experiences of residents with dementia in long-term care facilities. Journal of Aging Studies. 2011;25(1):1–12. [Google Scholar]
- 47.Williams KN, Perkhounkova Y, Herman R, Bossen A. A communication intervention to reduce resistiveness in dementia care: A cluster randomized controlled trial. The Gerontologist. 2016 doi: 10.1093/geront/gnw047. gnw047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sloane PD, Miller LL, Mitchell CM, Rader J, Swafford K, Hiatt SO. Provision of morning care to nursing home residents with dementia: opportunity for improvement? American journal of Alzheimer’s disease and other dementias. 2007;22(5):369–77. doi: 10.1177/1533317507305593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caris-Verhallen WM, Kerkstra A, van der Heijden PG, Bensing JM. Nurse-elderly patient communication in home care and institutional care: an explorative study. International journal of nursing studies. 1998;35(1):95–108. doi: 10.1016/s0020-7489(97)00039-4. [DOI] [PubMed] [Google Scholar]
- 50.Beck CK, Vogelpohl TS, Rasin JH, Uriri JT, O’Sullivan P, Walls R, et al. Effects of behavioral interventions on disruptive behavior and affect in demented nursing home residents. Nursing research. 2002;51(4):219–28. doi: 10.1097/00006199-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Morris JN, Nonemaker S, Murphy K, Hawes C, Fries BE, Mor V, et al. A commitment to change: revision of HCFA’s RAI. Journal of the American Geriatrics Society. 1997;45(8):1011–6. doi: 10.1111/j.1532-5415.1997.tb02974.x. [DOI] [PubMed] [Google Scholar]
- 52.Knoefel FD, Patrick L. Improving outcomes in geriatric rehabilitation: The impact of reducing cumulative illness. Geriatrics Today. 2003;6:153–7. [Google Scholar]
- 53.Sclan SG, Reisberg B. Functional assessment staging (FAST) in Alzheimer’s disease: reliability, validity, and ordinality. International psychogeriatrics. 1992;4(03):55–69. doi: 10.1017/s1041610292001157. [DOI] [PubMed] [Google Scholar]
- 54.Coyne ML, Hoskins L. Improving eating behaviors in dementia using behavioral strategies. Clinical nursing research. 1997;6(3):275–90. doi: 10.1177/105477389700600307. [DOI] [PubMed] [Google Scholar]
- 55.Goldstein H. Multilevel statistical models. London: Arnold; 2003. [Google Scholar]
- 56.Subramanian S. Multilevel methods, theory and analysis. Encyclopedia on health and behavior Thousand Oaks, CA: Sage Publications; 2004. pp. 602–8. [Google Scholar]
- 57.Keller HH, Carrier N, Duizer L, Lengyel C, Slaughter SE, Steele C. Making the most of mealtimes (m3): grounding mealtime interventions with a conceptual model. Journal of the American Medical Directors Association. 2014;15(3):158–61. doi: 10.1016/j.jamda.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]