This case report tests the effectiveness of short-acting corticotropin for refractory nephrotic syndrome, and through translational research, unravels the puzzle of acquired resistance to corticotropin therapy.
Abstract
There is increasing evidence supporting the use of corticotropin as an alternative treatment of refractory proteinuric glomerulopathies. The efficacy of short-acting corticotropin, however, remains unknown and was tested here in an adolescent with steroid-dependent nephrotic syndrome caused by minimal change disease. After developing Cushing syndrome and recently being afflicted with severe cellulitis, the patient was weaned off all immunosuppressants, including corticosteroids. This resulted in a relapse of generalized anasarca, associated with massive proteinuria and hypoalbuminemia. Subsequently, mono-therapy with short-acting animal-derived natural corticotropin was initiated and resulted in a rapid response, marked by substantial diuresis, reduction in body weight, and partial remission of proteinuria. Ten days later, the patient developed mild skin rash and subcutaneous nodules at injection sites. A relapse followed despite doubling the dose of corticotropin, consistent with delayed-onset resistance to treatment. Immunoblot-based antibody assay revealed de novo formation of antibodies in the patient’s serum that were reactive to the natural corticotropin. In cultured melanoma cells known to express abundant melanocortin receptors, addition of the patient’s serum strikingly mitigated dendritogenesis and cell signaling triggered by natural corticotropin, denoting neutralizing properties of the newly formed antibodies. Collectively, short-acting natural corticotropin seems effective in steroid-dependent nephrotic syndrome. De novo formation of neutralizing antibodies is likely responsible for acquired resistance to corticotropin therapy. The proof of concept protocols established in this study to examine the anticorticotropin neutralizing antibodies may aid in determining the cause of resistance to corticotropin therapy in future studies.
Intractable nephrotic syndrome continues to be a formidable challenge for clinical practice.1 A growing body of clinical and experimental evidence supports the use of corticotropin as an alternative treatment of proteinuric glomerulopathies.2,3 Corticotropin1-39 is an important component of the hypothalamic-pituitary-adrenal axis and plays a pivotal role in stress response.4 In addition, corticotropin is also a key endogenous agonist of the melanocortin hormone system, which regulates a diverse array of physiologic and neuroendocrinoimmunological functions.3,5 As the first US Food and Drug Administration–approved treatment of nephrotic syndrome, corticotropin was used in the 1950s for childhood nephrotic syndrome but fell out of favor with the advent of oral glucocorticoids.2,3,6 However, recent clinical observations demonstrating the successful use of corticotropin in steroid-resistant nephrotic glomerulopathies6–10 suggest a unique antiproteinuric activity of corticotropin that is steroidogenic-independent and may be attributable to its melanocortinergic activity.2,3 This has rekindled interest in corticotropin therapy for proteinuric glomerulopathies.2
Existing regimens of corticotropin therapy for glomerulopathies have solely used the sustained-release long-acting repository corticotropin, which is either extremely costly or unavailable in a number of regions and countries.11 In view of these drawbacks, we attempted to test the efficacy of short-acting corticotropin, which is inexpensive as an off-patent pharmaceutical and has been approved worldwide for corticotropin stimulation tests.11
Case Presentation
A 21-year-old adolescent male presented to the First Affiliated Hospital of Zhengzhou University in August 2015 with generalized anasarca. The patient first presented with nephrotic syndrome 5 years earlier at age 16 with a diagnosis of minimal change disease proven by kidney biopsy. In addition to benazepril, the patient had been treated with prednisone in combination with other immunosuppressants, including tacrolimus or mycophenolate mofetil (Supplemental Fig 4). Since the disease onset, the patient had experienced multiple relapses of nephrotic syndrome, which occurred during or shortly after the tapering of prednisone (Supplemental Fig 4). Two weeks before his presentation, all immunosuppressants, including prednisone, had been discontinued due to skin infections. A fulminant relapse of nephrotic syndrome subsequently ensued.
