Abstract
Objectives
The dynamic bone-periodontal ligament (PDL)-tooth fibrous joint consists of two adaptive functionally graded interfaces (FGI), the PDL-bone and PDL-cementum that respond to mechanical strain transmitted during mastication. In general, from a materials and mechanics perspective, FGI prevent catastrophic failure during prolonged cyclic loading. This review is a discourse of results gathered from literature to illustrate the dynamic adaptive nature of the fibrous joint in response to physiologic and pathologic simulated functions, and experimental tooth movement.
Methods
Historically, studies have investigated soft to hard tissue transitions through analytical techniques that provided insights into structural, biochemical, and mechanical characterization methods. Experimental approaches included two dimensional to three dimensional advanced in situ imaging and analytical techniques. These techniques allowed mapping and correlation of deformations to physicochemical and mechanobiological changes within volumes of the complex subjected to concentric and eccentric loading regimes respectively.
Results
Tooth movement is facilitated by mechanobiological activity at the interfaces of the fibrous joint and generates elastic discontinuities at these interfaces in response to eccentric loading. Both concentric and eccentric loads mediated cellular responses to strains, and prompted self-regulating mineral forming and resorbing zones that in turn altered the functional space of the joint.
Significance
A multiscale biomechanics and mechanobiology approach is important for correlating joint function to tissue-level strain-adaptive properties with overall effects on joint form as related to physiologic and pathologic functions. Elucidating the shift in localization of biomolecules specifically at interfaces during development, function, and therapeutic loading of the joint is critical for developing “functional regeneration and adaptation” strategies with an emphasis on restoring physiologic joint function.
I. Bone-Periodontal Ligament-Tooth Fibrous Joint
The bone-periodontal ligament (PDL)-tooth complex of the oral and craniofacial masticatory complex is a dynamic and biomechanically active fibrous joint (1). The primary function of the joint is to sustain cyclic chewing forces of varying magnitudes and frequencies. It is categorized as a fibrous joint by virtue of the PDL that 1) serves to attach teeth to the alveolar bone (1), a type of bone that is distinctly different from skeletal bone; 2) serves to consequently facilitate tooth displacement within the alveolar bony socket; 3) serves to distribute and dampen masticatory forces through the vascularized and innervated PDL; 4) contains differentiating zones at the ligament-cementum and ligament-bone entheses (attachment sites) (2, 3); 5) adjoins and interacts with cementum and alveolar bone through ligament-cementum and ligament-bone interfaces; 6) sustains and permits load-based reactionary forces from the tissues (enamel, dentin, cementum) and interfaces (enamel-dentin and cementum-dentin junctions) that makeup teeth exclusively; and 7) subsequently induce mechanical strain not limited to alveolar bone (4, 5). A multitude of structural components and tissues (categorized as biomaterials) join to form this nature’s well-lubricated load-bearing joint. Conceivably, this fibrous joint undergoes mechanical strain-mediated adaptation where the measured physical and chemical properties of load-bearing tissues per se and their interfaces are appreciated in the context of overall function. Understanding how tooth motion is guided within the alveolar socket in the presence of the PDL, and how the attachment process accommodates cyclic functional loads is critical as it would allow extracting strain-adaptive information that promotes tissue regeneration/remodeling to maintain biomechanical function of a joint.
To date, the biomechanical aspects of the bone-PDL-tooth fibrous joint have been investigated at discrete length-scales, that is, at the levels of the joint (6–13), tissues (3, 14–18), and cells (19–29). Investigations at a joint level have provided insights into the “coupled” nature of joint form and its masticatory function (11). At a tissue level, biological processes identified as interactions between the soft organic meshwork of the PDL and adjoining hard bone and cementum matrices, and subsequent strain-mediated mineralization of the inorganic hard tissue through active modeling and remodeling (30, 31), have provided insights into joint adaptation in response to physiologic and pathologic forces (2, 8, 9, 31–34). Through a reductionist approach, at a tissue and cellular-level, immunohistological approaches led to mapping of cell behavior and related matrix protein expressions to perturbations placed on tissues and cultured scaffolds (2, 3, 18, 26, 28, 32, 34–36). This also implies that cell and related tissue mechanics are seldom evaluated within the context of organ function. While probing using reductionist approach answers questions specific to tissues and cells respectively, it minimally addresses the importance of the measured physical and chemical properties, and thereby biological processes within the realm of function. In this manuscript, insights into adapted features within a complex in the context of function will be extracted using principles from biomechanics and mechanobiology. As a result, the manuscript will also highlight an interdisciplinary yet holistic approach (as opposed to reductionist) through the use of various imaging modalities to detail the effect of form and function relationship on strain adaptive properties of human alveolar bone, the PDL, cementum, and PDL-bone and PDL-cementum interfaces. Additionally, results will be discussed in the context of clinically relevant problems specifically related to orthodontics.
