Specific improvements in the design of medication labels and strategic provision of dosing tools can significantly reduce parental risk of dosing errors.
Abstract
BACKGROUND AND OBJECTIVES:
Poorly designed labels and dosing tools contribute to dosing errors. We examined the degree to which errors could be reduced with pictographic diagrams, milliliter-only units, and provision of tools more closely matched to prescribed volumes.
METHODS:
This study involved a randomized controlled experiment in 3 pediatric clinics. English- and Spanish-speaking parents (n = 491) of children ≤8 years old were randomly assigned to 1 of 4 groups and given labels and dosing tools that varied in label instruction format (text and pictogram, or text only) and units (milliliter-only ["mL"] or milliliter/teaspoon ["mL/tsp"]). Each parent measured 9 doses of liquid medication (3 amounts [2, 7.5, and 10 mL] and 3 tools [1 cup, 2 syringes (5- and 10-mL capacities)]) in random order. The primary outcome was dosing error (>20% deviation), and large error (>2× dose).
RESULTS:
We found that 83.5% of parents made ≥1 dosing error (overdosing was present in 12.1% of errors) and 29.3% of parents made ≥1 large error (>2× dose). The greatest impact on errors resulted from the provision of tools more closely matched to prescribed dose volumes. For the 2-mL dose, the fewest errors were seen with the 5-mL syringe (5- vs 10-mL syringe: adjusted odds ratio [aOR] = 0.3 [95% confidence interval: 0.2–0.4]; cup versus 10-mL syringe: aOR = 7.5 [5.7–10.0]). For the 7.5-mL dose, the fewest errors were with the 10-mL syringe, which did not necessitate measurement of multiple instrument-fulls (5- vs 10-mL syringe: aOR = 4.0 [3.0–5.4]; cup versus 10-mL syringe: aOR = 2.1 [1.5–2.9]). Milliliter/teaspoon was associated with more errors than milliliter-only (aOR = 1.3 [1.05–1.6]). Parents who received text only (versus text and pictogram) instructions or milliliter/teaspoon (versus milliliter-only) labels and tools made more large errors (aOR = 1.9 [1.1–3.3], aOR = 2.5 [1.4–4.6], respectively).
CONCLUSIONS:
Provision of dosing tools more closely matched to prescribed dose volumes is an especially promising strategy for reducing pediatric dosing errors.
What’s Known on This Subject:
Poorly designed labels and dosing tools contribute to dosing errors. Initial findings from the Safe Administration For Every Rx for Kids study identified high rates of error with cups compared with syringes, especially for small dose amounts, and greater confusion when labels included teaspoon-only instructions.
What This Study Adds:
Dosing tools should be provided that more closely match prescribed dose volumes, such that there is little room to overdose and no need to measure multiple instrument-fulls. Pictographic instructions and milliliter-only labels and tools can also reduce errors.
Suboptimal drug labeling and medication packaging have been identified as key contributors to outpatient medication errors.1–3 Over the past decade, most efforts to address this problem have focused on improving labeling for adult medications,4,5 despite high rates of pediatric dosing errors.6,7 The Safe Administration For Every Rx for Kids (SAFE Rx for Kids) study was initiated to fill gaps in knowledge regarding how to improve the labeling and packaging of pediatric medications. Findings from the first experiment supported the use of oral syringes over dosing cups (especially for smaller doses), supported avoidance of teaspoon terms as a strategy to reduce use of kitchen spoons, and suggested that error rates might be reduced when matched units on labels and dosing tools are used (ie, milliliter-only ["mL"] or milliliter/teaspoon["mL/tsp"] on both).8,9 Notably, error rates were significant even with syringes and matched labels and tools. This article presents findings from the second SAFE Rx for Kids experiment, which looks at the implications of additional strategies to reduce errors (specifically the role of pictograms and dosing-tool size) while continuing to examine implications of milliliter-only versus milliliter/teaspoon for additional dose amounts. No previous study has looked at these issues across a range of doses and with an experimental approach.
Pictograms represent a promising strategy for improving parental understanding of dosing instructions. Use of pictorial-enhanced written materials has been linked with improved comprehension and adherence with medical instructions,10–12 especially for those with low literacy.13–17 To date, no studies have examined the role of pictographic dosing diagrams on liquid medication prescription labels.
Many believe that moving to milliliter-only labels and tools would simplify the task of dosing liquid medications and reduce errors by parents as well as health care providers and pharmacists.18–22 A 2015 policy statement from the American Academy of Pediatrics recommended moving to a milliliter-exclusive system.18 Few studies have examined whether consistency in units is an equally effective strategy for error reduction compared with a milliliter-only approach; experiment 1 of the SAFE Rx study found no difference in error rates with matched labels and tools that were milliliter only compared with those containing both milliliter and teaspoon.8
Matching dosing-tool capacity to dose volume may further reduce errors.23 Providing too large of a tool means there is more space within the tool to give too much medication.24,25 Provision of a tool that is too small leads to the need to measure more than 1 instrument-full of medication. There has been limited previous study of this issue.13,26
To fill gaps in knowledge regarding best practices for the labeling and dosing of pediatric liquid medications, we examined the extent to which error rates could be reduced by: (1) inclusion of text only versus text and pictogram dosing instructions; (2) milliliter-only versus milliliter/teaspoon unit-concordant labels and tools; and (3) better matching of dosing-tool capacity to dose volume. We also investigated differences in impact of parental health literacy and language because low health literacy and limited English proficiency are factors known to place parents at a greater risk for errors.24,27–30
Methods
Participants, Recruitment, and Randomization
This was a randomized controlled study to examine ways to improve medication labels and dosing tools and reduce parental errors in dosing liquid medicines (SAFE Rx for Kids study). Recruitment took place in pediatric outpatient clinics at NYC Health + Hospitals/Bellevue (New York, NY), Gardner Packard Children’s Health Center (Atherton, CA), and Children’s Healthcare of Atlanta at Hughes Spalding Children’s Hospital (Atlanta, GA). Each site’s institutional review board approved the study.
Research assistants (RAs) determined parent and/or caregiver eligibility during regular clinic hours. Eligibility was met if the parent or legal guardian was ≥18 years old with a child ≤8 years old presenting for care, was English- or Spanish-speaking, and was the one who usually administers medications. Subjects were excluded if their visual acuity was worse than 20/50 (Rosenbaum screener), they were hearing impaired, their children were presenting for emergency care, or there was previous participation in a medication-related study. RAs obtained written, informed consent.
