Same-day access to EC is examined nationally by using mystery callers. Persistent barriers remain despite regulatory changes that started in 2013, which made it OTC.
Abstract
BACKGROUND:
Levonorgestrel emergency contraception (EC) is safe and effective for postcoital pregnancy prevention. Starting in 2013, the US Food and Drug Administration removed age restrictions, enabling EC to be sold over the counter to all consumers. We sought to compare the availability and access for female adolescents with the 2012 study, using the same study design.
METHODS:
Female mystery callers posing as 17-year-old adolescents in need of EC used standardized scripts to telephone 979 pharmacies in 5 US cities. Using 2015 estimated census data and the federal poverty level, we characterized income levels of pharmacy neighborhoods.
RESULTS:
Of 979 pharmacies, 827 (83%) indicated that EC was available. This proportion did not vary by pharmacy neighborhood income level, nor was significantly different from the 2012 study (P = .78). When examining access, 8.3% of the pharmacies reported it was impossible to obtain EC under any circumstances, which occurred more often in low-income neighborhoods (10.3% vs 6.3%, adjusted odds ratio 1.5; 95% confidence interval 1.20–1.94). This was not significantly different from 2012 (P = .66). Correct information regarding over-the-counter access was conveyed only 51.6% of the time; accuracy did not differ by pharmacy's neighborhood income (47.9% vs 55.3%, adjusted odds ratio 0.89; 95% confidence interval 0.71–1.11) and was not significantly different from 2012 (P = .37).
CONCLUSIONS:
A majority of pharmacies have EC available; however, barriers to and disparities in access for adolescents persist and have not changed since the previous study despite regulatory changes that were designed to improve access to EC.
What’s Known on This Subject:
Same-day access to emergency contraception (EC) is an important piece of effective pregnancy prevention for adolescents. Starting in 2013, the US Food and Drug Administration made EC available over the counter for consumers of all ages.
What This Study Adds:
Despite policy changes that started in 2013 that were intended to improve access to EC, there are still persistent barriers to access that are more prominent in low-income neighborhoods and have been unchanged since a 2012 study.
Great strides have been made in reducing teenage pregnancy in the United States since the 1990s.1 However, the United States still has the highest rate of unintended teenage pregnancies among similar high-income countries, which costs ∼$9.4 billion annually.1,2 Disparities by race and/or ethnicity and socioeconomic factors persist and are important to address given the long-term health and social consequences of unplanned pregnancies.1,3 Levonorgestrel emergency contraception (EC) is a safe and effective form of pregnancy prevention when used after unprotected sex or contraceptive failure.4 Access to EC is a core component of comprehensive pregnancy prevention in adolescents.
In a 2012 study, we showed that although EC was available at pharmacies, barriers to access for adolescents in low-income neighborhoods still existed.5 Such barriers are problematic because any delay in taking EC will impact its effectiveness.6,7 After a federal court case in 2013, the US Food and Drug Administration (FDA) removed all age restrictions and identification requirements so that brand-name EC (Plan B One-Step) could be sold over the counter (OTC) and without a prescription to anyone.8 Furthermore, in 2014, restrictions on generic forms of EC were removed; however, packaging still had to include a “use recommendation” that mentioned the intended users were women ≥17 years old until 2016. This decision was intended to improve access for consumers of all ages and enable EC to be taken sooner and more consistently when needed. However, no studies have determined whether this regulatory change has improved availability and access for adolescents.
We sought to examine if the FDA policy change resulted in increased availability of or access to EC for adolescents by using the same study design and population as the Wilkinson et al5 2012 study. We employed “mystery callers” posing as adolescents to conduct scripted telephone calls to pharmacies in 5 major US cities and determine the availability and accessibility of EC. We hypothesized that availability and access had improved since these policy changes but that barriers and disparities would still exist.
