Abstract
One in three people has been infected with Mycobacterium tuberculosis (MTB), and the risk for MTB infection in HIV-infected individuals is even higher. We hypothesized that HIV-positive individuals living in tuberculosis-endemic regions who do not get infected by Mycobacterium tuberculosis are genetically resistant. Using an “experiment of nature” design that proved successful in our previous work, we performed a genome-wide association study of tuberculin skin test positivity using 469 HIV-positive patients from prospective study cohorts of tuberculosis from Tanzania and Uganda to identify genetic loci associated with MTB infection in the context of HIV-infection. Among these individuals, 244 tested were tuberculin skin test (TST) positive either at enrollment or during the >8 year follow up, while 225 were not. We identified a genome-wide significant association between a dominant model of rs877356 and binary TST status in the combined cohort (Odds ratio = 0.2671, p = 1.22x10-8). Association was replicated with similar significance when examining TST induration as a continuous trait. The variant lies in the 5q31.1 region, 57kb downstream from IL9. Two-locus analyses of association of variants near rs877356 showed a haplotype comprised of rs877356 and an IL9 missense variant, rs2069885, had the most significant association (p = 1.59x10-12). We also replicated previously linked loci on chromosomes 2, 5, and 11. IL9 is a cytokine produced by mast cells and TH2 cells during inflammatory responses, providing a possible link between airway inflammation and protection from MTB infection. Our results indicate that studying uninfected, HIV-positive participants with extensive exposure increases the power to detect associations in complex infectious disease.
Author summary
Approximately one-third of the world’s population has been exposed to Mycobacterium tuberculosis, the bacterium that causes tuberculosis. A small number of those infected develop active disease; however, there is a substantial portion of exposed people who do not even show evidence of an immunological response. These people who appear to resist infection, as measured by a negative tuberculin skin test, represent a subpopulation from which we can learn about resistance. We used a genome-wide approach to study the genetic basis of this resistance in unique cohorts of hypervulnerable, HIV-positive individuals from Uganda and Tanzania, in which exposure was virtually assured. We identified one locus that was highly significantly associated and conferred more than 70% protection from infection. The most significant variant, rs8773656, was near IL9 and SLC25A48, and a haplotype including this variant and a missense mutation in IL9 was even more significantly associated with negative skin tests. Although it is impossible based solely on our data to determine the causal variant or genes, IL9 is an attractive candidate as its product has previously been associated with bronchial hyperresponsiveness, thereby providing a possible link between inflammation and protection from Mycobacterium tuberculosis infection.
Introduction
One third of the world’s population has been infected with Mycobacterium tuberculosis (MTB)[1, 2]. Subsequent tuberculosis disease (TB) occurs during the lifespan of about 10% of those infected[1–3]. Tuberculosis is a major cause of morbidity and mortality worldwide, with 1.5 million deaths and 9.6 million new cases of active disease reported in 2014[1]. Tuberculosis is the primary cause of death in people co-infected with the human immunodeficiency virus (HIV), and 400,000 of the global TB deaths in 2014 occurred in this patient population [1, 4]. The immunosuppression from HIV facilitates progression to active disease directly following infection, or by the reactivation of a latent MTB infection[5, 6]. While the clinical trajectory of a given MTB infection has many determinants and possible outcomes, infection per se is a necessary prerequisite. Of note, about 10–20% of people living in areas hyperendemic for MTB, which virtually guarantees repeated exposure, appear to be resistant to infection[7–10].
Historically, MTB infection has been evaluated with a tuberculin skin test (TST) measuring the induration caused by a delayed type hypersensitivity reaction to an intradermal injection of MTB purified protein derivative (PPD)[11, 12]. In endemic areas, induration ≥ 5mm measured between 48 and 72 hours post-injection is indicative of infection. A study of TST reactivity among siblings demonstrated high heritability, suggesting a possible genetic component to the MTB infection resistance phenotype[13, 14]. Several studies have capitalized on this finding and identified loci relevant to the MTB infection phenotype. A family-based linkage analysis of TST response identified SLC6A3 and a region on chromosome 11 (p14) as linked to infection[8]. A full genome microsatellite scan comparing persistent MTB negative patients to those with latent infections identified an association with the SLC11A1 gene, and candidate regions on chromosomes 2 (q14, q21-q24) and 5 (p13-q22)[15].
Recently, novel methods for evaluating MTB infection status have been developed. Interferon-gamma release assays (IGRAs) detect the concentration of IFN-γ in response to a mixture of MTB-specific antigens[16, 17]. The purified protein derivative used in TST has some antigenic overlap with the Bacille Calmette-Guérin (BCG) vaccine, although 10 years post-vaccination the confounding effect is minor; approximately 1% of adult subjects inoculated at birth with BCG are TST-false positive [18]. IGRAs’ antigens have no overlap with the BCG vaccine, and maintain excellent specificity in individuals who had childhood BCG vaccinations [16, 17]. However, in people with compromised immune systems and previously exposed to MTB, anergy due to immunodeficiency may prevent detection of a positive TST and/or IGRAs. Inclusion of negative and positive assay controls allows us to better assess this potential confounder.
We used a genome-wide approach to evaluate common variants for association with TST response in a patient population that hypothetically allows us to identify extreme genetic effects. Namely, we hypothesized that HIV-positive individuals who live in areas endemic for tuberculosis but who do not get infected, are strongly genetically resistant to MTB. Using two recently concluded prospective cohorts of tuberculosis disease from Tanzania and Uganda, with available TST and IFN-γ results, we identified a variant on chromosome 5q31.1, near SLC25A48 and IL9 that imparts resistance to MTB infection in immunocompromised individuals.
Results
Sex was significantly associated with TST status in the combined Ugandan and Tanzanian cohorts (Odds Ratio (OR) for males 1.91, 95% confidence interval (CI) 1.27–2.86, p = 0.002; Table 1), but it did not associate when studied in Uganda (p = 0.762; Table 2) or Tanzania (p = 0.349; Table 3) alone. Age was not significantly associated with TST status in the combined cohort (p = 0.108; Table 1), nor in Uganda (p = 0.384; Table 2) or Tanzania (p = 0.153; Table 3) alone. Therefore, all analyses below were adjusted for sex, 10 principal components, and cohort of origin when Tanzanian and Ugandan datasets were combined.
Table 1. Summary statistics of study participants in the combined Ugandan and Tanzanian cohorts.
Odds ratio result is from univariate logistic regression.
| Combined Data | TST+ | TST- | Odds Ratio | 95% Confidence Interval | p value | |
|---|---|---|---|---|---|---|
| n | 244 | 225 | ||||
| Age (st. dev.) | 32.43 (8.02) | 34.49 (7.96) | 0.108 | |||
| Sex | Male | 89 | 52 | |||
| Female | 155 | 173 | 1.91 | (1.27, 2.86) | 0.002 | |
| CD4* (st. dev) | 430.17 (262.45) | 430.12 (234.67) | 0.99 | |||
*CD4 data available for 151 TST+ patients and 198 TST- patients in the combined cohort
Table 2. Summary statistics of study participants in the household contact study in Uganda.