At presentation, the patient exhibited signs of Cushing syndrome, consistent with his long-term prednisone exposure. The cellulitis on the left upper thigh had been successfully controlled and recovered after intravenous infusion with penicillin G benzathine (4.8 million units/day) for 5 days. Laboratory testing showed massive proteinuria (urinary protein to creatinine ratio, 19.8 g/g), hypoalbuminemia (serum albumin, 15.2 g/L), and a serum creatinine level of 91 µmol/L (corresponding to estimated glomerular filtration rate of 103 mL/min/1.73 m2 as calculated using the CKD-EPI [CKD Epidemiology Collaboration]).12 A diagnosis of relapsing nephrotic syndrome was made.
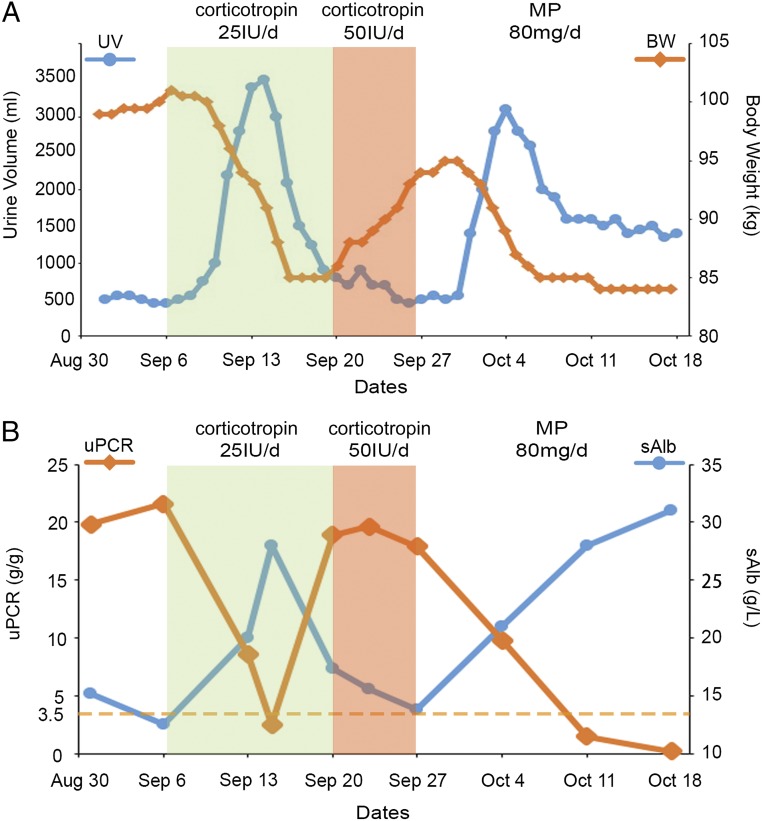
In view of the unique antiproteinuric effect of corticotropin in refractory nephrotic glomerulopathies as demonstrated by recent studies,6–10,13 corticotropin monotherapy was planned. Unfortunately, repository corticotropin is not available in the region where the patient was treated. Rather, an approved short-acting formulation of animal-derived natural corticotropin (Shanghai The First Biochemical & Pharmaceutical Co Ltd, Shanghai, China) was available14 and was used after the Institutional Review Board approved the proposal and the patient provided written informed consent. The initial regimen consisted of subcutaneous injections of 25 IU of short-acting natural corticotropin given daily at 9 am with reference to the Columbia corticotropin gel therapy (80 IU twice a week) regimen for nephrotic glomerulopathies.8–10 Three days after starting corticotropin treatment, the patient experienced a progressive reduction of body weight and a marked increase in urine output that peaked on day 7 (Fig 1A). In parallel, proteinuria, indicated by urinary protein to creatinine ratios, partially remitted, and serum albumin levels improved (Fig 1B). On day 14, the patient’s urine volume decreased, and he again developed progressive body weight gain along with an apparent rebound of proteinuria and worsening of hypoalbuminemia. The dose of corticotropin was doubled to 50 IU per day for another week with no noticeable improvement (Fig 1). Subsequently, corticotropin therapy was discontinued, and the patient’s condition was successfully controlled with daily intravenous infusions with methylprednisolone (80 mg/day) for another 2 weeks (Fig 1). Afterward, the patient maintained complete remission with oral prednisone (60 mg every other day) plus tacrolimus (2 mg/day). Over the entire disease course, the patient was continuously treated with benazepril (20 mg/day) and had normal blood pressure (130/80 mm Hg) and stable kidney function with estimated glomerular filtration rate ranging between 103 and 130 mL/min/1.73 m2. Corticotropin therapy had been well tolerated without any side effects except mild skin rash and subcutaneous nodules at injection sites that presented several days before treatment resistance.