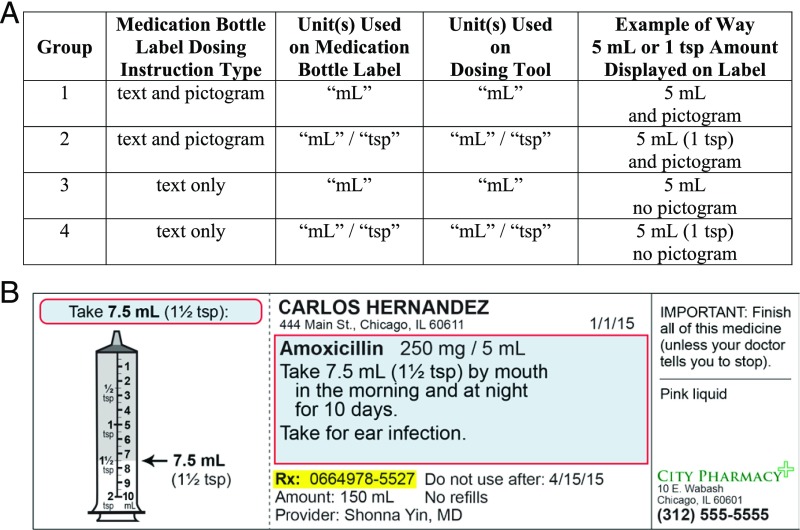
After enrollment, subjects were randomly assigned to 1 of 4 groups. Groups differed by type of instruction on the medication label and measurement units on the label and dosing tool: text and pictogram instructions on the label, "mL"-only label and tool (Group 1); text and pictogram instructions, "mL/tsp" label and tool (Group 2); text-only instructions, "mL"-only label and tool (Group 3); and text-only instructions, "mL/tsp" label and tool (Group 4) (Fig 1). Teaspoon-only labels and tools were not tested. Most dosing tools include milliliter units. A move to teaspoon only would also not be feasible because many prescribed doses are not teaspoon equivalents. A random-number generator, blocked by site, was used to randomly assign subjects; this was performed in sets of 100 with 25 subjects allocated per group. The lead project coordinator developed the allocation sequence. RAs remained blinded to assigned groups until after subject enrollment was finished. Once RAs began to administer dosing assessments, group assignments were evident from the labels and tools provided.
FIGURE 1.
Medication labels and units tested. A, comparison of randomization group characteristics. B, example of Group 2 medication label (English). Note: Group 4 labels were the same as Group 2 labels except for the inclusion of the pictogram “flap.”
Assessments
RAs performed assessments in English and Spanish depending on parents’ preferences. After the dosing assessments, RAs administered a survey to assess parents’ sociodemographics and health literacy. A $20 gift card was provided after completion of the assessments.
Dosing Accuracy
Study subjects were shown their assigned bottle labels and tools and were asked to respond to questions and demonstrate dosing. Each subject participated in 9 total trials; they were asked to measure 3 dose amounts (2, 7.5, and 10 mL) using 3 tools (5- and 10-mL–capacity oral syringes and dosing cups with 15 mL as the largest marking). To address potential learning effects, the order in which caregivers were given each tool and dose amount was randomized by using a random-number generator. Bottle labels were displayed in English or Spanish on the basis of the caregivers’ preferences. Tools were custom designed (Comar, Buena, NJ) to ensure that the only difference for each tool type across groups was the markings.
Caregivers were given as much time as they needed for the assessments. They were told the following: “Please use this [dosing tool handed to parent] to show me how much medicine the label tells you to give the child each time you give the medicine.” Caregivers dispensed the medication from standard bottles filled to the same level for each trial. A commonly administered pediatric medication (liquid suspension) was used for dosing assessments.
Dosing error was the primary outcome. To assess the magnitude of error, the weight of the measured dose (tool weight containing parent’s measured dose minus preassessment tool weight) was compared with a reference weight (eg, 5-mL dose defined as the average weight of 5 mL measured by 10 pediatricians using an oral syringe). A pharmacy-grade electronic prescription class II scale (Torbal DRX-4; Fulcrum Inc, Clifton, NJ) was used.
If the measured amount deviated from the amount listed on the label by >20%, the parent was considered to have made a clinically meaningful dosing error; this is an accepted threshold that has been used in previous studies.24,31–33 We also assessed errors of greater magnitude; a large dosing error was defined as measuring an amount more than 2 times the labeled dose.8
Sociodemographic Data, Health Literacy, and Child Health Status
Sociodemographics assessed included each child’s age and sex and each parent’s age, relationship to the child, income, country of birth, race/ethnicity, language, and education. Parental health literacy was measured by using the Newest Vital Sign (NVS), which is validated in English and Spanish and tests prose, document, and quantitative literacy skills by using a nutrition label. Parents were categorized as having low (score 0–1), marginal (2–3), or adequate (4–6) health literacy.34 Each child’s chronic disease status and associated medication use were assessed by using questions from the Children with Special Health Care Needs Screener.35
Statistical Analyses
Statistical analyses were performed by using SAS software version 9.4 (SAS Institute Inc, Cary, NC). χ2 and Fisher’s exact tests were conducted to compare parent characteristics among groups for categorical variables, and Kruskal-Wallis tests were used for continuous measurements. Similar analyses were performed to compare parents who did and did not enroll in the study.
Analyses were performed to compare error rates (>20% deviation, >2× dose) on the basis of (a) group and (b) tool type (model 1). Findings were analyzed by assigned group (all parents received assigned label and tool pairings). Analyses were also conducted by group characteristics: (a) dosing instruction type (text and pictogram versus text only) and (b) units used on labels and tools (milliliter only versus milliliter/teaspoon) (model 2). Multiple logistic regression was performed using generalized estimating equations to account for repeated measures (9 trials per subject). In addition to group and tool type, covariates selected a priori for inclusion in adjusted analyses were key study variables of dose amount, dosing order, health literacy, and label language. Stratified analyses and interaction tests were performed by using dose volume (ie, 2, 7.5, and 10 mL), health literacy, and language.
