Abstract
Attention deficits are prevalent among individuals with substance use disorders and may interfere with recovery. The present study evaluated the effectiveness of an automated electroencephalogram (EEG) biofeedback system in recovering illicit substance users who had attention deficits upon admission to a comprehensive residential treatment facility. All participants (n = 95) received group, family, and individual counseling. Participants were randomly assigned to 1 of 3 groups that either received 15 sessions of automated EEG biofeedback (AEB), 15 sessions of clinician guided EEG biofeedback (CEB), or 15 additional therapy sessions (AT). For the AEB and CEB groups, operant contingencies reinforced EEG frequencies in the 15–18 Hz (β) and 12–15 Hz (sensorimotor rhythm, “SMR”) ranges and reduce low frequencies in the 1–12 Hz (Δ, θ, and α) and 22–30 Hz (high β) ranges. The Test of Variables of Attention (TOVA), a “Go-NoGo” task, was the outcome measure. Attention scores did not change on any TOVA measure in the AT group. Reaction time variability, omission errors, commission errors, and d′ improved significantly (all p values < .01) in the AEB and CEB groups. AEB and CEB did not differ significantly from each other on any measure. The results demonstrate that automated neurofeedback can effectively improve attention in recovering illicit substance users in the context of a comprehensive residential substance abuse treatment facility.
Keywords: ADHD, attention, biofeedback, EEG, neurofeedback
Currently, there are no evidence-based guidelines for the treatment of attention deficits specific to individuals who are recovering from substance use disorders. Whereas approximately 4% of the general adult population has attention deficit hyperactivity disorder (AD/HD), as many as 35% of cocaine users seeking treatment meet the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM–IV) criteria for AD/HD and nearly one of every four individuals with a substance use disorder has AD/HD (Barkley, Murphy, & Fischer, 2010; Kessler, Lane, Stang, & Van Brunt, 2009; van Emmerik-van Oortmerssen et al., 2012). AD/HD is characterized by impulsivity, distractibility, and poor concentration such that people with AD/HD display a predisposition toward rapid, unplanned reactions to internal or external stimuli without regard to the negative consequences of these reactions (American Psychiatric Association, 2000).
Individuals who enter recovery programs with co-occurring substance use disorder and attention deficits more often fail to complete treatment programs, have lower abstinence rates, and have longer courses of substance use disorder than do those without attention deficits (Moeller et al., 2001; Wilens, 2004). Thus, for substance users with attention deficits, progress toward the goal of long-term sobriety may benefit from the effective treatment of the attention deficits and/or impulsivity.
Psychostimulant drugs are the primary medical treatment for attention deficits. Not surprisingly, people with substance use disorders are at risk for abusing psychostimulants prescribed for attention deficits (Kollins, MacDonald, & Rush, 2001). Additionally, key aims of substance abuse treatment programs are to increase individuals’ skills for emotional, cognitive, behavioral self-regulation and reduce chemical dependency.
EEG biofeedback (a.k.a., neurofeedback) has shown promise as a behavioral treatment for attention deficits (for a review, see Arns, de Ridder, Strehl, Breteler, & Coenen, 2009; Arns, Heinrich, & Strehl, 2014). The key aim of EEG biofeedback for attention deficits is to train people to self-generate a state of relaxed, alert, stable, focus (“effortless attention”) and enhance executive cognitive control by rewarding patterns of EEG activity associated with those mental states. Specific emotional, motivational, and cognitive processes and behaviors have been associated with particular EEG frequency bands (Onton & Makeig, 2009). During EEG biofeedback, electrical potentials generated by the neocortex are monitored using sensors on the scalp. EEG biofeedback incorporates principles of operant conditioning in which select EEG frequencies are differentially reinforced while the participant receives real-time information reflecting the ongoing cortical electrical activity.
The literature on EEG biofeedback effects on AD/HD symptoms is mixed, which is not surprising given the broad variety of training parameters and outcome measures that have been used by various investigators. One meta-analysis reported large effect sizes for inattention and impulsivity and a medium effect size for hyperactivity and concluded that EEG biofeedback is an “efficacious and specific” AD/HD treatment (Arns et al., 2009). Other reviews, however, have concluded that the overall reduction of AD/HD symptoms from neurofeedback is “probably efficacious” (Lofthouse, Arnold, Hersch, Hurt, & DeBeus, 2012) or only “trend toward efficacious” (Sonuga-Barke et al., 2013).
EEG biofeedback also has shown preliminary promise as a complementary treatment for substance use disorder (for a review see, Sokhadze, Cannon, & Trudeau, 2008). Peniston and Kulkosky (1989, 1990) treated alcoholics by employing auditory feedback of two slow brain wave frequencies, α (8–13 Hz) and θ (4–8 Hz) (the “Peniston protocol”). Alpha-theta feedback induces a hypnagogic state in which participants rehearse success imagery (e.g., being sober, refusing offers of alcohol, being confident, and happy). Repeated EEG biofeedback under the Peniston protocol was first found to improve long-term abstinence in alcoholics (Peniston & Kulkosky, 1990).
