In our large prospective cohort of febrile infants, neither the YOS score nor unstructured clinician suspicion reliably identified infants with serious bacterial infections.
Abstract
OBJECTIVES:
To assess the performance of the Yale Observation Scale (YOS) score and unstructured clinician suspicion to identify febrile infants ≤60 days of age with and without serious bacterial infections (SBIs).
METHODS:
We performed a planned secondary analysis of a prospective cohort of non–critically ill, febrile, full-term infants ≤60 days of age presenting to 1 of 26 participating emergency departments in the Pediatric Emergency Care Applied Research Network. We defined SBIs as urinary tract infections, bacteremia, or bacterial meningitis, with the latter 2 considered invasive bacterial infections. Emergency department clinicians applied the YOS (range: 6–30; normal score: ≤10) and estimated the risk of SBI using unstructured clinician suspicion (<1%, 1%–5%, 6%–10%, 11%–50%, or >50%).
RESULTS:
Of the 4591 eligible infants, 444 (9.7%) had SBIs and 97 (2.1%) had invasive bacterial infections. Of the 4058 infants with YOS scores of ≤10, 388 (9.6%) had SBIs (sensitivity: 51/439 [11.6%]; 95% confidence interval [CI]: 8.8%–15.0%; negative predictive value: 3670/4058 [90.4%]; 95% CI: 89.5%–91.3%) and 72 (1.8%) had invasive bacterial infections (sensitivity 23/95 [24.2%], 95% CI: 16.0%–34.1%; negative predictive value: 3983/4055 [98.2%], 95% CI: 97.8%–98.6%). Of the infants with clinician suspicion of <1%, 106 had SBIs (6.4%) and 16 (1.0%) had invasive bacterial infections.
CONCLUSIONS:
In this large prospective cohort of febrile infants ≤60 days of age, neither the YOS score nor unstructured clinician suspicion reliably identified those with invasive bacterial infections. More accurate clinical and laboratory predictors are needed to risk stratify febrile infants.
What’s Known on This Subject:
Most febrile infants have viral rather than bacterial infections. Clinical scores, such as the Yale Observation Scale (YOS) score, have been developed to predict bacterial infections in febrile children, however, there is less information regarding infants ≤60 days of age.
What This Study Adds:
Many febrile infants ≤60 days of age with invasive bacterial infections, such as bacteremia and bacterial meningitis, had normal YOS scores. Neither unstructured clinician suspicion nor the YOS reliably identified those febrile infants with invasive bacterial infections.
Fever is the most common reason for infants to present to emergency departments (ED) for evaluation.1 Between 5% and 10% of febrile infants ≤60 days of age evaluated in EDs will have serious bacterial infections (SBIs; defined as urinary tract infections [UTIs], bacterial meningitis, or bacteremia).2–5 The challenge for clinicians is to reliably recognize those infants with SBIs, particularly those with invasive bacterial infections (bacteremia or bacterial meningitis) without overtesting or treating empirically with antibiotics those infants with nonbacterial etiologies for their febrile illnesses.
The Yale Observation Scale (YOS) score is a clinical score developed to identify febrile children with bacterial infections.6,7 Prospectively derived in febrile children <2 years of age, the YOS score involves assessment across 6 behavioral domains, including quality of cry, reaction to parents, state variation, color, hydration, and response to social overtures.6 In clinical practice, instead of using a quantifiable score, such as the YOS score, clinicians often assign an implicit risk for SBI in an unstructured manner as part of their clinical decision-making,2,8 referred to in this article as “unstructured clinician suspicion.” Although the YOS score has been evaluated in young febrile infants in a few studies conducted over 2 decades ago,9,10 the ability of unstructured clinician suspicion to identify young febrile infants with SBIs has not been evaluated in the youngest infants.
In this planned subanalysis of a large multicenter prospective study of febrile infants ≤60 days of age presenting to the ED,11 we evaluated and report the accuracy of the YOS score as well as unstructured clinician suspicion to identify those with SBIs as well as those with invasive SBIs.
Methods
Study Design
We performed a planned secondary analysis of a prospective cross-sectional study of febrile infants who presented to any of the 26 EDs participating in the Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN) between December 2008 and May 2013.5 The institutional review board at each participating center approved the study. We obtained written informed consent from the parent or legal guardian of each infant before study enrollment. For the primary study, consent included permission to collect a research blood sample and to perform telephone follow-up.
Patient Eligibility
In the primary study,5 infants ≤60 days of age with temperature ≥38.0°C measured at home, by a referring provider, or in the ED were eligible if the treating ED clinician planned to obtain a blood culture. We excluded infants who were critically ill, such as those with signs of sepsis in the ED, or those with any of the following significant underlying illnesses: congenital heart disease, prematurity (≤36 weeks’ gestation), inherited or acquired immunodeficiency, or indwelling devices or catheters. Additionally, we excluded infants who had received any antibiotics within the 48 hours preceding ED presentation. We enrolled a convenience sample of eligible infants based on the availability of research staff for enrollment. Previously enrolled infants were not approached if they had additional eligible ED encounters. Because we did not collect clinical data for infants enrolled in the PECARN febrile infant parent study who did not have a research blood specimen collected, we considered these infants ineligible for this study.