Based on previous studies, including our previous experiment on dosing errors,8,13,28,31 we conservatively estimated a sample size of 120 patients per group or 480 total subjects. This sample size would allow us to detect an absolute difference of ∼15% with 80% power for our hypotheses related to pictographic dosing instructions as well as comparison of single versus multiple instrument-fulls.
Results
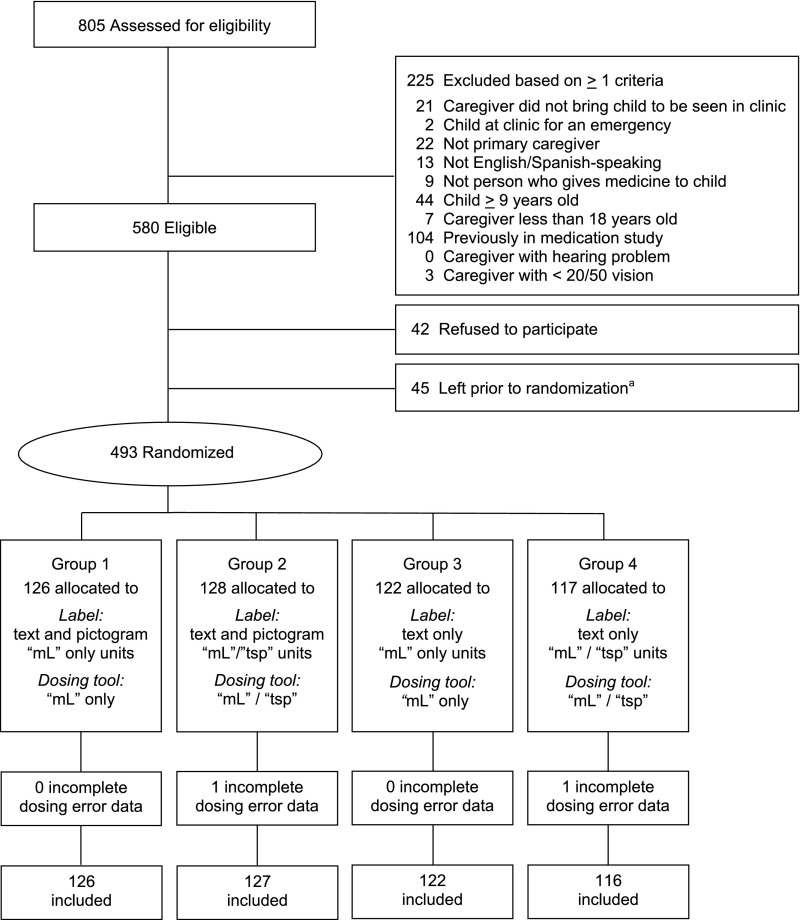
Between February 20, 2015 and July 23, 2015, 493 parents enrolled and were randomly assigned (Fig 2). Characteristics did not differ between enrolled and nonenrolled subjects. Dosing assessments were completed for the 491 parents included in this analysis (Table 1).
FIGURE 2.
Recruitment and enrollment of study participants. a Ran out of time after signing consent.
TABLE 1.
Characteristics of Study Population (N = 491)
| Entire Population | Group 1, N = 126 | Group 2, N = 127 | Group 3, N = 122 | Group 4, N = 116 | P | |
|---|---|---|---|---|---|---|
| Dosing instruction type | — | Text and pictogram | Text and pictogram | Text only | Text only | |
| Label unit | — | "mL" | "mL/tsp" | "mL" | "mL/tsp" | |
| Dosing tool unit | — | "mL" | "mL/tsp" | "mL" | "mL/tsp" | |
| Child characteristics | ||||||
| Age, mean (SD), y | 2.2 (2.4) | 2.5 (2.5) | 2.3 (2.5) | 1.8 (2.1) | 2.1 (2.3) | .2 |
| Girl, n (%) | 242 (49.3) | 54 (42.9) | 63 (49.6) | 66 (54.1) | 59 (50.9) | .3 |
| Chronic medical problem treated with medication, n (%)a | 81 (17.3) | 26 (21.8) | 23 (19.5) | 13 (11.0) | 19 (16.7) | .1 |
| Parent characteristics | ||||||
| Age, mean (SD), y | 30.3 (7.6) | 30.1 (7.7) | 30.5 (6.7) | 30.4 (8.4) | 30.3 (7.7) | .9 |
| Woman, n (%) | 456 (92.9) | 114 (90.5) | 120 (94.5) | 112 (91.8) | 110 (94.8) | .5 |
| Relationship to child, n (%) | ||||||
| Mother | 448 (91.2) | 111 (88.1) | 120 (94.5) | 110 (90.2) | 107 (92.2) | .3 |
| Marital status single, n (%)b | 192 (39.6) | 52 (41.9) | 45 (36.3) | 47 (38.8) | 48 (41.4) | .8 |
| Income, n (%) | .5 | |||||
| <$10 000 | 102 (20.8) | 32 (25.4) | 19 (15.0) | 26 (21.3) | 25 (21.6) | |
| $10 000–$19 999 | 120 (24.4) | 25 (19.8) | 34 (26.8) | 36 (29.5) | 25 (21.6) | |
| $20 000–$39 999 | 156 (31.8) | 43 (34.1) | 40 (31.5) | 34 (27.9) | 39 (33.6) | |
| ≥$40 000 | 63 (12.8) | 16 (12.7) | 19 (15.0) | 12 (9.8) | 16 (13.8) | |
| Unknown or missing | 50 (10.2) | 10 (7.9) | 15 (11.8) | 14 (11.5) | 11 (9.5) | |
| Country of birth outside of United States, n (%)c | 247 (50.8) | 59 (47.6) | 72 (57.6) | 58 (47.9) | 58 (50.0) | .4 |
| Race/ethnicity, n (%)d | .8 | |||||
| Hispanic | 264 (54.3) | 65 (52.4) | 70 (56.0) | 67 (55.4) | 62 (53.4) | |
| Non-Hispanic | ||||||
| White, non-Hispanic | 20 (4.1) | 6 (4.8) | 4 (3.2) | 6 (5.0) | 4 (3.4) | |
| Black, non-Hispanic | 166 (34.2) | 40 (32.3) | 40 (32.0) | 43 (35.5) | 43 (37.1) | |
| Other, non-Hispanic | 36 (7.4) | 13 (10.5) | 11 (8.8) | 5 (4.1) | 7 (6.0) | |
| Language Spanish, n (%)e | 161 (32.8) | 38 (30.2) | 43 (33.9) | 43 (35.3) | 37 (31.9) | .8 |
| Education, n (%)f | .5 | |||||
| Less than HS graduate | 133 (27.4) | 29 (23.4) | 41 (33.1) | 35 (28.9) | 28 (24.1) | |
| HS graduate or equivalent | 154 (31.8) | 37 (29.8) | 36 (29.0) | 40 (33.1) | 41 (35.3) | |
| Greater than HS graduate | 198 (40.8) | 58 (46.8) | 47 (37.9) | 46 (38.0) | 47 (40.5) | |
| Health literacy, n (%)g | .8 | |||||
| Low | 135 (28.2) | 31 (25.0) | 36 (29.8) | 37 (31.1) | 31 (27.0) | |
| Marginal | 211 (44.1) | 58 (46.8) | 53 (43.8) | 53 (44.5) | 47 (40.9) | |
| Adequate | 133 (27.8) | 35 (28.2) | 32 (26.4) | 29 (24.4) | 37 (32.2) | |
| Site characteristics | ||||||
| Site, n (%) | .97 | |||||
| Emory | 164 (33.4) | 41 (32.5) | 39 (30.7) | 43 (35.3) | 41 (35.3) | |
| NYU | 163 (33.2) | 43 (34.1) | 46 (36.2) | 39 (32.0) | 35 (30.2) | |
| Stanford | 164 (33.4) | 42 (33.3) | 42 (33.1) | 40 (32.8) | 40 (34.5) |
—, not applicable.