Odds ratio result is from univariate logistic regression.
| Uganda HHC Data | TST+ | TST- | Odds Ratio | 95% Confidence Interval | p value | |
|---|---|---|---|---|---|---|
| n | 150 | 49 | ||||
| Age (st. dev.) | 32.72 (7.78) | 31.57 (8.76) | 0.384 | |||
| Sex | Male | 68 | 21 | |||
| Female | 82 | 28 | 1.11 | (0.58, 2.12) | 0.762 | |
| CD4* (st. dev; range) | 504.76 (250.47; 15–906) |
463.19 (215.82; 1–673) |
0.16 | |||
*CD4 data available for 57 TST+ patients and 22 TST- patients in the Uganda cohort
Table 3. Summary statistics of study participants in the extended follow up of the DarDar vaccine trial in Tanzania.
Odds ratio result is from univariate logistic regression.
| Tanzania, DarDar Data Vaccine | TST+ | TST- | Odds Ratio | 95% Confidence Interval | p value | |
|---|---|---|---|---|---|---|
| n | 94 | 176 | ||||
| Age (st. dev.) | 33.50 (8.56) | 34.95 (7.61) | 0.153 | |||
| Sex | Male | 21 | 31 | |||
| Female | 73 | 145 | 1.35 | (0.72, 2.50) | 0.349 | |
| CD4* (st. dev; range) | 307.18 (235.77; 204–1490) |
165.56 (214.49; 204–1390) | 0.024 | |||
*CD4 data available for all patients in the Tanzania cohort
In logistic regression analysis adjusted for covariates, we observed a genome-wide significant association between a dominant genetic effect of rs877356 on chromosome 5q31.1 and binary TST status in the combined cohort (OR = 0.27, 95% CI 0.17–0.42, p = 1.22x10-8, Table 4, S1–S3 Figs). The variant had consistent effects in Uganda (OR = 0.17, 95% CI 0.08–0.37, p = 9.18x10-6; Table 5; S4 Fig) and Tanzania (OR = 0.33, 95% CI 0.18–0.59, p = 1.81x10-4; Table 6, S5 Fig). Linear regression analyses of continuous size of TST induration under a dominant genetic model produced similar results (combined cohort beta = -4.14, 95% CI -5.55 to -2.74, p = 1.45x10-8; S1 Table). Variant rs877356 met the multiple testing-adjusted threshold for this study (3.08x10-7) and was nearly genome-wide significant in an additive model using binary TST status (OR = 0.33, 95% CI 0.222–0.493, p = 5.45x10-8; S2 Table), and continuous size of TST induration (combined cohort beta = -3.34, 95% CI -4.53 to -2.14, p = 6.95x10-8; S3 Table). This SNP was in Hardy Weinberg equilibrium in Tanzania (p = 0.68) and Uganda (p = 0.21). No other unimputed SNPs were significant at the multiple testing corrected threshold in any of the genetic models tested (Tables 4–6, S1–S5 Tables).
Table 4. Single nucleotide polymorphisms associating with dichotomous tuberculin skin test status below a 5x10-5 p-value using a dominant genetic model in the combined cohort* (n = 469).
| SNP | Chr. | Minor Allele | MAF | Odds Ratio | 95% Confidence Interval | p value | Nearest gene |
|---|---|---|---|---|---|---|---|
| rs877356 | 5 | T | 0.2292 | 0.267 | (0.170, 0.422) | 1.22E-08 | SLC25A48/IL9 |
| rs7808481 | 7 | A | 0.2164 | 2.523 | (1.630, 3.910) | 3.33E-05 | Loc340268 |
| rs1880386 | 10 | A | 0.2132 | 2.462 | (1.594, 3.801) | 4.85E-05 | GRID1 |
* adjusted for 10 principal components, sex, and cohort of origin
Table 5. Single nucleotide polymorphisms associating with dichotomous tuberculin skin test status below a 5x10-5 p-value using a dominant genetic model in the Ugandan cohort* (n = 199).
| SNP | Chr. | Minor Allele | MAF | Odds Ratio | 95% Confidence Interval | p value | Nearest gene |
|---|---|---|---|---|---|---|---|
| rs877356 | 5 | T | 0.2337 | 0.171 | (0.078, 0.373) | 9.18E-06 | SLC25A48/IL9 |
| rs654718 | 11 | G | 0.2136 | 0.190 | (0.089, 0.406) | 1.81E-05 | MRE11A |
| rs7944514 | 11 | C | 0.4121 | 5.284 | (2.457, 11.360) | 2.03E-05 | POLD3 |
| rs7837658 | 8 | T | 0.4472 | 4.842 | (2.319, 10.110) | 2.67E-05 | RNF19A |
* adjusted for 10 principal components and sex
Table 6. Single nucleotide polymorphisms associating with dichotomous tuberculin skin test status below a 5x10-5 p-value using a dominant genetic model in the Tanzanian cohort* (n = 270).
| SNP | Chr. | Minor Allele | MAF | Odds Ratio | 95% Confidence Interval | p value | Nearest gene |
|---|---|---|---|---|---|---|---|
| rs17062122 | 6 | C | 0.3259 | 0.280 | (0.161, 0.487) | 6.20E-06 | Loc285735 |
| rs8142256 | 22 | C | 0.35 | 0.312 | (0.181, 0.539) | 2.87E-05 | FAM19A5 |
| rs10998959 | 10 | T | 0.2537 | 0.306 | (0.173, 0.540) | 4.33E-05 | Loc100129281 |
| rs11736841 | 4 | T | 0.2556 | 3.091 | (1.792, 5.332) | 4.96E-05 | ODZ3 |
| … | … | … | … | … | … | … | … |
| rs877356 | 5 | T | 0.2259 | 0.330 | (0.184, 0.589) | 1.81E-04 | SLC25A48/IL9 |
* adjusted for 10 principal components and sex
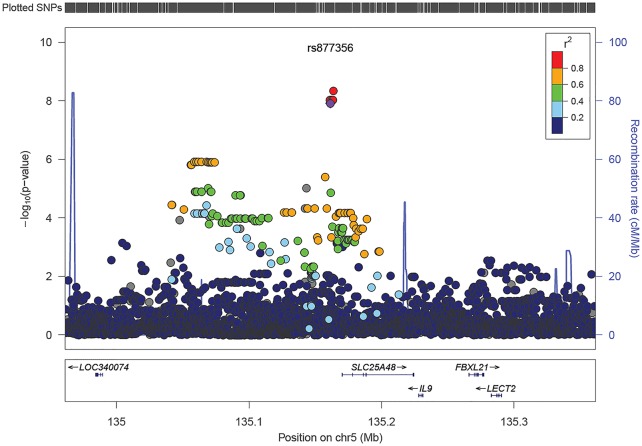
To evaluate SNPs in the region not included on our genotyping array, we imputed SNPs within 0.5 megabases of rs877356. One SNP, rs17169187, in high linkage disequilibrium (LD) with rs877356 (D’ = 1 in both cohorts, r2 = 0.99 in Tanzania, 0.98 in Uganda) and 2,340 bases away, is the variant with the most significant association to binary TST status using a dominant model (combined cohort OR = 0.26, 95% CI 0.16–0.40, p = 4.57x10-9; Fig 1, S6A Table). The results were consistent with those from linear regression on continuous size of TST induration (combined cohort beta = -4.29, 95% CI -5.69 to -2.88, p = 4.58x10-9; S7A Table). This variant is also genome-wide significant in additive modeling of both a binary TST designation (combined cohort OR = 0.320, 95% CI 0.214–0.478, p = 2.56x10-8; Fig 1, S6B Table) and continuous size of TST induration (combined cohort beta = -3.43, 95% CI -4.62 to -2.24, p = 2.84x10-8; S7B Table). Adjustment for CD4 count did not significantly affect our results (S8 Table).