Scott, Kaiser, Othmer, and Sideroff (2005) used the Peniston protocol and along with a SMR (12–15 Hz) -β (16–21 Hz) feedback protocol designed for treating attention deficits with 121 heroin, cocaine, methamphetamine, and polysubstance abusers. Scott et al.’s (2005) participants were randomized to EEG biofeedback (in addition to therapy, a 12-step program) versus therapy alone and they measured treatment retention, abstinence, attention (assessed via the Test of Variables of Attention [TOVA], a “Go-NoGo” task), and personality (assessed via the Minnesota Multiphasic Personality Inventory [MMPI]-2) changes. Attention improved significantly for the EEG biofeedback group but not for the therapy-only control group. Additionally, EEG biofeedback significantly changed seven of the 10 scales of the MMPI-2. Finally, subjects in the EEG biofeedback group remained engaged in the treatment program longer and were more successful at maintaining abstinence for 1 year than were those in the therapy alone control group (77% vs. 40%, respectively). Dehghani-Arani, Rostami, and Nadali (2013) reported that an EEG biofeedback protocols like the ones used by Scott et al. (2005) significantly reduce craving in opiate dependent individuals.
Until recently, the administration of EEG biofeedback required complex interpretations of baseline EEG, participants’ presenting symptoms, between-session changes in symptoms, and within-session reward criteria. Most clinicians who treat substance use disorders are not trained to operate EEG biofeedback equipment, potentially limiting the use EEG biofeedback in the context of substance use recovery programs.
In the present study we investigated the effects of EEG biofeedback that was provided using either a clinician-guided (by a board certified neurotherapist with more than 15 years of experience) or automated (BrainPaint®) EEG biofeedback systems on objective measures of attention, and impulsivity in treatment in 95 patients with substance use disorders co-occurring with attention deficits. A control group that received additional therapy time also was included. If the automated EEG biofeedback system performs as well as the clinician-guided system, EEG biofeedback may be feasible as an adjunctive therapy in substance use disorder treatments for patients with attention deficits.
Method
Design
The study was a randomized, mixed factorial design. The between groups factor included three levels of EEG biofeedback: 15 training sessions of clinician-guided EEG biofeedback (CEB), 15 training sessions of automated EEG biofeedback (AEB), or treatment as usual plus matched time receiving additional time counseling sessions instead of EEG biofeedback (AT). The within-subject factor included repeated attention assessments at baseline and after every five EEG biofeedback or additional counseling sessions. An EEG biofeedback sham-control group was not included for ethical reasons, limiting conclusions that can be made about the specificity of the EEG biofeedback effects.
In addition to the experimental treatments, all subjects received the standard program of therapy provided to all Cri-Help patients that is based on the Minnesota Model 12-step oriented program supported by group, family, and individual counseling (Stinchfield & Owen, 1998). Attention was measured using the visual form of the Test of Variables of Attention (TOVA, version 7).
Participants
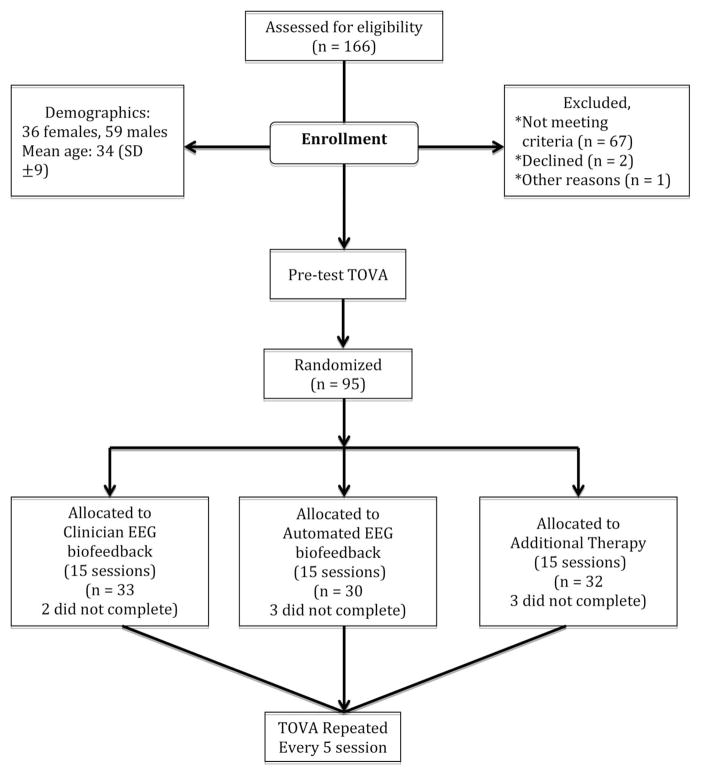
The participants were 36 female and 59 male subjects recruited from patients receiving substance abuse treatment at the Cri-Help, Inc. residential program in North Hollywood, California. The participants ranged in age from 18–56 with a mean age of 34 (SD ± 9). See Table 1 for a breakdown of demographic data by treatment group. The diagnosis of substance use disorder was established for each client during the clinical intake process via the DSM–IV, and TOVA scores were used to confirm attention abnormalities. Participants with attention deficits were not treated with ADHD medications while in residence at Cri-Help, Inc.
Table 1.