FIGURE 1.
Course of corticotropin therapy in the study patient with steroid-dependent nephrotic syndrome. The patient was converted to monotherapy with short-acting natural corticotropin after relapse of nephrotic syndrome. The changes in body weight (BW), urine volume (UV), urinary protein to creatinine ratios (uPCR), and serum albumin levels (sAlb) were documented and plotted (A and B). After a rapid response and partial remission of proteinuria in the first 2 weeks of corticotropin therapy (25 IU/day, subcutaneous injection), the patient exhibited delayed-onset resistance, and the dose of corticotropin was increased to 50 IU/day for another week without any improvement. Thereafter, corticotropin therapy was terminated and the patient was treated with methylprednisolone (MP; 80 mg/day, intravenous infusion), which induced a complete remission in 2 weeks. Corticotropin therapy was well tolerated without any side effects except mild skin rash and subcutaneous nodules that presented 10 days after corticotropin treatment. Throughout the disease course, the patient was continuously on benazepril (20 mg/day) and had a stable kidney function with estimated glomerular filtration rate ranging between 103 and 130 mL/min/1.73 m2.
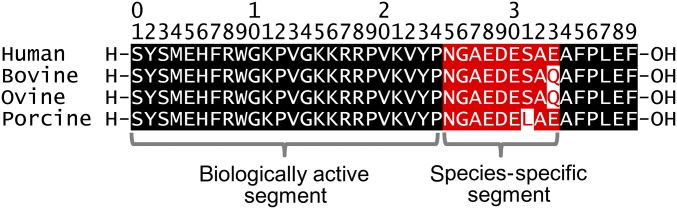
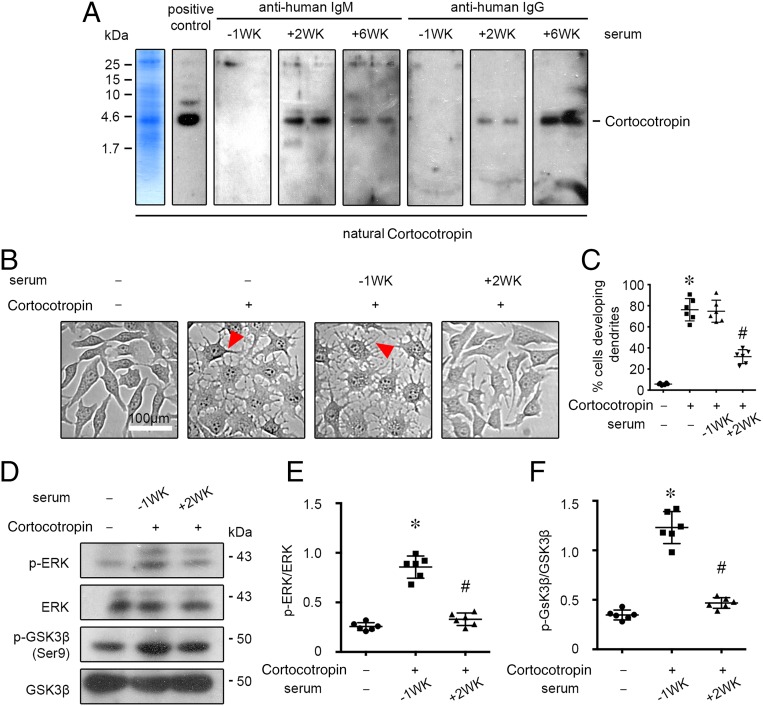
Animal-derived corticotropin1-39 has been shown to be antigenic in humans.15–20 Although the biologically active N-terminal 24-amino-acid segment is highly conserved and actually identical in all species, the C-terminal amino acids occupying positions 25 to 33 vary among species and are the most likely sources of the immunologic response16 (Fig 2). To determine if the patient’s resistance to corticotropin treatment was associated with possible immune reactions, as implied by dermatological signs, the patient’s serum was analyzed by the immunoblot-based antibody assay (Fig 3A). The short-acting formulation of natural corticotropin used for this patient has been prepared from pituitary extracts of mixed bovine, ovine, and porcine origins and contains a mixture of melanocortin peptides, including the predominant corticotropin1-39, as indicated by gradient sodium dodecyl sulfate polyacrylamide gel electrophoresis of the drug (Fig 3A, far left panel). Natural corticotropin was subjected to immunoblot analysis by incubating the blots overnight with a commercial rabbit anti-corticotropin antibody as positive control or with the patient’s serum. The blots were then developed by using an anti-rabbit secondary antibody or anti-human immunoglobulin (Ig)M or IgG antibodies. Shown in Fig 3A, blots developed by using serum collected 2 weeks after starting corticotropin treatment revealed abundant IgM antibodies and minimal IgG antibodies that were both reactive to natural corticotropin. Blots developed by using serum collected 6 weeks after starting corticotropin treatment revealed minimal IgM antibodies but abundant IgG antibodies against corticotropin. The IgM and IgG antibodies reactive to corticotropin were likely newly formed because blots developed by using serum collected 1 week before corticotropin treatment revealed no bands. The functionality of the newly developed antibodies was examined next (Fig 3 B–F). As a quintessential melanocortin peptide, corticotropin is a potent activator of virtually all of the 5 types of melanocortin receptors and downstream cell signaling cascades, including extracellular signal-regulated kinase (ERK) and glycogen synthase kinase (GSK)3β pathways as well as cyclic adenosine monophosphate response.3,5 In cultured B16 melanoma cells, which are known to intensely express MC1R,21 natural corticotropin potently induced generation of dendrites (Fig 3 B and C), a cellular morphogenic effect triggered by cyclic adenosine monophosphate responses.22 This effect was markedly diminished when corticotropin was premixed with the patient’s serum collected 2 weeks after starting corticotropin treatment but completely retained when corticotropin was premixed with the patient’s serum collected 1 week before corticotropin treatment. Likewise, the patient’s serum collected 2 weeks after corticotropin treatment evidently abrogated the corticotropin triggered phosphorylation of ERK and GSK3β, as shown by immunoblot analysis of melanoma cell lysates (Fig 3 D–F). Collectively, the newly formed antibodies against corticotropin are likely able to neutralize the biological activities of natural corticotropin.
FIGURE 2.
Homology in the amino acid sequence of natural bovine, ovine, porcine, and human corticotropin, showing the highly conserved biologically active segment (corticotropin1-24) as well as the species-specific segment (corticotropin25-33), which is the most likely source of the immunologic response. Amino acid residues colored black indicate the positions at which the amino acid sequence is homologous among various species. Those colored red indicate species-specific positions that likely harbor the immunologically active sites. Red letters indicate the variable amino acid residues that are different from those in human corticotropin.
FIGURE 3.