Methods
Two female research assistants posing as 17-year-old adolescents called every retail pharmacy in Nashville, Tennessee; Philadelphia, Pennsylvania; Cleveland, Ohio; Austin, Texas; and Portland, Oregon between July and December 2015. Lists of licensed pharmacies within the counties were obtained from local Boards of Pharmacy, and nonretail pharmacies (eg, home health care and hospitals) were removed. The age of 17 was chosen to replicate the previous study methods, and calls were made during weekdays between 9 am and 5 pm local time of each city called.5
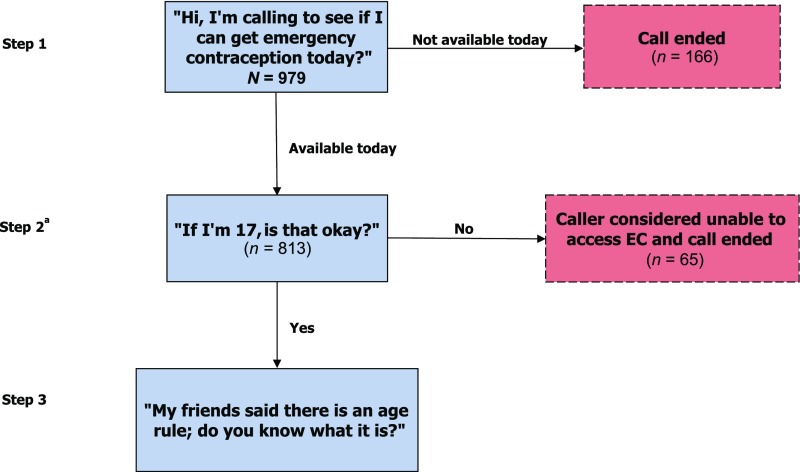
Callers followed standardized scripts (Fig 1) to elicit specific, uniform information on EC availability and access. The first question of the script examined same-day availability with the question (Step 1), “Hi, I’m calling to see if I can get emergency contraception today?” If EC was available, the next question examined whether a 17-year-old could access it with the question (Step 2), “If I am 17, is that okay?” If the pharmacy staff member said that she could not obtain EC because of her age, we considered this a denial of access and the call was ended. If the call continued and it had not already been discussed naturally, the final question was asked (Step 3), “My friends said there is an age rule, do you know what it is?” We deemed calls that reported no age restrictions on EC as being correct information; otherwise, they were coded as incorrect. Data were collected during calls in standardized abstraction forms.
FIGURE 1.
A schematic representation of study phone calls. a If (in Step 2) the caller was told that she was unable to access EC because of her age, we considered that a denial of OTC access and no additional questions were asked.
To examine the outcomes on the basis of neighborhood income, pharmacy addresses were merged with 2015 census block group estimates of median household income provided by GeoLytics, Inc (Somerville, NJ).9 Block groups with a median household income ≤200% of the 2015 federal poverty level for a household size of 3 were considered low-income neighborhoods.
We used logistic regression to examine how outcomes differed across low-income versus higher-income neighborhoods by clustering by city and adjusting for the study year and whether a pharmacy was part of a chain. To compare adjusted odds ratios (aORs) between the studies, we calculated the interaction between study year and neighborhood income for each outcome variable.
We examined the same 3 outcomes as the 2012 Wilkinson et al5 study as follows: (1) same-day availability, (2) whether the caller could access EC, and (3) whether the correct age for OTC access (ie, any age) was communicated by the pharmacy staff.
Data were analyzed using SAS, version 9.4 (SAS Institute Inc; Cary, NC). The Children’s Hospital Los Angeles Institutional Review Board deemed this study to be nonhuman subjects research.
Results
We eliminated 145 pharmacies from our initial sample of 1138 because they were noncommercial, which left a final sample of 993 pharmacies. As with the previous study, Philadelphia contributed the most (406, 40.9%) to our sample, and Portland contributed the fewest (96, 9.7%) (Table 1). A majority (71.4%) of pharmacies were chain pharmacies (≥4 locations), but only 5.2% were open 24 hours per day. Almost all (98.6%) of the pharmacies could be geocoded, which left a sample of 979 (42.7%) of them located in low-income neighborhoods.
TABLE 1.
Characteristics of the Selected Pharmacy Sample (N = 993)
| City, State (County) | N (%) |
|---|---|
| Austin, Texas (Travis) | 154 (15.5) |
| Cleveland, Ohio (Cuyahoga) | 211 (21.3) |
| Nashville, Tennessee (Davidson) | 126 (12.7) |
| Philadelphia, Pennsylvania (Philadelphia) | 406 (40.9) |
| Portland, Oregon (Multnomah) | 96 (9.7) |
| Chain pharmacies (≥4 locations) | 709 (71.4) |
| Open 24 h | 52 (5.2) |
| Median household income of census block group ≤200% FPLa | 418 (42.7) |
FPL, federal poverty level.
N = 979 geocoded pharmacies.