Demographic, TOVA, and Drug Preference Data for Additional Treatment (AT), Clinician-Operated EEG Biofeedback (CEB), and an Automated EEG Biofeedback (AEB) Group
| Variable | AT | CEB | AEB | p |
|---|---|---|---|---|
| n | 32 | 33 | 30 | |
| Sex (% female) | 37.5 | 30.5 | 37.0 | 0.30a |
| Age (M ± SD) | 30 ± 6 | 37 ± 10 | 32 ± 11 | 0.44b |
| Education (years) | 12.91 ± 1.70 | 13.29 ± 2.48 | 12.79 ± 2.44 | 0.64b |
| TOVA baseline | ||||
| RT variability | 85.5 ± 4.1 | 80.0 ± 4.0 | 78.7 ± 4.1 | 0.78b |
| Commission errors | 101.2 ± 3.0 | 93.3 ± 2.9 | 90.8 ± 3.1 | 0.16b |
| Omission errors | 77.9 ± 4 | 82.9 ± 4.0 | 77.0 ± 4.3 | 0.41b |
| d′ | 80.3 ± 4.4 | 84.3 ± 4.3 | 81.9 ± 4.5 | 0.69b |
| RT | 99.9 ± 4.3 | 102.7 ± 4.2 | 103.3 ± 4.4 | 0.80b |
| Race (%) | ||||
| African American | 3 | 3 | 0 | |
| Latino | 18 | 12 | 18 | |
| Caucasian | 67 | 82 | 67 | 0.87a |
| Other | 6 | 3 | 3 | |
| Primary drug (%) | ||||
| Methamphetamine | 32.2 | 36.5 | 55.2 | 0.38a |
| Heroin | 22.6 | 30.3 | 20.7 | |
| Other opiates | 16.1 | 6.1 | 3.4 | |
| Alcohol | 3.2 | 12.1 | 10.3 | |
| Marijuana/hashish | 6.5 | 9.1 | 3.4 | |
| Cocaine/crack | 9.7 | 6.1 | 3.4 | |
| Sedatives | 3.2 | 0 | 0 | |
| Other | 3.2 | 0 | 3.4 | |
Chi square test.
One-way ANOVA.
Attention Measurements
The TOVA is a continuous performance, Go-NoGo task that has been used by clinicians and researchers to assist in the diagnosis of attention deficits and to assess of AD/HD treatment efficacy (Monastra, Monastra, & George, 2002). The TOVA takes 21.6 minutes to complete, during which time monochrome visual targets (a square with a smaller square inside of it near the upper border) and nontargets (a square with a smaller square near the lower border) appear at irregular intervals. Participants were instructed to respond by clicking a microswitch when targets appeared and to refrain from clicking for nontargets. Response times were acquired with ± 1 millisecond (ms) accuracy. Stimuli were presented for 100 ms. Equal numbers (324) of targets and nontargets were presented during the 21.6-min session and interstimulus intervals ranged from 500 to 2000 ms. The first half of the TOVA contains a low ratio of targets to nontargets (1:3.5; ‘infrequent target condition’) and purports to measure inattentiveness/distractibility as reflected by errors of omission. The second half of the TOVA is characterized by a high target to nontarget ratio (3.5:1; ‘frequent target condition’) and is aimed at assessing impulsivity, as indicated by errors of commission. Dependent variables include response time (how quickly the microswitch is pressed), response time variability, commission errors (impulsivity), and omission errors (inattentiveness). A further measure that takes into account both of these error types is perceptual sensitivity or ‘d prime’ (d′), which expresses a ratio of hit rate (H) to false alarm rate (F), derived from signal detection theory (Swets & Green, 1978). d′ is not simply H-F; rather, it is the difference between the z transforms of these two rates: d′ = z(H) − z(F).
The scores are all reported as standard scores that reference a database of age- and sex-normative scores (Leark, Greenberg, Kindschi, & Dupuy, 2007).
Apparatus
The clinician-guided EEG biofeedback was conducted by a board certified neurofeedback therapist with 15 years of experience using Neurocybernetics® software. The hardware used with this system was a 3.2 GHz dual core PC and the Neurocybernetics® amplifier with a 120 Hz sampling rate. The monitor had a refresh rate of 32 Hz and speakers were attached to the PC soundcard. The system used for the automated EEG biofeedback incorporated the BrainMaster Atlantis II® with a sampling rate of 256 Hz with 3rd order Butterworth filters controlled by a laptop PC. Ten-20® EEG conductive paste was used to enhance conductivity between skin and electrodes.
Procedures
Cri-Help patients who agreed to receive inpatient treatment at the Cri-Help facility for a minimum of 2.5 weeks and had been abstinent from mood altering substances for two or more weeks were invited to volunteer for the study, including individuals who had been long-term residents at the Cri-Help facility. The program included both detoxification and residential treatment, and the recommended length of stay was individualized. All participants provided signed informed consent, and the Cri-Help, Inc. Institutional Review Board approved all study procedures.
All study candidates were first screened for psychological and psychiatric disorders using the DSM–IV. Subjects with a current diagnosis of depression or a previous diagnosis of psychosis, personality disorder, bipolar disorder, or seizure disorder were excluded. All volunteers were also screened for attention deficits using the TOVA (version 7) (Leark et al., 2007). Those who scored more than one standard deviation below normal on at least one TOVA variable during the screening assessment were enrolled and randomly assigned to either a control group that received additional therapy (AT; n = 32), clinician operated EEG biofeedback in addition to standard treatment (Clinician, CEB; n = 33), or automated EEG biofeedback in addition to standard treatment (Automated, AEB; n = 30). The randomization process was overseen by members of the research team who were blind to the clinical administration of the various forms of treatment. Three participants in the AT group, four in the CEB group, and two in the AEB withdrew from the study before completing EEG biofeedback training or additional therapy. The study results reported below included all randomized participants. Mixed models without any ad hoc imputation have been shown to provide more powerful tests than does mixed model analysis with last observation carried forward, best value replacement, or worst value replacement (Chakaraborty & Gu, 2009). Therefore, no ad hoc imputation methods were employed in the analyses presented below.