Short-acting natural corticotropin therapy results in de novo formation of corticotropin-reactive neutralizing antibodies. A, The short-acting formulation of natural corticotropin used for the study patient has been prepared from purified pituitary extracts of mixed bovine, ovine, and porcine origins and contains a mixture of melanocortin peptides, including the predominant corticotropin, as indicated by gradient sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) of the drug (left panel). Natural corticotropin was fractionated on SDS-PAGE gels, transferred to nitrocellulose membrane, and subjected to immunoblotting overnight with a commercial rabbit anticorticotropin antibody (positive control) or with the patient’s sera (1:1000 dilution), which were collected 1 week before (–1WK) or 2 or 6 weeks (2WK or 6WK) after starting corticotropin therapy. The blots were then developed by using an anti-rabbit secondary antibody or anti-human IgM or IgG antibodies. Bands for corticotropin were indicated. B, B16 melanoma cells were treated for 24 hours with vehicle, natural corticotropin (100 mIU/mL), or corticotropin (100 mIU/mL) premixed with an equal volume of sera collected 1 week before (–1WK) or 2 weeks (2WK) after starting corticotropin therapy. Representative phase contrast micrographs demonstrate the morphogenic effect of corticotropin in melanoma cells, highlighting the newly formed dendrites (arrowheads). C, Absolute counting of the number of cells with dendrites expressed as a percentage of the total number of cells. The cell culture experiment was repeated 6 times (n = 6); *P < .01 versus control group; #P < .01 versus ACTH group or –1WK + corticotropin group. D, Cell lysates were collected after different treatments and processed for immunoblot analysis for phosphorylated ERK (p-ERK), total ERK, phosphorylated GSK3β (p-GSK3β), and total GSK3β. E, Arbitrary units of p-ERK/ERK ratios and F, p-GSK3β/GSK3β ratios expressed as immunoblot densitometric ratios of the molecules as folds of the vehicle treated group. The cell culture experiment was repeated 6 times (n = 6); *P < .01 versus control group; #P < .01 versus –1WK + corticotropin group.
Discussion
To the best of our knowledge, this is the first report to evaluate the efficacy of short-acting corticotropin in refractory nephrotic syndrome.2 Short-acting corticotropin has been used in an off-label fashion and has successfully treated a variety of diseases, including multiple sclerosis and infantile spasms.11 Its effectiveness in kidney disease has barely been tested. In this study, our patient responded to short-acting corticotropin within several days. This is in stark contrast to the slow action of repository corticotropin, either synthetic6,7,23 or natural,8–10,13 on proteinuria. This disparity may be attributable to varying responses of different underlying glomerulopathies to corticotropin therapy but is more likely due to the distinct pharmacokinetics of the different formulations.
Formation of neutralizing antibodies is often implicated in resistance to treatment with biologics.24 The natural corticotropin used in this study was purified from pituitary extracts of mixed bovine, ovine, and porcine origins and thus may possess a considerable antigenicity. Nevertheless, even when using biologics of porcine origin, which are assumed to have minimal antigenicity,24 development of neutralizing antibodies have been reported.25 After prolonged treatment with depot porcine corticotropin to expedite recovery from pituitary-adrenal suppression, 8 of 9 patients developed corticotropin-specific antibodies.17 In another study, 13 of 19 children developed corticotropin-reactive antibodies after porcine corticotropin therapy for a variety of diseases, including asthma, Still disease, and nephrotic syndrome.18 In patients with rheumatoid arthritis, approximately one-third were found to have corticotropin antibodies after porcine corticotropin therapy.15 Even in the case of synthetic corticotropin1-24, which lacks the immunologically active sites of the natural corticotropin1-39,16 the long-acting formulation has also been demonstrated to be antigenic in man, resulting in formation of corticotropin-specific antibodies in 12 of 38 patients with rheumatoid arthritis,26 10 of 22 patients with asthma,27 and 10 of 13 patients with autoimmune Addison disease.28 So far, it remains uncertain whether the neutralizing antibodies against exogenous corticotropin affect the biophysiologic activities of native corticotropin produced by the patients per se. However, there is evidence that antibodies raised against corticotropin are able to bind to the biologically active corticotropin1-24 segment15,18 and diminish the lipolytic effect of corticotropin in vitro,20 thus possessing the potential to reduce the therapeutic potency and effectiveness of corticotropin. In addition, modification of circulating corticotropin concentrations via sequestration17 or increased enzymatic destruction19 mediated by circulating anti-corticotropin antibodies may also contribute to treatment failure or resistance.