Fig 1. Locus zoom plot of results from a logistic regression association of dichotomous tuberculin skin test status with a dominant genetic model of imputed SNPs in the SLC25A48/IL9 region in the combined cohort, adjusted for 10 principal components, sex, and cohort of origin; SNP with the most significant association of a genotyped SNP in the Exome Beadchip analysis in purple.
In the Tanzanian cohort, IFN-γ responses to positive control (PHA) and negative control (medium) antigens did not differ by TST results, but were significantly higher in TST cases for all mycobacterial antigens (S9A Table). In Uganda, we observed the same trends; however, due to smaller sample sizes, the comparisons were not statistically significant (S9B Table). Separately, we examined the prevalence of TST-positivity in the entire Ugandan household contact study cohort, and found that although the prevalence of TST+ in HIV+ is significantly lower, it is still very high (71% in HIV- versus 62% in HIV+, p = 0.003; S10 Table). Furthermore, the distribution of TST induration examined as a continuous variable did not differ by HIV status (p = 0.06). In the GWAS analysis, removing either potentially false negative subjects (n = 16), potentially false positive subjects (n = 20), or both did not affect the results substantially (S11–S14 Tables). The variant was also genome-wide significant when we included patients with prior tuberculosis in the analyses (S15 Table).
We found the strongest single variant association using a dominant model of rs877356; therefore, we used dominant coding of the SNP in 2-variant haplotype in the SLC25A48 region while using additive models of all other SNPs. An rs877356-rs2069885 haplotype had the strongest association in this analysis (omnibus p = 1.59x10-12 in the combined cohort; Table 7). The haplotypes had similar association in the Ugandan (p = 2.51x10-8; Table 8) and Tanzanian cohorts (p = 1.37x10-11; Table 9), with the T-G haplotype frequencies being 0.32/0.60 and 0.20/0.45 in TST+/TST- subjects, representing a similar enrichment in both cohorts (Tables 8 and 9). The haplotype, C-G, also had a consistent distribution between the cohorts, with a TST+/TST- frequency of 0.58/0.33 in Uganda and 0.68/0.48 in Tanzania (Tables 8 and 9). The results were consistent in additive modeling of both SNPs (p = 2.59x10-9 in the combined cohort; S16 Table). The haplotype had similar association in the Ugandan (p = 1.03x10-5; S16B Table) and Tanzanian cohorts (p = 6.35x10-5; S16C Table). In addition, patterns of linkage disequilibrium (LD) were strikingly similar across the whole region in both Ugandan and Tanzanian cohorts (S6 and S7 Figs), an unexpected result given the greater variation (and reduced extent) of LD among African populations. Remarkably, in the same cohorts, high similarity in LD structure was previously found near IL12B, encompassing a variant associated with resistance to active TB in HIV+ individuals and displaying signals of strong selection[19].
Table 7. Association of the 2-variant haplotype using a dominant model of rs877356 with an additive model of rs2069885 with dichotomous TST induration status in the SLC25A48/IL9 region in the combined cohort.
| Haplotype | TST+ | TST- | TST+ Freq | TST- Freq |
|---|---|---|---|---|
| C-A | 33 | 12 | 0.0679 | 0.02667 |
| C-G | 301 | 202 | 0.6193 | 0.4489 |
| T-A | 17 | 17 | 0.0350 | 0.0378 |
| T-G | 135 | 219 | 0.2778 | 0.4867 |
| Likelihood ratio χ2 = 57.97 df = 3 p-value = 1.59E-12* | ||||
* adjusted for principal components, sex, and cohort of origin
Table 8. Association of the 2-variant haplotype using a dominant model of rs877356 with an additive model of rs2069885 with dichotomous TST induration status in the SLC25A48/IL9 region in the Ugandan cohort.
| Haplotype | TST+ | TST- | TST+ Freq | TST- Freq |
|---|---|---|---|---|
| C-A | 19 | 2 | 0.0633 | 0.0204 |
| C-G | 175 | 32 | 0.5833 | 0.3265 |
| T-A | 9 | 5 | 0.0300 | 0.0510 |
| T-G | 97 | 59 | 0.3233 | 0.6020 |
| Likelihood ratio χ2 = 38.25 df = 3 p-value = 2.51E-08* | ||||
* adjusted for principal components and sex
Table 9. Association of the 2-variant haplotype using a dominant model of rs877356 with an additive model of rs2069885 with dichotomous TST induration status in the SLC25A48/IL9 region in the Tanzanian cohort.
| Haplotype | TST+ | TST- | TST+ Freq | TST- Freq |
|---|---|---|---|---|
| C-A | 14 | 10 | 0.0753 | 0.0284 |
| C-G | 126 | 170 | 0.6774 | 0.4830 |
| T-A | 8 | 12 | 0.0430 | 0.0341 |
| T-G | 38 | 160 | 0.2043 | 0.4545 |
| Likelihood ratio χ2 = 53.59 df = 3 p-value = 1.37E-11* | ||||
* adjusted for principal components and sex
We also determined whether previously associated or linked loci were significant in our results. Several regions previously shown to be linked to TST response were nominally significant in our study (10−3 > p > 10−4), including ones on chromosomes 2, 5 and 11 (S17 Table)[8, 15, 20]. Chromosome 11p14-15 associated with TST response in our analyses as it did previously[21]. Although our most significant region on chromosome 11 was distal to the linkage peak, the region directly under the peak was almost as significant (p~10−3) (S18 Table). Another previous association signal, IL-10, did not show signs of replication in our study (S17F Table). These results overall support the validity of our study design as most previous regions replicated.
Discussion
In this study we examined the association of common genetic variants with Mycobacterium tuberculosis infection in HIV+ patients from the extended follow-up of the DarDar vaccine trial in Tanzania and the Household Contact study in Uganda. By applying the “experiment of nature” strategy outlined in a genetic study of tuberculosis disease with the same cohorts [19], we hypothesized that these immunosuppressed patients who live in MTB endemic areas but do not get infected have strong innate resistance. This hypothesis and approach were validated as we identified a novel association between protection from MTB infection and rs877356 with a large effect size. This variant is 9,119 bases upstream of the coding region of SLC25A48[22], a Homo sapiens solute carrier family 25, member 48. SLC25A48 is a mitochondrial carrier of amino acids[23, 24]. This SNP is also 57,662 bases downstream from IL9, which we think is a particularly compelling candidate.