Participants randomized to the clinician- and automated-EEG biofeedback groups underwent two 30-min neurofeedback sessions per day, five days a week (weekdays) for a total of 15 sessions. β and SMR training were included in each individual session. The sensors for the training were placed at C5 and referenced to A1 with the ground sensor on the opposite ear. For SMR training we used C6 referenced to A2 with the ground on A1. The sensor sites were based on the International 10–20 system. Sensor impedance was tested and kept below 5K ohms. For both EEG biofeedback training groups, reward contingencies (points and sounds) were used to shape participants to increase EEG power in frequencies in the ranges between 15 and 18 Hz (β) and 12–15 Hz (SMR) and reduce power in frequencies in the 1–12 Hz (Δ, θ, and α) and 22–30 Hz (high β) ranges. Frequencies outside of these ranges did not influence feedback. Segments of EEG that contained noise exceeding ± 100 μV were classified as artifacts (e.g., movement or muscle) and filtered. Participants received a 20 second break after every two minutes of training. Thresholds were adjusted in a way that if the participant maintained the reinforcement band above the threshold for 80% of the time during at least 0.5 s, and the suppressed band under the threshold for 20% of the time, feedback was received. Whenever participants could maintain the reinforced EEG frequencies above the threshold for 90% of the time during two continuous trials, the threshold was changed so that participants had to produce more EEG in the target frequency range to score points (Scott et al., 2005). The CEB system required the therapist to watch for these percentages and manually adjust thresholds where the AEB system automated that procedure.
Each EEG biofeedback session consisted of individual periods of β training at C5 and SMR training at C6. The proportions of β and SMR, however, that comprised participants’ starting protocols were tailored according to TOVA baseline data. Those participants whose TOVA performances response times that both were 15% slower and 15% more omission errors (inattentiveness) than the normative sample received an EB protocol of 80% C5 β and 20% C6 SMR. Conversely, subjects with 15% below average commission errors and 15% faster response times (impulsiveness) than the normative sample received protocols in which 20% was C5 β and 80% C6 SMR. For all other participants (the majority) the starting protocols consisted of β training 50% of the time and SMR training 50% of the time. The software was programmed to adjust these percentages daily based on a 12-item questionnaire that collected information about symptoms likely related to over- and underarousal. The questions asked subjects to rate each of the following items as being average, better or worse: falling asleep, staying asleep, dreams, energy, irritability, attention, sadness, anxiety, fatigue, night sweats, body tension, and worrying. Responses to these questions that were consistent with anxiousness caused the software to administer less β training and more SMR. Responses consistent with lethargy caused the software to administer more β training and less SMR.
During the administration of the clinician-guided EEG biofeedback protocol, the clinician sat with the subject and observed artifacts such as muscle tension, foot tapping, and talking, and verbally instructed the subject to better participate with the feedback. The clinician manually paused the feedback until the subject remained still enough to resume training without corrupting the EEG signal with movement artifacts. The participants in this group received feedback in the form of a game (‘Mazes’) that had an appearance that was similar to the classic video game Pacman®. In the Mazes game, an icon moved through a maze eating dots. The speed and color of the icon were contingent on EEG parameters. Amplitude in the reward frequency determined the color and speed of the icon; it becomes darker and slower when the β or other reward frequency was too low in amplitude; as the amplitude in the reward frequency band increased, the icon became brighter and faster. When the amplitude equaled or exceeded the goal, the icon was at its maximum brightness and speed and points for success were displayed on the screen. When EEG amplitudes in the low or high frequency inhibit band exceeded the target window, then the icon stopped moving and turned black. When the icon reached the end of the maze, there was a brief rest for the client and a bar graph of his or her progress was shown. Then a new maze started. There were 30 different mazes; if more than 30 training periods were given, the early mazes were repeated. The clinician operating the system for reward and inhibits determined the EEG frequency threshold settings and manually readjusted them if one were beyond the desirable ranges for more than 30 s.
The automated, software-controlled neurofeedback system (Brain-Paint®) was programmed to set target EEG frequency thresholds, provide real-time within session coaching and instructions to the participant, and also detect movements, talking, and muscle tension artifacts. During the first 40 seconds of each automated EEG biofeedback session, participants were instructed to sit as still as possible and to relax muscle tension. This allowed the software to capture a period of artifact free data used as a reference. Muscle and movement artifacts produce large increases in the EEG amplitudes in the 22 to 30 Hz range. When the software detected increases in this frequency range that were 30% greater than the amplitudes recorded during the artifact free reference data, these were treated as muscle/movement artifacts and participants were instructed to relax and remain still via verbal feedback displayed on the computer monitor. The BrainPaint® system displayed a bar graph that was proportionately more green than red when the reward β, SMR, and θ conditions were met and more red than green when the EEG frequency and power when the reward conditions were not being met. An image of a geometrical figure with a fractal structure was displayed until on the left side of the screen for as long as participants maintained the target EEG parameters. A 20-s break period occurred after every two minutes of training. During the break, information based on the detection of artifacts in the EEG record commonly associated with muscle tension, talking, foot tapping, and “zoning out” were reported along with encouragement to reduce such behavior. When excessive movements were detected, the neurofeedback session was automatically paused and the computer verbally instructed the subject to remain still to resume the neurofeedback session.
