Abstract
BACKGROUND & AIMS
Oral sodium phosphate (OSP) is a common bowel purgative administered before colonoscopy; the Food and Drug Administration has warned against its use because of concerns about acute kidney injury (AKI) from the absorbed phosphate and dystrophic calcification. However, it is not clear if OSP is associated with AKI in the general population or in high-risk subgroups undergoing colonoscopy. We estimated the risk of AKI among patients undergoing a screening colonoscopy using OSP vs polyethylene glycol (PEG) for bowel cleansing in a large, US-based claims database.
METHODS
We used an insurance database to identify a cohort of patients ages 50 to 75 years who underwent screening colonoscopies as outpatients from January 2000 through November 2008 (before the Food and Drug Administration warning), receiving OSP (n [ 121,266) or PEG (n [ 429,430) within 30 days beforehand, without prior use of either drug. We collected data from patients for 6 months afterward to identify those who developed AKI or renal failure, or received dialysis. Adjusted and propensity score-matched hazard ratios (HR) and 95% confidence intervals (CI) were estimated using Cox proportional hazards models. We investigated the effects in subgroups with higher AKI risk (patients with chronic kidney disease, kidney stones, hypertension, or diabetes, or using antihypertensive or nonsteroidal anti-inflammatory drugs).
RESULTS
AKI occurred in 0.2% of OSP users and in 0.3% of PEG users (adjusted HR, 0.86; 95% CI, 0.75–0.99). OSP users matched well with PEG users, producing similar estimates (HR, 0.85; 95% CI, 0.72–1.01). We did not observe a consistent increase in the risk of AKI or other outcomes in any subgroups analyzed.
CONCLUSIONS
In a large database analysis, we did not associate administration of OSP before colonoscopy with increased risk of postprocedure AKI, even in high-risk clinical subgroups.
Keywords: Comparative Safety, Pharmacoepidemiology, Endoscopy, Bowel Preparations
Sodium phosphate preparations are effective agents for preprocedure bowel cleansing, although they may increase the risk of acute kidney injury (AKI). Healthy volunteers given oral sodium phosphate (OSP) solution showed enteric absorption of 50% of the phosphorus, an abrupt increase in serum phosphate concentration, and renal excretion of approximately 14% of the absorbed load.1 Retained phosphate may have systemic consequences because it binds and precipitates with calcium,2–4 leading to dystrophic soft-tissue deposition in various organ systems. Kidney biopsy series have detailed calcium phosphate deposition in the distal tubule and collecting duct of patients with AKI after sodium phosphate use.5–9 Observed cases of phosphate renal injury led the Food and Drug Administration (FDA) to place warnings of kidney injury on all over-the-counter and prescription sodium phosphate products used for bowel cleansing, including multicomponent preparation kits and prescription OSP tablets. The over-the-counter kits now contain reduced phosphate content and are marketed for treatment of constipation rather than precolonoscopy bowel cleansing. Most published case series have focused on multicomponent sodium phosphate preparation kits, although the FDA warning additionally was extended to OSP tablets. Although biopsy-confirmed renal injury from OSP has been observed in individuals, it is unclear how these individual-level effects translate to population-level risks. Without the context of appropriate population denominators, the relative burden of OSP-induced AKI compared with other agents is unknown.
The available epidemiologic data from large populations are inconclusive regarding the risk of AKI after OSP use in large populations,10 with roughly half of the studies suggesting increased risk,11–14 and the remainder suggesting no risk.15–19 A major limitation of all of these studies was inadequate power given the relatively small numbers of participants, compounded by the low frequency of AKI events. In addition, these studies may be subject to confounding by indication, include both inpatient and outpatient colonoscopies, and rely on poorly defined or inappropriate comparison groups. The single randomized study20 comparing OSP with polyethylene glycol (PEG), another commonly used bowel preparation agent, was funded by a maker of OSP and showed no difference in renal injury, but it also was limited by low power. The FDA warning on OSP notes that patients undergoing precolonoscopy bowel cleansing are at risk for dehydration, and it is possible that the observed AKI in OSP users may have resulted from inadequate rehydration rather than from the OSP directly.21 Given that OSP may be a more effective and less expensive purgative,22–26 quantifying the comparative risk of renal injury after its use may lead to more informed clinical decision making.
We conducted a large retrospective cohort study of middle-aged and older adults undergoing an outpatient colonoscopy from 2000 to 2008 to determine the risk of AKI associated with OSP tablet exposure compared with PEG. We examined the risk of AKI among all participants as well as among high-AKI-risk subgroups, including those with alterations in renal calcium metabolism, which potentially can increase the risk from high-phosphate products.
Methods
We conducted a cohort study using a large, US-based administrative claims database. All analyses were performed with SAS 9.2 (SAS Institute, Inc, Cary, NC). This secondary analysis of de-identified administrative claims data was exempt from further review by the University of North Carolina at Chapel Hill Institutional Review Board.