YOS Score
All participating physicians received standardized in-person training in the application of the YOS score (Table 1).6,7 Study staff provided annual or as-needed retraining in the application of YOS score to every eligible physician. Treating physicians assigned the YOS score to infants using a structured case report form. As can be seen from Table 1, there are 6 domains in the YOS, each assigned a score of 1, 3, or 5. Therefore, the total YOS score ranges from 6 for the most well-appearing infant to 30 for the most ill-appearing infant.6 If any individual domain score was not recorded, we were unable to assign a total YOS score, and these infants were excluded from the analysis. Based on previous studies, a YOS score of 6 was considered a “perfect” score and a YOS score of ≤10 a normal score.7
TABLE 1.
YOS Score
| Normal | Moderate Impairment | Severe Impairment | |
|---|---|---|---|
| 1 point | 3 points | 5 points | |
| Quality of cry | Strong with normal tone, or content and not crying | Whimpering or sobbing | Weak, moaning, or high-pitched |
| Reaction to parents | Cries briefly and then stops, or content and not crying | Cries off and on | Continual cry or hardly responds |
| State variation | If awake, stays awake, or if asleep and stimulated, wakes up quickly | Eyes close briefly, awakes with prolonged stimulation | Falls to sleep or will not rouse |
| Color | Pink | Pale extremities or acrocyanosis | Pale, cyanotic, mottled, or ashen |
| Hydration | Skin normal, eyes normal and mucous membranes moist | Skin and eyes normal, and mouth slightly dry | Skin doughy or tented and dry mucous membranes and/or sunken eyes |
| Response (talk, smile) to social overtures | Smiles or alert | Brief smile or alert briefly | No smile or face anxious, dull, expressionless, or no alerting |
Unstructured Clinician Suspicion
The same physician who applied the YOS score was then asked to estimate the infant’s risk of having any SBI by selecting 1 of the following 5 risk categories: <1%, 1% to 5%, 6% to 10%, 11% to 50%, or >50%.
Data Collection
The treating clinician also completed standardized case forms to collect patient history and physical examination findings. Study staff at each participating site abstracted results of bacterial cultures, ED disposition, and other treatment decisions from the medical record. We performed telephone follow-up to assess for the presence or development of bacterial meningitis for infants who were discharged from the ED without lumbar punctures. We excluded from the analysis those in this cohort whom we were unable to reach by telephone.
Outcome Measures
Our primary outcome was any SBI defined as the presence of a UTI, bacteremia, or bacterial meningitis.12 Our secondary outcome was an invasive bacterial infection defined as bacteremia or bacterial meningitis. For catheterized urine specimens, UTI was defined as urine culture growth of a urinary pathogen ≥50 000 colony-forming units (CFUs) per mL, or growth of ≥10 000 CFUs per mL in association with a positive urinalysis (>5 white blood cells [WBCs] per high power field, positive nitrite, or leukocyte esterase).13 For suprapubic aspiration urine specimens, UTI was defined as growth of a pathogen of ≥1000 CFUs per mL.13 We defined bacteremia as growth of pathogenic bacteria in blood culture and bacterial meningitis as growth of pathogenic bacteria in the cerebrospinal fluid.12 Bacterial species we considered to be contaminants included viridans streptococci, coagulase-negative staphylococci, and Cornebacterium species.5
Statistical Analysis
We compared medians using the Mann–Whitney test and proportions using the χ2 test. First, we compared infants with SBIs to those without SBIs. We then constructed receiver operating characteristic curves to measure the ability of the YOS score and unstructured clinician suspicion to discriminate between infants with and without SBIs, as well as those with and without invasive bacterial infections. We also used established YOS score dichotomous cut-offs (>6 and >10) to determine the discriminative ability of the YOS score. Furthermore, in our analysis of unstructured clinician suspicion, we also conducted the analysis using conservative cut-offs of clinician suspicion (<1% and <5% risk for SBI) to parallel the YOS score cut-offs of 6 and 10.
We used SAS software version 9.4 for all statistical analyses (SAS Institute, Inc, Cary, NC).