Missing for 22 children overall (7 in Group 1, 9 in Group 2, 4 in Group 3, and 2 in Group 4).
Missing for 6 parents (2 in Group 1, 3 in Group 2, 1 in Group 3, and 0 in Group 4).
Missing for 5 parents (2 in Group 1, 2 in Group 2, 1 in Group 3, and 0 in Group 4).
Missing for 5 parents (2 in Group 1, 2 in Group 2, 1 in Group 3, and 0 in Group 4).
Language of survey administration.
Missing for 6 parents (2 in Group 1, 3 in Group 2, 1 in Group 3, and 0 in Group 4).
Health literacy measured by using the NVS (low = 0–1; marginal = 2–3; adequate = 4–6). Data are missing for 12 subjects who did not complete the NVS (2 in Group 1, 6 in Group 2, 3 in Group 3, and 1 in Group 4).
Dosing Accuracy
Overall, 410 (83.5%) parents made at least 1 dosing error (in 9 trials); on average, parents made errors in 23.2% of trials. Overdosing was present in 12.1% of errors. There were more errors with the 2- and 7.5-mL doses tested compared with the 10-mL dose (2 mL vs 10 mL: aOR = 3.7 [3.1–4.4]; 7.5 mL vs 10 mL: aOR = 1.4 [1.2–1.6]). One hundred and forty-four (29.3%) parents made at least 1 large error (>2× dose); on average, parents made large errors in 4.5% of trials.
Dosing Instructions
Parents who received text and pictogram dosing instructions with "mL"-only labels and tools (Group 1) (a) had decreased odds of making a dosing error compared with parents who received "mL/tsp" labels and tools with or without pictographic dosing instructions (Groups 2 and 4) and (b) had lower odds of making a large dosing error compared with Group 4 (Table 2). No interactions among study groups and either health literacy or language were found to be significant.
TABLE 2.
Dosing Errors by Group and Group Characteristics, and Role of Dosing-Tool Type, Health Literacy, and Language (N = 479)
| Dosing Error (>20% deviation) | Large Dosing Error (>2× dose) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % Trials with Errors per Parenta | Pb | aORc | 95% CI | P | % Trials with Errors per Parenta | Pb | aORc | 95% CI | P | ||
| By group (model 1) | |||||||||||
| Group | Instruction type; units on label; dosing tool | ||||||||||
| 1 | Text and pictogram; "mL" | 18.7 | .03 | 1.0d | Ref | Ref | 2.6 | .001 | 1.0d | Ref | Ref |
| 2 | Text and pictogram; "mL/tsp" | 24.8 | 1.4 | 1.07–1.9 | .01 | 4.8 | 3.4 | 0.8–14.2 | .1 | ||
| 3 | Text only; "mL" | 24.4 | 1.3 | 0.95–1.8 | .1 | 4.0 | 2.6 | 0.6–11.2 | .2 | ||
| 4 | Text only; "mL/tsp" | 25.0 | 1.6 | 1.1–2.1 | .01 | 6.6 | 5.6 | 1.4–23.0 | .02 | ||
| By group characteristics (model 2) | |||||||||||
| Dosing instruction type | |||||||||||
| Text and pictogram | 21.7 | .1 | 1.0 | Ref | Ref | 3.7 | .01 | 1.0 | Ref | Ref | |
| Text only | 24.7 | 1.2 | 0.96–1.5 | .09 | 5.3 | 1.9 | 1.1–3.3 | .02 | |||
| Units on label, dosing tool | |||||||||||
| "mL" only | 21.5 | .02 | 1.0d | Ref | Ref | 3.3 | .0003 | 1.0d | Ref | Ref | |
| "mL/tsp" | 24.9 | 1.3 | 1.05–1.6 | .02 | 5.6 | 2.5 | 1.4–4.6 | .002 | |||
| By other variablese | |||||||||||
| Dosing tool type | |||||||||||
| Dosing cup | 33.6 | <.001 | 3.3 | 2.7–4.0 | <.001 | 8.1 | <.001 | 3.2 | 2.3–4.5 | <.001 | |
| Syringe: 5 mL | 21.2 | 1.6 | 1.4–2.0 | <.001 | 1.9 | 0.5 | 0.3–0.8 | .001 | |||
| Syringe: 10 mL | 14.8 | 1.0 | Ref | Ref | 3.4 | 1.0 | Ref | Ref | |||
| Health literacyf | |||||||||||
| Low | 33.9 | <.001 | 2.5 | 1.9–3.4 | <.001 | 7.2 | .13 | 2.4 | 1.1–5.4 | .03 | |
| Marginal | 21.2 | 1.5 | 1.1–1.9 | .01 | 3.8 | 1.5 | 0.8–2.8 | .2 | |||
| Adequate | 15.4 | 1.0 | Ref | Ref | 2.7 | 1.0 | Ref | Ref | |||
| Language | |||||||||||
| English | 20.2 | .14 | 1.0 | Ref | Ref | 3.7 | .08 | 1.0 | Ref | Ref | |
| Spanish | 29.2 | 1.2 | 0.95–1.5 | .1 | 6.0 | 1.7 | 0.9–3.0 | .1 | |||
CI, confidence interval. Ref, reference.