The two central objectives to highlight the critical importance of regenerative capacity of PDL-bone and PDL-cementum interfaces within the context of multi-scale biomechanics of the bone-PDL-tooth fibrous joint will be as follows:
- The changes in form of a human tooth relative to the socket-shape and vice versa can prompt local adaptations through strain concentrations within the PDL, PDL-bone and PDL-cementum interfaces that facilitate the dynamic function of the bone-PDL-tooth fibrous joint (8, 31). Within this objective the following salient points will be discussed.
-
aThe structural integration in tandem with the gradual variation in physical and chemical characteristics at the PDL-bone and PDL-cementum interfaces exist within a bone-PDL-cementum complex (32).
-
bDeviation from normal PDL-space (150–380 μm) to adapted regions of (5–50 μm) (33) at the original ligament-bone and ligament-cementum attachment are to resist functional demands.
-
cOver time, these constriction sites within the PDL-space are new load-bearing sites and could eventually impair joint function (31).
-
a
- In vivo models can be used to manipulate and help understand the adaptive capacities of entheses/interfaces. “Natural intelligence” of interfaces can be determined by extracting mechanoresponsive signals from cells within local regions specifically within the context of a clinical setting such as orthodontic tooth movement (OTM) (37, 38).
- d
In all cases, insights into the functional information for the PDL-bone and PDL-cementum interfaces will be extracted from in situ experiments (6, 39), and mapping of physical and chemical characteristics of interfaces will be performed through high resolution complementary and correlative microscopy techniques.
1. The changes in form of a human tooth relative to the socket-shape and vice versa can prompt local adaptations through strain concentrations within the PDL, PDL-bone and PDL-cementum interfaces that facilitate the dynamic nature of the bone-PDL-tooth fibrous joint function (8, 31)
The mammalian tooth attachment is a part and parcel of the oral and craniofacial masticatory complex, possesses the unique characteristic of short range motion compared to long range motion observed in diarthrodial joints of the musculoskeletal system. Such short-range motion is provided by a soft, fibrous PDL that attaches cementum to the alveolar bone, and acts as a shock absorber to distribute occlusal loads during mastication (11, 32, 40). Absorption of functional loads decreases hard tooth-tooth impact and enamel wear, alleviates stress/strain concentrations across PDL-cementum, PDL-bone attachment sites and interfaces, and allows for decades of cyclical loading (40, 41). Tooth movement due to chewing within physiological limits drives joint function through maintenance of the functional PDL-space via mechanobiological processes (42). The goal of this section is to elucidate tooth movement within the alveolar socket and to gain insights into the contribution and adaptive capacity of the constitutive properties of soft/hard tissues and interfaces to maintain joint biomechanics. Experimental approaches included a validated holistic method using noninvasive imaging technique through micro-X-ray computed tomography (μ-XCT) coupled to a mechanical loading device (6, 8). Ex vivo experimentation enabled visualization and evaluation of the effect of physical shifts within the intact complex of a loaded fibrous joint (6, 8).
1.1. Entheses, Entheses Organs and Interfaces: Materials Science, Biomechanics, and Mechanobiology Perspectives (Figure 1) (8, 32, 43)
Figure 1. Load-bearing periodontal ligament space (PDL-space), PDL-bone and PDL-cementum attachment sites, and PDL-inserts.