The experimental subjects received two sessions per day five days per week, Mondays through Fridays between the hours of 9:00 a.m. to 4:00 p.m. These subjects received a total of 15 sessions in 1.5 weeks. The TOVA was administered at baseline (Session 0), and after five, 10, and 15 sessions or in the case of AT participants, the corresponding passage of time. Each group’s data are shown as a function of the number of EEG biofeedback sessions.
Results
The results of the TOVA administered at four testing intervals were analyzed and the results are presented in Figures 1 and 2.
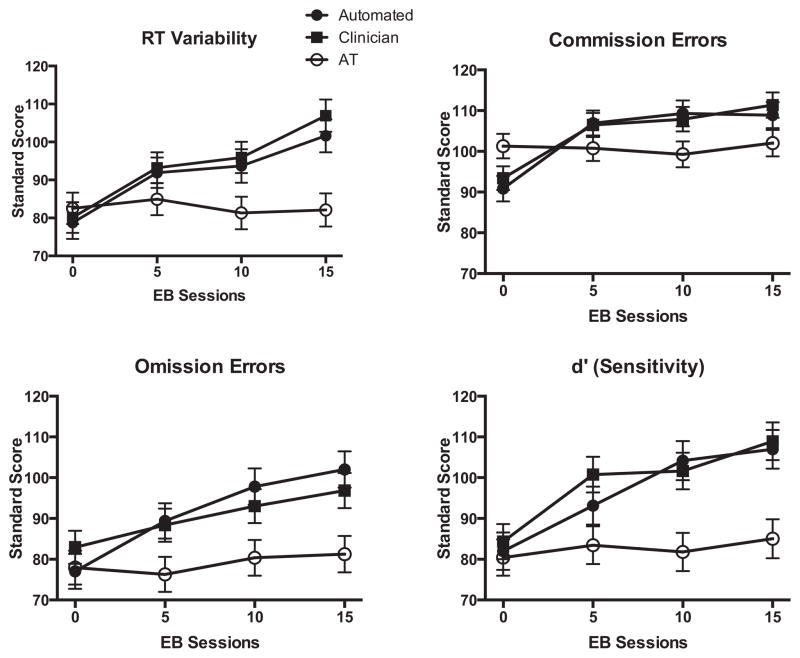
Figure 1.
TOVA RT variability, commission errors, omission errors, and d′ as a function of group and EEG biofeedback sessions, or equivalent time intervals for treatment as usual (AT) group. Clinician- and Automated-EEG biofeedback groups are abbreviated as Clinician and Automated, respectively.
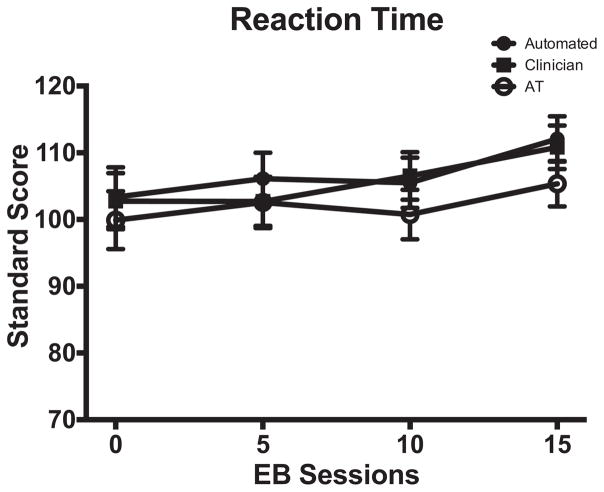
Figure 2.
TOVA RTs as a function of group and EEG biofeedback sessions, or equivalent time intervals for additional therapy (AT) group. Clinician- and Automated-EEG biofeedback groups are abbreviated as Clinician and Automated, respectively.
Baseline scores did not significantly differ between the three groups on any of the TOVA measures [all p values ranged from 0.16 to 0.80, see Table 1]. We found improvement in the reaction time (RT) variability (RTV), omission errors, and d′ in both of the EEG biofeedback groups over the four administrations of the TOVA. The AT group showed no change in any of the measures of TOVA during the four administrations (see Figure 1). Linear mixed models reported significant effects of group for RTV (F = 3.155, p = .047), omission errors (F = 4.40, p = .015), and d′ (F = 7.48, p = .001) and pairwise comparisons revealed significant (p < .05) differences between the groups that received EEG biofeedback and AT but not between the two neurofeedback conditions on these variables. EEG biofeedback group differences in commission errors failed to reach statistical significance (F = 0.79, p = .45). Group × session interactions were also reported for RTV (F = 3.55, p = .002), commission errors (F = 4.29, p < .0001), and d′(F = 2.24, p = .04). For each of these variables, the 95% confidence interval (CI) upper and lower bounds for the measurements taken at baseline were outside the 95% CIs taken during the terminal TOVA evaluation for the EEG biofeedback groups. The slopes of changes observed across sessions were computed and the means and standard deviations of each group’s slopes were used to compute effect sizes (Cohen’s d). For RTV, Cohen’s d = 0.93 (AEB vs. AT) and 0.82 (CEB vs. AT). For commission errors, Cohen’s d = 0.74 (AEB vs. AT) and 0.62 (CEB vs. AT). For d′, Cohen’s d = 0.52 (AEB vs. AT) and 0.53 (CEB vs. AT). The group × session interaction for TOVA omission errors did not reach statistical significance (F = 1.83, p = .09).