Percent of trials (out of 9 trials for all except dosing tools, which had 3 trials each) with errors per parent, averaged across all parents.
Type 3 χ2 from full model.
Full model adjusting for randomization group, tool type, dose volume, dosing order, language, and health literacy. No group by health literacy interaction found for dosing error (P = .2). Model failed to converge for large dosing error because of the small number of events. No group by language interaction found for dosing error (P = .8). Model failed to converge for large dosing error because of the small number of events.
Used as referent group because the American Academy of Pediatrics and other organizations support a move to milliliter-exclusive dosing.
Results were the same for models 1 and 2 for dosing tool, health literacy, and language analyses.
Health literacy measured by using the NVS.
In analyses based on group characteristics, parents who received dosing instructions that were text only (versus text and pictogram) made more large errors (aOR = 1.9 [1.1–3.3]); no statistically significant difference was seen for any error (P = .09). Parents who received units on labels and tools that were "mL/tsp" (versus "mL" only) also made more large errors (aOR = 2.5 [1.4–4.6]); more errors were also seen for any error (aOR = 1.3 [1.05–1.6]).
Dosing Tool
Error rates varied for tools depending on the volume of the dose tested, with the fewest errors present when the dosing-tool capacity more closely matched the dose volume (Table 3). For the 2-mL dose, the most errors were seen with the cup and 10-mL syringe (5- vs 10-mL syringe: aOR = 0.3 [0.2–0.4]; cup versus 10-mL syringe: aOR = 7.5 [5.7–10.0]). For the 7.5-mL doses, the most errors were seen with the 5-mL syringe (5- vs 10-mL syringe: aOR = 4.0 [3.0–5.4]; cup versus 10-mL syringe: aOR = 2.1 [1.5–2.9]). Similar findings were seen for the 10-mL dose.
TABLE 3.
Dosing Errors by Dose Amount: Role of the Dose Volume to Tool Capacity Relationship and Health Literacy
| Dosing Error (>20% Deviation) | |||||
|---|---|---|---|---|---|
| % Trials with Errors per Parenta | Pb | aORc | 95% CI | P | |
| Dose volume: 2 mL | |||||
| Overall (N = 479) | |||||
| Dosing tool type | |||||
| Dosing cup | 71.0 | <.001 | 7.5 | 5.7–10.0 | <.001 |
| Syringe: 5 mLd | 10.9 | 0.3 | 0.2–0.4 | <.001 | |
| Syringe: 10 mL | 26.5 | 1.0 | Ref | Ref | |
| Low HL (n = 135) | |||||
| Dosing tool type | |||||
| Dosing cup | 80.7 | <.001 | 11.9 | 6.5–21.9 | <.001 |
| Syringe: 5 mLd | 18.5 | 0.5 | 0.3–0.8 | .004 | |
| Syringe: 10 mL | 32.6 | 1.0 | Ref | Ref | |
| Marginal HL (n = 211) | |||||
| Dosing tool type | |||||
| Dosing cup | 70.1 | <.001 | 6.9 | 4.5–10.5 | <.001 |
| Syringe: 5 mLd | 8.5 | 0.2 | 0.1–0.4 | <.001 | |
| Syringe: 10 mL | 28.4 | 1.0 | Ref | Ref | |
| Adequate HL (n = 133) | |||||
| Dosing tool type | |||||
| Dosing cup | 62.4 | <.001 | 8.7 | 4.9–15.3 | <.001 |
| Syringe: 5 mLd | 6.8 | 0.3 | 0.2–0.6 | .0009 | |
| Syringe: 10 mL | 17.3 | 1.0 | Ref | Ref | |
| Dose volume: 7.5 mL | |||||
| Overall (N = 479) | |||||
| Dosing tool type | |||||
| Dosing cup | 17.8 | <.001 | 2.1 | 1.5–2.9 | <.001 |
| Syringe: 5 mL | 28.4 | 4.0 | 3.0–5.4 | <.001 | |
| Syringe: 10 mLd | 9.8 | 1.0 | Ref | Ref | |
| Low HL (n = 135) | |||||
| Dosing tool type | |||||
| Dosing cup | 24.4 | <.001 | 1.3 | 0.8–2.2 | .2 |
| Syringe: 5 mL | 54.1 | 5.1 | 3.3–7.9 | <.001 | |
| Syringe: 10 mLd | 19.3 | 1.0 | Ref | Ref | |
| Marginal HL (n = 211) | |||||
| Dosing tool type | |||||
| Dosing cup | 17.5 | <.001 | 3.0 | 1.5–5.8 | .001 |
| Syringe: 5 mL | 23.7 | 4.6 | 2.7–8.1 | <.001 | |
| Syringe: 10 mLd | 6.7 | 1.0 | Ref | Ref | |
| Adequate HL (n = 133) | |||||
| Dosing tool type | |||||
| Dosing cup | 11.3 | .002 | 2.3 | 1.2–4.6 | .02 |
| Syringe: 5 mL | 9.8 | 2.1 | 1.3–3.5 | .003 | |
| Syringe: 10 mLd | 5.3 | 1.0 | Ref | Ref | |
| Dose volume: 10 mL | |||||
| Overall (N = 479) | |||||
| Dosing tool type | |||||
| Dosing cup | 12.1 | <.001 | 1.7 | 1.2–2.4 | .004 |
| Syringe: 5 mL | 24.2 | 4.1 | 2.9–5.8 | <.001 | |
| Syringe: 10 mLd | 7.9 | 1.0 | Ref | Ref | |
| Low HL (n = 135) | |||||
| Dosing tool type | |||||
| Dosing cup | 17.0 | <.001 | 1.1 | 0.7–1.7 | .6 |
| Syringe: 5 mL | 42.2 | 4.3 | 2.7–7.0 | <.001 | |
| Syringe: 10 mLd | 16.3 | 1.0 | Ref | Ref | |
| Marginal HL (n = 211) | |||||
| Dosing tool type | |||||
| Dosing cup | 10.4 | <.001 | 2.7 | 1.2–5.9 | .01 |
| Syringe: 5 mL | 21.3 | 6.5 | 3.3–13.1 | <.001 | |
| Syringe: 10 mLd | 4.3 | 1.0 | Ref | Ref | |
| Adequate HL (n = 133) | |||||
| Dosing tool type | |||||
| Dosing cup | 9.8 | .1 | 2.0 | 0.9–4.4 | .1 |
| Syringe: 5 mL | 10.5 | 2.1 | 0.9–4.8 | .1 | |
| Syringe: 10 mLd | 5.3 | 1.0 | Ref | Ref | |
Tool type by health literacy interaction found for 7.5 mL dose (P = .004) and 10 mL dose (P = .04), but not for 2 mL dose (P = .4). CI, confidence interval. HL, health literacy.