Data Source
We used Truven MarketScan database (Truven Health Analytics Inc, Ann Arbor, MI), which is composed of 2 portions: (1) Commercial Claims and Encounters, employer-based commercial insurance plans for employees, spouses and dependents aged younger than 65 years from large insurers throughout the United States; and (2) Medicare Supplementary and Coordination of Benefit, employer-based Medicare supplementary insurance for individuals aged 65 years or older. These databases contain enrollment information, inpatient and outpatient diagnosis and procedure claims, and pharmacy dispensing information. Participants have unique identifiers that permit longitudinal follow-up evaluation through linkage of claims.
Study Population
We identified individuals aged 50 to 75 years undergoing an outpatient screening colonoscopy between January 1, 2000, and November 11, 2008—1 month before the FDA warning to avoid channeling of high-risk AKI patients away from OSP—using Current Procedural Terminology codes (4523, 45355, 45378, 45379, 45380, 45381, 45382, 45384, 45385, 45386, 45387, 45391, 45392, G0105, and G0121). We required at least 1 year of continuous enrollment before colonoscopy. If a patient had multiple eligible colonoscopies, only the first was considered. Individuals with AKI, end-stage renal disease, unspecified renal failure, rhabdomyolysis, dialysis, or renal transplantation in the baseline year before colonoscopy were excluded. To ensure utilization of the observed insurance plan for pharmacy benefits, patients were required to fill at least one other medication during the baseline period, providing assurance that medication use and medical interactions all would be observable in the billing claims database.
Exposure Information
The 30 days before colonoscopy were considered the exposure period (Figure 1), during which pharmacy claims were queried for dispensing of prescription OSP tablets or PEG bowel preparation solutions. We could observe only pharmacy-dispensed OSP use; over-the-counter products would not appear in claims and thus were unavailable for analysis. Supplementary Table 1 lists the included OSP and PEG formulations of interest. It was assumed that regardless of when the prescription was dispensed in the 30-day exposure window, it would be ingested on the day before or the day of the colonoscopy. Thus, the date of the colonoscopy was the beginning of the follow-up period. To restrict the analysis to new users of the medications, we excluded individuals with use of either agent in the baseline year before the exposure period. The drug exposure status at the index date was carried throughout the follow-up evaluation in an intent-to-treat analysis because we were interested in only one initial drug exposure.
Figure 1.
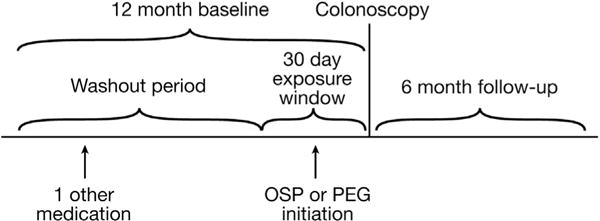
Cohort schematic of new users of OSP or PEG before colonoscopy.
Outcome Information
We followed individuals for 6 months for renal outcomes. We defined AKI as an inpatient or outpatient diagnosis code for acute renal failure (International Classification of Diseases, 9th revision, Clinical Modification codes 584.5–584.9). As sensitivity analyses, we used additional definitions of renal injury, as follows: (1) a procedure code for dialysis; (2) a diagnosis of AKI plus a procedure code for dialysis—the most restrictive although the most sensitive definition identifying the most extreme cases; (3) a composite definition of any kidney failure, which included International Classification of Diseases, 9th revision codes of AKI, unspecified kidney failure (586), end-stage renal disease (585.6), or a procedure code for dialysis, which was the most broad, inclusive definition.
Covariate Information
We defined covariates from submitted procedure claims, diagnoses, and medication dispensing, which occurred during the baseline year. Covariates included patient demographics, renal risk factors and other comorbid conditions, markers of health care use, prevalent medication use, and other medications initiated during the exposure period. Table 1 shows a complete list of covariates. Supplementary Table 2 shows the definitions of our included covariates using administrative claims coding. Because of the nature of our claims-based data, if a claim with a diagnosis or procedure was not submitted during the covariate assessment period, we considered the condition not present.
Table 1.
