Abstract
The aim of this article is to present a systematic review of magnetic resonance spectroscopy (MRS) studies of substance use disorders. As a noninvasive and nonionizing imaging technique, MRS is being widely used in substance abuse research to evaluate the effects substances of abuse have on brain chemistry. Nearly 40 peer-reviewed research articles that focused on the utility of MRS in alcohol, methamphetamine, 3,4-methylenedioxymethamphetamine, cocaine, opiates, opioids, marijuana, and nicotine use disorders were reviewed. Findings indicate inconsistencies with respect to alterations in brain chemistry within each substance of abuse, and the most consistent finding across substances was decreased N-acetylaspartate and choline levels with chronic alcohol, methamphetamine, and nicotine use. Variation in the brain regions studied, imaging technique, as well as small sample sizes might explain the discrepancies in findings within each substance. Future well-designed MRS studies offer promise in examining novel treatment approaches in substance use disorders.
Keywords: alcohol and drug abuse, neurochemistry, neuroimaging, magnetic resonance spectroscopy
Introduction
Magnetic resonance spectroscopy (MRS) is gaining popularity in substance abuse research as a tool for identifying neurochemical biomarkers and monitoring response to treatment. This noninvasive imaging technique does not use ionizing radiation, making it low risk for research on a number of populations including children. MRS provides in vivo data on the concentration of neurometabolites in human tissue found in specific regions of the body. Brain tissue is of particular interest for understanding neurocognitive disorders and psychiatric disorders including substance use disorders. Neurometabolites are products or derivatives of normal chemical metabolism and function as indicators of neurochemical activity. Information about the level of a specific metabolite or balance between metabolites determined by MRS can be used to track disease and assist in developing novel treatments targeted at bioenergetics or brain energy metabolism. The utility of MRS in substance use disorders is derived from important insights into etiology and cerebral consequences of substance abuse in addition to observing changes over time during abstinence.
There have been a growing number of MRS studies in substance abuse research over the past several years. Thus, we aimed to systematically review the MRS substance abuse literature to evaluate the effect substances have on brain chemistry and the relationship of brain chemistry alterations and cognitive function. The substance of this review is divided into two sections. The first section provides an overview of MRS technique as well as the neurometabolites that are commonly studied with MRS in the human brain. The second section focuses on the methods and results of the systematic review of the substance abuse MRS literature. In addition, we provide a synthesis of the recent MRS research efforts, outline the gaps in the knowledge, and discuss the future of MRS in substance use disorders.
Magnetic Resonance Spectroscopy
Methods
As a derivative of magnetic resonance imaging (MRI), the fundamentals of MRS are based on the principles of nuclear magnetic resonance (Castillo, Kwock, & Mukherji, 1996). The distinction between MRI and MRS, though, is that MRI produces cross-sectional images of water within the human body, whereas the observed peaks in MRS represent metabolite concentrations. Chemists have applied in vitro spectroscopy as a technique for evaluating compounds in solution since around the 1950s, but the application of MRS to study humans is relatively novel. The U.S. Food and Drug Administration approved the use of MRS as a clinical tool in 1995 (Gujar, Maheshwari, Bjorkman-Burtscher, & Sundgren, 2005), and while data can be collected from human tissue in nearly any part of the body, the brain has been most frequently studied. Most published studies of human brain biochemistry report findings using proton and phosphorus-31 (31P) MRS; however, other methods used include carbon-13 and fluorine-19 (Lyoo & Renshaw, 2002).
Proton MRS focuses on signals arising from hydrogen protons within small molecules that are present in the brain at relatively high concentrations. Proton MRS is the most commonly reported method in MRS studies of the brain. Proton MRS uses the same hardware that is used for routine clinical MRI, and as a result, it is readily accessible and the associated costs are relatively low (Barker & Lin, 2006). Another advantage to using proton MRS is that it is relatively straightforward to obtain and interpret, given the relative abundance of proton nuclei in human tissue. Phosphorus MRS, referred to as 31P MRS, detects the signal of the phosphorus nuclei and is also used in brain MRS studies but due to its technical limitations, it is not used as commonly as proton MRS. An advantage, however, of 31P MRS is the extensive information it provides with regard to bioenergetics as well as cellular membrane precursors and break down products (Reddy & Keshavan, 2003). In contrast, technical aspects of 31P MRS, such as low spatial resolution, which results in lower resolution images, and low signal-to-noise ratios (i.e., the ratio between the magnitude of a resonance and the magnitude of noise observed in the spectrum (Blüml & Panigrahy, 2012), limit its clinical applicability (Barker & Lin, 2006).
Like 31P, carbon-13 and fluorine-19, which detect the signal of the carbon-13 and fluorine-19 nuclei, respectively, are not commonly reported MRS methods compared with proton because they require specialized hardware and expertise. The main advantage, though, of employing carbon-13 MRS is the rich information it offers with respect to glialneuronal interactions (Mason & Krystal, 2006). Fluorine-19 MRS is most commonly used to detect and quantify fluorinated medications in the brain (Mason & Krystal, 2006).
Technical Considerations
There are different magnetic field strengths that are generated by a current and measured in Tesla that have been used in human brain MRS studies, ranging from 0.5 to 7 Tesla (Barker & Lin, 2006). The magnetic field strength is simply the strength or magnitude of the magnetic field. Higher field strengths have the advantage of increasing signal-to-noise ratios, enhancing signal detection and improving spatial resolution (i.e., more defined images; Lyoo & Renshaw, 2002).
A superconducting magnet creates a strong magnetic field and serves as the common factor in both MRI and MRS. The hardware used for MRS studies is similar to MRI with the exception of extra time that is required for data acquisition, shimming, and analysis. Shimming, the first step in acquiring clinical data from MRS, is a process used to maximize homogeneity of the magnetic field (Gujar et al., 2005). As the concentration of water exceeds the concentration of neurometabolites by a factor of more than 10,000, a second important step in spectroscopy is suppression of the water signal. Other MRS considerations include selection of specific acquisition methods, such as single-voxel spectroscopy versus chemical shifting imaging, which are methods that refer to the volume(s) of tissue being evaluated. For example, single-voxel spectroscopy requires less time but it limits investigations to the examination of single small volumes of tissue, whereas chemical shifting imaging permits evaluation of larger volumes of tissue at the expense of longer acquisition times (Gujar et al., 2005). Furthermore, parameter applications, such as echo time, are selected based on the neurometabolites of interest. A short echo time is used to detect metabolites with shorter relaxation times (e.g., glutamate and myo-inositol), and a long echo time is selected for the detection of metabolites with longer relaxation times (e.g., N-acetylaspartate, choline, and creatine; Gujar et al., 2005).
In summary, planning and performing MRS research differs from MRI research. It requires selection of appropriate data acquisition methods and parameters to answer a specific research question.
Measurable Compounds
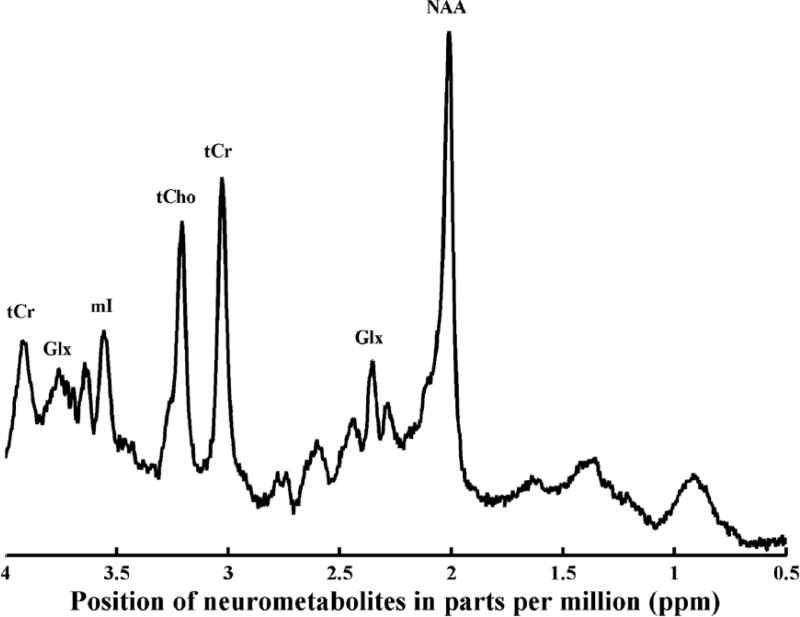
In MRI, the signal derives primarily from the hydrogen atoms of water and data are displayed as images. In MRS, the signal derives from compounds that are present at much lower concentrations, and results are typically represented as a collection of peaks (referred to as resonances) within a spectrum (Gujar et al., 2005) on the x-axis of line graph (see Figure 1). The line shown on the x-axis is continuous with peaks and valleys based on the metabolite position. The x-axis, or chemical shift, is shown as a ppm scale (parts per million) with the peaks identifying the location of a specific metabolite and the y-axis is proportional to the concentration of the metabolite being measured. The most commonly observed resonance peaks in proton MRS are N-acetylaspartate, creatine, choline, myo-inositol, gamma-aminobutyric acid (GABA), glutamate, and glutamine. As shown in Table 1, each of these metabolites is found at a specific ppm on the spectrum. Furthermore, each metabolite provides specific information about the function of the neurons, the cellular structures as well as some molecules that are involved with neurotransmission (see Table 1). The commonly observed resonance peaks in phosphorus MRS include phosphomonoesters, inorganic phosphate, phosphodiester, phosphocreatine, and alpha-, beta-, gamma-nucleoside triphosphate. The phosphorus spectrum of resonances provides information regarding phospholipid metabolism, bioenergetics, and pH (see Table 2).
Figure 1.
Proton magnetic resonance spectrum from human anterior cingulate cortex using a short echo time (30 seconds).
Note. Metabolites are labeled total creatine (tCr), glutamine + glutamate (Glx), myo-inositol (ml), total choline (tCho), total creatine (tCr), glutamine + glutamate (Glx), N-acetylaspartate (NAA).
Table 1.
Metabolites Displayed in a Resonance of Spectrum Using Proton Magnetic Resonance Spectroscopy.
| Metabolite | Spectrum location (ppm) | Role | Physiologic significance |
|---|---|---|---|
| N-acetylaspartate | 2.02 | Marker of neuronal integrity | Lower N-acetylaspartate concentrations are considered a sign of neuronal loss or damage |
| Creatine and phosphocreatine | 3.03 and 3.94 | Brain energy metabolism | Decreased creatine seen in liver disease and decreased phosphocreatine observed in depression |
| Choline | 3.20 | Cell membrane turnover | Higher levels of choline with demyelination and tumors |
| Myo-inositol | 3.56 | Glial marker | Increased myo-inositol seen in Alzheimer’s disease, diabetes mellitus, and multiple sclerosis; and decreased myo-inositol in chronic hepatic and hypoxic encephalopathies, stroke, Graves’ disease, and lymphoma |
| Glutamate and glutamine | 2.40 | Glutamate is the brain’s primary excitatory neurotransmitter and glutamine is a precursor to amino acid neurotransmitters | Increased glutamate with brain injury and in encephalopathy/hyperammonemias and reduced Glx noted in ADHD |
| gamma-aminobutyric acid | 3.03 | Primary inhibitory neurotransmitter | Decreased gamma-aminobutyric acid reported in schizophrenia |
Table 2.