Results
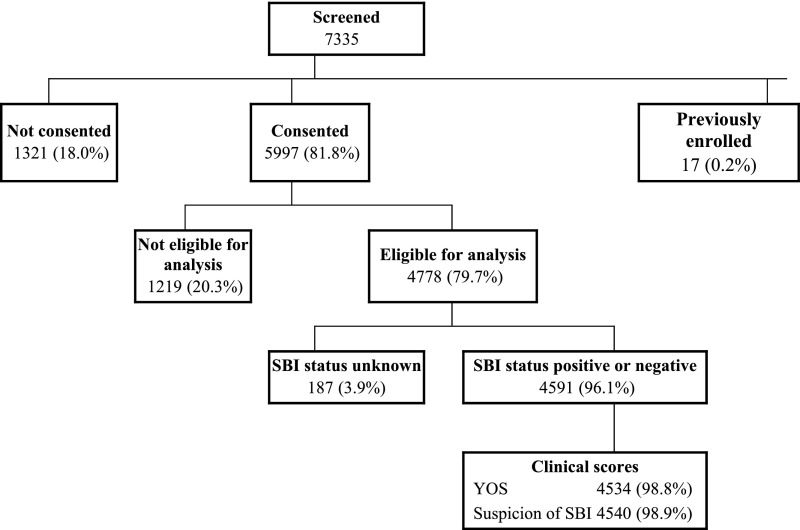
Of the 7335 screened infants, we enrolled a total of 5997 infants in the PECARN febrile infant parent study. Of these, 4778 (79.7% of enrolled) were eligible for this study, and 4591 (96.1% of eligible) had their SBI status assessed (Fig 1). Of the 4591 infants with a known SBI status, 1466 (31.9%) were ≤28 days of age and 2595 (56.5%) were boys.
FIGURE 1.
Patient enrollment.
Of the 4591 study infants, 444 infants (9.7%) had SBIs and 97 (2.1%) had invasive bacterial infections (Table 2). The 24 cases of bacterial meningitis were caused by the following pathogens: group B Streptococcus (9 cases), Escherichia coli (4 cases), Klebsiella species (2 cases), Listeria monocytogenes (2 cases) Neisseria meningitides (1 case), Enterococcus faecalis (3 cases), Enterobacter cloacae (1 case), Streptococcus pneumoniae (1 case), and Staphylococcus aureus (1 case).
TABLE 2.
Frequency of UTI, Bacteremia, and Bacterial Meningitis in Infants With Any SBI
| N (% of the 444 Infants With Any SBI) | |
|---|---|
| Any SBI | 444 (100) |
| UTI | 384 (86.5) |
| With bacteremia | 36 (8.1) |
| With bacterial meningitis | 3 (0.7) |
| With bacteremia and bacterial meningitis | 2 (0.5) |
| Invasive SBI | 97 (21.8) |
| Bacteremia | 84 (18.9) |
| With bacterial meningitis | 11 (2.5) |
| Bacterial meningitis | 24 (5.4) |
Infants could have >1 type of bacterial infection.
We then compared the characteristics of infants with and without SBIs, as well as those with and without invasive bacterial infections (Table 3). Infants with SBIs were younger (for patients with SBIs: median age: 32 days; interquartile range [IQR]: 19–47 days; compared with patients without SBIs: median age: 38 days; IQR: 26–48 days; P < .01) and were more likely to have had lumbar punctures performed (399 [89.9% of infants with SBIs] vs 3232 [77.9%] for patients without SBIs; P < .01). Five infants with bacteremia (age range: 35–59 days) did not have a lumbar puncture performed.
TABLE 3.
Characteristics of Febrile Infants With and Without SBIs
| Any SBI, N = 444, n/N (%) | Invasive Bacterial Infection, N = 97 n/N (%) | No SBI, N = 4147, n/N (%) | |
|---|---|---|---|
| Age, da | 32.0 (19.0–47.0) | 25.0 (13.0–42.0) | 38.0 (26.0–48.0) |
| Boy | 269/444 (60.6) | 50/97 (51.5) | 2326/4147 (56.1) |
| Temperature, °Ca | 38.6 (38.3–39.0) | 38.6 (38.3–39.0) | 38.3 (38.1–38.7) |
| Peripheral WBC, cells/mm3a | 13.5 (9.9–17.6) | 10.7 (7.0–15.4) | 9.6 (7.1–12.6) |
| Peripheral ANC, cells/mm3a | 6.9 (4.2–9.7) | 5.4 (3.5–8.7) | 3.1 (2.0–4.8) |
| Urine obtained | 429/444 (96.6) | 91/97 (93.8) | 3991/4147 (96.2) |
| LP performed | 399/444 (89.9) | 92/97 (94.8) | 3232/4147 (77.9) |
| CSF WBC, cells/mm3a | 5.0 (2.0–11.9) | 5.4 (2.0–22.0) | 3.0 (2.0–7.8) |
| Positive CSF Gram-stain | 9/399 (2.3) | 8/92 (8.7) | 9/3232 (0.3) |
| Initial hospitalization | 415/444 (93.5) | 94/97 (96.9) | 3037/4147 (73.2) |
ANC, absolute neutrophil count; CSF, cerebrospinal fluid.
Median (IQR).