Baseline Demographics and Outcomes by Statin Initiation Status
| Full cohort
|
Matched cohort
|
|||
|---|---|---|---|---|
| PEG (N = 429,430) |
Sodium phosphate (N = 121,266) |
PEG (N = 121,203) |
Sodium phosphate (N = 121,203) |
|
| Demographics | ||||
| Male, % | 46.9 | 39.4 | 39.6 | 36.4 |
| Mean age, y (SD) | 59.8 (7.1) | 58.3 (6.4) | 58.4 (6.4) | 58.3 (6.4) |
| Renal risk factors | ||||
| Diabetes, % | 15.9 | 12.5 | 12.4 | 12.5 |
| CKD, % | 1.2 | 0.7 | 0.7 | 0.7 |
| Other kidney disease, % | 0.3 | 0.2 | 0.2 | 0.2 |
| Proteinuria, % | 0.4 | 0.3 | 0.4 | 0.3 |
| Kidney stones, % | 1.9 | 1.8 | 1.8 | 1.8 |
| Hypercalciuria, % | 0.4 | 0.5 | 0.4 | 0.5 |
| Mean number of creatinine measurements (SD) | 0.01 (0.20) | 0.01 (0.15) | 0.01 (0.18) | 0.01 (0.15) |
| CVD and comorbid conditions | ||||
| Hypertension, % | 39.6 | 36.3 | 36.1 | 36.3 |
| Hyperlipidemia, % | 29.8 | 39.2 | 38.7 | 39.2 |
| Other ischemic heart disease, % | 10.7 | 7.9 | 7.8 | 7.9 |
| Atrial fibrillation, % | 2.4 | 1.5 | 1.6 | 1.5 |
| Heart failure, % | 1.9 | 1.0 | 1.1 | 1.0 |
| Chronic liver disease and cirrhosis, % | 2.9 | 2.6 | 2.6 | 2.6 |
| Multiple myeloma, % | 0.1 | 0.1 | 0.1 | 0.1 |
| Systemic lupus erythematosus, % | 0.7 | 0.7 | 0.7 | 0.7 |
| Metabolic disorders, % | 1.0 | 0.9 | 0.9 | 0.9 |
| Prevalent medication use during baseline | ||||
| Statins, % | 36.4 | 33.8 | 33.9 | 33.8 |
| ACE inhibitors, % | 23.1 | 19.0 | 19.1 | 19.0 |
| ARBs, % | 13.7 | 13.3 | 13.3 | 13.3 |
| β-blockers, % | 22.1 | 18.2 | 18.2 | 18.2 |
| Calcium channel blockers, % | 16.0 | 13.3 | 13.0 | 13.3 |
| Antiplatelet agents, % | 4.7 | 3.6 | 3.5 | 3.6 |
| α-blockers, % | 4.3 | 2.9 | 3.0 | 2.9 |
| Thiazides, % | 24.6 | 22.8 | 22.9 | 22.8 |
| Potassium-sparing diuretics, % | 6.8 | 6.2 | 6.1 | 6.2 |
| Loop diuretics, % | 5.6 | 4.0 | 3.9 | 4.0 |
| Niacin, % | 2.0 | 1.9 | 1.8 | 1.9 |
| Fibrates, % | 4.3 | 3.9 | 3.9 | 3.9 |
| Ezetimibe, % | 6.4 | 6.8 | 6.7 | 6.8 |
| Anticoagulants, % | 3.4 | 2.2 | 2.2 | 2.2 |
| NSAIDs, % | 26.3 | 26.2 | 25.9 | 26.2 |
| Medications initiated during exposure window | ||||
| Statins, % | 1.3 | 1.4 | 1.4 | 1.4 |
| ACE inhibitors, % | 0.7 | 0.6 | 0.6 | 0.6 |
| ARBs, % | 0.4 | 0.4 | 0.4 | 0.4 |
| β-blockers, % | 0.5 | 0.4 | 0.4 | 0.4 |
| Calcium channel blockers, % | 0.4 | 0.3 | 0.3 | 0.3 |
| Antiplatelet agents, % | 0.1 | 0.1 | 0.1 | 0.1 |
| α-blockers, % | 0.1 | 0.1 | 0.1 | 0.1 |
| Thiazides, % | 0.7 | 0.7 | 0.7 | 0.7 |
| Potassium-sparing diuretics, % | 0.2 | 0.2 | 0.2 | 0.2 |
| Loop diuretics, % | 0.2 | 0.2 | 0.2 | 0.2 |
| Niacin, % | 0.1 | 0.1 | 0.1 | 0.1 |
| Fibrates, % | 0.2 | 0.2 | 0.2 | 0.2 |
| Ezetimibe, % | 0.4 | 0.4 | 0.4 | 0.4 |
| Anticoagulants, % | 0.1 | 0.0 | 0.0 | 0.0 |
| NSAIDs, % | 1.2 | 1.3 | 1.2 | 1.3 |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CVD, cardiovascular disease; NSAIDs, nonsteroidal anti-inflammatory drugs.
Statistical Analysis
We calculated adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) using multivariable Cox proportional hazards models. Follow-up time was censored at the first occurrence of the following: 6 months after the index date, plan un-enrollment, or the end of the study (May 10, 2009, which was 180 days after the last possible index date).
Propensity Score Matching
Analyses were repeated using propensity score (PS) matching. The predicted probability of initiating OSP vs PEG—the PS for treatment—was estimated with multivariable logistic regression from the measured covariates. We created an exchangeable comparison group of PEG users by 1:1 matching them to sodium phosphate users using a greedy matching algorithm that matches up to the fifth decimal of the PS, if possible, and excluding those who fail to match.27 Cox proportional hazards models then were run in the matched cohorts. In subgroup analyses, the PS was re-estimated, and the matching algorithm was run within each subgroup.
Sensitivity Analysis
To avoid bias caused by channeling of high-risk patients away from OSP in response to early reports of OSP-associated AKI, we performed a restricted analysis in colonoscopy patients before 2005, and then in 2005 to 2008.