Both genes have supporting evidence that may implicate them. With respect to SLC25A48, there is evidence from GTEX that this SNP is an eQTL for a lncRNA closer to it than IL9 (http://gtexportal.org/home/eqtls/bySnp?snpId=rs877356&tissueName=All). In contrast, the involvement of IL9 as the potentially causal gene in our association study was supported by our haplotype analyses. The rs877356-rs2069885 haplotype had the most significant association in this region. The SNP, rs2069885, is 66kb away from rs877356, and is a missense variant in IL9 (Threonine (ACG) ->Methionine (ATG))[22]. While rs2069885 was not significant in univariate analyses (p = 0.091 in the combined cohort for TST as a binary outcome and with an additive model), the association of the haplotype was several orders of magnitude more significant than that of rs877356 alone.
Although we cannot at the present distinguish which of these two genes, if either, is the truly associating one, IL9 is an attractive candidate for resistance to MTB infection because of its association with bronchial hyperresponsiveness [25], which is hereditary and a risk factor for asthma[25–27]. Of note, the prevalence of asthma in East Africa is high, especially in urban settings[28], childhood MTB infection protects from asthma, and an inverse relationship between incidence of active TB and asthma has been reported [29, 30]. IL9 was originally described as a T cell and mast cell growth factor, but has since been found to have pleiotropic effects on the immune system[31–33]. IL9 promotes IL4-mediated production of IgE and IgG antibodies[34, 35], and bronchial hyper-responsiveness is associated with elevated serum IgE levels[25, 36]. IL9 also promotes proliferation of hematopoietic progenitor cells[37, 38], and it has specific effects on lungs. In airway smooth muscle cells, IL9 induces the expression of chemokine CCL11, thereby inducing eosinophil chemotaxis and allergic reactions, and in airway epithelial cells, IL9 directly induces mucous production and stimulates IL13, which leads to further airway inflammation and perhaps reduced risk of MTB infection [31, 32, 39–41].
The TST phenotype can be studied both as a binary variable, < versus ≥ 5mm induration, or as a continuous outcome. Our single-SNP association results were consistent using both outcomes. Variant rs877356 was genome-wide significant in both logistic and linear regression models in the combined cohort using a dominant genetic model as well as at a multiple testing corrected level in an additive model. The most significant imputed variant in the region, rs17169187, was genome-wide significant for both outcomes in additive and dominant modeling.
One possible limiting factor of these conclusions is immune anergy, which is a potential confounder in studies of TST reactivity, especially in an HIV+ context. TST responses can be < 5mm because a patient has not been infected with MTB, or in case of anergy, is unable to mount a hypersensitivity reaction to PPD even if infected. However, we believe our results are unlikely to be confounded by anergy for several reasons. First, if anergy existed, it would result in misclassifying cases as controls, which would decrease power and underestimate effect sizes. Since we observed significant effects, this was not the case in our data. Second, we leveraged existing interferon-γ response data in both cohorts to evaluate confounding by immunosuppression. We removed all patients suspected of immune anergy prior to analysis, and further adjustment for a missing response variable did not affect the association of our variant, demonstrating the robustness of our findings. Particularly in the Tanzania data, where the reported rate of TST-positivity in HIV-infected is lower than in HIV-uninfected individuals[42], the PHA responses were quite high and did not differ by TST status, demonstrating that individuals do indeed mount immune responses. Analyses utilizing these immunologic data, where available, showed significant effects for the same SNP, suggesting our results are robust to immunological differences between subjects. Third, data from the entire household contact study in Uganda indicates that anergy is not an issue in that cohort: the prevalence of TST-positivity in HIV-infected individuals is 62%, compared to 71% in HIV-uninfected individuals and ~34% in HIV-uninfected community controls (S10 Table and [43]). This high rate of TST-positivity in the HIV-infected subjects is inconsistent with anergy being a major confounder in this population. Furthermore, since we see similar genetic effects in the Ugandan and Tanzanian cohort, it is unlikely that anergy is a problem in Tanzania and not in Uganda. Lastly, we replicated loci that had been previously associated with TST in independent HIV- cohorts, further validating our design. Unfortunately, data on PHA and CD4+ count were unavailable for some of the subjects in this study, so we were unable to fully explore some of these potential explanations. In summary, the aforementioned sensitivity analyses and other factors make anergy an unlikely cause of the observed association in these data, though we cannot absolutely exclude this possibility. Future studies should examine this locus as a candidate for association with TST.
As we have previously shown for tuberculosis disease[19], the present study confirms that the choice of an extreme phenotype, HIV+ patients who live in MTB endemic areas but do not get infected, enriches for major, homogeneous genetic effects. This design permits the use of relatively small sample size even in a genome-wide association study. Although the small sample size is the biggest weakness in this study, the large and replicated effect size observed in this unique study design and populations allowed us to find significant associations in an apparently relevant region of the genome. The variant with the most significant association is near IL9, a gene with a substantial role in airway inflammation, bronchial asthma, and other respiratory infections[44, 45]. This, along with observational studies of the inverse incidence of asthma and tuberculosis, leads to the conclusion that the same gene whose over-expression plays a significant role in the pathogenesis of asthma, could also prevent MTB infection by the same mechanism.
Material and methods
Study populations
HIV+ subjects from a cohort in Tanzania and one in Uganda were included in this study. A complete description of the study cohorts and genetic analysis methods is provided in our previous work [19].
Tanzania
Patients from the extended follow-up cohort of the DarDar vaccine trial in Dar es Salaam, Tanzania were recruited for this study. The full cohort has been described elsewhere[46]. Briefly, the DarDar trial was a phase III randomized trial of SRL 172, an inactivated whole cell mycobacterial vaccine booster to a childhood Bacille Calmette-Guérin (BCG) vaccination. Subjects were enrolled between 2001 and 2005. Follow-up continued until the study was concluded in 2008. Recruited patients were HIV+-positive adults (≥18 years old) with a BCG scar, a CD4 count >200 cells/μl and were TB-negative at the time of enrollment. TST reactivity was measured at enrollment, preventing any confounding by the effects of the vaccine. A saline placebo was administered to 1007 patients, while 1006 patients received 5 doses of the vaccine. A routine follow-up for active TB (physical examination, chest radiograph, sputum samples for culture and acid fast bacilli (AFB) stain, and phlebotomy for an automated mycobacterial blood culture) was performed every 3 months for the duration of the study. Upon conclusion of the trial, an extended follow-up cohort of 800 participants from both the placebo and vaccine arm was selected for annual evaluation for active TB. Between September and December of 2013, 304 patients from the extended follow-up were recruited during their routine visits.
Uganda
We obtained 263 samples from HIV-positive participants from the Household Contact Study (HHC), conducted in Kampala, Uganda. This cohort has been previously described in detail[47, 48]. Briefly, the Uganda National Tuberculosis and Leprosy Programme referred patients diagnosed with new active tuberculosis to the study, and patients who consented were enrolled as index cases. Household contacts were defined as individuals living in the same household as the index case for at least 7 consecutive days in the 3 month period leading up to the diagnosis of the index case[10]. Household contacts were subsequently enrolled and evaluated for active TB, latent TB, and HIV. Recommended therapy was administered to all cases of active TB[49]. In contrast to the cohort from Tanzania, CD4 counts were not available until 2004 when antiretroviral drugs became available in Uganda. In subjects enrolled prior to 2004, antiretrovirals were not given to HIV-positive subjects. In subjects enrolled when antiretrovirals were available in Uganda, these drugs were administered after the diagnosis of HIV, which occurred after basic clinical data, including TST, and blood samples were obtained. Of note, the HHC study design guarantees exposure of the controls to MTB during the follow up[47]. We only analyzed adult participants (≥18 years old) of the HHC.