Figure 2 shows RT data for all groups. Unlike the other TOVA variables where baseline scores were generally poorer at baseline than normative scores (M = 100, SD = 10), baseline (Session 0) RTs were normal for all groups and did not change significantly over TOVA administrations for any group (ps > .05). This is important in reference to the TOVA variables discussed above in that stable RT scores across administrations indicates that improvements in RT variability, commission errors, omission errors, and d′ observed over the course of the study in the neurofeedback groups was not achieved at the cost of slower responding.
Discussion
The present study addressed a pragmatic question; can automated EEG biofeedback training be incorporated into a comprehensive residential substance use recovery program in a manner that effectively addressed attention deficits in recovering substance users? We found clear support for an affirmative answer to the above question. At baseline, all three groups scored approximately one standard deviation below the TOVA normative sample on three of four key attention variables. Participants who received clinician guided EEG biofeedback improved significantly on RT variability, impulsivity (commission errors), inattentiveness (omission errors), and signal detection to be on par with nonimpaired individuals relative to the TOVA normative sample and these effects were replicated in the automated EEG biofeedback group. Patients who did not receive EEG biofeedback showed no significant improvements on the TOVA subscores. The novel result of the present study is that the automated EEG biofeedback improved attention performance on the TOVA like the clinician-controlled system. Given that individuals with co-occurring substance use disorder and attention deficits have lower success rates in recovery than substance users without attention deficits (Moeller et al., 2001; Wilens, 2004), automated EEG biofeedback may provide an economically feasible approach to enhancing self-regulation of attention and impulsivity in the context of recovery treatment.
Importantly, at baseline TOVA RTs were normal for all groups and did not change significantly, indicating that the improvements observed in commission errors (impulsivity), omission errors (inattentiveness), and RT variability scores by the EEG biofeedback groups were not because those participants made their choices more slowly after receiving EEG biofeedback (i.e., participants did not sacrifice speed to achieve better accuracy). As mentioned above, RTs were more variable in all groups at baseline but variability normalized in the groups that received EEG biofeedback. Like TOVA omission errors, abnormal RT variability may reflect inefficient deactivation of the brain’s default mode network which manifests as brief lapses in attention and small clusters of slow responses (Klein, Wendling, Huettner, Ruder, & Peper, 2006; Sergeant, 2000; Weissman, Roberts, Visscher, & Woldorff, 2006). The term default mode network refers to a set of interconnected cortical regions, including the ventral and dorsal medial prefrontal cortex, the posterior cingulate/retrosplenial cortex, the inferior parietal lobule, lateral temporal cortex, and the hippocampal formation (Buckner, Andrews-Hanna, & Schacter, 2008). This default mode network is actively involved in self-referential mental activities (that arise spontaneously during “rest” periods), which are commonly involved in daydreaming, fantasizing, and planning (Mason et al., 2007). An inability to suppress the default mode network has been linked to distractibility (Fassbender et al., 2009).
The EEG biofeedback training employed in the present study rewarded participants for increasing EEG frequencies in the β and SMR ranges while down-training Δ, θ, and α. There is evidence that this training can alter activity in fronto-striatal brain circuits during tasks that engage executive cognitive function. For example, Lévesque, Beauregard, and Mensour (2006) studied SMR and β EEG biofeedback in AD/HD participants who were tested on the counting Stroop test before and after receiving EEG biofeedback. Participants who received SMR and β neurofeedback, but not the control group, showed task-related increases in activation levels in bilateral caudate, and left substantia nigra, structures important for attention and conflict monitoring (Botvinick, Cohen, & Carter, 2004; Lévesque et al., 2006). Interestingly, dysfunctional activity in brain circuits implicated in attention deficits also have been hypothesized to play a role in substance dependence (Keramati & Gutkin, 2013).
In view of the growing interest among psychologists in the therapeutic uses of meditation techniques, including mindfulness-based relapse prevention, some readers may be interested in similarities between meditation and EEG biofeedback (Brewer, Bowen, Smith, Marlatt, & Potenza, 2010; Witkiewitz, Lustyk, & Bowen, 2013; Zylowska et al., 2008). Interestingly, Brandmeyer and Delorme (2013) recently hypothesized that the core features are the same. In both situations one sits quietly for extended periods, practices focusing attention on specific stimuli; the breath and bodily sensations, in the case of meditation, or sounds and visual stimuli correlated with brain activity in the case of neuro-feedback. Both techniques aim to reduce mind wandering, obsessive thinking, fidgeting, and muscular tension and to cultivate awareness of these bodily, cognitive, and emotional states.
The present study was narrowly focused on evaluating whether an automated EEG biofeedback system could be integrated effectively into a comprehensive substance use recovery program. As such, there are numerous issues that remain to be addressed in future research. One important issue is whether the effectiveness of EEG biofeedback is related to individual differences in how well participant learned to produce the rewarded EEG frequencies, changes in EEG across or within session, or time over threshold. The software used to administer neurofeedback in this experiment was commercial software and was used in the present study “as is.” Unfortunately, the software did not include the option to record and report these variables. Such features would be extremely useful both for researchers and clinicians. Yet this should not detract from the fact that these programs used in their commercially available forms produced significant improvements in attention performance in the context of a residential treatment facility and that issue was the primary focus of the present study. Future studies should be conducted that investigate individual differences in neurofeedback effectiveness, and for such studies the above mentioned features would be crucial. Additionally, electromyogram biofeedback can produce effects on attention deficits, so it is possible that training muscle relaxation alone may be sufficient to produce the effects observed in the present study (Maurizio et al., 2014).