Results
We identified 550,696 eligible participants undergoing outpatient colonoscopies, 429,430 of whom were prescribed PEG and 121,266 were prescribed OSP before the procedure. Table 1 shows the characteristics of the study participants according to the choice of bowel purgative. There was a slight predominance of females in both groups, and the proportion of females was higher in the OSP group vs the PEG group. Individuals in the PEG group were slightly older than those in the OSP group and had a higher prevalence of comorbid conditions such as diabetes mellitus, chronic kidney disease (CKD), hypertension, and cardiovascular disease. The frequency of recent kidney stones and diagnoses of hypercalciuria were similar in the 2 groups. Prevalent medication use for a variety of agents was higher among the PEG group, including the frequency of angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, diuretics, and nonsteroidal anti-inflammatory drugs. Medicines initiated during the exposure period for the 2 groups were comparable. We matched OSP to PEG users on the PS very successfully, and the distribution of covariates was much more similar between the 2 matched groups.
Patients were followed for up to 180 days for the outcome or censoring. Patients had a mean follow-up time of 170.7 days (SD, 32.0 d). There was a total of 1595 episodes of AKI in the cohort: 241 (0.2%) in the OSP group, and 1354 (0.3%) in the PEG group. Other outcomes, including any kidney failure or the need for dialysis, were distributed similarly between the 2 groups.
The unadjusted HR of AKI for the OSP group compared with PEG was 0.63 (95% CI, 0.55–0.72) (Table 2). After adjustment for potential confounders, the HR was attenuated to 0.86 (95% CI, 0.75–0.99). Among the subgroups reported to have an increased risk of AKI, OSP use was not associated with an increased risk of AKI compared with PEG in the adjusted analyses. The clinically relevant subgroups included individuals with baseline CKD (HR, 0.91; 95% CI, 0.58–1.42), advanced age (≥60 y) (HR, 0.83; 95% CI, 0.70–0.99), diabetes mellitus (HR, 0.77; 95% CI, 0.60–0.98), and the concurrent use of nonsteroidal anti-inflammatory drugs (HR, 0.84; 95% CI, 0.65–1.08) or angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers (HR, 0.89; 95% CI, 0.75–1.05). Among individuals with a history of hypercalciuria, the observed point estimate was increased (HR, 2.80; 95% CI, 0.84–9.29), although the 95% CIs were wide from the small number of events in either treatment group; this association was not observed in any of the sensitivity analyses using other definitions of AKI (Supplementary Tables 3–5). As a whole, the sensitivity analyses for dialysis, any renal failure outcome, and AKI requiring dialysis agreed with the primary analysis very well. However, some subgroups suffered from a very small number of events per treatment group, leading to unstable estimates.
Table 2.
HRs of AKI in OSP Users Compared With PEG Users
| Crude
|
Adjusted
|
PS matched
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Events | (%) HR | 95% CI | HR | 95% CI | N | Events (%) | HR | 95% CI | ||
| Whole sample | PEG | 429,430 | 1354 (0.3) | – | – | – | – | 121,203 | 283 (0.2) | – | – |
| Sodium phosphate | 121,266 | 241 (0.2) | 0.63 | 0.55–0.72 | 0.86 | 0.75–0.99 | 121,203 | 241 (0.2) | 0.85 | 0.72–1.01 | |
| With CKD | PEG | 5201 | 194 (3.7) | – | – | – | – | 788 | 22 (2.8) | – | – |
| Sodium phosphate | 791 | 22 (2.8) | 0.74 | 0.48–1.15 | 0.91 | 0.58–1.42 | 788 | 22 (2.8) | 1.00 | 0.55–1.80 | |
| ≥60 y | PEG | 198,486 | 989 (0.5) | – | – | – | – | 45,803 | 172 (0.4) | – | – |
| Sodium phosphate | 45,895 | 152 (0.3) | 0.67 | 0.56–0.79 | 0.83 | 0.70–0.99 | 45,803 | 152 (0.3) | 0.88 | 0.71–1.10 | |
| With diabetes | PEG | 68,371 | 558 (0.8) | – | – | – | – | 15,101 | 99 (0.7) | – | – |
| Sodium phosphate | 15,117 | 74 (0.5) | 0.60 | 0.47–0.76 | 0.77 | 0.60–0.98 | 15,101 | 73 (0.5) | 0.74 | 0.54–1.00 | |
| With kidney stones | PEG | 7969 | 45 (0.6) | – | – | – | – | 2202 | 13 (0.6) | – | – |
| Sodium phosphate | 2210 | 10 (0.5) | 0.80 | 0.41–1.60 | 1.01 | 0.49–2.07 | 2202 | 10 (0.5) | 0.77 | 0.34–1.76 | |
| Hypercalciuria | PEG | 1864 | 10 (0.5) | – | – | – | – | 547 | 2 (0.4) | – | – |
| Sodium phosphate | 558 | 5 (0.9) | 1.67 | 0.57–4.88 | 2.80 | 0.84–9.29 | 547 | 5 (0.9) | 2.50 | 0.49–12.89 | |
| On NSAIDs | PEG | 118,078 | 408 (0.4) | – | – | – | – | 33,300 | 77 (0.2) | – | – |
| Sodium phosphate | 33,321 | 73 (0.2) | 0.63 | 0.49–0.81 | 0.84 | 0.65–1.08 | 33,300 | 73 (0.2) | 0.95 | 0.69–1.31 | |
| On thiazides | PEG | 108,878 | 564 (0.5) | – | – | – | – | 28,433 | 126 (0.4) | – | – |
| Sodium phosphate | 28,468 | 96 (0.3) | 0.65 | 0.52–0.81 | 0.82 | 0.66–1.02 | 28,433 | 95 (0.3) | 0.75 | 0.58–0.95 | |
NSAID, nonsteroidal anti-inflammatory drug.