Metabolites Displayed in a Resonance of Spectrum Using Phosphorus Magnetic Resonance Spectroscopy.
| Metabolite | Spectrum location (ppm) | Role | Physiologic significance |
|---|---|---|---|
| Phosphomonoesters | 5.15–7.02 | Precursor of membrane phospholipid synthesis | Increased phosphomonoester observed in tumors, neonate brain, and regenerating liver, and decreased concentrations seen in schizophrenia and bipolar disorder |
| Inorganic phosphate | 5.02 | Intracellular pH and metabolic pathways | Brain pH involved in maintaining optimal central nervous system; decreased inorganic phosphate reported in bipolar disorder |
| Phosphodiester | 2.67–3.20 | Breakdown products of membrane phospholipids | Decreased phosphodiester levels seen in schizophrenia |
| Phosphocreatine | 0 | Brain energy metabolism | Decreased phosphocreatine observed in depression |
| Gamma-, alpha-, and beta- nucleoside triphosphate | −2.48, −7.52, and −16.26, respectively | High-energy phosphate metabolism | Reduced nucleoside triphosphate seen in depression |
Proton Magnetic Resonance Spectroscopy Metabolites
N-Acetylaspartate
N-acetylaspartate is observed at 2.02 ppm in the spectrum and is the most prominent signal in the water suppressed proton spectrum. The precise physiological role of N-acetylaspartate is not known, but one of its functions relates to neuronal and axonal integrity while another role involves mitochondrial bioenergetics (Gujar et al., 2005). Lower concentrations of N-acetylaspartate have been interpreted as a sign of neuronal loss or damage in neurological (Achtnichts et al., 2013; Verma et al., 2013), sleep (Yadav et al., 2014), and psychiatric disorders (Kraguljac et al., 2012; Maltezos et al., 2014).
Creatine
The creatine resonances are composite peaks arising from both creatine and phosphocreatine, and they are observed at 3.03 and 3.94 ppm. Creatine and phosphocreatine are believed to be sensitive to changes in brain energy metabolism, and similar to N-acetylaspartate, alterations in brain creatine concentrations are considered to reflect mitochondrial dysfunction (Sung et al., 2013) as well as changes in cerebral energy metabolism (Iosifescu et al., 2008). The energy buffering system in the brain involves phosphocreatine and adenosine triphosphate. When an adenosine triphosphate molecule is consumed, a phosphate group is transferred from phosphocreatine to adenosine diphosphate by the enzyme creatine kinase, thereby replenishing adenosine triphosphate (Kondo, Hellem, et al., 2011). Given that there are changes in neuronal energy requirements, mitochondrial dysfunction may lead to reductions in the formation of phosphocreatine transferred by mitochondrial creatine kinase (Sung et al., 2013).
Choline
The cytosolic choline-containing compounds glycerophosphocholine and phosphocholine contribute to the choline peak observed at 3.2 ppm. Concentrations of glycerophospho choline and phosphocholine vary depending on brain region with higher levels found in myelin (Licata & Renshaw, 2010). Clioline-containing compounds play an important role in the synthesis and degradation of cellular membranes and are considered sensitive to changes in membrane turnover (Gujar et al., 2005). Furthermore, choline is considered an index of cellular density in brain tumors, as well as potentially serving as a marker of increased glial density relative to aging and disease processes (Nordahl et al., 2005).
Myo-inositol
The chemical shift of the myo-inositol peak is 3.56 ppm. The exact role of myo-inositol within the brain is not clear, but it is considered to be a marker of glial proliferation. Furthermore, myo-inositol is a sugar with involvement in regulating neuronal osmolarity, as well as metabolism of membrane-bound phospholipids (Kondo, Hellem, et al., 2011).
Glutamate and Glutamine
Both glutamate and glutamine resonate closely together as peaks located between 2.10 and 2.50 ppm. The peak seen at 2.40 ppm arises from the combined glutamate and glutamine resonances and is referred to as Glx (Licata & Renshaw, 2010). The concentration of glutamate in the brain exceeds the concentration of glutamine and therefore the glutamate signal often confounds measures of glutamine (Maddock & Buonocore, 2012). These two metabolites can be measured independently at field strengths greater than 3 Tesla with appropriate methods (Hurd et al., 2004), but Glx has been more commonly reported, especially in older reports. These metabolites are central to the glutamate–glutamine cycle, which plays a critical role in brain metabolism. In addition, glutamate is the brain’s primary excitatory neurotransmitter, and glutamine is a major precursor and fuel supply of amino acid neurotransmitters, like glutamate and GABA, as well as involvement in regulating the metabolism of brain ammonia (Maddock & Buonocore, 2012). Abnormal function of the glutamatergic system has been implicated in the pathophysiology of mood disorders (Sanacora, Zarate, Krystal, & Manji, 2008).
gamma-aminobutyric Acid
GABA is detected as a peak at 3.03 ppm. GABA is the primary inhibitory neurotransmitter in the mammalian central nervous system. Decreased brain GABA concentrations may suggest neuronal dysfunction (Long et al., 2013) and have been noted in several neuropsychiatric disorders (Chang, Cloak, & Ernst, 2003). Glutamate, glutamine, and GABA resonances are notorious for being difficult to measure (Licata & Renshaw, 2010), despite several methods that have been used to more easily obtain data from these metabolites. As a consequence, studies reporting these metabolites typically only appear in more recent publications.
Phosphorus Magnetic Resonance Spectroscopy Metabolites
Phosphomonoesters
The phosphomonoester resonance is seen at 5.15 to 7.02 ppm and contains the phosphomonoesters phosphorylethanolamine and phosphorylcholine as well as glycerophosphate and inositol phosphates. Phosphorylethanolamine and phosphorylcholine are the most abundant metabolites in this resonance, and they are precursors of membrane phospholipid synthesis (Kato, Inubushi, & Kato, 1998). The information provided from the phosphomonoester peak relates to neuronal membrane metabolism (Frangou & Williams, 1996).
Inorganic Phosphate
The chemical shift of the inorganic phosphate peak is 5.02 ppm, and given that it is detected between the phosphomonoester and phosphodiester peaks, it is challenging to quantitatively analyze (Kato et al., 1998). The inorganic peak reflects the pH dependent equilibrium between PO4− and PO4−2 ions, which allows the calculation of brain pH from the chemical shift of inorganic phosphate (Kondo, Hellem, et al., 2011). Brain pH is critically involved in maintaining healthy function of the central nervous system, and inorganic phosphate plays a role in several metabolic pathways (Kato et al., 1998).
Phosphodiester
The phosphodiester peak appears at 2.67 to 3.20 ppm on the phosphorus MRS spectra. The phosphodiester peak arises from both glycerophosphate and glycerophosphoethanolamine, and these metabolites reflect the breakdown products of membrane phospholipids (Kondo, Hellem, et al., 2011). Like the phosphomonoesters resonance, the phosphodiester peak provides information regarding neuronal membrane metabolism (Frangou & Williams, 1996).
Phosphocreatine
The phosphocreatine peak is the tallest peak and positioned in the middle of the phosphorus MRS spectra at 0 ppm. Phosphocreatine is abundant in tissues with variable energy demands, such as the brain and muscle tissue. Its role, through the creatine kinase reaction, is to replenish an adenosine triphosphate molecule after it is consumed by transferring a high-energy phosphate group to adenosine diphosphate (Kondo, Hellem, et al., 2011).
Alpha-, Beta-, and Gamma-Nucleoside Triphosphates
Nucleoside triphosphates form three distinct peaks: alpha (a; −7.52 ppm), beta (β; −16.26 ppm), and gamma (γ; −2.48 ppm). The β-nucleoside triphosphate peak reflects adenosine triphosphate, whereas the γ- and a-nucleoside triphosphate peaks include contributions from adenosine triphosphate as well as adenosine di- and monophosphate (Trksak et al., 2013). Because adenosine triphosphate is involved in many biochemical processes in the brain, the β-nucleoside triphosphate resonance is present at a higher concentration than the α- and γ-nucleoside triphosphate peaks (Kondo, Hellem, et al., 2011).
In summary, proton and 31P MRS are used to measure brain chemistry. Proton MRS evaluates the function of neuronal and cellular structures as well as measuring some molecules that are involved in neurotransmission, and 31P MRS examines neurometabolites that are involved in phospholipid metabolism, high-energy metabolism, and pH.
Method
A literature search was conducted using the U.S. National Library of Medicine’s PubMed and Cumulative Index to Nursing and Allied Health Literature databases to identify peer-reviewed substance abuse neuroimaging MRS studies by substance (e.g., alcohol, methamphetamine, 3,4-methylenedioxymethamphetamine [MDMA], cocaine, opiates or opioids, marijuana, and nicotine). The terms that were included in the search are outlined in Table 3. The following were used as inclusion criteria: adult (age > 18 years of age), proton or 31P MRS studies of alcohol, methamphetamine, MDMA, cocaine, opiates, opioids, marijuana, or nicotine use disorders, the use of a comparison group, studies written in English language, and to understand the progress in substance abuse research in the last decade, human studies published since 2004 were included. The following were used as exclusion criteria: review papers, intervention studies with a pharmacological treatment, and the chronic use1 of two or more substances of abuse (with the exception of nicotine). Furthermore, because psychiatric diagnoses may also have a relationship with changes in brain chemistry (Maddock & Buonocore, 2012), this review excluded studies that included substance use disorders with a known diagnosis of a comorbid mood disorder and, finally, MRS studies of HIV and substances of abuse were not included since HIV may also have an impact on brain chemistry (Masters & Ances, 2014).
Table 3.
Terms Included in the Search.