Immune assays
Tanzania
Intradermal injections of purified protein derivative (0.1 ml, RT-23, Staten Serum Institute, Copenhagen) on the forearm were administered to all enrolled patients prior to vaccination, and resultant skin induration size was measured by trained personnel after 48–72 hours. Preventative isoniazid treatment (300 mg daily for 6 months) was offered to subjects with a positive TST using the criterion for HIV+ patients (≥5 mm as positive) as recommended by a consensus statement [50].
Immune response to Mycobacterial antigens was assessed with an interferon gamma (IFN-γ) enzyme linked immunosorbent assay (ELISA), a tritiated thymidine lymphocyte proliferation assay (LPA) and an ELISA for antibodies to the glycolipid lipoarabinomannan of MTB (LAM). The assays used in this study have been described in detail elsewhere[51]. Briefly, phlebotomy was performed prior to vaccination and at the conclusion of the study, and peripheral blood mononuclear cells (PBMCs) were isolated by ficoll density gradient centrifugation for IFN-γ and LPA assays, performed on site. Centrifuged, frozen serum was sent to Dartmouth College for LAM assays.
IFN-γ and LPA assays used four different antigens: Mycobacterium Vaccae sonicate (2 mcg/ml), MTB Antigen 85 (Ag85; 1 mcg/ml), MTB early secretory antigenic target 6 (ESAT-6; 2 mcg/ml), and MTB whole cell lysate (WCL; 1 mcg/ml)[51]. Importantly, ESAT-6 is not present in the childhood Bacille Calmette-Guérin (BCG) vaccine that is commonplace in East Africa; therefore, confounding by BCG status can be controlled with this additional data. Media alone was used as a negative control and phytohemagglutinin (PHA, 2.5 mcg/mL; Sigma, St. Louis, MO) was used as a positive control[51].
Uganda
Intradermal injections of purified protein derivative (5 tuberculin units) on the forearm were administered to study participants at enrollment, and 3, 6, 12, and 24 months post-enrollment, if the tests were negative at earlier time points[10]; PPD was also obtained from Serum Staten Institute as in Tanzania. The size of skin induration was measured by trained personnel 48–72 hours after each injection. For patients measured at multiple time points, the largest TST reaction was used in the analysis, and a 5 mm cutoff was used to define TST+, as recommended by a consensus statement[50]. In this phase of the HHC study, daily isoniazid preventative treatment was offered to all HIV+ subjects for 6–9 months [10, 15].
Briefly, phlebotomy was performed at enrollment. Whole blood was stimulated with MTB antigens: MTB culture filtrate CXFT, ESAT-6, and CFP10[10, 52], and the IFN-γ response was measured by ELISA (Thermo Scientific, Rockford, IL). Whole blood cultured without antigen stimulation served as a negative control. Phytohemagglutinin (PHA; Sigma, St. Louis, MO) was used as a positive control, while the IFN-γ response to media was subtracted from antigen-stimulated readings[10, 52]. Negative differences were considered a 0.
DNA isolation and genotyping
For participants from the extended follow-up of the DarDar vaccine trial, 5ml of whole blood was drawn upon enrollment, and DNA was extracted the day of the phlebotomy using the Gentra Puregene Blood kit (QIAGEN) in accordance with the manufacturer’s recommendations. For participants of the Household Contact Study, buffy coats were isolated on site and shipped to Dartmouth College for DNA extraction. The QIAamp DNA Blood Mini Kit (QIAGEN) was used to isolate DNA from the buffy coats. DNA samples were stored at -80°C before genotyping. DNA quality was evaluated with the 260/280 ratio using a NanoDrop 2000 spectrophotometer at Dartmouth College (Thermo Scientific) and an Electrophoresis Quality Score at the University of Miami.
Samples from the DarDar vaccine trial (n = 304) and the Household Contact Study (n = 263) were submitted for genotyping at the Hussman Institute for Human Genomics, Miami, Florida. A total of 567 samples passed quality control measures and were genotyped using the Illumina Human Core Exome Beadchip (542,585 SNPs). SNPs with a genotyping call rate < 0.95 and a Hardy-Weinberg equilibrium p-value < 1x10-4 were excluded. Participants with a per individual genotyping call rate < 0.95 were excluded. Concordance of reported and genotypic sex was verified. In case of relatedness among study participants (pi-hat > 0.20), one individual was randomly removed. The final study population included 270 participants from the extended follow up of the DarDar vaccine trial and 199 participants from the Household Contact Study. All quality control analyses were performed in PLINK(v1.07)[53]. Results for the most significant SNP are shown in S8 Fig.
Statistical analyses
Statistical genetics methods
To adjust for possible admixture within each cohort, principal components were calculated using SNPs with r2 < 0.1 and MAF > 0.2 using the SNPRelate package in R[54, 55]. The qqman package in R was used to generate Manhattan and qq plots[56]. Locus zoom was used to plot the regions with the strongest association[57].
TST data were evaluated using additive, dominant, and recessive genetic models both as a continuous variable using linear regression, and as a binary variable (TST positive, ≥ 5mm vs. TST negative, < 5mm) with logistic regression in PLINK(v1.07)[53]. A total of 162,228 SNPs passed the inclusion criteria at a MAF > 0.20 (chosen to provide adequate power in our studies using QUANTO[58], S19 Table), corresponding to a Bonferroni corrected multiple testing threshold of 3.08x10-7. Summary statistics and univariate logistic regression models of TST+/TST- status with available covariates were calculated in STATA(v11.2)[59]. Covariates associating with TST positivity (0.05 level) were included in final models.
All analyses were adjusted for 10 principal components to account for possible population structure, and analyses of the cohorts combined were adjusted for a cohort variable. SNPs in the regions of interest were imputed with IMPUTE2 (v2.3.1), using one phased reference panel from the 1000 Genomes project[60–62]. We used UNPHASED(v3.1.7)[63] to perform 2 SNP haplotype association analyses, adjusting for the same covariates as in the single SNP association analyses above. We studied all pairwise haplotypes that included the most significantly associating SNP in the combined cohort and an additional 30 available SNPs within 250 kb of this SNP that had a minor allele frequency >0.05. Haplotype plots were generated using Haploview[64].