Percent of trials (out of 3 trials per parent per tool) with errors per parent, averaged across parents.
Type 3 χ2 from full model.
Full model adjusting for randomization group, tool type, dose amount, dosing order, and language.
Tool with most closely matched dosing-tool capacity to prescribed dose volume, without the need for measurement of more than 1 instrument-full for a single dose.
Impact of the dose volume to tool capacity relationship was greatest for those with low and marginal health literacy, particularly when there was a need for measurement of multiple instrument-fulls (P = .004 for tool type by health literacy interaction with 7.5-mL dose; P = .04 for interaction with 10-mL dose) (Table 3). No tool type by language interaction was found.
Discussion
This study is unique in that it sought to identify a range of strategies to improve pediatric liquid medication labels and associated dosing tools by examining the role of dosing pictograms, matched units on labels and tools, and provision of dosing tools based on prescribed dose volumes. Overall, we found a high rate of dosing errors, with >80% of parents making at least 1 error (± >20% deviation) in 9 dosing trials. The greatest reduction in errors was seen when the dosing tool provided more closely matched the prescribed dose volume, such that there was little room to overdose and no need to measure multiple instrument-fulls for a single dose. Smaller impacts on errors were seen with pictograms and with a change in units from "mL/tsp" to "mL" only. Although fewer large dosing errors were seen (representing ∼5% of all trials), nearly 30% of the parents in our study made at least 1 large error (>2× deviation). Our findings suggest that inclusion of pictographic dosing diagrams and "mL"-only units on labels and tools may be especially effective strategies to reduce large errors.
The high rate of dosing errors we found is consistent with previous studies involving pediatric medications.28,36 Confusion about how to properly choose and use dosing tools as well as confusion related to units of measure contribute to errors.37 Similar to previous studies, we found that underdosing accounted for the majority of errors,13,31 although ∼1 in 8 errors involved overdosing. Whereas overdosing is concerning with respect to toxicity, underdosing can contribute to poor clinical outcomes because of treatment failure.
It is well established that the use of standardized tools is important for optimal dosing accuracy and that use of nonstandard kitchen spoons is associated with errors; the US Food and Drug Administration recommends inclusion of standardized tools with all liquid over-the-counter products.3 Consistent with previous findings, including those from a previous SAFE Rx for Kids experiment, more errors were associated with cups than with syringes.8,9,24,38 We found in this study, however, that the optimal dosing tool varied based on the dose volume tested and the capacity of the tool. When we tested a small 2-mL dose, cups were associated with the greatest number of errors, compared with both 5- and 10-mL syringes, with most errors involving overdosing. For the 2-mL dose, having a larger syringe than necessary was also associated with more overdosing. Notably, when we tested larger doses (ie, 7.5 mL and 10 mL), cups were superior to the 5-mL syringes, which required parents to measure more than 1 instrument-full; parents typically underdosed when given a tool that was too small to fit a single dose. Overall, the fewest errors were seen when the desired measured volume was closest to the dosing tool capacity without exceeding it.
Our findings support the use of a specific algorithm to help health care providers and pharmacists determine which dosing tool is most optimal to provide to parents. The current lack of specific guidance regarding dosing tools could be one reason why tool provision practices are often suboptimal. A previous study found in one pharmacy setting that almost one-third of tools given to families with pediatric prescription liquid medications required measurement of multiple instrument-fulls.26
Our study is unique in that it is the first to explore the use of pictographic dosing diagrams on pediatric medication labels. We found that pictograms were associated with statistically significant reductions in large overdosing errors, with a trend for reduction in any error.
Interestingly, although we were not initially powered to look at under- and overdosing separately, secondary analyses which used >20% deviation above the dose as a cutpoint for any overdosing error and >20% deviation below the dose as a cutpoint for any underdosing error yielded similar findings to analyses involving large overdosing errors, which suggests that pictograms may be helpful for preventing both underdosing and overdosing errors. The benefit of pictograms, especially for preventing large errors, indicates that this is an important strategy to consider for reducing multifold errors. Pictograms visually highlight what should be dosed in an obvious way and can provide a dramatic contrast when the pictorially represented dose and what the parent measures are vastly different.
In this study, we found that parents who received labels and tools with only "mL" units were less likely to make errors compared with those who received labels and tools with "mL/tsp"; however, reductions in overall errors were small, with a similar number of underdosing and overdosing errors. Error reductions were greater for large overdosing errors. This is unsurprising because confusion between milliliter and teaspoon units, or misunderstanding of teaspoon ("tsp") as tablespoon ("tbsp"), contributes to multifold errors. Interestingly, in an earlier study by our team, we found error rates to be comparable for parents who received labels and tools with "mL" only versus "mL/tsp" units.8
Parents who were categorized as having low health literacy were at greatest risk for errors, which is consistent with previous studies.39 Notably, parents with the lowest levels of health literacy were most impacted by closer matching of dosing-tool capacity to prescribed dose volumes. Interestingly, the impact of pictograms and units on risk of dosing errors did not vary by health literacy; this suggests that these strategies could be used as part of a universal precautions approach to prevent medication errors.