| Substance | Key search terms (# search results) | Number included in review |
|---|---|---|
| Alcohol | Alcohol and magnetic resonance spectroscopy (7,330) | 7 |
| Alcohol and MRS (1078) | ||
| Alcohol use disorder and magnetic resonance spectroscopy (93) | ||
| Alcohol use disorder and MRS (41) | ||
| Recovery from alcohol and magnetic resonance spectroscopy (119) | ||
| Recovery from alcohol and MRS (57) | ||
| Alcoholism and magnetic resonance spectroscopy (74) | ||
| Alcoholism and MRS (54) | ||
| Methamphetamine | Methamphetamine and magnetic resonance spectroscopy (59) | 11 |
| Methamphetamine and MRS (21) | ||
| Methamphetamine use disorder and magnetic resonance spectroscopy (8) | ||
| Methamphetamine use disorder and MRS (4) | ||
| Recovery from methamphetamine and magnetic resonance spectroscopy (4) | ||
| Recovery from methamphetamine and MRS (1) | ||
| 3,4-Methylenedioxymethamphetamine | 3,4-Methylenedioxymethamphetamine or MDMA and magnetic resonance spectroscopy (28) | 4 |
| 3,4-Methylenedioxymethamphetamine or MDMA and MRS (9) | ||
| 3,4-Methylenedioxymethamphetamine or MDMA use disorder and magnetic resonance spectroscopy (23) | ||
| 3,4-Methylenedioxymethamphetamine or MDMA use disorder and MRS (9) | ||
| Recovery from 3,4-methylenedioxymethamphetamine or MDMA and magnetic resonance spectroscopy (28) | ||
| Cocaine | Cocaine and magnetic resonance spectroscopy (57) | 3 |
| Cocaine and MRS (19) | ||
| Cocaine use disorder and magnetic resonance spectroscopy (10) | ||
| Cocaine use disorder and MRS (6) | ||
| Recovery from cocaine and magnetic resonance spectroscopy (4) | ||
| Recovery from cocaine and MRS (3) | ||
| Opiates and opioids | Opiates or opioids or heroin and magnetic resonance spectroscopy (122) | 2 |
| Opiates or opioids or heroin and MRS (25) | ||
| Opiate or opioid use disorder and magnetic resonance spectroscopy (22) | ||
| Opiate or opioid use disorder and MRS (11) | ||
| Recovery from opiates or opioids or heroin and magnetic resonance spectroscopy (120) | ||
| Marijuana | Marijuana or THC and magnetic resonance spectroscopy (60) | 2 |
| Marijuana or THC and MRS (18) | ||
| Marijuana use disorder and magnetic resonance spectroscopy (6) | ||
| Marijuana use disorder and MRS (3) | ||
| Recovery from marijuana and magnetic resonance spectroscopy (0) | ||
| Recovery from marijuana and MRS (0) | ||
| Nicotine | Nicotine and magnetic resonance spectroscopy (65) | 7 |
| Nicotine and MRS (11) | ||
| Nicotine use disorder and magnetic resonance spectroscopy (7) | ||
| Nicotine use disorder and MRS (3) | ||
| Recovery from nicotine and magnetic resonance spectroscopy (1) | ||
| Recovery from nicotine and MRS (1) | ||
| Total | 36 |
Sample
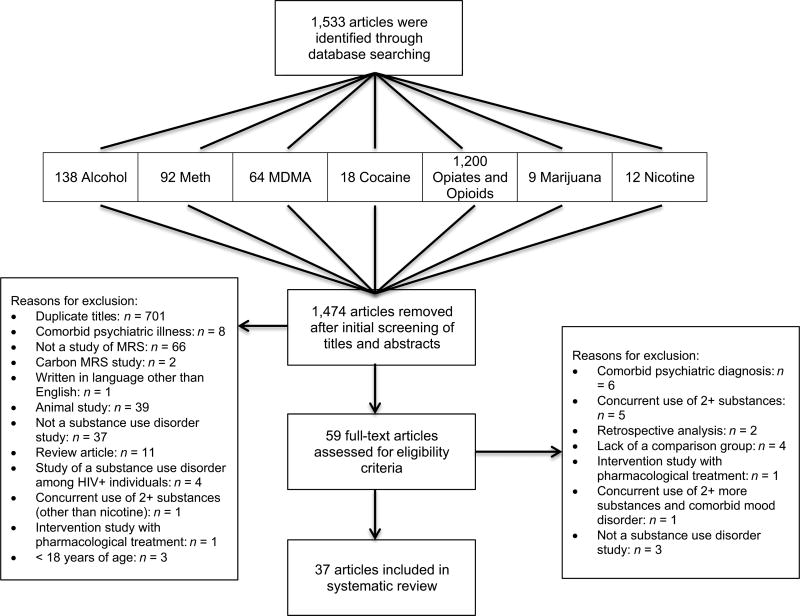
The initial computerized database search produced 1,533 articles. Titles were assessed for duplicate titles, in addition to reviewing abstracts for relevance, which reduced the sample to 59 articles. Each of the articles was reviewed, and after applying the eligibility criteria, 37 articles reporting MRS data in substance abuse research were included (see Figure 2). Of these 37, seven of them were studies in alcohol dependence, 11 in methamphetamine dependence, 4 in MDMA use, 3 in cocaine dependence, 3 in opiate and opioid dependence, 2 in chronic marijuana use, and 7 in nicotine dependence. Table 4 presents the published MRS studies that were included in this review.
Figure 2.
Flow of information through the different phases of the systematic review.
Table 4.
Magnetic Resonance Spectroscopy Studies of Substance Use Disorders.
| Study | Sample (n) | Study design | Scan type (field strength) |
Region (s) of interest |
Cross-sectional MRS findings | Longitudinal MRS findings |
|---|---|---|---|---|---|---|
| Alcohol | ||||||
| Durazzo, Gazdzinski, Banys, & Meyerhoff, 2004 | Recent abstinent (1 week) alcohol-dependent subjects (24) | Cross-sectional | Proton (1.5 T) | Major cerebral lobes | The alcohol-dependent group showed lower NAA (p = .02) and choline levels (p = .02) in frontal GM and WM compared to the light-drinking subjects. | |
| Light drinking subjects (26) | Subcortical nuclei | The alcohol-dependent group had lower GM, parietal lobe (both p = .02) and thalamic (p < .003) choline than the light-drinking subjects. | ||||
| Smoking alcohol-dependent subjects had reduced NAA in frontal WM (p = .007) and midbrain (p < .02) as well as midbrain choline (p < .02) compared with nonsmoking alcohol-dependent subjects. | ||||||
| The smoking alcohol-dependent group demonstrated lower NAA than the nonsmoking light-drinking group in frontal GM (p < .05), frontal WM (p < .005) and lower NAA in the smoking alcohol-dependent group than the nonsmoking light-drinking group and the smoking light-drinking group in frontal WM (p < .005). | ||||||
| The smoking alcohol-dependent and nonsmoking alcohol-dependent groups had lower choline than the nonsmoking light-drinking group in frontal GM (p = .001), parietal WM (p = .002) and frontal WM (p = .001). The smoking alcohol-dependent group had lower choline than the non-smoking and smoking light-drinking groups, and the nonsmoking alcohol-dependent group had lower choline than the smoking light-drinking group (p = .01). | ||||||
| Thalamic choline was also lower in the smoking and nonsmoking alcohol-dependent groups compared to the smoking and nonsmoking light-drinking groups (p = .008). | ||||||
| Meyerhoff et al., 2004 | Heavy drinking alcohol-dependent subjects (46) | Cross-sectional | Proton (1.5 T) | Major cerebral lobes | NAA in frontal WM was 5% lower in the heavy drinking group compared with light drinking group (p = .04) | |
| Light drinking subjects (52) | Subcortical nuclei | Frontal WM NAA was lower in family history-negative heavy drinking group compared to family history-positive heavy drinking group (p = .04), and also lower in family history-negative light drinking group (p = .01). | ||||
| Brainstem | Creatine in parietal GM was 8% higher in heavy drinking subjects than light drinking subjects (p = .005). | |||||
| Brainstem myo-inositol was 22% higher in family history-negative heavy drinking subjects versus family history-positive (p = .01). | ||||||
| Independent of drinking status, family history-negative individuals had 18% higher brain myo-inositol than family history-positive participants (p = .0004). | ||||||
| Parietal GM myo-inositol (p = .05) and choline (p = .07) were also higher in the heavy drinking group versus the light drinking group. | ||||||
| Lower frontal WM NAA observed in individuals older than 38 years in heavy drinking group (p = .02), as well as higher parietal GM myo-inositol (p = .009). | ||||||
| Ende et al., 2005 | Alcohol-dependent subjects with recent (mean = 16.5, SD = 7.3 days) abstinence (33) | Longitudinal | Proton (1.5 T) | Anterior superior cerebellar vermis | The alcohol-dependent group showed significantly lower choline values in the frontal WM (p = .002), cerebellar cortex (p < .001), and the cerebellar vermis (p = .001) compared to healthy controls at scan 1. | Abstinent subjects demonstrated a significant increase in choline in all sub regions at scan 2 (p < .001). |
| Cerebellum and pons | There were no differences in NAA and creatine between the two groups at scan 1 in the three frontal and cerebellar subregions. | There were changed in the choline and NAA values in alcohol-dependent subjects between scan 2 and scan 3. | ||||
| Healthy control subjects (30) | Junction of pons and midbrain | |||||
| Cerebellar vermis | ||||||
| Frontal lobe GM and WM | ||||||
| Superior frontal gyrus | ||||||
| Anterior cingulate gyrus | ||||||
| Mason & Krystal, 2006 | Alcohol-dependent males with recent (mean = 5, SD = 4 days) abstinence (12) | Longitudinal | Proton (2.1 T) | Occipital cortex | There were no group differences or smoking-related differences in NAA, choline or creatine at scan 1, in addition to no longitudinal differences. | |
| Healthy control males (8) | Glx was reported as higher in smoking than nonsmoking participants but did not differ between controls and alcohol-dependent subjects. | |||||
| In the alcohol-dependent group, NAA-adjusted mean levels of Glx in smokers were significantly higher than in nonsmokers (p value not provided). | ||||||
| Concentrations of GABA were higher in nonsmoking alcohol-dependent subjects at ~1 week abstinent compared to smoking alcohol-dependent subjects (p value not provided) | GABA levels decreased at scan 2 compared with scan 1 in nonsmoking alcohol-dependent subjects (p = .004), while no significant change was observed in smoking alcohol-dependent subjects (p = .75). However, there were no differences observed between these two groups at ~ 1 month of abstinence nor did they differ from the healthy controls. | |||||
| Bartsch et al., 2007 | Alcohol-dependent subjects with recent (mean = 3, SD = 1 days) abstinence (24) | Longitudinal | Proton (1.5 T) | Infratentorially in the left cerebellar hemisphere | There were no significant group differences of infra- or supretentorial NAA, choline, creatine and myo-inositol quantifications between alcohol-dependent subjects and controls in scans 1 or 2 | |
| Healthy control subjects (10) | Supratentorial level for the mesial frontal lobes | At scan 2, cerebellar choline (p < .01) and frontomesial NAA (p = .01) levels increased in alcohol-dependent subjects. | ||||
| Hermann et al., 2011 | Treatment-seeking alcohol-dependent subjects (47) | Longitudinal | Proton (3 T) | ACC | Decreased glutamate in the ACC of alcohol-dependent subjects at scan 2 compared with scan 1 (p < .05) and to controls (p < .01). | |
| Healthy control subjects (57) | ||||||
| Modi et al., 2011 | Alcohol-dependent males with recent (7 days) abstinence (9) | Cross-sectional | Proton (1.5 T) | PVC | A significantly elevated choline/creatine ratio (p < .015) was found in the occipital lobe of the alcohol-dependent group as compared to controls. | |
| Healthy control males (13) | ||||||
| Methamphetamine (METH) | ||||||
| Nordahl et al., 2005 | METH-using subjects with sustained (mean = 37.5, SEM = 5.9 months) abstinence (8) | Cross-sectional | Proton (1.5 T) | ACC | Creatine values did not differ between the groups in the ACC. | |
| METH-using subjects with recent (mean = 3.0, SEM = 0.4 months) abstinence (16) | PVC | Lower values for ACC NAA/creatine in both the short- and long-term abstinent groups of METH users compared with controls (p < .001). | ||||
| Healthy controls (13) | Choline/NAA ratios in the ACC were higher in the METH users with short-term abstinence compared to both long-term abstinent METH users (p = .01) and controls (p < .001), but not between long-term abstinent METH users and controls (p = .82). | |||||
| No differences in NAA, choline, or creatine were observed in the PVC between the two groups. | ||||||
| Salo et al., 2007 | METH-using subjects with recent (mean = 20.0, SEM = 5.4 months) abstinence (36) | Cross-sectional | Proton (1.5 T) | ACC | Creatine values did not differ between the groups in either the ACC or the PVC. | |
| Healthy control subjects (16) | PVC | Lower values for ACC NAA/creatine (p = .007) and higher values of ACC choline/NAA (p = .004) were noted in the METH-using group compared to controls. | ||||
| No metabolite differences were observed in the PVC. | ||||||
| Sung et al., 2007 | METH-using subjects with mean = 26.1, SD = 44.1 months abstinence (30) | Cross-sectional | Proton (3 T) | Frontal WM | No differences were found in NAA concentrations in either regions between groups, with the exception of In frontal WM, NAA concentration was significantly lower for METH users that self-reported using > 100 grams lifetime compared to METH users that self-reported using < 100 grams lifetime and controls (p = .004), but no differences relative to the abstinence duration. | |
| Healthy control subjects (20) | Midfrontal GM | Higher myo-inositol values were noted in left frontal white matter of METH-using subjects relative to controls (p = .02). | ||||
| NAA concentration in the frontal WM of male METH users was lower than that of healthy male controls (p = .009). | ||||||
| Ernst & Chang, 2008 | METH-using subjects with mean = 2.1, SD = 3.0 months abstinent (25) | Longitudinal | Proton (1.5 T) | ACC | At baseline, concentrations of Glx or NAA were not significantly different between METH-using subjects and controls subjects in any of the brain regions. | GLX values in frontal GM showed a trend to correlate inversely with the log of duration of abstinence (p = .07) in 92% of METH-using subjects scanned at 5-months abstinence. |