Figure 2 shows the distribution of the PS for the 2 treatments in the overall population. The high degree of overlap for the majority of users of either agent suggests good exchangeability between the OSP and PEG users and low confounding by measured characteristics. The OSP treatment group matched the PEG users very well, with almost all (99.9%) OSP users being retained in all analyses. The distribution of covariates in the PS-matched users is shown in Table 1; very good balance of covariates was achieved between the 2 matched treatments. The PS-matched HRs agreed very well with the adjusted estimates. The matched HR was 0.85 (95% CI, 0.72–1.01) for the entire group. All other matched subgroup estimates can be seen in Table 2.
Figure 2.
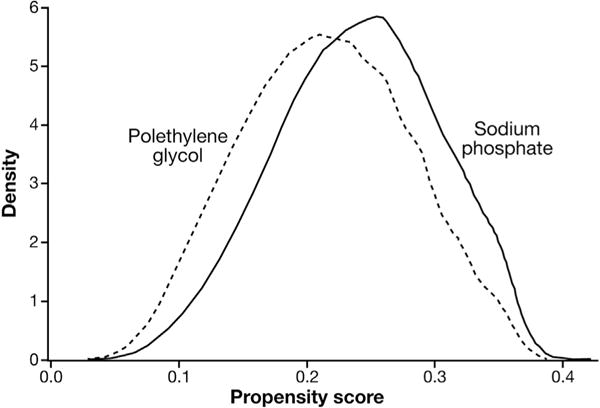
Propensity score distributions by precolonoscopy bowel preparation treatment.
When the sensitivity analyses were run in the years 2000 to 2004 and 2005 to 2008, there was virtually no change in adjusted estimates from the overall sample (2000–2004: HR, 0.87; 95% CI, 0.64–1.19; and 2005–2008; HR, 0.86; 95% CI, 0.73–1.00), suggesting minimal channeling of high-risk patients to PEG in the later periods of the study.
Discussion
In this cohort study of nearly 555,000 middle-aged men and women undergoing outpatient colonoscopies, we examined the comparative risk of AKI associated with OSP vs PEG. We did not find a consistent increased risk of AKI associated with the use of OSP compared with PEG in the full cohort, in the PS-matched analysis, or in any subgroup such as individuals with CKD, advanced age, diabetes mellitus, or concurrent user of medications known to increase AKI risk. Rather, there appeared to be a consistent finding of null or slightly decreased risk of AKI associated with OSP vs PEG.
Previous studies regarding the risk of AKI after OSP cathartic use have shown mixed findings. A meta-analysis summarizing data from 7 studies with more than 13,000 patients reported inconclusive findings that could neither support or refute that OSP was associated with AKI.10 Our analysis had distinct advantages over these earlier studies. First, the design, which identified new users, anchored on an outpatient screening colonoscopy, and utilized well-defined exposure and follow-up periods, was meant to identify a cohort of patients equally likely to receive either OSP or PEG in a homogenous group of colonoscopy procedures. Second, the PS matching analysis showed a high degree of exchangeability between the 2 groups, evidenced both by the distribution of the covariates in the matched cohort and the large amount of overlap of the PS distributions; this allowed for a comparison of the 2 exposures with minimal confounding by measured characteristics. Third, the large granular data set allowed for robust adjustment for numerous clinical risk factors, measures of health care use, and concurrent medication use in a very large cohort of patients.
We had postulated that higher urine calcium excretion may identify a subset of individuals at higher risk for renal tubular injury from calcium phosphate precipitation. Although all patients receiving OSP are likely to have sudden and massive renal tubular excretion of phosphate, high urinary calcium excretion theoretically may place them at higher risk for developing deposition of calcium phosphate in the renal interstitium and distal tubules, the same location noted on pathologic examination from renal biopsy specimens from patients with AKI after OSP exposure.5–9 Furthermore, ambient conditions, such as high urine pH or high osmolality, would strongly favor precipitation of luminal calcium and phosphate.28,29 We did not see a consistent association of OSP with AKI among individuals with a diagnosis of hypercalciuria or nephrolithiasis, but the use of diagnosis codes to identify individuals with abnormal calcium excretion by the kidney is arguably insensitive, and a minority of individuals also may have had disease-associated hypercalciuria. Ultimately, we identified very few individuals with the condition; further investigation may be warranted in cohorts with larger numbers of patients with confirmed hypercalciuria.