In aggregate, our findings may open up a new avenue for further exploration of short-acting corticotropin as a novel, pragmatic, and affordable therapeutic modality for refractory proteinuric glomerulopathies. The proof-of-concept protocols established here to examine anticorticotropin neutralizing antibodies may aid in determining the cause of resistance to corticotropin therapy in future studies.
Acknowledgments
The authors are indebted to Drs Minglei Lu and Sijie Zhou for their technical support during the cell culture study.
Glossary
- ERK
extracellular signal-regulated kinase
- GSK
glycogen synthase kinase
- Ig
immunoglobulin
Footnotes
Drs P. Wang and Gong conceptualized and designed the study; Dr Gong drafted the manuscript; Drs Liu and Brem carried out the initial analyses and revised the manuscript; Drs Zhang and Y. Wang coordinated and supervised data collection and critically reviewed the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported in part by the Foundation for Health, the Medicine Science Foundation of Henan (grant 201601002), and by the US National Institutes of Health (grant DK092485). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Greenbaum LA, Benndorf R, Smoyer WE. Childhood nephrotic syndrome—current and future therapies. Nat Rev Nephrol. 2012;8(8):445–458 [DOI] [PubMed] [Google Scholar]
- 2.Gong R. The renaissance of corticotropin therapy in proteinuric nephropathies. Nat Rev Nephrol. 2011;8(2):122–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gong R. Leveraging melanocortin pathways to treat glomerular diseases. Adv Chronic Kidney Dis. 2014;21(2):134–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dores RM. Adrenocorticotropic hormone, melanocyte-stimulating hormone, and the melanocortin receptors: revisiting the work of Robert Schwyzer: a thirty-year retrospective. Ann N Y Acad Sci. 2009;1163:93–100 [DOI] [PubMed] [Google Scholar]
- 5.Gantz I, Fong TM. The melanocortin system. Am J Physiol Endocrinol Metab. 2003;284(3):E468–E474 [DOI] [PubMed] [Google Scholar]
- 6.Berg AL, Nilsson-Ehle P, Arnadottir M. Beneficial effects of ACTH on the serum lipoprotein profile and glomerular function in patients with membranous nephropathy. Kidney Int. 1999;56(4):1534–1543 [DOI] [PubMed] [Google Scholar]
- 7.Berg AL, Arnadottir M. ACTH-induced improvement in the nephrotic syndrome in patients with a variety of diagnoses. Nephrol Dial Transplant. 2004;19(5):1305–1307 [DOI] [PubMed] [Google Scholar]
- 8.Hogan J, Bomback AS, Mehta K, et al. . Treatment of idiopathic FSGS with adrenocorticotropic hormone gel. Clin J Am Soc Nephrol. 2013;8(12):2072–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bomback AS, Tumlin JA, Baranski J, et al. . Treatment of nephrotic syndrome with adrenocorticotropic hormone (ACTH) gel. Drug Des Devel Ther. 2011;5:147–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bomback AS, Canetta PA, Beck LH Jr, Ayalon R, Radhakrishnan J, Appel GB. Treatment of resistant glomerular diseases with adrenocorticotropic hormone gel: a prospective trial. Am J Nephrol. 2012;36(1):58–67 [DOI] [PubMed] [Google Scholar]