| Healthy control subjects (28) | Frontal WM | METH-using subjects with ≤1 month of abstinence showed lower Glx values in frontal GM compared to controls (p = .01) but not in the basal ganglia or frontal WM. | ||||
| Basal ganglia | Prefrontal GM GLX concentration was lower in METH-using subjects who reported craving symptoms compared to METH-using subjects who did not report craving symptoms (p = .05). | |||||
| Salo, Buonocore, et al., 2011 | METH-using subjects with sustained (mean = 46.9, SD = 27.6 months) abstinence (17) | Cross-sectional | Proton (1.5 T) | ACC | ACC choline/NAA ratios were high in the short-term abstinent group compared to controls (p < .001), but no differences were noted between the long-term abstinent group and controls. | |
| METH-using subjects with recent (mean = 2.9, SD = 1.7 months) abstinence (17) | PVC | Lower NAA/creatine levels in the ACC were found in the short-term abstinent METH-using group compared to controls (p < .0001) and long-term abstinent METH users (p = .01). | ||||
| Healthy control subjects (24) | No differences were observed between long-term abstinent METH-using subjects and controls. | |||||
| Salo, Nordahl, et al., 2011 | METH-dependent subjects (32) | Cross-sectional | Proton (1.5 T) | ACC | There was no difference in ACC or PVC creatine values between the two groups. | |
| Healthy control subjects (13) | PVC | Lower values for ACC NAA/creatine were observed in the METH-dependent subjects compared with controls (p = .02). | ||||
| Sung et al., 2013 | METH-dependent subjects (51) | Cross sectional | Phosphorus (3 T) | Frontal lobe | PCr levels in the METH-dependent group were reduced in the frontal lobe compared with those in the healthy control group (p = .001). | |
| Healthy control subjects (23) | Frontal PCr levels in female METH users were lower than male METH users (p = .04). | |||||
| Crocker et al., 2014 | Abstinent (median = 370 days) METH-using subjects (29) | Cross-sectional | Proton (3 T) | Medial prefrontal cortex | Reduced levels of glutamate (p = .000) and NAA (p = .02) were observed in the METH group relative to the unmedicated patients with first episode of psychosis and also relative to the control group (p < .001 and p = .002, respectively). | |
| Unmedicated subjects with first episode of psychosis (29) | ||||||
| Healthy control subjects (45) | ||||||
| Howells et al., 2014 | METH-dependent subjects abstinent from METH for a mean = 56, SD = 60 days (16) | Cross-sectional | Proton (3 T) | ACC | The METH-dependent + METH-induced psychosis group showed decreased NAA in the right ACC (p < .005) and right DLPFC (p < .05) when compared to controls. | |
| METH-dependent + METH-induced psychosis subjects abstinent from METH for a mean = 60, SD = 53 days (10) | DLPFC | The METH-dependent group demonstrated decreased NAA levels in the right ACC (p < .005) and right DLPFC (p < .05) when compared to controls. | ||||
| Healthy control subjects (19) | The METH-dependent group had decreased right DLPFC choline compared with the control group (p < .05). | |||||
| Lin, Jan, Kydd, & Russell, 2015 | METH-dependent subjects (18) | Cross-sectional | Proton (1.5 T) | Basal ganglia | There were no differences observed in NAA/total creatine, choline/total creatine and choline/NAA between the two groups in the basal ganglia or PVC. | |
| Healthy control subjects (22) | PVC | |||||
| O’Neill, Tobias, Hudkins, & London, 2015 | METH-using subjects with recent (range of 4–7 days) abstinence (44) | Cross-sectional | Proton (1.5 T) | Midline posterior cingulate | Lower mean Glx values were noted for the METH-using group in the precuneus (p = .031) and right inferior frontal cortex (p = .013) but not in the left inferior frontal cortex compared to the control group. | |
| Healthy control subjects (23) | Midline precuneus | |||||
| Bilateral inferior frontal cortex | ||||||
| 3,4-Methylenedioxymethamphetamine (MDMA or Ecstasy) | ||||||
| Daumann et al., 2004 | MDMA-using subjects (13) | Cross-sectional | Proton (1.5 T) | Left hippocampus | There were no significant differences in NAA, choline, or creatine observed in any of the three brain regions between the MDMA using subjects and controls. The hippocampal ratio of NAA/Cr showed a trend toward significance (p = .007). | |
| Healthy control subjects (13) | GM of the midfrontal and midoccipital cortex | |||||
| Cowan et al., 2007 | MDMA-using subjects (9) | Proton (4 T) | Midline occipital cortex | There were no significant differences observed in absolute mean levels of myo-inositol, NAA, or NAA/creatine and myo-inositol/creatine ratios between the two groups. | ||
| Healthy control subjects (7) | ||||||
| de Win et al., 2008 | Ecstasy-naïve young adults (188) | Longitudinal | Proton (1.5 T) | Left frontoparietal WM | At baseline, the two groups did not differ on NAA/creatine, choline/creatine or myo-inositol/creatine ratios in any brain region. | No significant effect of ecstasy was observed on brain metabolite ratios at the follow-up scan. |
| Incident ecstasy-using subjects (59) | Midfrontal and midoccipital GM | |||||
| Liu et al., 2011 | MDMA users (31) | Cross-sectional | Proton (3 T) | Basal ganglia | myo-inositol levels was noted to be higher in MDMA-using subjects compared to healthy controls (p < .001). | |
| Healthy control subjects (33) | ||||||
| Cocaine | ||||||
| Yang et al., 2009 | Chronic crack-cocaine subjects (14) | Cross-sectional | Proton (3 T) | ACC | There were no differences found in ratios of NAA/total creatine and total choline/total creatine between the two groups. | |
| ealthy control subjects (14) | Glutamate/total creatine ratio was lower in the crack-cocaine-using group relative to controls (p < .005). | |||||
| Trksak et al., 2013 | Non–treatment seeking cocaine-dependent subjects (8) | Longitudinal | Phosphorus (4 T) | Whole brain | At baseline, there were no differences in any of the phosphorus neurometabolites between cocaine-dependent subjects and healthy controls. | Cocaine-dependent subjects had higher α-NTP after recovery sleep than after sleep deprivation (p = .008), higher β-NTP after recovery sleep than both baseline (p = .016) and sleep deprivation (p = .001), and higher total NTP after recovery sleep than both baseline (p = .043) and sleep deprivation (p = .001). |
| Healthy control subjects (14) | ||||||
| Martinez et al., 2014 | Cocaine-dependent subjects (15) | Cross-sectional | Proton (3 T) | Left striatum | No differences were seen in glutamate or glutamine between the cocaine-dependent subjects and healthy controls. | |
| Healthy control subjects (15) | ||||||
| Opiates and opioids | ||||||
| Silveri et al., 2004 | Heroin-dependent subjects during their first month of methadone treatment (43) | Cross-sectional | Phosphorus (1.5 T) | Orbitofrontal and occipital cortices including basal ganglia and frontal cortex and containing GM and WM | PCr levels were lower in heroin-dependent subjects compared with healthy controls (p < .005). | |
| Healthy control subjects (15) | Heroin-dependent subjects had higher PDE than healthy controls (p = .05). | |||||
| PCr was reduced in the heroin-dependent subjects who were on methadone maintenance for 8–14 days (p < .05) and 15–28 days (p < .05) compared to those on methadone for 0–7 days and to healthy controls. | ||||||
| PME was elevated in the heroin-dependent subjects who were on methadone maintenance for 15–28 days compared to those on methadone for 0–7 days and to healthy controls (p < .05). | ||||||
| Hermann et al., 2011 | Opiate-dependent subjects currently on opioid maintenance therapy (17) | Cross-sectional | Proton (3 T) | ACC | In the ACC, no differences between the opiate-dependent subjects and controls were found with respect to NAA, choline, creatine, glutamate and Glx. | |
| Healthy control subjects (20) | Frontal WM | In frontal WM, higher choline was observed for the opiate-dependent group compared to healthy controls (p = .004). | ||||
| Marijuana | ||||||
| Silveri, Jensen, Rosso, Sneider, & Yurgelun-Todd, 2011 | Chronic marijuana-using subjects (15) | Cross-sectional | Proton (4 T) | Basal ganglia | Global myo-inositol/creatine ratios were lower in the chronic marijuana-using subjects relative to healthy controls (p = .003). No other significant group differences in global metabolite ratios were observed. | |
| Healthy control subjects (11) | Thalamus | myo-inositol/creatine decreases as GM content increases in healthy control subjects (p = .05) but not in the marijuana-using group (p = .49). | ||||
| Temporal and parietal lobes | The marijuana-using group showed lower estimated myo-inositol/creatine in pure WM than the healthy control group (p = .005). | |||||
| Occipital WM and GM | ||||||
| Mashhoon, Jensen, Sneider, Yurgelun-Todd, & Silveri, 2013 | Chronic marijuana-using subjects (13) | Cross-sectional | Proton (4 T) | Thalamus | Left thalami myo-inositol/creatine ratios were lower in chronic marijuana-using subjects relative to healthy controls (p = .05). No other significant group differences in metabolite ratios were observed. | |
| Healthy control subjects (10) | Temporal lobe | |||||
| POC | ||||||
| Nicotine | ||||||
| Epperson et al., 2005 | Nicotine-dependent subjects (16) | Longitudinal | Proton (2.1 T) | Midline of the occipital cortex | There were no differences pre-versus post-abstinence within each gender and when controlling for phase of menstrual cycle in females with respect to cortical GABA levels. | |
| Healthy control subjects (20) | Healthy nonsmoking females showed a decline in cortical GABA levels from the follicular to the midluteal menstrual phase (p = .0002), whereas the healthy nicotine-dependent females did not show a decline in cortical GABA levels. | |||||
| Gallinat et al., 2007 | Nicotine-dependent subjects (13) | Cross-sectional | Proton (3 T) | Left hippocampus | Glutamate concentrations did not differ between groups in either brain region. | |
| Former nicotine-dependent subjects (9) | ACC | |||||
| Healthy control subjects (16) | ||||||
| Gallinat & Schubert, 2007 | Nicotine-dependent subjects (13) | Cross-sectional | Proton (3 T) | Left hippocampus | Compared to healthy control subjects, nicotine-dependent subjects showed significantly lower hippocampal NAA (p = .023) but in the ACC. | |
| Healthy control subjects (13) | ACC | No differences between groups with respect to choline or creatine in the left hippocampus or ACC. | ||||
| Mashhoon et al., 2011 | Nicotine-dependent females who maintained abstinence with nicotine replacement therapy (4) | Proton (4 T) | dACC | The females who did not maintain abstinence showed reduced dACC glutamate/creatine ratios (p < .03), GABA(p< .01) and choline/creatine ratios (p < .01) relative to females who maintained abstinence. | ||
| Nicotine-dependent females who did maintain abstinence with nicotine replacement therapy (5) | POC | |||||
| Gutzeit et al., 2013 | Male nicotine-dependent subjects (14) | Longitudinal | Proton (3 T) | Left insular cortices | At scan 2, the nicotine-dependent group showed an increase in glutamine compared with scan 1 (p = .023). | |
| Male healthy control subjects (10) | The control group did not show any metabolic changes over the three scans. | |||||
| Mennecke et al., 2014 | Nicotine-dependent subjects (12) | Longitudinal | Proton (3 T) | Right and left anterior hippocampus | The ratio of Glx/total creatine (p = .01) was increased while total choline/total creatine ratios were decreased in the left ACC in nicotine-dependent subjects compared to controls (p = .01) at scan 2. | |
| Healthy control subjects (12) | dACC | |||||
| O’Neil et al., 2014 | Nicotine-dependent subjects (18) | Cross-sectional | Proton (1.5 T) | Thalamus | Thalamic glutamate levels did not differ between groups. | |
| Healthy control subjects (16) |
Note. T = Tesla; NAA = N-acetylaspartate; WM = white matter; GM = gray matter; Glx = glutamate + glutamine; GABA = gamma-aminobutyric acid; ACC = anterior cingulate cortex; PVC = primary visual cortex; DLPFC = dorsolateral prefrontal cortex; NTP = nucleoside triphosphate; POC = parieto-occipital cortex; dACC = dorsal anterior cingulate cortex; α-NTP = alpha nucleoside triphosphate; β-NTP = beta nucleoside triphosphate.