When examining same-day availability, we found a similar rate when compared with the 2012 study (83.3% vs 80.5%), with no significant differences on the basis of the neighborhood income of a pharmacy in either study (Table 2) or between the aORs (P = .78). However, 8.3% of the pharmacies in this study denied access to EC and told the caller that there was no possible way to obtain EC because of their stated age of 17. This denial of access happened more often in pharmacies located in low-income neighborhoods (aOR 1.53, 95% confidence interval 1.20–1.94) (Table 2) and with no significant differences over time (P = .66).
TABLE 2.
Outcomes Examined on the Basis of Census Block Group Median Household Income and Between Studies
| Income Group | aORa (95% CI) | ||
|---|---|---|---|
| ≤200% FPL | >200% FPLb | ||
| EC available on day of call for patient | |||
| 2016c | 340 (81.3%) (n = 418) | 473 (84.3%) (n = 561) | 0.97 (0.75–1.25) |
| 2012d | 338 (78.2%) (n = 432) | 398 (82.2%) (n = 484) | 0.98 (0.77–1.45) |
| Unable to obtain medication at all because of age | |||
| 2016 | 35 (10.3%) (n = 340) | 30 (6.3%) (n = 473) | 1.53 (1.20–1.94) |
| 2012 | 80 (23.7%) (n = 338) | 58 (14.6%) (n = 398) | 1.93 (1.53–2.43) |
| Correct age given to dispense EC OTC | |||
| 2016 | 163 (47.9%) (n = 340) | 257 (55.3%) (n = 473) | 0.89 (0.71–1.11) |
| 2012 | 169 (50.0%) (n = 338) | 250 (62.8%) (n = 398) | 0.59 (0.45–0.79) |
CI, confidence interval; FPL, federal poverty level.
City is a clustering variable adjusted for chain status.
Reference group.
Eight addresses were not able to be geocoded, and 6 addresses had a median household income of $0 and were excluded.
Eleven addresses were not able to be geocoded, and 16 addresses had a median household income of $0 and were excluded.
Finally, when callers assessed the pharmacy staff’s understanding of dispensing regulations, they found 48.4% of pharmacies incorrectly reported that EC was not available without a prescription to consumers of any age. In these instances, pharmacy staff mentioned some type of restriction based on age or a prescription requirement to the caller. This misinformation did not vary by neighborhood income level of a pharmacy like in the previous study (0.89, aOR 0.71–1.11) (Table 2) and had not changed significantly over time (P = .37).
Discussion
Same-day availability of EC in selected metropolitan cities in the United States (as reported by pharmacists) has remained unchanged for adolescent callers since the last study in 2012 despite policy changes in 2013 and 2014. In the 2 years since the FDA removed age restrictions for brand-name EC access, adjusted analysis shows no significant changes regarding access or misinformation. Furthermore, denial of access to nonprescription EC to minors continues to be communicated more often by pharmacies located in low-income neighborhoods, and that has not changed since 2012. In these instances, pharmacy staff told the callers that there was no possible way to obtain EC (with or without a prescription) because of their reported age of 17, which is concerning because an adolescent may not attempt to get EC after being told this. Other research has been published on EC availability since the 2012 study.10–16 However, to our knowledge, this is the first study to replicate the study design and examine whether the policy changes that began in 2013 may have resulted in changes to barriers to availability or access over time.
There are several limitations of our study that warrant consideration. First, although the sampling frame for the 2 studies was identical, the individual pharmacies were not exactly the same. Therefore, we could not make comparisons between the studies on an individual-pharmacy level and so only compared the outcomes on an aggregate level. Second, as in the previous study, we did not ask for the identities of the pharmacy staff members and thus cannot comment on the direct source of the misinformation. Third, information provided over the phone does not necessarily reflect what would happen during in-person inquiries. Now that EC is available OTC, pharmacies often stock EC on the store shelf, and thus, consumers can obtain EC without interacting with a pharmacy staff member. Furthermore, during our data collection, labeling for generic EC continued to incorporate age-related user recommendations, which could propagate confusion for pharmacy staff members and possibly be interpreted as a restriction.
Despite these limitations, we believe that our study demonstrates persistent barriers in access to EC since the 2013 policy change that removed age restrictions. The FDA made this regulatory decision to expand access of EC to all consumers, but especially to adolescents in need of unplanned-pregnancy prevention. Unfortunately, that change was necessary but not sufficient. Misinformation about and denial of access to EC for adolescents endures and is more common in low-income neighborhoods. Additional education and information for pharmacy staff and adolescents regarding availability and access could help eliminate these barriers, especially in low-income neighborhoods. This education could come in the form of uniform package labels for all levonorgestrel EC that clearly states the regulations regardless of the brand being obtained as well as education tailored to pharmacy staff members. Clinicians, particularly those who treat adolescents, can continue to provide education for their patients regarding availability and access when discussing pregnancy prevention.