The findings of the study should be interpreted in the context of important limitations. First, administrative claims for AKI outcomes may be insensitive.30,31 Serum creatinine–based estimates of glomerular filtration rate or urinary biomarkers have a better ability to assess early changes in renal function, whereas claims-based measures are better suited to detect established events with good specificity. Although having such measures may have increased the detection of kidney injury, the frequency of AKI in this study was not notably different from previous studies of community-acquired AKI.32–35 In addition, measures of relative effect, such as the reported HRs, still may be unbiased36,37 given the high specificity of billing codes for AKI30; thus, we reported relative, rather than absolute, measures of effect.
A second limitation was the low sensitivity of claims to estimate baseline levels of kidney function; we used diagnosis codes for CKD, and these diagnoses were very rare and did not vary substantially by treatment group. In this sample from the general population of middle-aged and older adults undergoing screening colonoscopies, it is unlikely that glomerular filtration rate is widely and consistently monitored. If glomerular filtration rate is unknown to the prescribing physician, then treatment choice cannot be influenced by it or confound the outcome association. To avoid the potential for differential prescribing based on renal function, we restricted our sample to colonoscopies occurring before AKI warnings were placed on OSP because we were concerned that after warnings were made, patients at higher risk of AKI would be channeled toward PEG, confounding the treatment-AKI association. The result remained consistent even after further restriction of the time period from 2000 to 2004, long before reports of OSP-induced AKI were widespread.
Third, our study used pharmacy dispensing claims to define exposure rather than direct measures of patients taking the medication. This prevented us from estimating the dose administered. Furthermore, we could only observe prescription medication use, not over-the-counter sodium phosphate–containing kits, which patients may have used during the exposure period. Thus, there is the potential for exposure misclassification by over-the-counter use. However, we would assume that because all our included patients had filled prescriptions for a bowel preparation agent, they would use the OSP or PEG in their possession, minimizing the risk of misclassification. Similarly to other studies, we could not assess the adequacy of patient hydration practices while using the medications.
In addition, as with any observational study, there is the possibility of residual confounding by unmeasured covariates. We adjusted for many covariates, and PS matching provided highly similar groups with a similar distribution of covariates. Yet, remaining differences in unmeasured potential confounders may have masked the true association of OSP with AKI.
Finally, this study used US-based health care claims from individuals with employer-based commercial or Medicare supplementary insurance coverage, perhaps making the reported estimates less generalizable to uninsured populations, those with governmental insurance coverage, or other countries outside the United States.
In summary, this study did not show an increased AKI risk associated with OSP vs PEG in this large sample, generally representative of the insured, adult screening population. This result agrees with many previously published studies that have shown no difference between OSP and PEG15,17,20 or any non-OSP preparation agent.18 Nonetheless, calcium phosphate deposition and AKI have been shown by kidney biopsy in some individuals recently exposed to OSP. Even though these individual risks did not translate to a population risk in this study, future research should be directed toward understanding the occurrence of renal injury in specific individuals after OSP exposure.
Supplementary Material
Supplementary Table 1. Included Formulations by Treatment
Supplementary Table 2. Covariate Definitions
Supplementary Table 3. HRs of the Risk of the Need for Dialysis in OSP Users Compared With PEG Users
Supplementary Table 4. HRs of the Risk of AKI Requiring Dialysis in OSP Users Compared With PEG Users
Supplementary Table 5. HRs of the Risk of Any Renal Failure in OSP Users Compared With PEG Users
Acknowledgments
Presented orally at the 29th International Convention on Pharmacoepidemiology and Therapeutic Risk Management, Montreal, Canada, August 25–28, 2013.
Abbreviations used
- AKI
acute kidney injury
- CI
confidence interval
- CKD
chronic kidney disease
- FDA
Food and Drug Administration
- HR
hazard ratio
- OSP
oral sodium phosphate
- PEG
polyethylene glycol
- PS
propensity score
Footnotes
Conflicts of interest
This author discloses the following: Abhijit Kshirsagar has served on an advisory board for Amgen. The remaining authors disclose no conflicts.
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://dx.doi.org/10.1016/j.cgh.2014.01.034.