Unlike most prior studies of EEG biofeedback effects on attention, the participants involved in the present study were in treatment for substance use disorders. As such, the study was designed to execute the EEG biofeedback regimen as quickly and efficiently as possible. Pilot research conducted before the present study was initiated indicated that participants tolerated twice daily sessions well and that large effects on attention could be achieved rapidly relative to spacing sessions out over a longer timeframe. Thus, compared with other studies that spaced sessions out over several months, administered only a few sessions per week and required up to 40 EEG biofeedback sessions to achieve optimal results, the present achieved large effects on attention using a twice daily training schedule (Arns et al., 2009). Of course, outside of a residential facility twice daily training sessions may not be feasible. Finally, it should be noted that the study was not powered to investigate whether the two EEG biofeedback approaches used in the present study produce equivalent effects.
In summary, automated neurofeedback can effectively improve symptoms of attention deficits in recovering illicit substance users in the context of a comprehensive residential substance abuse treatment facility. Moderate to large improvements on objective indices of attention performance were observed in participants who received EEG biofeedback within 15 sessions. Therefore, EEG biofeedback should continue to be investigated as an adjunctive therapy for substance use disorder patients with attention deficits.
Acknowledgments
We thank Marcus Sola (CRI-Help, Chairman of the Board), Jack Bernstein (CRI-Help, CEO), and Marlene Nadel (CRI-Help, Clinical Supervisor) for their participation and willingness to add an innovative approach to their existing treatment model. Thanks also to the CRI-Help Board of Directors for providing funding for this project. We also thank EEG Spectrum International for their donation of a Neurocybernetics EEG biofeedback system, and Don Theodore, MA, MFT (Research Coordinator), who administered all EEG biofeedback protocols and coordinated subject sessions with the traditional treatment team. Universal Attention Disorders, Inc. contributed administrations of the Test of Variables of Attention (TOVA). The authors are not affiliated with Neurocybernetics®, BrainPaint®, or The TOVA Company®, financial or otherwise. Julian R. Keith received support from NCCAM (R15AT007226) during the analysis and authorship of this article.
Appendix. Study Flow Chart
Figure A1.
Contributor Information
Julian R. Keith, University of North Carolina, Wilmington
Lobsang Rapgay, University of California, Los Angeles.
Don Theodore, CRI-Help, Inc., Los Angeles, California.
Jeffrey M. Schwartz, University of California, Los Angeles
Jae L. Ross, Alliant International University
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. [Google Scholar]
- Arns M, de Ridder S, Strehl U, Breteler M, Coenen A. Efficacy of neurofeedback treatment in ADHD: The effects on inattention, impulsivity and hyperactivity: A meta-analysis. Clinical EEG and Neuroscience. 2009;40:180–189. doi: 10.1177/155005940904000311. [DOI] [PubMed] [Google Scholar]
- Arns M, Heinrich H, Strehl U. Evaluation of neurofeedback in ADHD: The long and winding road. Biological Psychology. 2014;95:108–115. doi: 10.1016/j.biopsycho.2013.11.013. [DOI] [PubMed] [Google Scholar]
- Barkley R, Murphy K, Fischer M. ADHD in adults: What the science says. New York, NY: Guilford Press; 2010. [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brandmeyer T, Delorme A. Meditation and neurofeedback. Frontiers in Psychology. 2013;4:688. doi: 10.3389/fpsyg.2013.00688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Bowen S, Smith JT, Marlatt GA, Potenza MN. Mindfulness-based treatments for co-occurring depression and substance use disorders: What can we learn from the brain? Addiction. 2010;105:1698–1706. doi: 10.1111/j.1360-0443.2009.02890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Chakaraborty H, Gu H. A mixed model approach for intent-to-treat analysis in longitudinal clinical trials with missing values. Research Triangle Park, NC: RTI International; 2009. RTI Press publication No. MR-0009–0903. Retrieved June 9, 2014 from http://www.rti.org/rtipress. [PubMed] [Google Scholar]
- Dehghani-Arani F, Rostami R, Nadali H. Neurofeedback training for opiate addiction: Improvement of mental health and craving. Applied Psychophysiology and Biofeedback. 2013;38:133–141. doi: 10.1007/s10484-013-9218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender C, Zhang H, Buzy WM, Cortes CR, Mizuiri D, Beckett L, Schweitzer JB. A lack of default network suppression is linked to increased distractibility in ADHD. Brain Research. 2009;1273:114–128. doi: 10.1016/j.brainres.2009.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keramati M, Gutkin B. Imbalanced decision hierarchy in addicts emerging from drug-hijacked dopamine spiraling circuit. PLoS ONE. 2013;8:e61489. doi: 10.1371/journal.pone.0061489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Lane M, Stang PE, Van Brunt DL. The prevalence and workplace costs of adult attention deficit hyperactivity disorder in a large manufacturing firm. Psychological Medicine. 2009;39:137–147. doi: 10.1017/S0033291708003309. [DOI] [PubMed] [Google Scholar]