Surprisingly, there was no impact of language on the overall risk of making errors. Furthermore, language had no interaction with tool type for errors. The potential for language disparities within our study was likely minimized because assessments and labels were provided in Spanish. Spanish-speaking parents may have also had previous exposure to metric measurements in their native countries, resulting in a decreased risk of error.
The current study has limitations that are common for this type of controlled experiment. We employed a hypothetical dosing scheme, which might not reflect how parents dose medications at home. We tested a limited number of dosing tools and capacities and dose volumes. We used 1 label format and 1 type of pictogram. Because this study sought to examine the impact of multiple potential strategies to reduce errors, and because there were fewer large dosing errors in our study, it was difficult to obtain stable model estimates for subanalyses, including analyses by dose amount. Additional studies that specifically focus on strategies to prevent large errors would be helpful. Because we only enrolled patients from 3 urban, university-affiliated pediatric clinic sites, findings may not be generalizable.
Conclusions
Our study findings demonstrate that matching dosing tools more closely with prescribed dose volumes is an important strategy for reducing pediatric medication errors. Inclusion of pictographic dosing diagrams and milliliter-only units on labels and tools may be especially helpful in preventing large dosing errors. Findings are being used to inform the development of a comprehensive labeling and dosing strategy for pediatric liquid medications, which will be tested in a real-world randomized trial. Notably, even with the optimization of labels and tools, a significant proportion of parents with low health literacy still made errors. Thus, to ensure that literacy-related disparities are not exacerbated, implementation of our comprehensive labeling and dosing strategy will likely need to be combined with additional public health approaches, including population-wide educational campaigns and promotion of health literacy–informed provider counseling strategies.
Acknowledgments
We thank our research staff and the staff of the pediatric outpatient clinics at NYC Health + Hospitals/Bellevue, Gardner Packard Children’s Health Center, and Children’s Healthcare of Atlanta at Hughes Spaulding Children’s Hospital for their support. We thank Comar for helping our team develop customized medication-dosing tools for this project.
Glossary
- aOR
adjusted odds ratio
- RA
research assistant
- SAFE Rx for Kids
Safe Administration for Every Rx for Kids
- tsp
teaspoon
Footnotes
Dr Yin conceptualized and designed the study, analyzed and interpreted the data, drafted the initial manuscript, critically revised the manuscript, and provided study supervision; Drs Wolf, Dreyer, and Mendelsohn helped conceptualize and design the study, analyzed and interpreted the data, critically revised the manuscript, and provided study supervision; Drs Parker, Sanders, and Bailey helped conceptualize and design the study, were involved in the analysis and interpretation of the data, critically revised the manuscript, and provided study supervision; Dr Kim participated in the design of the study, analyzed and interpreted the data, and critically revised the manuscript; Ms Patel and Ms Jimenez participated in the design of the study and assisted in acquisition of data, analysis, interpretation of the data, and drafting of the manuscript; Ms Meyers participated in the design of the study and assisted in analysis and interpretation of the data and drafting of the manuscript; Ms Jacobson, Ms Smith, Ms Hedlund, and Dr McFadden participated in the design of the study, assisted in acquisition of data, analysis, and interpretation of the data, and critically revised the manuscript; and all authors approved the final manuscript as submitted.
This trial has been registered at www.clinicaltrials.gov (identifier NCT01854151).
FINANCIAL DISCLOSURE: Drs Bailey, Parker, and Wolf and Ms Jacobson have served as consultants to and received grant funding from Merck, Sharp, and Dohme Federal Credit Union for work unrelated to this study. Drs Bailey and Wolf have also received grant funding by way of their institutions from Eli Lilly and Company and have served as consultants to Luto Research, Ltd. The other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded by the National Institutes of Health/National Institute of Child Health and Human Development (R01HD070864). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Institute of Medicine Standardizing Medication Labels: Confusing Patients Less, Workshop Summary. Washington, DC: Roundtable on Health Literacy Board on Population Health and Public Health Practice; 2008 [Google Scholar]
- 2.Institute of Medicine Preventing Medication Errors. Washington, DC: The National Academies Press; 2006 [Google Scholar]
- 3.U.S. Department of Health and Human Services; Center for Drug Evaluation and Research (CDER) . Guidance for Industry: Dosage Delivery Devices for Orally Ingested OTC Liquid Drug Products. Silver Spring, MD: Food and Drug Administration; 2011 [Google Scholar]
- 4.Shrank WH, Parker R, Davis T, et al. . Rationale and design of a randomized trial to evaluate an evidence-based prescription drug label on actual medication use. Contemp Clin Trials. 2010;31(6):564–571 [DOI] [PubMed] [Google Scholar]
- 5.Wolf MS, Davis TC, Curtis LM, et al. . Effect of standardized, patient-centered label instructions to improve comprehension of prescription drug use. Med Care. 2011;49(1):96–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conroy S, Sweis D, Planner C, et al. . Interventions to reduce dosing errors in children: a systematic review of the literature. Drug Saf. 2007;30(12):1111–1125 [DOI] [PubMed] [Google Scholar]
- 7.Smith MD, Spiller HA, Casavant MJ, Chounthirath T, Brophy TJ, Xiang H. Out-of-hospital medication errors among young children in the United States, 2002-2012. Pediatrics. 2014;134(5):867–876 [DOI] [PubMed] [Google Scholar]