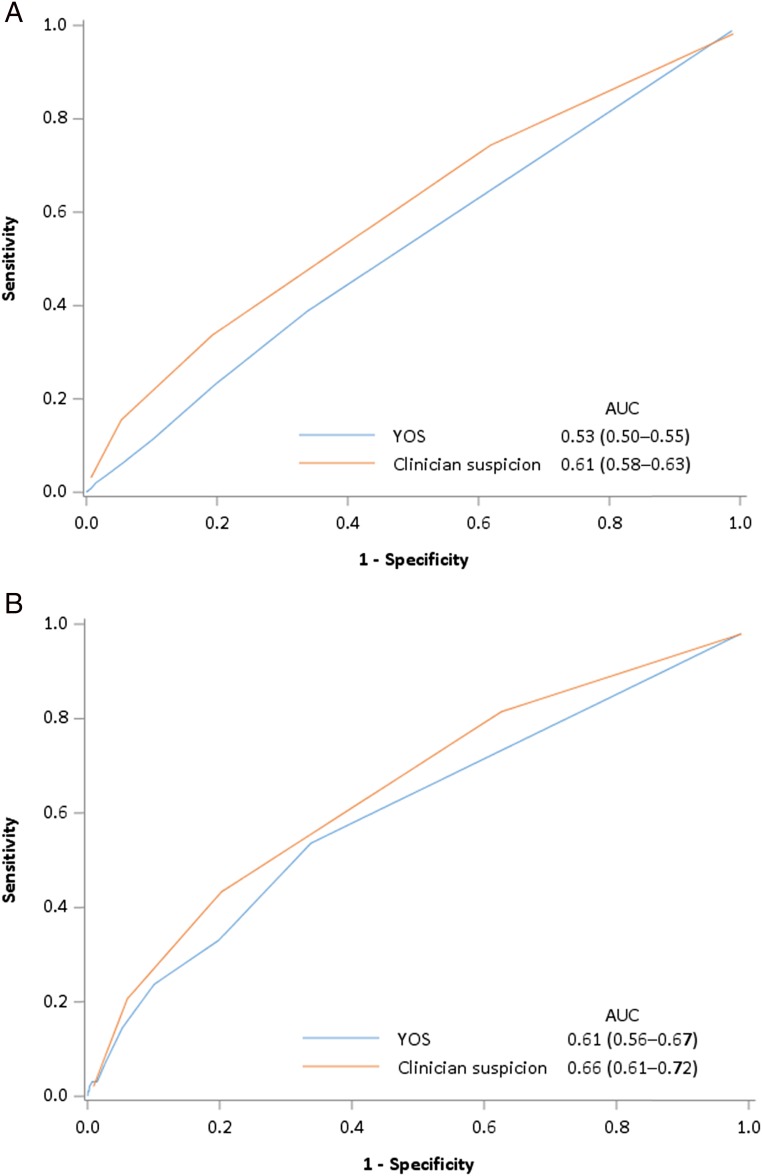
The YOS score was documented in 4534 infants (98.8% of study patients). Infants with SBIs had similar median YOS scores as those without SBIs (median YOS score of 6 for patients with and without SBIs; P = .57). The YOS score did not discriminate between infants with and without SBIs (area under the curve [AUC]: 0.53; 95% confidence interval [CI]: 0.50–0.55) and had only modest discriminative ability for invasive bacterial infections (AUC: 0.61; 95% CI: 0.56–67) (Fig 2).
FIGURE 2.
Receiver operator curves for YOS and unstructured clinician suspicion to distinguish between febrile infants with and without any SBI (A) or invasive bacterial infections (B).
Treating physicians recorded their unstructured clinician suspicion for 4540 infants (98.9% of the study infants). As clinician suspicion increased, the rate of SBI and invasive bacterial infections both increased modestly (Table 4). Unstructured clinician suspicion showed only a modest ability to distinguish infants with either an SBI (AUC: 0.61; 95% CI: 0.58–0.63) or invasive bacterial infection (AUC: 0.66; 95% CI: 0.61–0.72) from those without SBIs (Fig 2).
TABLE 4.
Clinician Suspicion and Risk of SBI
| Clinician Suspicion for SBI, % | Any SBI, N = 436, n (%) (95% CI) | Invasive Bacterial Infection, N = 95, n (%) (95% CI) | No SBI, N = 4104, n (%) (95% CI) |
|---|---|---|---|
| <1 | 106 (6.4) (5.3–7.7) | 16 (1.0) (0.6–1.6) | 1542 (93.6) (92.3–94.7) |
| 1–5 | 180 (9.3) (8.0–10.7) | 37 (1.9) (1.3–2.6) | 1760 (90.7) (89.3–92.0) |
| 6–10 | 81 (12.2) (9.8–15.0) | 22 (3.3) (2.1–5.0) | 581 (87.8) (85.0–90.2) |
| 11–50 | 55 (22.3) (17.2–28.0) | 18 (7.3) (4.4–11.3) | 192 (77.7) (72.0–82.8) |
| >50 | 14 (32.6) (19.1–48.5) | 2 (4.7) (0.6–15.8) | 29 (67.4) (51.5–80.9) |
Using standard cut-off points for the YOS score and unstructured clinician suspicion, we calculated the test performance of these scores for identifying infants with SBIs (Table 5) and with invasive bacterial infections (Supplemental Table 6). Neither the YOS score nor unstructured clinician suspicion (at either of the cut-off points) identified all 24 infants with bacterial meningitis. Nine (37.5%) infants with bacterial meningitis had perfect YOS scores of 6, and 14 (58.3%) infants had normal YOS scores of ≤10. Of the 9 infants with bacterial meningitis and perfect YOS scores, 2 were ≥29 days of age. Of the 23 infants with bacterial meningitis and an available unstructured clinician suspicion score, 2 (8.7%) infants with bacterial meningitis had an unstructured clinician suspicion score of <1%, and 10 (43.5%) infants had scores of ≤5%.