References
- 1.Patel V, Emmett M, Santa Ana CA, et al. Pathogenesis of nephrocalcinosis after sodium phosphate catharsis to prepare for colonoscopy: intestinal phosphate absorption and its effect on urine mineral and electrolyte excretion. Hum Pathol. 2007;38:193–194. doi: 10.1016/j.humpath.2006.10.008. author reply 194–195. [DOI] [PubMed] [Google Scholar]
- 2.Biberstein M, Parker BA. Enema-induced hyperphosphatemia. Am J Med. 1985;79:645–646. doi: 10.1016/0002-9343(85)90064-6. [DOI] [PubMed] [Google Scholar]
- 3.Korzets A, Dicker D, Chaimoff C, et al. Life-threatening hyperphosphatemia and hypocalcemic tetany following the use of fleet enemas. J Am Geriatr Soc. 1992;40:620–621. doi: 10.1111/j.1532-5415.1992.tb02115.x. [DOI] [PubMed] [Google Scholar]
- 4.Vukasin P, Weston LA, Beart RW. Oral Fleet Phospho-Soda laxative-induced hyperphosphatemia and hypocalcemic tetany in an adult: report of a case. Dis Colon Rectum. 1997;40:497–499. doi: 10.1007/BF02258399. [DOI] [PubMed] [Google Scholar]
- 5.Desmeules S, Bergeron MJ, Isenring P. Acute phosphate nephropathy and renal failure. N Engl J Med. 2003;349:1006–1007. doi: 10.1056/NEJM200309043491020. [DOI] [PubMed] [Google Scholar]
- 6.Markowitz GS, Nasr SH, Klein P, et al. Renal failure due to acute nephrocalcinosis following oral sodium phosphate bowel cleansing. Hum Pathol. 2004;35:675–684. doi: 10.1016/j.humpath.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Markowitz GS, Stokes MB, Radhakrishnan J, et al. Acute phosphate nephropathy following oral sodium phosphate bowel purgative: an underrecognized cause of chronic renal failure. J Am Soc Nephrol. 2005;16:3389–3396. doi: 10.1681/ASN.2005050496. [DOI] [PubMed] [Google Scholar]
- 8.Connor A, Sykes L, Roberts IS, et al. Acute phosphate nephropathy after sodium phosphate preparations. BMJ. 2008;337:a182. doi: 10.1136/bmj.a182. [DOI] [PubMed] [Google Scholar]
- 9.Ori Y, Herman M, Tobar A, et al. Acute phosphate nephropathyan emerging threat. Am J Med Sci. 2008;336:309–314. doi: 10.1097/MAJ.0b013e318167410c. [DOI] [PubMed] [Google Scholar]
- 10.Brunelli SM. Association between oral sodium phosphate bowel preparations and kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:448–456. doi: 10.1053/j.ajkd.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 11.Hurst FP, Bohen EM, Osgard EM, et al. Association of oral sodium phosphate purgative use with acute kidney injury. J Am Soc Nephrol. 2007;18:3192–3198. doi: 10.1681/ASN.2007030349. [DOI] [PubMed] [Google Scholar]
- 12.Russmann S, Lamerato L, Motsko SP, et al. Risk of further decline in renal function after the use of oral sodium phosphate or polyethylene glycol in patients with a preexisting glomerular filtration rate below 60 ml/min. Am J Gastroenterol. 2008;103:2707–2716. doi: 10.1111/j.1572-0241.2008.02201.x. [DOI] [PubMed] [Google Scholar]
- 13.Khurana A, McLean L, Atkinson S, et al. The effect of oral sodium phosphate drug products on renal function in adults undergoing bowel endoscopy. Arch Intern Med. 2008;168:593–597. doi: 10.1001/archinte.168.6.593. [DOI] [PubMed] [Google Scholar]
- 14.Ehrenpreis ED, Parakkal D, Semer R, et al. Renal risks of sodium phosphate tablets for colonoscopy preparation: a review of adverse drug reactions reported to the US Food and Drug Administration. Colorectal Dis. 2011;13:e270–e275. doi: 10.1111/j.1463-1318.2011.02679.x. [DOI] [PubMed] [Google Scholar]
- 15.Russmann S, Lamerato L, Marfatia A, et al. Risk of impaired renal function after colonoscopy: a cohort study in patients receiving either oral sodium phosphate or polyethylene glycol. Am J Gastroenterol. 2007;102:2655–2663. doi: 10.1111/j.1572-0241.2007.01610.x. [DOI] [PubMed] [Google Scholar]
- 16.Singal AK, Rosman AS, Post JB, et al. The renal safety of bowel preparations for colonoscopy: a comparative study of oral sodium phosphate solution and polyethylene glycol. Aliment Pharmacol Ther. 2008;27:41–47. doi: 10.1111/j.1365-2036.2007.03558.x. [DOI] [PubMed] [Google Scholar]
- 17.Abaskharoun R, Depew W, Vanner S. Changes in renal function following administration of oral sodium phosphate or polyethylene glycol for colon cleansing before colonoscopy. Can J Gastroenterol. 2007;21:227–231. doi: 10.1155/2007/630639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunelli SM, Lewis JD, Gupta M, et al. Risk of kidney injury following oral phosphosoda bowel preparations. J Am Soc Nephrol. 2007;18:3199–3205. doi: 10.1681/ASN.2007040440. [DOI] [PubMed] [Google Scholar]