Findings
Alcohol
Magnetic resonance spectroscopy studies of alcohol dependence appear to be inconsistent with respect to an association between chronic alcohol use and changes in brain chemistry. For instance, N-acetylaspartate and choline were noted as lower in the frontal gray matter and in the frontal white matter at 1 week of abstinence from alcohol in alcohol-dependent subjects relative to light drinking subjects (Durazzo et al., 2004). In contrast, compared with healthy control subjects, there were no differences observed in concentrations of N-acetylaspartate and choline in the occipital cortex (Mason & Krystal, 2006) and infratentorially in the left cerebellar hemisphere or the mesial frontal lobe (Bartsch et al., 2007). Similarly, changes in N-acetylaspartate with longer periods of abstinence appear to be inconsistent with some studies reporting no longitudinal N-acetylaspartate changes with 1 to 3 months of abstinence (Ende et al., 2005; Mason & Krystal, 2006), while one study noted an increase in N-acetylaspartate values at 6 to 7 weeks of abstinence (Bartsch et al., 2007) compared with baseline. Consistently, though, relative to baseline measurements, an increase in choline levels with 1 to 3 months of abstinence was noted in the cerebellum (Bartsch et al., 2007; Ende et al., 2005) and the frontal lobe (Ende et al., 2005), indicating the potential of some recovery in the brain with abstinence from alcohol. Furthermore, creatine was reported as either elevated in alcohol-dependent subjects but not in light drinking subjects in the parietal gray matter (Meyerhoff et al., 2004), or as not differing from the control group in the frontal lobe and cerebellum (Ende et al., 2005) or occipital cortex (Mason & Krystal, 2006). Finally, longitudinal changes in creatine with recovery were not observed (Ende et al., 2005; Mason & Krystal, 2006).
The remaining proton metabolites (i.e., myo-inositol, glutamate, glutamine, and GABA) were not commonly reported. Two of the studies reported myo-inositol, and one of these two studies suggested that a family history of alcohol dependence influenced differences in brainstem myo-inositol levels between controls and alcohol-dependent individuals (Meyerhoff et al., 2004). The other study that reported myo-inositol noted that there were no changes in infra- or supretentorial myo-inositol values with 4 to 5 weeks of abstinence from alcohol compared with myo-inositol values at 1 week of alcohol abstinence (Bartsch et al., 2007). With respect to glutamate, anterior cingulate cortex glutamate decreased in alcohol-dependent males after 2 weeks of abstinence compared with baseline and compared with healthy controls (Hermann et al., 2011). Finally, cortical GABA concentrations decreased with 1 month of abstinence from alcohol in nonsmoking alcohol-dependent subjects but not smoking alcohol-dependent subjects, suggesting that smoking may influence GABA’s role in alcohol dependence and withdrawal (Mason & Krystal, 2005).
While changes in neurochemistry with alcohol use have been relatively well documented over the past 11 years, less is known about how these alterations influence cognition. Two studies employed neuropsychological testing to understand the association between brain chemistry and attention and concentration in alcohol users (Bartsch et al., 2007). The authors of one of the studies reported an increase in frontomesial N-acetylaspartate with 6 to 7 weeks of abstinence accompanied by improvements in attention, but no correlation between cognitive function and cerebral choline levels, or between long-term memory, nonverbal and verbal cognitive abilities, and N-acetylaspartate or choline (Bartsch et al., 2007). Similarly, independent of smoking status, Durazzo et al. (2004) found a positive correlation between cerebral vermis N-acetylaspartate levels and visuomotor scanning speed and incidental learning in alcohol-dependent subjects with 1 week of abstinence. There were no associations among other regional metabolite concentrations and measures of neurocognition.
Even though there are inconsistencies in the alcohol and MRS literature, these findings overall suggest that the relationship between brain chemistry and cognition may be region specific and perhaps provide evidence of repair to neuronal viability with early abstinence. Given the limited number of MRS studies of alcohol dependence that administered neuropsychological testing, more work in this area would be able to provide a better understanding of these relationships. Since alcohol dependence diagnosis was based on DSM criteria in all studies and the mean duration of dependence was relatively consistent across studies, these sources of individual difference do not account for the inconsistent pattern of findings. One possible explanation for the inconsistencies in study findings is the varying lengths of time abstinent (ranged from 0 to 17 days) from alcohol at the time of MRS procedures. It is also possible that demographic variables, such as age of onset of dependence and age of abstinence, also contributed to inconsistencies, but these variables were not consistently reported across all studies.
Methamphetamine
Results from proton MRS studies noted neuronal injury and cholinergic changes in a variety of brain regions. For example, a number of studies demonstrated reduced N-acetylaspartate values in the anterior cingulate cortex in persons with methamphetamine (METH) dependence with short-term abstinence relative to long-term abstinence (Salo, Buonocore, et al., 2011), compared with healthy controls (Howells et al., 2014; Nordahl et al., 2005; Salo, Buonocore, et al., 2011; Salo, Nordahl, et al., 2011) and in long-term abstinence compared with healthy controls (Salo et al., 2007), implying sustained neuronal injury with chronic METH use. On the contrary, two of the studies did not observe N-acetylaspartate differences between recovering (Ernst & Chang, 2008) and active (Lin et al., 2015) METH-dependent and healthy control subjects in the anterior cingulate cortex (Ernst & Chang, 2008), the frontal white matter (Ernst & Chang, 2008), the basal ganglia (Ernst & Chang, 2008; Lin et al., 2015), and the primary visual cortex (Lin et al., 2015). Moreover, a few studies documented an elevated ratio of choline/N-acetylaspartate in the anterior cingulate cortex in recent abstinence compared with longer periods of abstinence (Ernst & Chang, 2008; Salo et al., 2007; Salo, Buonocore, et al., 2011), whereas one study reported decreased choline values in the right dorsolateral prefrontal cortex in both active and METH-dependent subjects abstinent for nearly 3 months relative to controls (Howells et al., 2014). Finally, another study found no differences in choline/N-acetylaspartate ratios between active METH-users and controls in the basal ganglia or in the primary visual cortex (Lin et al., 2015).
Consistent proton MRS findings documented no differences in concentrations of creatine between METH-dependent subjects and controls (Lin et al., 2015; Nordahl et al., 2005; Salo, Nordahl, et al., 2011). However, a phosphorous MRS study showed a decrease in frontal lobe phosphocreatine values in METH-dependent subjects relative to healthy controls, and the effect was greater in females compared with males (Sung et al., 2013), suggesting a possible gender-specific brain energy changes with chronic METH use. Furthermore, one study reported changes in myo-inositol with METH use, with higher levels of myo-inositol in frontal white matter of METH-users but not in controls, which might provide evidence for increased cell proliferation in response to METH-induced neurotoxicity (Sung et al., 2007).
Reports of changes in the glutamateric system were also consistent. For example, glutamate and Glx levels were shown to be lower in short-term METH-dependent subjects in the medial prefrontal cortex (Crocker et al., 2014), in the frontal gray matter (Ernst & Chang, 2008), as well as the precuneus and the right inferior frontal cortex (O’Neill et al., 2015). Finally, with longer periods of abstinence from METH, there is some evidence of brain normalization demonstrated by longitudinal increases in Glx values at 1 to 2 months abstinent and a further increase at 5 months abstinent (Ernst & Chang, 2008).
The association between changes in brain chemistry with chronic METH use and cognition is understudied. Two out of the 11 studies included in this review examined the relationship between cognitive performance and MRS findings. The results from these two studies demonstrate an association between poorer cognitive performance, specifically attentional control (Salo et al., 2007) and ability to resolve spatial conflict (Salo, Nordahl, et al., 2011) and lower levels of N-acetylaspartate in the anterior cingulate cortex (Salo et al., 2007, Salo, Nordahl, et al., 2011) and the primary visual cortex (Salo, Nordahl, et al., 2011).
The inconsistencies in the METH and MRS study findings, specifically the N-acetylaspartate and choline results, are difficult to explain. The majority of the studies evaluated similar brain regions and the discrepant findings were reported in studies using the same field strength. Other variables to consider are length of time using METH as well as dose and frequency of use. The ranges of these variables, however, are wide in the studies reporting inconsistent findings related to N-acetylaspartate and choline, which does not provide a clear explanation for the differences in findings. Nonetheless, these data do indicate that chronic METH use results in neuronal injury with evidence of brain normalization with abstinence from METH.
3,4-Methylenedioxymethamphetamine
Findings from MRS studies in MDMA use have not demonstrated a consistent pattern of the effects of MDMA on the brain (Cowan et al., 2007; Daumann et al., 2004; de Win et al., 2008), even when employing a higher field strength at 3 Tesla (Cowan et al., 2007). Indeed, only one study reported significant differences in neurometabolites in MDMA users relative to healthy volunteers, and in this study, the authors noted elevated basal ganglia myo-inositol with MDMA use but not in a non-MDMA using comparison group, which might suggest a glial response to MDMA use (Liu et al., 2011). Also, none of the studies used neuropsychological testing as a method to understand the relationship between brain chemistry and cognition in MDMA users.
It is not clear why reports over the past 11 years have produced conflicting results from studies that were reported in prior decades, which do suggest that MDMA has possible neurotoxic effects (Chang, Ernst, Grob, & Poland, 1999). One possibility for this discrepancy is the challenges associated with studying pure MDMA users. The majority of MDMA users are polysubstance users with concurrent marijuana use reported more frequently than other drugs of abuse (Singer, Linares, Ntiri, Henry, & Minnes, 2004), and it has been suggested that marijuana may provide neuroprotective effects (Costa, 2007). Another consideration is the potential that proton MRS is not sensitive enough to detect changes in brain chemistry related to MDMA use.