TABLE 5.
Test Characteristics of the YOS Score >6 and >10 as Well as Unstructured Clinician Suspicion ≥1% and ≥5% for the Identification of Infants With any SBI Overall Stratified by Patient Age (0–28 vs 29–60 d of Age)
| Sensitivity, % (95% CI) | Specificity, % (95% CI) | Negative Predictive Value, % (95% CI) | Likelihood Ratio +, % (95% CI) | Likelihood Ratio, −, % (95% CI) | |
|---|---|---|---|---|---|
| YOS > 6 | 0.39 (0.35–0.44) | 0.66 (0.64–0.67) | 0.91 (0.90–0.92) | 1.15 (1.01–1.30) | 0.92 (0.85–1.00) |
| 0–28 d | 0.40 (0.33–0.48) | 0.65 (0.62–0.68) | 0.88 (0.85–0.90) | 1.16 (0.96–1.40) | 0.91 (0.81–1.03) |
| 29–60 d | 0.38 (0.32–0.45) | 0.66 (0.64–0.68) | 0.93 (0.91–0.94) | 1.13 (0.96–1.33) | 0.93 (0.84–1.03) |
| YOS > 10 | 0.12 (0.09–0.15) | 0.90 (0.89–0.91) | 0.90 (0.89–0.91) | 1.12 (0.85–1.47) | 0.99 (0.95–1.02) |
| 0–28 d | 0.14 (0.09–0.20) | 0.89 (0.87–0.91) | 0.87 (0.85–0.89) | 1.30 (0.89–1.91) | 0.96 (0.91–1.02) |
| 29–60 d | 0.10 (0.06–0.14) | 0.90 (0.89–0.91) | 0.92 (0.91–0.93) | 0.96 (0.64–1.42) | 1.01 (0.96–1.05) |
| Suspicion > 1% | 0.76 (0.71–0.80) | 0.38 (0.36–0.39) | 0.94 (0.92–0.95) | 1.21 (1.14–1.29) | 0.65 (0.55–0.77) |
| 0–28 d | 0.74 (0.68–0.80) | 0.33 (0.31–0.36) | 0.90 (0.86–0.92) | 1.11 (1.01–1.22) | 0.77 (0.60–1.00) |
| 29–60 d | 0.77 (0.71–0.82) | 0.40 (0.38–0.41) | 0.95 (0.94–0.96) | 1.27 (1.18–1.37) | 0.59 (0.47–0.74) |
| Suspicion > 5% | 0.34 (0.30–0.39) | 0.80 (0.79–0.82) | 0.92 (0.91–0.93) | 1.76 (1.52–2.03) | 0.82 (0.76–0.87) |
| 0–28 d | 0.38 (0.31–0.45) | 0.76 (0.74–0.79) | 0.89 (0.87–0.91) | 1.59 (1.29–1.96) | 0.82 (0.73–0.92) |
| 29–60 d | 0.32 (0.26–0.38) | 0.82 (0.81–0.84) | 0.93 (0.92–0.94) | 1.80 (1.47–2.20) | 0.83 (0.76–0.90) |
Discussion
We prospectively assessed the YOS score and unstructured clinician suspicion to identify febrile infants ≤60 days old with SBIs presenting to the EDs in PECARN. Neither the YOS score nor unstructured clinician suspicion provided accurate discrimination between infants with either SBIs or invasive bacterial infections versus those without SBIs. Importantly, a substantial proportion of infants with bacterial meningitis were classified as low risk by both the YOS score and by unstructured clinician suspicion.
Clinical scoring systems to evaluate febrile children combine physical examination, observation, and/or clinician gestalt to determine the risk of bacterial infections. The application of a clinical score has the potential to reduce invasive diagnostic testing by identifying infants at low and high risk of bacterial infections.14,15 The YOS score was derived over 3 decades ago in a cohort of 300 febrile children 0 to 24 months of age.6 Although a few young infants were included, this score was primarily designed to stratify the risk of serious illness in older febrile infants.7 The primary outcome was serious illnesses defined as any SBI, electrolyte abnormality, bronchiolitis, or pneumonia.6 Because only 3% of children with scores ≤10 (ie, a normal YOS score) had serious illnesses, the investigators suggested a cut-off point of a YOS score ≤10 to risk stratify febrile children. In a subsequent prospective validation study of 100 febrile children <2 years of age, children with YOS scores ≤10 were substantially less likely to have serious illnesses (15% vs 64%; P < .001).7
Although the predictive ability of the YOS score has been previously evaluated, most of the studies were conducted using older infants (ie, ≥3 months of age).16–18 The YOS score was first applied to a prospective cohort of 126 febrile infants 29 to 56 days of age who presented to a single pediatric ED between 1987 and 1988.9 Of the 27 infants with serious illnesses, 20 had a normal YOS score ≤10 (sensitivity: 25.9%; 95% CI: 13.2%–44.7%). Given the substantial risk of serious illness in the “low-risk” group, the authors concluded that the YOS score, even when applied by experienced pediatricians, was not sufficiently accurate to identify significant illness in the youngest infants.9 A larger prospective study designed to evaluate outpatient management of low-risk infants enrolled 747 infants 29 to 56 days of age who presented to a single ED with temperatures of ≥38.2°C between 1987 and 1992.10 Of the 65 children with SBIs, 43 had a normal YOS score of ≤10 (sensitivity: 33.8%; 95% CI: 23.5%–46.0%). In our substantially larger multicenter cohort of febrile infants <2 months of age, we found that a normal YOS score reliably excluded SBIs.