- 19.Seol DC, Hong SN, Kim JH, et al. Change in renal function after sodium phosphate preparation for screening colonoscopy. World J Gastroenterol. 2010;16:2010–2016. doi: 10.3748/wjg.v16.i16.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johanson JF, Popp JW, Jr, Cohen LB, et al. A randomized, multicenter study comparing the safety and efficacy of sodium phosphate tablets with 2L polyethylene glycol solution plus bisacodyl tablets for colon cleansing. Am J Gastroenterol. 2007;102:2238–2246. doi: 10.1111/j.1572-0241.2007.01363.x. [DOI] [PubMed] [Google Scholar]
- 21.FDA requires new safety measures for oral sodium phosphate products to reduce risk of acute kidney injury risk associated with both prescription and over-the-counter (OTC) products. Vol. 2013. Silver Spring, MD: US Food and Drug Administration; 2008. [Google Scholar]
- 22.Brunelli SM, Feldman HI, Latif SM, et al. A comparison of sodium phosphosoda purgative to polyethylene glycol bowel preparations prior to colonoscopy. Fam Med. 2009;41:39–45. [PMC free article] [PubMed] [Google Scholar]
- 23.Juluri R, Eckert G, Imperiale TF. Polyethylene glycol vs. sodium phosphate for bowel preparation: a treatment arm meta-analysis of randomized controlled trials. BMC Gastroenterol. 2011;11:38. doi: 10.1186/1471-230X-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juluri R, Eckert G, Imperiale TF. Meta-analysis: randomized controlled trials of 4-L polyethylene glycol and sodium phosphate solution as bowel preparation for colonoscopy. Aliment Pharmacol Ther. 2010;32:171–181. doi: 10.1111/j.1365-2036.2010.04326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belsey J, Crosta C, Epstein O, et al. Meta-analysis: the relative efficacy of oral bowel preparations for colonoscopy 1985–2010. Aliment Pharmacol Ther. 2012;35:222–237. doi: 10.1111/j.1365-2036.2011.04927.x. [DOI] [PubMed] [Google Scholar]
- 26.Lawrance IC, Willert RP, Murray K. Bowel cleansing for colonoscopy: prospective randomized assessment of efficacy and of induced mucosal abnormality with three preparation agents. Endoscopy. 2011;43:412–418. doi: 10.1055/s-0030-1256193. [DOI] [PubMed] [Google Scholar]
- 27.Parsons LS. Reducing bias in a propensity score matched-pair sample using greedy matching techniques Twenty-Sixth Annual SAS® Users Group International Conference. Cary, NC: SAS Institute, Inc; 2001. [Google Scholar]
- 28.Nordin BEC. Calcium, phosphate, and magnesium metabolism: clinical physiology and diagnostic procedures. New York: Longman Group Limited; 1976. Urinary excretion. [Google Scholar]
- 29.Patel V, Nicar M, Emmett M, et al. Intestinal and renal effects of low-volume phosphate and sulfate cathartic solutions designed for cleansing the colon: pathophysiological studies in five normal subjects. Am J Gastroenterol. 2009;104:953–965. doi: 10.1038/ajg.2008.124. [DOI] [PubMed] [Google Scholar]
- 30.Winkelmayer WC, Schneeweiss S, Mogun H, et al. Identification of individuals with CKD from Medicare claims data: a validation study. Am J Kidney Dis. 2005;46:225–232. doi: 10.1053/j.ajkd.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 31.Vlasschaert ME, Bejaimal SA, Hackam DG, et al. Validity of administrative database coding for kidney disease: a systematic review. Am J Kidney Dis. 2011;57:29–43. doi: 10.1053/j.ajkd.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 32.Kaufman J, Dhakal M, Patel B, et al. Community-acquired acute renal failure. Am J Kidney Dis. 1991;17:191–198. doi: 10.1016/s0272-6386(12)81128-0. [DOI] [PubMed] [Google Scholar]
- 33.Feest TG, Round A, Hamad S. Incidence of severe acute renal failure in adults: results of a community based study. BMJ. 1993;306:481–483. doi: 10.1136/bmj.306.6876.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liano F, Pascual J. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int. 1996;50:811–818. doi: 10.1038/ki.1996.380. [DOI] [PubMed] [Google Scholar]
- 35.Obialo CI, Okonofua EC, Tayade AS, et al. Epidemiology of de novo acute renal failure in hospitalized African Americans: comparing community-acquired vs hospital-acquired disease. Arch Intern Med. 2000;160:1309–1313. doi: 10.1001/archinte.160.9.1309. [DOI] [PubMed] [Google Scholar]
- 36.Greenland S, Lash TL. Bias analysis. In: Rothman KJ, Greenland S, Lash TL, editors. Modern epidemiology. 3rd. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 37.Chubak J, Pocobelli G, Weiss NS. Tradeoffs between accuracy measures for electronic health care data algorithms. J Clin Epidemiol. 2012;65:343–349.e2. doi: 10.1016/j.jclinepi.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Included Formulations by Treatment
Supplementary Table 2. Covariate Definitions
Supplementary Table 3. HRs of the Risk of the Need for Dialysis in OSP Users Compared With PEG Users
Supplementary Table 4. HRs of the Risk of AKI Requiring Dialysis in OSP Users Compared With PEG Users
Supplementary Table 5. HRs of the Risk of Any Renal Failure in OSP Users Compared With PEG Users