Cocaine
The cocaine and MRS study findings largely indicate that there are no differences in brain chemistry among cocaine-dependent and healthy control subjects with the exception of glutamatergic and energy metabolism changes. For example, one study found lower levels of anterior cingulate cortex glutamate concentrations in chronic crack cocaine users when compared with healthy controls (Yang et al., 2009). Furthermore, using phosphorus MRS, whole brain a-nucleoside triphosphate levels increased after a night of sleep compared with sleep deprivation, and β- and total-nucleoside triphosphate values were higher after a night of sleep compared with sleep deprivation and baseline in cocaine-dependent subjects relative to healthy controls (Trksak et al., 2013). On the whole, the results from these studies demonstrate that glutamatergic changes are present with cocaine use as well as changes to brain bioenergetics.
None of the cocaine and MRS studies included in this review explored the relationship between neurochemistry and cognitive performance. One of the studies (Trksak et al., 2013) compared cognitive performance between cocaine-dependent and healthy control subjects, but the authors did not report associations between cognitive testing and MRS data.
Opiates and Opioids
The effects of long-term opiate and opioid use on brain chemistry are understudied. There were only three studies that were included in this review, and they primarily focused on opioid maintenance therapy in opiate users, and neither of these studies administered cognitive performance testing. Results from the proton MRS study suggest an imbalance of the glutamate system with higher levels of anterior cingulate cortex Glx noted in older opiate users relative to healthy controls, as well as increased choline concentrations in the left frontal white matter observed across all age groups of opiate users compared with controls (Hermann et al., 2012). Also, alterations in brain bioenergetics were conflicting using phosphorus MRS in studies of subjects maintained on methadone therapy. One study noted reduced levels of phosphocreatine found in the orbitofrontal and occipital cortices in heroin users initiating methadone maintenance therapy but not in a healthy comparison group (Silveri et al., 2004), whereas another study demonstrated no differences in concentrations of whole brain phosphocreatine in individuals on methadone maintenance therapy compared with healthy controls (Trksak et al., 2010). Length of methadone maintenance therapy might explain the discrepancy in these findings, as in one of the studies, methadone was recently initiated (Silveri et al., 2004), while in the other study, the duration of methadone maintenance ranged from 8 to 28 months (Trksak et al., 2010). Because of the limited number of MRS studies in opiate use, it is difficult to assess the impact of opiate use on the brain; however, the few published reports provide some evidence that there are differences in neurochemistry in opiate users starting opiate maintenance therapy in comparison to nonopiate users.
Marijuana
The effects of marijuana use on brain chemistry in adults without concurrent use of another substance of abuse or HIV+ serostatus are limited. The two studies that were eligible for this review reported no group differences in most neurometabolites among marijuana users and healthy control subjects (Mashhoon et al., 2011; Silveri et al., 2011), with the exception of alterations in myo-inositol. Notably, changes in the thalamus (Mashhoon et al., 2011) and globally (Silveri et al., 2011) were noted with lower myo-inositol (Mashhoon et al., 2013) and myo-inositol/creatine ratio (Silveri et al., 2011) in chronic marijuana users with respect to healthy volunteers. These limited findings indicate that marijuana use may result in decreased glial proliferation. Finally, none of the studies that were included in this review administered neuropsychological testing as a study procedure.
Nicotine
MRS studies of chronic nicotine use have mostly targeted the anterior cingulate cortex. The main neurometabolites of interest have been glutamate, Glx, and GABA, and although there are inconsistencies noted in the findings, they generally suggest that nicotine use results in changes in brain chemistry. However, none of the studies included neuropsychological testing to investigate the impact of brain chemistry changes on cognitive performance. Regardless, when compared with females who did not relapse while using nicotine replacement therapy, dorsal anterior cingulate cortex glutamate/creatine levels were found to be reduced in females who relapsed (Mashhoon et al., 2011). On the other hand, relative to healthy controls, there were no differences in anterior cingulate cortex and hippocampal glutamate levels in nicotine-dependent subjects (Gallinat & Schubert, 2007). It is possible that glutamate serves as a biomarker for relapse considering changes in glutamate were not found in treatment of naïve nicotine users yet were identified in females seeking treatment. As an alternative, perhaps gender influences glutamate since reduced concentrations were noted in a female-specific group only.
Along the same lines of the glutamate finding, alterations in cortical GABA concentrations were also noted in nicotine using females versus nicotine using males (Epperson et al., 2005), suggesting that GABA levels may also vary by gender. Furthermore, MRS studies of nicotine have also evaluated changes in N-acetylaspartate and choline, and these findings indicate the possibility of nicotine use resulting in neurotoxicity as evidenced by reduced N-acetylaspartate levels in the left hippocampus among nicotine users compared with nonnicotine users as well as higher anterior cingulate cortex choline concentrations with increased lifetime nicotine exposure (Gallinat et al., 2007). The association between choline and lifetime nicotine use suggests that nicotine use may cause changes in cell density or accelerate the rate of cortical membrane turnover (Gallinat et al., 2007).
Synthesis of Research Efforts During the Last Decade
The MRS studies of substance use disorders included in this review demonstrate some overlap in brain chemistry changes across drug classes, thus suggesting that changes in neurometabolites may be substance of abuse specific rather than a universal impact from drug use. The most consistent finding, though, across all substances evaluated was no differences in brain chemistry with chronic substance use compared with non–substance using controls (see Table 5). For example, in one or more alcohol, METH, MDMA, cocaine, opiates, opioids, marijuana, and nicotine MRS studies, the authors reported no differences in N -acetylaspartate values, particularly in the anterior cingulate cortex between substance users and controls. This finding was surprising considering that the anterior cingulate cortex is rich with dopamine (Allman, Hakeem, Erwin, Nimchinsky, & Hof, 2001), and a few of the substances examined have an effect on dopaminergic system. Thus, we expected that studies would consistently report reduced N-acetylaspartate values with chronic substance use, specifically METH and cocaine dependence. There were, however, a few studies that did note decreased N-acetylaspartate levels, notably in studies of chronic alcohol, METH, and nicotine use. A possible explanation for the discrepancy in these findings is the field strength used as well as the varying brain regions of interest.
Table 5.
Overview of Cross-Sectional Magnetic Resonance Spectroscopy Findings Across Substances.
| Metabolite | Decrease | Increase | No differences |
|---|---|---|---|
| Proton MRS | |||
| N-acetylaspartate | Alcohol, METH, nicotine | Alcohol, METH, MDMA, cocaine, opiates and opioids, marijuana, nicotine | |
| Creatine | Alcohol | Alcohol, METH, MDMA, opiates and opioids, marijuana, nicotine | |
| Choline | Alcohol, METH, nicotine | Alcohol, METH, opiates | Alcohol, METH, MDMA, cocaine, opiates and opioids, marijuana, nicotine |
| Myo-inositol | Marijuana | Alcohol, METH, MDMA | METH, MDMA |
| Glutamate | METH, cocaine, nicotine | METH, cocaine, opiates and opioids, marijuana, nicotine | |
| Glutamine | Cocaine, marijuana | ||
| Glx | METH | Alcohol | METH, opiates and opioids |
| gamma-aminobutyric acid | Nicotine | Alcohol | |
| Phosphorus MRS | |||
| Phosphomonoesters | Opiates and opioids | Cocaine | |
| Inorganic phosphate | Cocaine | ||
| Phosphodiester | Opiates and opioids | Cocaine | |
| Phosphocreatine | METH, opiates and opioids | Cocaine | |
| Alpha-nucleoside triphosphate | Cocaine | ||
| Beta-nucleoside triphosphate | Cocaine | ||
| Gamma-nucleoside triphosphate | Cocaine |
Another consistent finding that was reported across substances related to no differences in creatine levels between substance users (e.g., alcohol, METH, MDMA, opiates and opioids, marijuana, and nicotine) using proton MRS. In contrast, studies that performed phosphorus MRS consistently demonstrated lower concentrations of phosphocreatine, suggesting brain energy metabolism alterations in METH and opiate and opioid users. Given that phosphorus MRS is a more advanced and sensitive technique (Blüml & Panigrahy, 2012), it may detect alterations in energy metabolism better than proton MRS, which might explain why phosphorus MRS studies reported changes in phosphocreatine while proton MRS studies did not.
The report of increased choline values with abstinence was consistent among alcohol and METH users, thus suggesting that the rate of cellular membrane production may increase with brain recovery. Next, fewer consistent findings were noted from studies of glutamate, glutamine, and Glx concentrations. Compared with controls, no differences in glutamate were commonly reported in METH, cocaine, opiates and opioids, marijuana, and nicotine dependence, in glutamine levels with cocaine and marijuana use and in Glx in METH and opiate and opioid users. In contrast, however, glutamate was documented as decreased in METH, cocaine, and nicotine dependence and Glx was lower in METH users relative to control, which may reflect alterations in glutamatergic metabolism or transmission with substance use. Importantly, though, not all studies evaluated glutamate or glutamine, specifically studies using a 1.5 Tesla field strength, as these neurometabolites are challenging to detect with lower field strengths (Hurd et al., 2004). Finally, GABA was not commonly studied across substances. In fact, only two studies reported GABA, and these findings consistently showed a decreased in GABA concentrations with nicotine use.
What Are the Gaps?
The majority of the studies used proton MRS methodology to understand changes in brain chemistry in substance use disorders. However, there is a gap in knowledge with respect to how substances of abuse impact brain metabolic energy, which can be quantified using phosphorus MRS imaging. Only three of the published articles included in this review applied phosphorus MRS imaging, and while the findings from these studies suggest that substance use compromises high-energy phosphate metabolism, the small number of publications limits generalizability.
There is limited information regarding how changes in neurochemistry relate to cognitive function. Out of the 37 studies reviewed, only three of them conducted neuropsychological testing in conjunction with MRS scanning and two of them were studies of METH, MRS, and cognitive function. Since N-acetylaspartate has been identified as a marker of neuronal integrity, it is not surprising that the cognitive performance findings suggested lower concentrations of N-acetylaspartate with poorer cognitive function among METH users. The lack of information with respect to the relationship between brain chemistry and cognition underscore the need for more attention in this area.
Studies of MRS and substance abuse are notorious for small sample sizes. The largest sample size noted in this systematic review included 188 MDMA-naïve young adults (de Win et al., 2008). This is a remarkably large sample size compared with the majority of the other studies, which on average included approximately 30 participants. Concerns regarding external validity and low statistical power are raised with a small sample size. In fact, in a review of neuroimaging meta-analyses, the median statistical power of 461 individual studies was 8% (Button et al., 2013). If low statistical power is typical in neuroimaging research, the likelihood of statistically significant findings reflecting a true effect is jeopardized (Button et al., 2013).
Where We Are Headed?
Information gleaned from MRS studies of substance users has provided useful insight on the neurobiological effects of substance use and abstinence. Because MRS scans reveal concentrations of target chemicals in brain regions, its utility is becoming more widespread, particularly in examining pharmacological treatment approaches. This review, however, excluded studies that evaluated the effects of a medication on brain chemistry changes in substance use disorders because one of our goals was to understand the neurochemistry changes in substance use disorders. With that said, we excluded three studies from this review based on the exclusionary criterion of a medication investigation, but it is important to note that these types of studies have the potential to increase the effectiveness of medication management in substance abuse. If future pharmacological development studies adapt the approach of including MRS, our understanding of how medications work in substance use disorders may allow for more targeted treatment modalities. In addition to pharmaceutical development, MRS may be used in the specialty of substance abuse for diagnostic purposes.