Additional analyses to account for potential anergy
To help mitigate the confounding influence of anergy, we utilized available IFN-γ data. Subjects can remain TST-negative given an exposure to M. tuberculosis in multiple ways that may need to be accounted for analytically. MTB infection can be of an insufficient dose, inhaled but mechanically prevented from seeding the lungs, seed the lungs but be cleared before immune memory is invoked, potentially localized without a systemic response, and importantly for our analyses MTB can establish a latent infection but host immunosuppression and an inability to mount a delayed type hypersensitivity response can prevent a positive TST test, i.e. anergy[65]. To adjust for possible anergy in Tanzania, we removed all patients who had negative IFN-γ responses to the positive control antigen, PHA (defined as a PHA < 300pg/mL), which indicates low T cell counts or activity and lymphocyte proliferation assays (LPA) (defined as a proliferative index < 3), indicating few T cells exist in the individual. LPA data was not available for the Ugandan cohort; therefore, we removed all patients who had negative responses to all available antigens and to PHA (PHA < 300pg/mL). Patients who were PHA positive but negative for all other antigens remained in the study. ELISA assays were not performed on 102 patients from the HHC cohort and 33 patients from the DarDar vaccine trial extended follow up. Of the patients with missing assays, 71 had TST measurements ≥ 5mm, and 31 < 5mm in Uganda, and 10 had TST ≥ 5mm, and 23 < 5mm in Tanzania. Logistic regression models of TST status adjusting for missing ELISA data were performed to prevent confounding by missing data. Patients who stated that they had previous active TB, but had a TST of 0mm were excluded from the analyses presented below.
To evaluate possible false negative TST responses on our association results, additional logistic regression analyses were performed removing individuals with a 0mm TST induration who had a substantial IFN-γ response (> mean in TST positives) to any of the tested antigens at the time of the TST induration measurement (S20 Table). The effect of possible false positive TST results due to BCG vaccination was evaluated by performing logistic regression analyses removing individuals with positive TST scores but low ELISA response (< mean of TST negatives) to any of the tested antigens. Numbers of individuals excluded from the analyses and the criteria are presented in S21 Table.
Functional annotation
The ENCODE Project[66] was accessed via the UCSC Genome Browser[22] and used for functional annotation.
Ethics
Informed consent was obtained from all patients in the extended DarDar follow-up cohort. The research ethics committee at the Muhimbili University of Health and Allied Sciences and the Committee for the Protection of Human Subjects at Dartmouth College and the Dartmouth-Hitchcock Medical Center approved this study. Informed consent was obtained from all subjects in the Household Contact study in Kampala, Uganda. Ethics committees that approved this work were at Muhimbili University of Health and Allied Sciences, Committee for the Protection of Human Subjects at Dartmouth College (#14606), Uganda Council for Science and Technology, and University Hospitals of Cleveland (10-01-25)
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Cases and controls were run together to minimize batch effects.
(DOCX)
Acknowledgments
We would like to acknowledge the invaluable contribution made by the study medical officers, health visitors, laboratory and data personnel: Dr. Lorna Nshuti, Dr. Roy Mugerwa, Dr. Sarah Zalwango, Allan Chiunda, Bonnie Thiel, Mark Breda, Dennis Dobbs, Hussein Kisingo, Mary Rutaro, Albert Muganda, Richard Bamuhimbisa, Yusuf Mulumba, Deborah Nsamba, Barbara Kyeyune, Faith Kintu, Dr. Mary Nsereko, Gladys Mpalanyi, Janet Mukose, Grace Tumusiime, Pierre Peters, Annet Kawuma, Saidah Menya, Joan Nassuna, Alphonse Okwera, Keith Chervenak, Karen Morgan, Alfred Etwom, Micheal Angel Mugerwa, Dr. Brenda Okware, and Lisa Kucharski. We would like to acknowledge and thank Dr. Francis Adatu Engwau, Head of the Uganda National Tuberculosis and Leprosy Program, for his support of this project. We would like to acknowledge the medical officers, nurses and counselors at the National Tuberculosis Treatment Centre, Mulago Hospital, the Ugandan National Tuberculosis and Leprosy Program and the Uganda Tuberculosis Investigation Bacteriological Unit, Wandegeya, for their contributions to this study. We would also like to thank the staff at the Infectious Disease Center in Dar es Salaam, Tanzania, and Betty Mchaki at Muhimbili University of Health and Allied Sciences. This study would not be possible without the generous participation of the Ugandan and Tanzanian patients and their families.
Data Availability
All the summary level data, as well as the individual level data for Tanzania are available from DRYAD (doi:10.5061/dryad.cq183). Because of the IRB restriction on the data from Uganda, individual level data are only available upon request from the Uganda Genetics of TB Data Access Committee by contacting Dr. Sudha Iyengar (ski@case.edu).
Funding Statement
RSS was supported by Public Health Service award T32 GM07347 from the National Institute of General Medical Studies for the Vanderbilt Medical-Scientist Training Program. RSS, NK, and SMW were partially supported by National Institutes of Health grant P20 GM103534. CMS, LLM, KC, and WHB were partially supported by Tuberculosis Research Unit (grant N01-AI95383) and UO1-AI-09-001 from the National Institutes of Health. CMS, WHB, and RPI were also supported by National Institutes of Health grant R01HL096811. JHM and SMW were supported by National Institutes of Health grants LM010098. JHM was supported by National Institutes of Health grants AI59694, AI116794, and LM009012. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Programme WHOGT. Global tuberculosis report 2014. 2014.
- 2.Raviglione MC, Snider DE Jr., Kochi A. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. Jama. 1995;273(3):220–6. . [PubMed] [Google Scholar]
- 3.Stewart GR, Robertson BD, Young DB. Tuberculosis: a problem with persistence. Nature reviews Microbiology. 2003;1(2):97–105. doi: 10.1038/nrmicro749 . [DOI] [PubMed] [Google Scholar]
- 4.UNAIDS. UNAIDS report on the global AIDS epidemic. 2013.
- 5.Selwyn PA, Schoenbaum EE, Davenny K, Robertson VJ, Feingold AR, Shulman JF, et al. Prospective study of human immunodeficiency virus infection and pregnancy outcomes in intravenous drug users. Jama. 1989;261(9):1289–94. Epub 1989/03/03. . [PubMed] [Google Scholar]
- 6.Di Perri G, Cruciani M, Danzi MC, Luzzati R, De Checchi G, Malena M, et al. Nosocomial epidemic of active tuberculosis among HIV-infected patients. Lancet. 1989;2(8678–8679):1502–4. Epub 1989/12/23. . [PubMed] [Google Scholar]
- 7.Rieder HL. Epidemiologic Basis of Tuberculosis Control. International Union Against Tuberculosis and Lung Disease, Paris. 1999:162. [Google Scholar]
- 8.Cobat A, Gallant CJ, Simkin L, Black GF, Stanley K, Hughes J, et al. Two loci control tuberculin skin test reactivity in an area hyperendemic for tuberculosis. The Journal of experimental medicine. 2009;206(12):2583–91. doi: 10.1084/jem.20090892 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rose DN, Schechter CB, Adler JJ. Interpretation of the tuberculin skin test. Journal of general internal medicine. 1995;10(11):635–42. . [DOI] [PubMed] [Google Scholar]
- 10.Ma N, Zalwango S, Malone LL, Nsereko M, Wampande EM, Thiel BA, et al. Clinical and epidemiological characteristics of individuals resistant to M. tuberculosis infection in a longitudinal TB household contact study in Kampala, Uganda. BMC infectious diseases. 2014;14:352 doi: 10.1186/1471-2334-14-352 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar V AA, Fausto N, Aster JC. Robbins & Cotran Pathologic Basis of Disease. 8th ed: Saunders; 2010. [Google Scholar]
- 12.Vukmanovic-Stejic M, Reed JR, Lacy KE, Rustin MH, Akbar AN. Mantoux Test as a model for a secondary immune response in humans. Immunology letters. 2006;107(2):93–101. doi: 10.1016/j.imlet.2006.08.002 . [DOI] [PubMed] [Google Scholar]
- 13.Sepulveda RL, Heiba IM, King A, Gonzalez B, Elston RC, Sorensen RU. Evaluation of tuberculin reactivity in BCG-immunized siblings. American journal of respiratory and critical care medicine. 1994;149(3 Pt 1):620–4. doi: 10.1164/ajrccm.149.3.8118628 . [DOI] [PubMed] [Google Scholar]
- 14.Sepulveda RL, Heiba IM, Navarrete C, Elston RC, Gonzalez B, Sorensen RU. Tuberculin reactivity after newborn BCG immunization in mono- and dizygotic twins. Tuber Lung Dis. 1994;75(2):138–43. doi: 10.1016/0962-8479(94)90043-4 . [DOI] [PubMed] [Google Scholar]
- 15.Stein CM, Zalwango S, Malone LL, Won S, Mayanja-Kizza H, Mugerwa RD, et al. Genome scan of M. tuberculosis infection and disease in Ugandans. PloS one. 2008;3(12):e4094 doi: 10.1371/journal.pone.0004094 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CDC. Interferon-Gamma Release Assays (IGRAs)—Blood Tests for TB Infection 2011. Available from: http://www.cdc.gov/tb/publications/factsheets/testing/IGRA.html.