- 8.Yin HS, Parker RM, Sanders LM, et al. . Liquid medication errors and dosing tools: a randomized controlled experiment. Pediatrics. 2016;138(4):e20160357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin HS, Parker RM, Sanders LM, et al. . Effect of medication label units of measure on parent choice of dosing tool: a randomized experiment. Acad Pediatr. 2016;16(8):734–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houts PS, Doak CC, Doak LG, Loscalzo MJ. The role of pictures in improving health communication: a review of research on attention, comprehension, recall, and adherence. Patient Educ Couns. 2006;61(2):173–190 [DOI] [PubMed] [Google Scholar]
- 11.Katz MG, Kripalani S, Weiss BD. Use of pictorial aids in medication instructions: a review of the literature. Am J Health Syst Pharm. 2006;63(23):2391–2397 [DOI] [PubMed] [Google Scholar]
- 12.Dowse R, Ehlers M. Medicine labels incorporating pictograms: do they influence understanding and adherence? Patient Educ Couns. 2005;58(1):63–70 [DOI] [PubMed] [Google Scholar]
- 13.Yin HS, Mendelsohn AL, Fierman A, van Schaick L, Bazan IS, Dreyer BP. Use of a pictographic diagram to decrease parent dosing errors with infant acetaminophen: a health literacy perspective. Acad Pediatr. 2011;11(1):50–57 [DOI] [PubMed] [Google Scholar]
- 14.Delp C, Jones J. Communicating information to patients: the use of cartoon illustrations to improve comprehension of instructions. Acad Emerg Med. 1996;3(3):264–270 [DOI] [PubMed] [Google Scholar]
- 15.Hanson E. Evaluating cognitive services for non-literate and visually impaired patients in community pharmacy rotation sites. Am J Pharm Educ. 1995;59(1):48–54 [Google Scholar]
- 16.Austin PE, Matlack R II, Dunn KA, Kesler C, Brown CK. Discharge instructions: do illustrations help our patients understand them? Ann Emerg Med. 1995;25(3):317–320 [DOI] [PubMed] [Google Scholar]
- 17.Michielutte R, Bahnson J, Dignan MB, Schroeder EM. The use of illustrations and narrative text style to improve readability of a health education brochure. J Cancer Educ. 1992;7(3):251–260 [DOI] [PubMed] [Google Scholar]
- 18.Paul I; Committee on Drugs . Metric units and the preferred dosing of orally administered liquid medications. Pediatrics. 2015;135(4):784–789 [Google Scholar]
- 19.National Council for Prescription Drug Programs NCPDP Recommendations and Guidance for Standardizing the Dosing Designations on Prescription Container Labels of Oral Liquid Medications. Scottsdale, AZ: NCPDP White Papers; 2014 [Google Scholar]
- 20.American Association of Poison Control Centers AAPCC Resolution - Standardizing Volumetric Measures for Oral Medications Intended for Use by Children. NCPDP White Papers. Alexandria, VA: American Association of Poison Control Centers; 2010 [Google Scholar]
- 21.Institute for Safe Medication Practices ISMP calls for elimination of “Teaspoonful” and other non-metric measurements to prevent errors 2009. Available at: http://www.ismp.org/pressroom/PR20090603.pdf. Accessed June 1, 2016
- 22.American Academy of Family Physicians Preferred unit of measurement for liquid medications. Available at: www.aafp.org/about/policies/all/preferred-unit.html. Accessed June 1, 2016
- 23.US Department of Health and Human Services Guidance for industry: safety considerations for product design to minimize medication errors. Available at: www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm331810.pdf. Accessed November 7, 2016
- 24.Yin HS, Mendelsohn AL, Wolf MS, et al. . Parents’ medication administration errors: role of dosing instruments and health literacy. Arch Pediatr Adolesc Med. 2010;164(2):181–186 [DOI] [PubMed] [Google Scholar]
- 25.Litovitz T. Implication of dispensing cups in dosing errors and pediatric poisonings: a report from the American Association of Poison Control Centers. Ann Pharmacother. 1992;26(7–8):917–918 [DOI] [PubMed] [Google Scholar]
- 26.Wallace LS, Keenum AJ, DeVoe JE. Evaluation of consumer medical information and oral liquid measuring devices accompanying pediatric prescriptions. Acad Pediatr. 2010;10(4):224–227 [DOI] [PubMed] [Google Scholar]
- 27.Leyva M, Sharif I, Ozuah PO. Health literacy among Spanish-speaking Latino parents with limited English proficiency. Ambul Pediatr. 2005;5(1):56–59 [DOI] [PubMed] [Google Scholar]
- 28.Yin HS, Dreyer BP, Ugboaja DC, et al. . Unit of measurement used and parent medication dosing errors. Pediatrics. 2014;134(2). Available at: www.pediatrics.org/cgi/content/full/134/2/e354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bailey SC, Agarwal N, Sleath B, Gumusoglu S, Wolf MS. Improving drug labeling and counseling for limited English proficient adults. J Health Care Poor Underserved. 2011;22(4):1131–1143 [DOI] [PubMed] [Google Scholar]
- 30.Bailey SC, Pandit AU, Yin S, et al. . Predictors of misunderstanding pediatric liquid medication instructions. Fam Med. 2009;41(10):715–721 [PubMed] [Google Scholar]
- 31.Yin HS, Dreyer BP, van Schaick L, Foltin GL, Dinglas C, Mendelsohn AL. Randomized controlled trial of a pictogram-based intervention to reduce liquid medication dosing errors and improve adherence among caregivers of young children. Arch Pediatr Adolesc Med. 2008;162(9):814–822 [DOI] [PubMed] [Google Scholar]
- 32.Kozer E, Scolnik D, Macpherson A, et al. . Variables associated with medication errors in pediatric emergency medicine. Pediatrics. 2002;110(4):737–742 [DOI] [PubMed] [Google Scholar]
- 33.Simon HK, Weinkle DA. Over-the-counter medications. Do parents give what they intend to give? Arch Pediatr Adolesc Med. 1997;151(7):654–656 [DOI] [PubMed] [Google Scholar]
- 34.Weiss BD, Mays MZ, Martz W, et al. . Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med. 2005;3(6):514–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bethell CD, Read D, Stein RE, Blumberg SJ, Wells N, Newacheck PW. Identifying children with special health care needs: development and evaluation of a short screening instrument. Ambul Pediatr. 2002;2(1):38–48 [DOI] [PubMed] [Google Scholar]
- 36.Yin HS, Dreyer BP, Moreira HA, et al. . Liquid medication dosing errors in children: role of provider counseling strategies. Acad Pediatr. 2014;14(3):262–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frush KS, Luo X, Hutchinson P, Higgins JN. Evaluation of a method to reduce over-the-counter medication dosing error. Arch Pediatr Adolesc Med. 2004;158(7):620–624 [DOI] [PubMed] [Google Scholar]
- 38.Sobhani P, Christopherson J, Ambrose PJ, Corelli RL. Accuracy of oral liquid measuring devices: comparison of dosing cup and oral dosing syringe. Ann Pharmacother. 2008;42(1):46–52 [DOI] [PubMed] [Google Scholar]
- 39.Davis TC, Wolf MS, Bass PF III, et al. . Literacy and misunderstanding prescription drug labels. Ann Intern Med. 2006;145(12):887–894 [DOI] [PubMed] [Google Scholar]