Overall, in psychiatry research, the diagnostic potential of MRS remains unfulfilled, but the recent advances in MRS offers the possibility of identifying brain biomarkers for disease. For instance, in Parkinson’s disease, reduced cingulate N-acetylaspartate/creatine ratios have been noted in individuals with Parkinson’s disease and, thus, identified as a clinical biomarker of disease progression (Sharma et al., 2013). Finally, future uses of MRS also include evaluating treatment response, and a study by Kondo, Sung, et al. (2011) demonstrates an application of this approach. Phosphorus MRS was conducted before and after depressed adolescents were treated with an investigational medication, creatine, which was hypothesized to increase brain phosphocreatine content. The researchers noted a significant increase in phosphocreatine concentrations at the end of treatment scan in parallel with a decrease in depressive symptoms (Kondo, Sung, et al., 2011). The results from this study offer the opportunity for substance abuse researchers to consider study designs that take the neurobiology of addiction coupled with targeted treatment approaches into consideration.
With the current emphasis on translational research in a variety of disciplines, including nursing and medicine, the question of how neuroimaging research translates to clinicians is important to consider. Led by an initiative from the National Institute on Drug Abuse and National Institute of Alcohol Abuse and Alcoholism (Volkow, Koob, & Baler, 2015), the search for biomarkers in substance use disorders is becoming increasingly more important for the development of personalized treatment. This review illustrates the potential of MRS providing valuable insight into the effect substance use disorders have on brain bioenergetics, the function of neuronal and cellular structures, and neurotransmitter pathways. These discoveries may allow for the identification of better therapeutic targets, and as a result, using MRS in substance use disorders has the potential to make a profound impact on treatment outcomes.
Limitations and Recommendations
Some of the MRS findings reviewed here showed differences in neurochemistry between subjects with a substance use disorder and healthy control subjects. Even though we carefully considered eligibility criteria for inclusion in this review, including excluding studies that allowed concurrent use of two or more substances (with the exception of nicotine), comorbid psychiatric illness, and HIV+ serostatus, to reduce confounding variables, the metabolite differences observed between patients and controls does not necessarily indicate injury or damage. There are additional factors to consider, such as but not limited to, substance use history, past medical history, medication history, demographic variables, and so forth, which might explain neurochemistry differences between the two groups.
As shown in Table 3, the MRS literature in substance use disorders has used a variety of anatomical reference points and some diversity of technique (e.g., field strength and type of scan). Furthermore, the length of time abstinent from a substance at time of the MRS procedure ranged considerably. It would be advantageous if future studies adopted a standardized protocol for data acquisition and reporting.
Conclusion
In conclusion, proton and 31P MRS are emerging translational research tools for the study of substance use disorders. The majority of the published MRS and substance use literature has used proton MRS, and furthermore, much of the focus has been on METH dependence. A number of studies revealed that there are no differences between substance use disorders and healthy controls, and the findings that did demonstrate differences between groups suggest potential neuronal injury and changes in cellular membranes. There is limited knowledge, though, on the impact of changes in brain chemistry on cognitive performance. Even though there are limitations of MRS in substance abuse research, such as small sample size and low statistical power, this methodology could continue as a pivotal role for improving treatment outcomes, but further improvements in design and analysis are recommended.
Acknowledgments
Funding
This article was supported by NIDA grant R36DA036767 awarded to Tracy Hellem and NIDA grant K05DA031247 awarded to Perry Renshaw.
Footnotes
This did not apply to MDMA and concurrent marijuana use since most MDMA users regularly smoke marijuana (Daumann et al., 2004). Also, applying this exclusion criterion to MRS studies of MDMA would have resulted in excluding all MRS studies or MDMA since 2004.
Author Roles
Hellem was the lead author and wrote the majority of the content. Shi provided figures and editing. Latendresse edited content and Renshaw provided MRS expertise and editing.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Achtnichts L, Gonen O, Rigotti DJ, Babb JS, Naegelin Y, Penner IK, Gass A. Global N-acetylaspartate concentration in benign and non-benign multiple sclerosis patients of long disease duration. European Journal of Radiology. 2013;82:848–852. doi: 10.1016/j.ejrad.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P. The anterior cingulate cortex. Annals of the New York Academy of Sciences. 2001;935:107–117. [PubMed] [Google Scholar]
- Barker PB, Lin DDM. In vivo proton MR spectroscopy of the human brain. Progress in Nuclear Magnetic Resonance Spectroscopy. 2006;49:99–128. [Google Scholar]
- Bartsch AJ, Homola G, Biller A, Smith SM, Weijers HG, Wiesbeck GA, Bendszus M. Manifestations of early brain recovery associated with abstinence from alcoholism. Brain. 2007;130(Pt 1):36–47. doi: 10.1093/brain/awl303. [DOI] [PubMed] [Google Scholar]
- Blüml S, Panigrahy A, editors. MR spectroscopy of pediatric brain disorders. New York, NY: Springer Science + Business Media; 2012. [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafo MR. Power failure: Why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Castillo M, Kwock L, Mukherji SK. Clinical applications of proton MR spectroscopy. American Journal of Neuroradiology. 1996;17(1):1–15. [PMC free article] [PubMed] [Google Scholar]
- Chang L, Cloak CC, Ernst T. Magnetic resonance spectroscopy studies of GABA in neuropsychiatric disorders. Journal of Clinical Psychiatry. 2003;64(Suppl. 3):7–14. [PubMed] [Google Scholar]
- Chang L, Ernst T, Grob CS, Poland RE. Cerebral 1H–MRS alterations in recreational 3,4-methy-lenedioxymethamphetamine (MDMA, “ecstasy”) users. Journal of Magnetic Resonance Imaging. 1999;10:521–526. doi: 10.1002/(sici)1522-2586(199910)10:4<521::aid-jmri4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Costa B. On the pharmacological properties of A9-tetrahydrocannabinol (THC) Chemistry & Biodiversity. 2007;4:1664–1677. doi: 10.1002/cbdv.200790146. [DOI] [PubMed] [Google Scholar]
- Cowan RL, Bolo NR, Dietrich M, Haga E, Lukas SE, Renshaw PF. Occipital cortical proton MRS at 4 Tesla in human moderate MDMA polydrug users. Psychiatry Research. 2007;155:179–188. doi: 10.1016/j.pscychresns.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker CE, Bernier DC, Hanstock CC, Lakusta B, Purdon SE, Seres P, Tibbo PG. Prefrontal glutamate levels differentiate early phase schizophrenia and methamphetamine addiction: A 1H MRS study at 3 Tesla. Schizophrenia Research. 2014;157:231–237. doi: 10.1016/j.schres.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Daumann J, Fischermann T, Pilatus U, Thron A, Moeller-Hartmann W, Gouzoulis-Mayfrank E. Proton magnetic resonance spectroscopy in ecstasy (MDMA) users. Neuroscience Letters. 2004;362:113–116. doi: 10.1016/j.neulet.2004.03.004. [DOI] [PubMed] [Google Scholar]
- de Win MM, Jager G, Booij J, Reneman L, Schilt T, Lavini C, van den Brink W. Sustained effects of ecstasy on the human brain: a prospective neuroimaging study in novel users. Brain. 2008;131(Pt 11):2936–2945. doi: 10.1093/brain/awn255. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Banys P, Meyerhoff DJ. Cigarette smoking exacerbates chronic alcohol-induced brain damage: A preliminary metabolite imaging study. Alcoholism: Clinical and Experimental Research. 2004;28:1849–1860. doi: 10.1097/01.alc.0000148112.92525.ac. [DOI] [PubMed] [Google Scholar]
- Ende G, Welzel H, Walter S, Weber-Fahr W, Diehl A, Hermann D, Mann K. Monitoring the effects of chronic alcohol consumption and abstinence on brain metabolism: A longitudinal proton magnetic resonance spectroscopy study. Biological Psychiatry. 2005;58:974–980. doi: 10.1016/j.biopsych.2005.05.038. [DOI] [PubMed] [Google Scholar]
- Epperson CN, O’Malley S, Czarkowski KA, Gueorguieva R, Jatlow P, Sanacora G, Mason GF. Sex, GABA, and nicotine: The impact of smoking on cortical GABA levels across the menstrual cycle as measured with proton magnetic resonance spectroscopy. Biological Psychiatry. 2005;57:44–48. doi: 10.1016/j.biopsych.2004.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Chang L. Adaptation of brain glutamate plus glutamine during abstinence from chronic methamphetamine use. Journal of Neuroimmune Pharmacology. 2008;3:165–172. doi: 10.1007/s11481-008-9108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangou S, Williams SCR. Magnetic resonance spectroscopy in psychiatry: Basic principles and applications. British Medical Bulletin. 1996;52:474–485. doi: 10.1093/oxfordjournals.bmb.a011561. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Lang UE, Jacobsen LK, Bajbouj M, Kalus P, von Haebler D, Schubert F. Abnormal hippocampal neurochemistry in smokers: evidence from proton magnetic resonance spectroscopy at 3 T. Journal of Clinical Psychopharmacology. 2007;27:80–84. doi: 10.1097/JCP.0b013e31802dffde. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Schubert F. Regional cerebral glutamate concentrations and chronic tobacco consumption. Pharmacopsychiatry. 2007;40:64–67. doi: 10.1055/s-2007-970144. [DOI] [PubMed] [Google Scholar]
- Gujar SK, Maheshwari S, Bjorkman-Burtscher I, Sundgren PC. Magnetic resonance spectroscopy. Journal of Neuro-Opthalmology. 2005;25:217–226. doi: 10.1097/01.wno.0000177307.21081.81. [DOI] [PubMed] [Google Scholar]
- Gutzeit A, Froehlich JM, Hergan K, Graf N, Binkert CA, Meier D, Mutschler J. Insula-specfic 1H magnetic resonance spectroscopy reactions in heavy smokers under acute nicotine withdrawal and after oral nicotine substitution. European Addiction Research. 2013;19:184–193. doi: 10.1159/000345915. [DOI] [PubMed] [Google Scholar]
- Hermann D, Frischknecht U, Heinrich M, Hoerst M, Vollmert C, Vollstadt-Klein S, Ende G. MR spectroscopy in opiate maintenance therapy: Association of glutamate with the number of previous withdrawals in the anterior cingulate cortex. Addiction Biology. 2012;17:659–667. doi: 10.1111/j.1369-1600.2010.00290.x. [DOI] [PubMed] [Google Scholar]
- Hermann D, Weber-Fahr W, Sartorius A, Hoerst M, Frischknecht U, Tunc-Skarka N, Sommer WH. Translational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats. Biological Psychiatry. 2011;71:1015–1021. doi: 10.1016/j.biopsych.2011.07.034. [DOI] [PubMed] [Google Scholar]
- Howells FM, Uhlmann A, Temmingh H, Sinclair H, Meintjes E, Wilson D, Stein DJ. (1) H-magnetic resonance spectroscopy ((l)H-MRS) in methamphetamine dependence and methamphetamine induced psychosis. Schizophrenia Research. 2014;153:122–128. doi: 10.1016/j.schres.2014.01.029. [DOI] [PubMed] [Google Scholar]