The appropriate approach to the diagnostic evaluation of febrile infants is an area of clinical debate.19 Recent investigations have shown substantial variability in ED management of febrile infants, especially for older infants.1 Over the past several decades, the rate of invasive bacterial disease has decreased, even in the youngest infants.20 Both routine maternal perinatal antibiotic prophylaxis21 and herd immunity from widespread conjugate vaccine programs20 have reduced the incidence of invasive bacterial infections in young infants. Although infants with invasive bacterial infections require prompt initiation of parenteral antibiotics, low-risk infants may not require invasive diagnostic testing, such as lumbar punctures, or hospitalization.22
An accurate approach to risk stratification of febrile infants could assist clinician decision-making to optimize diagnostic evaluation and therapeutic management of these infants. The current study demonstrates that neither structured clinical scoring via the YOS score nor unstructured clinician suspicion were sufficiently sensitive in identifying young febrile infants with SBIs. Highly accurate multivariate predictive models that incorporate newer laboratory tests and biomarkers, such as procalitonin,4,23–25 are needed to identify low-risk infants for whom unnecessary invasive diagnostic testing, empirical antibiotics, and hospitalization may be obviated.19 Looking ahead, host expression patterns, such as RNA biosignatures, provide a new diagnostic paradigm,5,26 although these tools will require additional refinement and validation before introduction to clinical practice.
Our study has several limitations. First, we enrolled a convenience sample of infants based on the availability of research staff at the participating sites. However, the SBI rate was similar in the missed eligible patients (data not shown), suggesting our enrollment was representative.5 Second, because we excluded infants who were critically ill, our findings should not be applied to those infants. Third, the performance of lumbar punctures was at the discretion of the treating clinicians, and we therefore could have potentially missed identifying bacterial meningitis in some infants. Although we performed clinical follow-up for all infants who did not have lumbar punctures performed, we ultimately had to exclude those infants without available follow-up information. Fourth, we did not assess the reliability of the YOS score assessment by either measuring clinicians’ proficiency or measuring interrater reliability. However, we did provide standardized clinician training to reduce variability in the application of the YOS. Nevertheless, the YOS score has been available in practice for over 30 years, and most pediatric emergency physicians are familiar with it. Fifth, because the same clinicians assigned unstructured clinician suspicion after applying the YOS score, we cannot determine if the structured assessment of the YOS score introduced biases in the assessment. Finally, our study included a relatively small number of infants with bacterial meningitis, reducing our certainty around the score performance for these infants. In the current era of widespread conjugate vaccines and maternal antibiotic prophylaxis, bacterial meningitis has become rare, even in the youngest infants.20,21
Conclusions
In our large prospective cohort of febrile infants <60 days of age, neither the YOS score nor unstructured clinician suspicion reliably identified febrile infants with SBIs, including bacteremia and bacterial meningitis, and should therefore not be relied on to guide clinician decision-making regarding the management of young febrile infants.
Acknowledgments
The participating centers and investigators in alphabetical order by center are as follows: Elizabeth C. Powell, MD, MPD, Ann & Robert H. Lurie Children's Hospital; Deborah A. Levine, MD, Michael G. Tunik, MD, Bellevue Hospital Center; Lise E. Nigrovic, MD, MPH, Boston Children's Hospital; Genie Roosevelt, MD, Children's Hospital of Colorado; Prashant Mahajan, MD, MPH, MBA, Children's Hospital of Michigan; Elizabeth R. Alpern, MD, MSCE, Children's Hospital of Philadelphia; Lorin Browne, DO, Mary Saunders, MD, Children's Hospital of Wisconsin; Shireen M. Atabaki, MD, MPH, Children's National Medical Center; James G. Linakis, MD, PHD, Hasbro Children's Hospital; John D. Hoyle, Jr., MD, Helen DeVos Children's Hospital; Dominic Borgialli, DO, MPH, Hurley Medical Center; Stephen Blumberg, MD, Ellen F. Crain, MD, PhD, Jacobi Medical Center; Jennifer Anders, MD, Johns Hopkins Children's Center; Bema Bonsu, MD, Daniel M. Cohen, MD, Nationwide Children's Hospital; Jonathan E. Bennett, MD, Nemours/Alfred I. DuPont Hospital for Children; Peter S. Dayan, MD, MSc, New York Presbyterian-Morgan Stanley Children's Hospital; Richard Greenberg, MD, Primary Children's Medical Center; David M. Jaffe, MD, Jared Muenzer, MD, St. Louis Children's Hospital; Andrea T. Cruz, MD, MPH, Charles Macias, MD, Texas Children's Hospital; Nathan Kuppermann, MD, MPH, Leah Tzimenatos, MD, University of California Davis Medical Center; Rajender Gattu, MD, University of Maryland; Alexander J. Rogers, MD, University of Michigan; Anne Brayer, MD, University of Rochester; and Kathleen Lillis, MD, Women and Children's Hospital of Buffalo.