Acknowledgments
We thank Alissa Sharp and Lisa Grismer, who greatly contributed to the data collection as paid research assistants, and Dr George Eckert for his biostatistic support. We also thank Dr Kathleen Nelson for her mentorship on this project.
Glossary
- aOR
adjusted odds ratio
- EC
emergency contraception
- FDA
US Food and Drug Administration
- OTC
over the counter
Footnotes
Dr Wilkinson conceptualized and designed the study, conducted the analysis, and drafted the initial manuscript; Dr Rafie contributed to the study design and data collection and reviewed and revised the manuscript; Ms Clark participated in data collection and initial data analysis and reviewed and revised the manuscript; Drs Carroll and Miller reviewed the data analysis and reviewed and revised the manuscript; and all authors approved of the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: This study was made possible by the Young Investigator Award from the Academic Pediatric Association. In addition, Dr Miller received support from National Institutes of Health grant K24HD075862. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Romero L, Pazol K, Warner L, et al. . Reduced disparities in birth rates among teens aged 15-19 years - United States, 2006-2007 and 2013-2014. MMWR Morb Mortal Wkly Rep. 2016;65(16):409–414 [DOI] [PubMed] [Google Scholar]
- 2.Sedgh G, Finer LB, Bankole A, Eilers MA, Singh S. Adolescent pregnancy, birth, and abortion rates across countries: levels and recent trends. J Adolesc Health. 2015;56(2):223–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng AS, Kaye K. Why it Matters: Teen Childbearing, Education and Economic Wellbeing. Washington, DC: The National Campaign to Prevent Teen and Unplanned Pregnancy; 2012 [Google Scholar]
- 4.Davidoff F, Trussell J. Plan B and the politics of doubt. JAMA. 2006;296(14):1775–1778 [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson TA, Fahey N, Suther E, Cabral HJ, Silverstein M. Access to emergency contraception for adolescents. JAMA. 2012;307(4):362–363 [DOI] [PubMed] [Google Scholar]
- 6.von Hertzen H, Piaggio G, Ding J, et al. ; WHO Research Group on Post-Ovulatory Methods of Fertility Regulation . Low dose mifepristone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomised trial. Lancet. 2002;360(9348):1803–1810 [DOI] [PubMed] [Google Scholar]
- 7.Task Force on Postovulatory Methods of Fertility Regulation Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet. 1998;352(9126):428–433 [PubMed] [Google Scholar]
- 8.Food and Drug Administration-Center for Drug Evaluation and Research FDA approves Plan B One-Step emergency contraceptive for use without a prescription for all women of child-bearing potential-Application NDA 021998/S-002. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/021998Orig1s002.pdf
- 9.GeoLytics, Inc Available at: www.geolytics.com/. Accessed July 20, 2016
- 10.Orr KK, Lemay VA, Wojtusik AP, Opydo-Rossoni M, Cohen LB. Availability and accuracy of information regarding nonprescription emergency contraception. J Pharm Pract. 2016;29(5):454–460 [DOI] [PubMed] [Google Scholar]
- 11.Samson FD, Loren R, Downing N, Schroeppel S, Kelly PJ, Ramaswamy M. Availability of emergency contraception in rural and urban pharmacies in Kansas. J Rural Health. 2013;29(1):113–118 [DOI] [PubMed] [Google Scholar]
- 12.Legare K, Bakshi S, Keyhani S, Howell EA. Availability of over-the-counter emergency contraception in 2 disparate New York City neighborhoods. Am J Public Health. 2012;102(11):e45–e47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaffaney M, Stamm C, Borgelt L, et al. . 67. Barriers to emergency contraception access in the state of Wyoming. J Adolesc Health. 2015;56(2):S36 [Google Scholar]
- 14.Bullock H, Steele S, Kurata N, et al. . Pharmacy access to ulipristal acetate in Hawaii: is a prescription enough? Contraception. 2016;93(5):452–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cleland K, Bass J, Doci F, Foster AM. Access to emergency contraception in the over-the-counter era. Womens Health Issues. 2016;26(6):622–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chau VM, Stamm CA, Borgelt L, et al. . Barriers to Single-Dose Levonorgestrel-Only Emergency Contraception Access in Retail Pharmacies [published online ahead of print April 23, 2017]. Womens Health Issues. 10.1016/j.whi.2017.03.010 [DOI] [PubMed] [Google Scholar]