- Klein C, Wendling K, Huettner P, Ruder H, Peper M. Intra-subject variability in attention-deficit hyperactivity disorder. Biological Psychiatry. 2006;60:1088–1097. doi: 10.1016/j.biopsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Kollins SH, MacDonald EK, Rush CR. Assessing the abuse potential of methylphenidate in nonhuman and human subjects: A review. Pharmacology, Biochemistry, and Behavior. 2001;68:611–627. doi: 10.1016/S0091-3057(01)00464-6. [DOI] [PubMed] [Google Scholar]
- Leark RA, Greenberg LK, Kindschi CL, Dupuy TR. Test of variables of attention: Clinical manual. Los Alamitos, CA: 2007. [Google Scholar]
- Lévesque J, Beauregard M, Mensour B. Effect of neurofeed-back training on the neural substrates of selective attention in children with attention-deficit/hyperactivity disorder: A functional magnetic resonance imaging study. Neuroscience Letters. 2006;394:216–221. doi: 10.1016/j.neulet.2005.10.100. [DOI] [PubMed] [Google Scholar]
- Lofthouse N, Arnold LE, Hersch S, Hurt E, DeBeus R. A review of neurofeedback treatment for pediatric ADHD. Journal of Attention Disorders. 2012;16:351–372. doi: 10.1177/1087054711427530. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: The default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurizio S, Liechti MD, Heinrich H, Jäncke L, Steinhausen HC, Walitza S, … Drechsler R. Comparing tomographic EEG biofeedback and EMG biofeedback in children with attention-deficit/hyperactivity disorder. Biological Psychology. 2014;95:31–44. doi: 10.1016/j.biopsycho.2013.10.008. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. Journal of Substance Abuse Treatment. 2001;21:193–198. doi: 10.1016/S0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- Monastra VJ, Monastra DM, George S. The effects of stimulant therapy, EEG biofeedback, and parenting style on the primary symptoms of attention-deficit/hyperactivity disorder. Applied Psycho-physiology and Biofeedback. 2002;27:231–249. doi: 10.1023/A:1021018700609. [DOI] [PubMed] [Google Scholar]
- Onton J, Makeig S. High-frequency broadband modulations of electroencephalographic spectra. Frontiers in Human Neuroscience. 2009;3:61. doi: 10.3389/neuro.09.061.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peniston EG, Kulkosky PJ. Alpha-theta brainwave training and beta-endorphin levels in alcoholics. Alcoholism: Clinical and Experimental Research. 1989;13:271–279. doi: 10.1111/j.1530-0277.1989.tb00325.x. [DOI] [PubMed] [Google Scholar]
- Peniston EG, Kulkosky PJ. Alcoholic personality and alpha-theta brainwave training. Medical Psychotherapy. 1990;3:37–55. [Google Scholar]
- Scott WC, Kaiser D, Othmer S, Sideroff SI. Effects of an EEG biofeedback protocol on a mixed substance abusing population. The American Journal of Drug and Alcohol Abuse. 2005;31:455–469. doi: 10.1081/ADA-200056807. [DOI] [PubMed] [Google Scholar]
- Sergeant J. The cognitive-energetic model: An empirical approach to Attention-Deficit Hyperactivity Disorder. Neuroscience and Biobehavioral Reviews. 2000;24:7–12. doi: 10.1016/S0149-7634(99)00060-3. [DOI] [PubMed] [Google Scholar]
- Sokhadze TM, Cannon RL, Trudeau DL. EEG biofeedback as a treatment for substance use disorders: Review, rating of efficacy, and recommendations for further research. Applied Psychophysiology and Biofeedback. 2008;33:1–28. doi: 10.1007/s10484-007-9047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Brandeis D, Cortese S, Daley D, Ferrin M, Holtmann M, … Sergeant J. Nonpharmacological interventions for ADHD: Systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. The American Journal of Psychiatry. 2013;170:275–289. doi: 10.1176/appi.ajp.2012.12070991. [DOI] [PubMed] [Google Scholar]
- Stinchfield R, Owen P. Hazelden’s model of treatment and its outcome. Addictive Behaviors. 1998;23:669– 683. doi: 10.1016/S0306-4603(98)00015-X. [DOI] [PubMed] [Google Scholar]
- Swets JA, Green DM. Applications of Signal Detection Theory. In: Pick HL Jr, Leibowitz HW, Singer JE, Steinschneider A, Stevenson HW, editors. Psychology: From research to practice. chap. 19. New York, NY: Plenum Press; 1978. pp. 311–331. [Google Scholar]
- van Emmerik-van Oortmerssen K, van de Glind G, van den Brink W, Smit F, Crunelle CL, Swets M, Schoevers RA. Prevalence of attention-deficit hyperactivity disorder in substance use disorder patients: A meta-analysis and meta-regression analysis. Drug and Alcohol Dependence. 2012;122:11–19. doi: 10.1016/j.drugalcdep.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nature Neuroscience. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Wilens TE. Impact of ADHD and its treatment on substance abuse in adults. Journal of Clinical Psychiatry. 2004;65:38–45. [PubMed] [Google Scholar]
- Witkiewitz K, Lustyk MKB, Bowen S. Retraining the addicted brain: A review of hypothesized neurobiological mechanisms of mindfulness-based relapse prevention. Psychology of Addictive Behavior. 2013;27:351–365. doi: 10.1037/a0029258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylowska L, Ackerman DL, Yang MH, Futrell JL, Horton NL, Hale TS, … Smalley SL. Mindfulness meditation training in adults and adolescents with ADHD: A feasibility study. Journal of Attention Disorders. 2008;11:737–746. doi: 10.1177/1087054707308502. [DOI] [PubMed] [Google Scholar]