The authors thank the research coordinators in PECARN, the Microarray Core at Baylor Institute for Immunology Research; Walt Schalick, MD, PhD, bioethicist consultant (no funding), Department of Pediatrics, Medical College of Wisconsin; Phuong Nguyen, lab assistant (no funding), Microarray Core at Baylor Institute for Immunology Research.
Glossary
- AUC
area under the curve
- CFU
colony-forming unit
- CI
confidence interval
- ED
emergency department
- IQR
interquartile range
- PECARN
Pediatric Emergency Care Applied Research Network
- SBI
serious bacterial infection
- UTI
urinary tract infection
- WBC
white blood cell
- YOS
Yale Observation Scale
Footnotes
Dr Nigrovic helped conceive and design the study, supervised patient enrollment and data abstraction, conducted the primary data analysis, and drafted the initial manuscript; Dr Mahajan conceived and designed the study, obtained funding, supervised patient enrollment and data abstraction, contributed to data analysis, and revised the manuscript; Drs Blumberg, Browne, Linakis, Ruddy, Bennett, Rogers, Tzimenatos, Powell, and Alpern supervised patient enrollment and data abstraction, contributed to study design, and revised the manuscript; Dr Casper had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; Dr Ramilo conceptualized and designed the study, obtained funding, and revised the manuscript; Dr Kuppermann conceived and designed the study, obtained funding, supervised patient enrollment and data abstraction, conducted the primary data analysis, and revised the manuscript; and all authors approved the final manuscript.
FINANCIAL DISCLOSURE: Octavio Ramilo, MD, Division of Pediatric Infectious Diseases and Center for Vaccines and Immunity, Nationwide Children's Hospital and The Ohio State University, reports personal fees from HuMabs, Abbvie, Janssen, Medimmune and Regeneron, and grants from Janssen. All these fees and grants are not related to the current work.
FUNDING: The research reported in this publication was supported in part by grant H34MCO8509 from Health Resources and Services Administration, Emergency Services for Children and by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R01HD062477. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This project is also supported in part by the Health Resources and Services Administration (HRSA), Maternal and Child Health Bureau (MCHB), Emergency Medical Services for Children (EMSC) Network Development Demonstration Program under cooperative agreements U03MC00008, U03MC00001, U03MC00003, U03MC00006, U03MC00007, U03MC22684, and U03MC22685. This information or content and conclusions are those of the author and should not be construed as the official position or policy of, nor should any endorsements be inferred by HRSA, HHS or the US Government. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found online at www.pediatrics.org/cgi/doi/10.1542/peds.2017-1100.
References
- 1.Aronson PL, Thurm C, Williams DJ, et al. ; Febrile Young Infant Research Collaborative . Association of clinical practice guidelines with emergency department management of febrile infants ≤56 days of age. J Hosp Med. 2015;10(6):358–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pantell RH, Newman TB, Bernzweig J, et al. Management and outcomes of care of fever in early infancy. JAMA. 2004;291(10):1203–1212 [DOI] [PubMed] [Google Scholar]
- 3.Bachur RG, Harper MB. Predictive model for serious bacterial infections among infants younger than 3 months of age. Pediatrics. 2001;108(2):311–316 [DOI] [PubMed] [Google Scholar]
- 4.Milcent K, Faesch S, Gras-Le Guen C, et al. Use of procalcitonin assays to predict serious bacterial infection in young febrile infants. JAMA Pediatr. 2016;170(1):62–69 [DOI] [PubMed] [Google Scholar]
- 5.Mahajan P, Kuppermann N, Mejias A, et al. ; Pediatric Emergency Care Applied Research Network (PECARN) . Association of RNA biosignatures with bacterial infections in febrile infants aged 60 days or younger. JAMA. 2016;316(8):846–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCarthy PL, Sharpe MR, Spiesel SZ, et al. Observation scales to identify serious illness in febrile children. Pediatrics. 1982;70(5):802–809 [PubMed] [Google Scholar]
- 7.McCarthy PL, Lembo RM, Baron MA, Fink HD, Cicchetti DV. Predictive value of abnormal physical examination findings in ill-appearing and well-appearing febrile children. Pediatrics. 1985;76(2):167–171 [PubMed] [Google Scholar]