- 17.Gold CQ-T. [Feb 2015]. Available from: http://www.cellestis.com/IRM/content/aust/qtfproducts_tbgoldintube.html.
- 18.Farhat M, Greenaway C, Pai M, Menzies D. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis. 2006;10(11):1192–204. . [PubMed] [Google Scholar]
- 19.Sobota RS, Stein CM, Kodaman N, Scheinfeldt LB, Maro I, Wieland-Alter W, et al. A Locus at 5q33.3 Confers Resistance to Tuberculosis in Highly Susceptible Individuals. Am J Hum Genet. 2016;98(3):514–24. doi: 10.1016/j.ajhg.2016.01.015 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall NB, Igo RP Jr., Malone LL, Truitt B, Schnell A, Tao L, et al. Polymorphisms in TICAM2 and IL1B are associated with TB. Genes Immun. 2015;16(2):127–33. doi: 10.1038/gene.2014.77 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cobat A, Poirier C, Hoal E, Boland-Auge A, de La Rocque F, Corrard F, et al. Tuberculin skin test negativity is under tight genetic control of chromosomal region 11p14-15 in settings with different tuberculosis endemicities. J Infect Dis. 2015;211(2):317–21. doi: 10.1093/infdis/jiu446 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujita PA, Rhead B, Zweig AS, Hinrichs AS, Karolchik D, Cline MS, et al. The UCSC Genome Browser database: update 2011. Nucleic acids research. 2011;39(Database issue):D876–82. doi: 10.1093/nar/gkq963 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu X, Cheng R, Verbitsky M, Kisselev S, Browne A, Mejia-Sanatana H, et al. Genome-wide association study identifies candidate genes for Parkinson's disease in an Ashkenazi Jewish population. BMC medical genetics. 2011;12:104 doi: 10.1186/1471-2350-12-104 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmieri F. The mitochondrial transporter family SLC25: identification, properties and physiopathology. Molecular aspects of medicine. 2013;34(2–3):465–84. doi: 10.1016/j.mam.2012.05.005 . [DOI] [PubMed] [Google Scholar]
- 25.Postma DS, Bleecker ER, Amelung PJ, Holroyd KJ, Xu J, Panhuysen CI, et al. Genetic susceptibility to asthma—bronchial hyperresponsiveness coinherited with a major gene for atopy. The New England journal of medicine. 1995;333(14):894–900. doi: 10.1056/NEJM199510053331402 . [DOI] [PubMed] [Google Scholar]
- 26.Hopp RJ, Townley RG, Biven RE, Bewtra AK, Nair NM. The presence of airway reactivity before the development of asthma. The American review of respiratory disease. 1990;141(1):2–8. doi: 10.1164/ajrccm/141.1.2 . [DOI] [PubMed] [Google Scholar]
- 27.Longo G, Strinati R, Poli F, Fumi F. Genetic factors in nonspecific bronchial hyperreactivity. An epidemiologic study. American journal of diseases of children. 1987;141(3):331–4. . [DOI] [PubMed] [Google Scholar]
- 28.Shimwela M, Mwita JC, Mwandri M, Rwegerera GM, Mashalla Y, Mugusi F. Asthma prevalence, knowledge, and perceptions among secondary school pupils in rural and urban coastal districts in Tanzania. BMC public health. 2014;14:387 doi: 10.1186/1471-2458-14-387 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Hertzen L, Klaukka T, Mattila H, Haahtela T. Mycobacterium tuberculosis infection and the subsequent development of asthma and allergic conditions. The Journal of allergy and clinical immunology. 1999;104(6):1211–4. . [DOI] [PubMed] [Google Scholar]
- 30.von Mutius E, Pearce N, Beasley R, Cheng S, von Ehrenstein O, Bjorksten B, et al. International patterns of tuberculosis and the prevalence of symptoms of asthma, rhinitis, and eczema. Thorax. 2000;55(6):449–53. ; doi: 10.1136/thorax.55.6.449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goswami R, Kaplan MH. A brief history of IL-9. Journal of immunology. 2011;186(6):3283–8. doi: 10.4049/jimmunol.1003049 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Temann UA, Laouar Y, Eynon EE, Homer R, Flavell RA. IL9 leads to airway inflammation by inducing IL13 expression in airway epithelial cells. International immunology. 2007;19(1):1–10. doi: 10.1093/intimm/dxl117 . [DOI] [PubMed] [Google Scholar]
- 33.Uyttenhove C, Simpson RJ, Van Snick J. Functional and structural characterization of P40, a mouse glycoprotein with T-cell growth factor activity. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(18):6934–8. ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dugas B, Renauld JC, Pene J, Bonnefoy JY, Peti-Frere C, Braquet P, et al. Interleukin-9 potentiates the interleukin-4-induced immunoglobulin (IgG, IgM and IgE) production by normal human B lymphocytes. European journal of immunology. 1993;23(7):1687–92. doi: 10.1002/eji.1830230743 . [DOI] [PubMed] [Google Scholar]
- 35.Petit-Frere C, Dugas B, Braquet P, Mencia-Huerta JM. Interleukin-9 potentiates the interleukin-4-induced IgE and IgG1 release from murine B lymphocytes. Immunology. 1993;79(1):146–51. ; [PMC free article] [PubMed] [Google Scholar]
- 36.Sears MR, Burrows B, Flannery EM, Herbison GP, Hewitt CJ, Holdaway MD. Relation between airway responsiveness and serum IgE in children with asthma and in apparently normal children. The New England journal of medicine. 1991;325(15):1067–71. doi: 10.1056/NEJM199110103251504 . [DOI] [PubMed] [Google Scholar]
- 37.Donahue RE, Yang YC, Clark SC. Human P40 T-cell growth factor (interleukin-9) supports erythroid colony formation. Blood. 1990;75(12):2271–5. . [PubMed] [Google Scholar]
- 38.Williams DE, Morrissey PJ, Mochizuki DY, de Vries P, Anderson D, Cosman D, et al. T-cell growth factor P40 promotes the proliferation of myeloid cell lines and enhances erythroid burst formation by normal murine bone marrow cells in vitro. Blood. 1990;76(5):906–11. . [PubMed] [Google Scholar]
- 39.Longphre M, Li D, Gallup M, Drori E, Ordonez CL, Redman T, et al. Allergen-induced IL-9 directly stimulates mucin transcription in respiratory epithelial cells. The Journal of clinical investigation. 1999;104(10):1375–82. doi: 10.1172/JCI6097 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamasaki A, Saleh A, Koussih L, Muro S, Halayko AJ, Gounni AS. IL-9 induces CCL11 expression via STAT3 signalling in human airway smooth muscle cells. PloS one. 2010;5(2):e9178 doi: 10.1371/journal.pone.0009178 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cambier CJ, Falkow S, Ramakrishnan L. Host evasion and exploitation schemes of Mycobacterium tuberculosis. Cell. 2014;159(7):1497–509. doi: 10.1016/j.cell.2014.11.024 . [DOI] [PubMed] [Google Scholar]