- 11.Gettig J, Cummings JP, Matuszewski K. H.p. Acthar gel and cosyntropin review: clinical and financial implications. P&T. 2009;34(5):250–257 [PMC free article] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hladunewich MA, Cattran D, Beck LH, et al. . A pilot study to determine the dose and effectiveness of adrenocorticotrophic hormone (H.P. Acthar® Gel) in nephrotic syndrome due to idiopathic membranous nephropathy. Nephrol Dial Transplant. 2014;29(8):1570–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jia F, Jiang H, Du L, Li N, Sun J, Niu C. An effective initial polytherapy for children with West syndrome. Neural Regen Res. 2013;8(17):1623–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glass D, Daly JR. Development of antibodies during long-term therapy with corticotrophin in rheumatoid arthritis. I. Porcine ACTH. Ann Rheum Dis. 1971;30(6):589–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imura H, Sparks LL, Grodsky GM, Forsham PH. Immunologic studies of adrenocorticotropic hormone (ACTH): dissociation of biologic and immunologic activities. J Clin Endocrinol Metab. 1965;25(10):1361–1369 [DOI] [PubMed] [Google Scholar]
- 17.Fleischer N, Abe K, Liddle GW, Orth DN, Nicholson WE. ACTH antibodies in patients receiving depot porcine ACTH to hasten recovery from pituitary-adrenal suppression. J Clin Invest. 1967;46(2):196–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landon J, Friedman M, Greenwood FC. Antibodies to corticotrophin and their relation to adrenal function in children receiving corticotrophin therapy. Lancet. 1967;1(7491):652–655 [DOI] [PubMed] [Google Scholar]
- 19.Norman AP, Sanders S. Effect of corticotorphin on skeletal maturation and linear growth in six patients with severe asthma. Lancet. 1969;1(7589):287–289 [DOI] [PubMed] [Google Scholar]
- 20.Felber JP, Ashcroft SH, Villanueva A, Vannotti A. Antibodies to synthetic corticotrophin. Nature. 1966;211(5049):654–655 [DOI] [PubMed] [Google Scholar]
- 21.Smith AG, Luk N, Newton RA, Roberts DW, Sturm RA, Muscat GE. Melanocortin-1 receptor signaling markedly induces the expression of the NR4A nuclear receptor subgroup in melanocytic cells. J Biol Chem. 2008;283(18):12564–12570 [DOI] [PubMed] [Google Scholar]
- 22.Scott G, Leopardi S. The cAMP signaling pathway has opposing effects on Rac and Rho in B16F10 cells: implications for dendrite formation in melanocytic cells. Pigment Cell Res. 2003;16(2):139–148 [DOI] [PubMed] [Google Scholar]
- 23.Ponticelli C, Passerini P, Salvadori M, et al. . A randomized pilot trial comparing methylprednisolone plus a cytotoxic agent versus synthetic adrenocorticotropic hormone in idiopathic membranous nephropathy. Am J Kidney Dis. 2006;47(2):233–240 [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg AS. Immunogenicity of biological therapeutics: a hierarchy of concerns. Dev Biol (Basel). 2003;112:15–21 [PubMed] [Google Scholar]
- 25.Schellekens H. The immunogenicity of therapeutic proteins. Discov Med. 2010;9(49):560–564 [PubMed] [Google Scholar]
- 26.Glass D, Nuki G, Daly JR. Development of antibodies during long-term therapy with corticotrophin in rheumatoid arthritis. II. Zinc tetracosactrin (Depot Synacthen). Ann Rheum Dis. 1971;30(6):593–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ratcliffe JG, Pritchard M, El-Shaboury AH. The production of antibodies to porcine corticotrophin and to Synacthen. J Endocrinol. 1969;43(2):l–li [PubMed] [Google Scholar]
- 28.Gan EH, MacArthur K, Mitchell AL, Joshi A, Crock P, Pearce SH. Spontaneous and tetracosactide-induced anti-ACTH antibodies in man. Clin Endocrinol (Oxf). 2016;84(4):489–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.