- 8.Bergman DA, Mayer ML, Pantell RH, Finch SA, Wasserman RC. Does clinical presentation explain practice variability in the treatment of febrile infants? Pediatrics. 2006;117(3):787–795 [DOI] [PubMed] [Google Scholar]
- 9.Baker MD, Avner JR, Bell LM. Failure of infant observation scales in detecting serious illness in febrile, 4- to 8-week-old infants. Pediatrics. 1990;85(6):1040–1043 [PubMed] [Google Scholar]
- 10.Baker MD, Bell LM, Avner JR. Outpatient management without antibiotics of fever in selected infants. N Engl J Med. 1993;329(20):1437–1441 [DOI] [PubMed] [Google Scholar]
- 11.Mahajan P, Kuppermann N, Suarez N, et al. ; Febrile Infant Working Group for the Pediatric Emergency Care Applied Research Network (PECARN) . RNA transcriptional biosignature analysis for identifying febrile infants with serious bacterial infections in the emergency department: a feasibility study. Pediatr Emerg Care. 2015;31(1):1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine DA, Platt SL, Dayan PS, et al. ; Multicenter RSV-SBI Study Group of the Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics . Risk of serious bacterial infection in young febrile infants with respiratory syncytial virus infections. Pediatrics. 2004;113(6):1728–1734 [DOI] [PubMed] [Google Scholar]
- 13.Hoberman A, Wald ER. Urinary tract infections in young febrile children. Pediatr Infect Dis J. 1997;16(1):11–17 [DOI] [PubMed] [Google Scholar]
- 14.Thompson M, Van den Bruel A, Verbakel J, et al. Systematic review and validation of prediction rules for identifying children with serious infections in emergency departments and urgent-access primary care. Health Technol Assess. 2012;16(15):1–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van den Bruel A, Haj-Hassan T, Thompson M, Buntinx F, Mant D; European Research Network on Recognising Serious Infection investigators . Diagnostic value of clinical features at presentation to identify serious infection in children in developed countries: a systematic review. Lancet. 2010;375(9717):834–845 [DOI] [PubMed] [Google Scholar]
- 16.Teach SJ, Fleisher GR; Occult Bacteremia Study Group . Efficacy of an observation scale in detecting bacteremia in febrile children three to thirty-six months of age, treated as outpatients. J Pediatr. 1995;126(6):877–881 [DOI] [PubMed] [Google Scholar]
- 17.Yilmaz HL, Yildizdas RD, Alparslan N, Ozcan K, Yaman A, Kibar F. Screening tools for bacteraemia in a selected population of febrile children. Ann Acad Med Singapore. 2008;37(3):192–199 [PubMed] [Google Scholar]
- 18.Bang A, Chaturvedi P. Yale observation scale for prediction of bacteremia in febrile children. Indian J Pediatr. 2009;76(6):599–604 [DOI] [PubMed] [Google Scholar]
- 19.Kuppermann N, Mahajan P. Role of serum procalcitonin in identifying young febrile infants with invasive bacterial infections: one step closer to the holy grail? JAMA Pediatr. 2016;170(1):17–18 [DOI] [PubMed] [Google Scholar]
- 20.Poehling KA, Talbot TR, Griffin MR, et al. Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. JAMA. 2006;295(14):1668–1674 [DOI] [PubMed] [Google Scholar]
- 21.Schrag SJ, Zywicki S, Farley MM, et al. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N Engl J Med. 2000;342(1):15–20 [DOI] [PubMed] [Google Scholar]
- 22.Pingree EW, Kimia AA, Nigrovic LE. The effect of traumatic lumbar puncture on hospitalization rate for febrile infants 28 to 60 days of age. Acad Emerg Med. 2015;22(2):240–243 [DOI] [PubMed] [Google Scholar]
- 23.Maniaci V, Dauber A, Weiss S, Nylen E, Becker KL, Bachur R. Procalcitonin in young febrile infants for the detection of serious bacterial infections. Pediatrics. 2008;122(4):701–710 [DOI] [PubMed] [Google Scholar]
- 24.Dauber A, Weiss S, Maniaci V, Nylen E, Becker KL, Bachur R. Procalcitonin levels in febrile infants after recent immunization. Pediatrics. 2008;122(5). Available at: www.pediatrics.org/cgi/content/full/122/5/e1119 [DOI] [PubMed] [Google Scholar]
- 25.Gomez B, Mintegi S, Bressan S, Da Dalt L, Gervaix A, Lacroix L; European Group for Validation of the Step-by-Step Approach . Validation of the “Step-by-Step” approach in the management of young febrile infants. Pediatrics. 2016;138(2):e20154381. [DOI] [PubMed] [Google Scholar]
- 26.Herberg JA, Kaforou M, Wright VJ, et al. ; IRIS Consortium . Diagnostic test accuracy of a 2-transcript host RNA signature for discriminating bacterial vs viral infection in febrile children. JAMA. 2016;316(8):835–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.