- Hurd R, Sailasuta N, Srinivasan R, Vigneron DB, Pelletier D, Nelson SJ. Measurement of brain glutamate using TE-averaged PRESS at 3T. Magnetic Resonance in Medicine. 2004;51:435–440. doi: 10.1002/mrm.20007. [DOI] [PubMed] [Google Scholar]
- Iosifescu DV, Bolo NR, Nierenberg AA, Jensen JE, Fava M, Renshaw PF. Brain bioenergetics and response to triiodothyronine augmentation in major depressive disorder. Biological Psychiatry. 2008;63:1127–1134. doi: 10.1016/j.biopsych.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Kato T, Inubushi T, Kato N. Magnetic resonance spectroscopy in affective disorders. Journal of Neuropsychiatry and Clinical Neurosciences. 1998;10:133–147. doi: 10.1176/jnp.10.2.133. [DOI] [PubMed] [Google Scholar]
- Kondo DG, Hellem TL, Sung YH, Kim N, Jeong EK, DelMastro KK, Renshaw PF. Review: Magnetic resonance spectroscopy studies of pediatric major depressive disorder. Depression Research and Treatment. 2011;2011:1–13. doi: 10.1155/2011/650450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo DG, Sung YH, Hellem TL, Fiedler KK, Shi X, Jeong EK, Renshaw PF. Open-label adjunctive creatine for female adolescents with SSRI-resistant major depressive disorder: A 31 -phosphorus magnetic resonance spectroscopy study. Journal of Affective Disorders. 2011;135:354–361. doi: 10.1016/j.jad.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraguljac NV, Reid M, White D, Jones R, den Hollander J, Lowman D, Lahti AC. Neurometabolites in schizophrenia and bipolar disorder—A systematic review and meta-analysis. Psychiatry Research. 2012;203:111–125. doi: 10.1016/j.pscychresns.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licata SC, Renshaw PF. Neurochemistry of drug action: Insights from proton magnetic resonance spectroscopic imaging and their relevance to addiction. Annals of the New York Academy of Sciences. 2010;1187:148–171. doi: 10.1111/j.1749-6632.2009.05143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JC, Jan RK, Kydd RR, Russell BR. Investigating the microstructural and neurochemical environment within the basal ganglia of current methamphetamine abusers. Drug and Alcohol Dependence. 2015;149:122–127. doi: 10.1016/j.drugalcdep.2015.01.026. [DOI] [PubMed] [Google Scholar]
- Liu HS, Chou MC, Chung HW, Cho NY, Chiang SW, Wang CY, Chen CY. Potential long-term effects of MDMA on the basal ganglia thalamocortical circuit: A proton MR spectroscopy and diffusion-tensor imaging study. Radiology. 2011;260:531–540. doi: 10.1148/radiol.11101918. [DOI] [PubMed] [Google Scholar]
- Long Z, Medlock C, Dzemidzic M, Shin YW, Goddard AW, Dydak U. Decreased GABA levels in anterior cingulate cortex/medial prefrontal cortex in panic disorder. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2013;44:131–135. doi: 10.1016/j.pnpbp.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyoo IK, Renshaw PF. Magnetic resonance spectroscopy—Current and future applications in psychiatric research. Biological Psychiatry. 2002;51:195–207. doi: 10.1016/s0006-3223(01)01313-0. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Buonocore MH. MR spectroscopic studies of the brain in psychiatric disorders. Current Topics in Behavioral Neurosciences. 2012;11:199–251. doi: 10.1007/7854_2011_197. [DOI] [PubMed] [Google Scholar]
- Maltezos S, Horder J, Coghlan S, Skirrow C, O’Gorman R, Lavender TJ, Murphy DG. Glutamate/ glutamine and neuronal integrity in adults with ADHD: A proton MRS study. Translational Psychiatry. 2014;18(4):e373. doi: 10.1038/tp.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Nabulsi N, Grassetti A, Urban NBL, Perez A, Huang Y. Imaging glutamate homeostasis in cocaine addiction with the metabotropic glutamate receptor 5 positron emission tomography radiotracer [11C]ABP688 and Magnetic Resonance Spectroscopy. Biological Psychiatry. 2014;75:165–171. doi: 10.1016/j.biopsych.2013.06.026. http://dx.doi.org/10.1016/j.biopsych.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashhoon Y, Janes AC, Jensen JE, Prescot AP, Pachas G, Renshaw PF, Kaufman MJ. Anterior cingulate proton spectroscopy glutamate levels differ as a function of smoking cessation outcome. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2011;35:1709–1713. doi: 10.1016/j.pnpbp.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashhoon Y, Jensen JE, Sneider JT, Yurgelun-Todd DA, Silveri MM. Lower left thalamic myo-inositol levels associated with greater cognitive impulsivity in marijuana-dependent young men: Preliminary spectroscopic evidence at 4T. Journal of Addiction Research & Therapy. 2013;20(Suppl. 4) doi: 10.4172/2155-6105.S4-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason GF, Krystal JH. MR spectroscopy: Its potential role for drug development for the treatment of psychiatric diseases. NMR Biomedicine. 2006;19:690–701. doi: 10.1002/nbm.1080. [DOI] [PubMed] [Google Scholar]
- Masters MC, Ances BM. Role of neuroimaging in HIV-associated neurocognitive disorders. Seminars in Neurology. 2014;34:89–102. doi: 10.1055/s-0034-1372346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennecke A, Gossler A, Hammen T, Dörfler A, Stadlbauer A, Rosch J, Thürauf N. Physiological effects of cigarette smoking in the limbic system revealed by 3 tesla magnetic resonance spectroscopy. Journal of Neural Transmission. 2014;121:1211–1219. doi: 10.1007/s00702-014-1190-6. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ, Blumenfeld R, Truran D, Lindgren J, Flenniken D, Cardenas V, Weiner MW. Effects of heavy drinking, binge drinking, and family history of alcoholism on regional brain metabolites. Alcoholism: Clinical & Experimental Research. 2004;28:650–661. doi: 10.1097/01.ALC.0000121805.12350.CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi S, Bhattacharya M, Kumar P, Deshpande SN, Tripathi RP, Khushu S. Brain metabolic changes in alcoholism: Localized proton magnetic resonance spectroscopy study of the occipital lobe. European Journal of Radiology. 2011;79:96–100. doi: 10.1016/j.ejrad.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Nordahl TE, Salo R, Natsuaki Y, Galloway GP, Waters C, Moore CD, Buonocore MH. Methamphetamine users in sustained abstinence. Archives of General Psychiatry. 2005;62:444–452. doi: 10.1001/archpsyc.62.4.444. [DOI] [PubMed] [Google Scholar]
- O’Neil J, Tobias MC, Hudkins M, Oh EY, Helleman GS, Nurmi EL, London ED. Thalamic glutamate decreases with cigarette smoking. Psychopharmacology. 2014;231:2717–2724. doi: 10.1007/s00213-014-3441-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill J, Tobias MC, Hudkins M, London ED. Glutamatergic neurometabolites during early abstinence from chronic methamphetamine abuse. International Journal of Neuropsychopharmacology. 2015;18(3):1–9. doi: 10.1093/ijnp/pyu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy R, Keshavan MS. Phosphorus magnetic resonance spectroscopy: Its utility in examining the membrane hypothesis of schizophrenia. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 2003;69(6):401–405. doi: 10.1016/j.plefa.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Salo R, Buonocore MH, Leamon M, Natsuaki Y, Waters C, Moore CD, Nordahl TE. Extended findings of brain metabolite normalization in MA-dependent subjects across sustained abstinence: A proton MRS study. Drug and Alcohol Dependence. 2011;113:133–138. doi: 10.1016/j.drugalcdep.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Buonocore MH, Natsuaki YT, Moore CD, Waters C, Leamon MH. Spatial inhibition and the visual cortex: A magnetic resonance spectroscopy imaging study. Neuropsychologia. 2011;49:830–838. doi: 10.1016/j.neuropsychologia.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Natsuaki Y, Leamon MH, Galloway GP, Waters C, Buonocore MH. Attentional control and brain metabolite levels in methamphetamine abusers. Biological Psychiatry. 2007;61:1272–1280. doi: 10.1016/j.biopsych.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Zarate CA, Krystal J, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nature Reviews Drug Discovery. 2008;7:426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Moon CS, Khogali A, Haidous A, Chabenne A, Ojo C, Ebadi M. Biomarkers in Parkinson’s disease (recent update) Neurochemistry International. 2013;63:201–229. doi: 10.1016/j.neuint.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Jensen JE, Rosso IM, Sneider JT, Yurgelun-Todd DA. Preliminary evidence for white matter metabolite differences in marijuana-dependent young men using 2D J-resolved magnetic resonance spectroscopic imaging at 4 Tesla. Psychiatry Research. 2011;191:201–211. doi: 10.1016/j.pscychresns.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Pollack MH, Diaz CI, Nassar LE, Mendelson JH, Yurgelun-Todd DA, Kaufman MJ. Cerebral phosphorus metabolite and transverse relaxation time abnormalities in heroin-dependent subjects at onset of methadone maintenance treatment. Psychiatry Research. 2004;131:217–226. doi: 10.1016/j.pscychresns.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Singer LT, Linares TJ, Ntiri S, Henry R, Minnes S. Psychosocial profiles of older adolescent MDMA users. Drug and Alcohol Dependence. 2004;74:245–252. doi: 10.1016/j.drugalcdep.2003.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung YH, Carey PD, Stein DJ, Ferrett HL, Spottiswoode BS, Renshaw PF, Yurgelun-Todd DA. Decreased frontal N-acetylaspartate levels in adolescents concurrently using both methamphetamine and marijuana. Behavioural Brain Research. 2013;246:154–161. doi: 10.1016/j.bbr.2013.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung YH, Cho SC, Hwang J, Kim SJ, Kim H, Bae S, Lyoo IK. Relationship between N-acetyl-aspartate in gray and white matter of abstinent methamphetamine abusers and their history of drug abuse: A proton magnetic resonance spectroscopy study. Drug and Alcohol Dependence. 2007;88:28–35. doi: 10.1016/j.drugalcdep.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Trksak GH, Bracken BK, Jensen JE, Plante DT, Penetar DM, Tartarini WL, Lukas SE. Effects of sleep deprivation on brain bioenergetics, sleep, and cognitive performance in cocaine-dependent individuals. Scientific World Journal. 2013;2013:10. doi: 10.1155/2013/947879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trksak GH, Jensen JE, Plante DT, Penetar DM, Tartarini WL, Maywalt MA, Lukas SE. Effects of sleep deprivation in sleep homeostatis and restoration during methadone-maintenance: A [31]P MRS brain imaging study. Drug and Alcohol Dependence. 2010;106:79–91. doi: 10.1016/j.drugalcdep.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma G, Woo JH, Chawla S, Wang S, Sherriff S, Elman LB, Poptani H. Whole-brain analysis of amyotrophic lateral sclerosis by using echo-planar spectroscopic imaging. Radiology. 2013;267:851–857. doi: 10.1148/radiol.13121148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Koob G, Baler R. Biomarkers in substance use disorders. ACS Chemical Neuroscience. 2015;6:522–525. doi: 10.1021/acschemneuro.5b00067. [DOI] [PubMed] [Google Scholar]
- Yadav SK, Kumar R, Macey PM, Woo MA, Yan-Go FL, Harper RM. Insular cortex metabolite changes in obstructive sleep apnea. Sleep. 2014;37:951–958. doi: 10.5665/sleep.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Salmeron BJ, Ross TJ, Xi ZX, Stein EA, Yang Y. Lower glutamate levels in rostral anterior cingulate of chronic cocaine users—A (1)H-MRS study using TE-averaged PRESS at 3 T with an optimized quantification strategy. Psychiatry Research. 2009;174:171–176. doi: 10.1016/j.pscychresns.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]