- 42.Seshadri C, Uiso LO, Ostermann J, Diefenthal H, Shao HJ, Chu HY, et al. Low sensitivity of T-cell based detection of tuberculosis among HIV co-infected Tanzanian in-patients. East Afr Med J. 2008;85(9):442–9. ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whalen CC, Zalwango S, Chiunda A, Malone L, Eisenach K, Joloba M, et al. Secondary attack rate of tuberculosis in urban households in Kampala, Uganda. PloS one. 2011;6(2):e16137 Epub 2011/02/23. doi: 10.1371/journal.pone.0016137 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siezen CL, Bont L, Hodemaekers HM, Ermers MJ, Doornbos G, Van't Slot R, et al. Genetic susceptibility to respiratory syncytial virus bronchiolitis in preterm children is associated with airway remodeling genes and innate immune genes. The Pediatric infectious disease journal. 2009;28(4):333–5. Epub 2009/03/05. doi: 10.1097/INF.0b013e31818e2aa9 . [DOI] [PubMed] [Google Scholar]
- 45.Janssen R, Bont L, Siezen CL, Hodemaekers HM, Ermers MJ, Doornbos G, et al. Genetic susceptibility to respiratory syncytial virus bronchiolitis is predominantly associated with innate immune genes. J Infect Dis. 2007;196(6):826–34. Epub 2007/08/19. doi: 10.1086/520886 . [DOI] [PubMed] [Google Scholar]
- 46.von Reyn CF, Mtei L, Arbeit RD, Waddell R, Cole B, Mackenzie T, et al. Prevention of tuberculosis in Bacille Calmette-Guerin-primed, HIV-infected adults boosted with an inactivated whole-cell mycobacterial vaccine. Aids. 2010;24(5):675–85. Epub 2010/02/02. doi: 10.1097/QAD.0b013e3283350f1b . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stein CM, Hall NB, Malone LL, Mupere E. The household contact study design for genetic epidemiological studies of infectious diseases. Front Genet. 2013;4:61 Epub 2013/05/04. doi: 10.3389/fgene.2013.00061 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hall NB, Igo RP Jr., Malone LL, Truitt B, Schnell A, Tao L, et al. Polymorphisms in TICAM2 and IL1B are associated with TB. Genes Immun. 2014. Epub 2014/12/19. doi: 10.1038/gene.2014.77 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. American journal of respiratory and critical care medicine. 2003;167(4):603–62. Epub 2003/02/18. doi: 10.1164/rccm.167.4.603 . [DOI] [PubMed] [Google Scholar]
- 50.Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Recomm Rep. 2000;49(RR-6):1–51. . [PubMed] [Google Scholar]
- 51.Lahey T, Mitchell BK, Arbeit RD, Sheth S, Matee M, Horsburgh CR, et al. Polyantigenic interferon-gamma responses are associated with protection from TB among HIV-infected adults with childhood BCG immunization. PloS one. 2011;6(7):e22074 doi: 10.1371/journal.pone.0022074 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahan CS, Zalwango S, Thiel BA, Malone LL, Chervenak KA, Baseke J, et al. Innate and adaptive immune responses during acute M. tuberculosis infection in adult household contacts in Kampala, Uganda. The American journal of tropical medicine and hygiene. 2012;86(4):690–7. doi: 10.4269/ajtmh.2012.11-0553 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–75. Epub 2007/08/19. doi: 10.1086/519795 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.RDC T. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. 2007.
- 55.Zheng X, Levine D, Shen J, Gogarten SM, Laurie C, Weir BS. A high-performance computing toolset for relatedness and principal component analysis of SNP data. Bioinformatics. 2012;28(24):3326–8. Epub 2012/10/13. doi: 10.1093/bioinformatics/bts606 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turner SD. qqman: an R package for visualizing GWAS results using Q-Q and manhattan plots. bioRxiv beta. 2014. doi: 10.1101/005165 [Google Scholar]
- 57.Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP, et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26(18):2336–7. doi: 10.1093/bioinformatics/btq419 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gauderman WJ. Sample size requirements for matched case-control studies of gene-environment interaction. Stat Med. 2002;21(1):35–50. Epub 2002/01/10. . [DOI] [PubMed] [Google Scholar]
- 59.StataCorp. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP; 2009.
- 60.Genomes Project C, Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, et al. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491(7422):56–65. Epub 2012/11/07. doi: 10.1038/nature11632 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS genetics. 2009;5(6):e1000529 Epub 2009/06/23. doi: 10.1371/journal.pgen.1000529 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Howie B, Marchini J, Stephens M. Genotype imputation with thousands of genomes. G3. 2011;1(6):457–70. Epub 2012/03/03. doi: 10.1534/g3.111.001198 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dudbridge F. Likelihood-based association analysis for nuclear families and unrelated subjects with missing genotype data. Human heredity. 2008;66(2):87–98. Epub 2008/04/03. doi: 10.1159/000119108 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–5. Epub 2004/08/07. doi: 10.1093/bioinformatics/bth457 . [DOI] [PubMed] [Google Scholar]
- 65.O'Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP. The immune response in tuberculosis. Annu Rev Immunol. 2013;31:475–527. doi: 10.1146/annurev-immunol-032712-095939 . [DOI] [PubMed] [Google Scholar]
- 66.Consortium EP. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489(7414):57–74. doi: 10.1038/nature11247 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Cases and controls were run together to minimize batch effects.
(DOCX)
Data Availability Statement
All the summary level data, as well as the individual level data for Tanzania are available from DRYAD (doi:10.5061/dryad.cq183). Because of the IRB restriction on the data from Uganda, individual level data are only available upon request from the Uganda Genetics of TB Data Access Committee by contacting Dr. Sudha Iyengar (ski@case.edu).