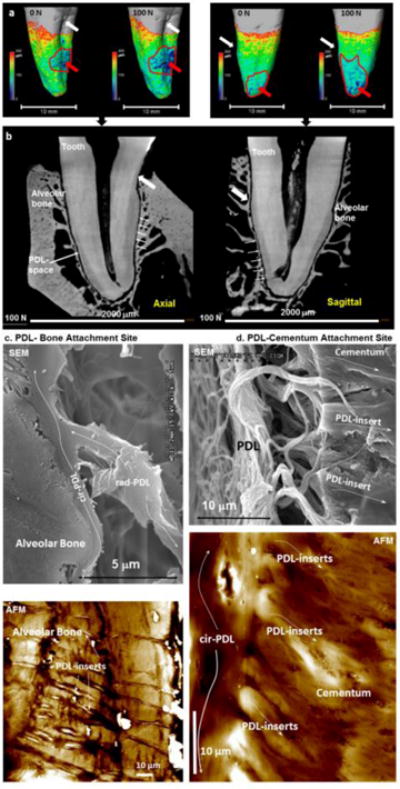
(a) Periodontal ligament (PDL)-space represented on the root surface of a human premolar at 0 N (mostly uniform PDL-space) and at 100 N (concentrated as pointed by the red arrow). Axial and sagittal virtual sections in two-dimensional (2D) space (b) are extrapolated from three-dimensional (3D) space (a). White arrows point to equivalent landmarks in both 3D (a) and 2D (b) spaces. Regions with significantly narrowed PDL-space specifically under loaded conditions are encircled in by a red curve (a) and corresponding regions in 2D space are indicated by arrows (b). The attachment of PDL to the alveolar bone (PDL-bone) and cementum (PDL-cementum) as visualized using scanning electron and atomic force microscopy (SEM, AFM) techniques is shown in panel c (PDL-bone) and d (PDL-cementum) respectively. Note a change in radial-PDL (rad-PDL) orientation to circumferential PDL (cir-PDL) as it “skirts” along alveolar bone and cementum before it inserts (PDL-inserts) into respective mineralized tissues within the load-bearing complex. In both cases these inserts are hygroscopic as denoted by their increased height profile when imaged under hydrated conditions (white regions correlate to topographical peaks of 2000–3000 nm compared to darker regions, which correlate to 0 nm).
Similar to other diarthrodial joints in the body, the load-bearing bone-PDL-tooth fibrous joint has the ability to react and sustain cyclic load through self-regulated biological processes. Under loaded conditions, mechanical strains can stimulate cells at various sites of the complex (Figs. 1a and 1b), and promote strain-adaptive properties within periodontal tissues and their interfaces (Figs. 1c and 1d). From a materials perspective, the strain-adaptive properties are termed as physicochemical properties.
Functional aspects related to interfaces within the context of biomechanics and mechanobiology were eloquently stated by Darcy Thompson and were adapted to fit within manuscript’s content. Thompson wrote “…and the beauty and strength of the mechanical construction (joint) lie not in one part or in another (tissues per se), but in the harmonious concatenation (interfaces) which all the parts, soft and hard, rigid and flexible, tension-bearing and pressure-bearing, make up together (44).” An interface is a “concatenation” of dissimilar materials. Various analytical techniques, similar to materials science lessons from nature, have taught that an interface can be converted to a homophone; interphase in that it is a region that encapsulates changes in physical and chemical characteristics that permit transition of one material to another (soft to hard (vice versa), hard to hard, soft to soft – all of which are dissimilar tissues) in a seamless fashion. This implies that an interphase is a transition zone and challenges identification of the end and beginning of two dissimilar tissues.
In biology, a term analogous to an interface is an enthesis organ. This term was first used in the musculoskeletal system to define interfaces between dissimilar tissues such as bone and tendon (osteotendinous), or bone and ligament (osteoligamentous) (45, 46). There is yet another term known as the “junction”. However, as used in the context of an interface, it is limited in meaning as it alludes to a physical demarcation between two different materials/tissues as seen by the naked eye, or limited to a low resolution light microscope. Several such junctions known as the dentin-enamel, cementum-dentin, and cementum-enamel junctions were also observed (47–52). From mechanics and materials science perspectives, the topic of particular interest to researchers is “fracture toughness” of a seemingly discontinuous system, that is, that which can be defined as an abrupt change from one material to another. However, interdisciplinary approaches, including various analytical techniques and histological analyses indicated natural interfaces to consist of gradients owing to gradual changes in structure, elemental and molecular compositions, and elastic modulus within a finite width between the dissimilar tissues including the cementum-dentin, cementum-enamel, and enamel-dentin regions (47–53). In a similar fashion, fibrocartilaginous joints showed that as tendons inserted into bone, they transitioned through zones containing gradients in organic and inorganic concentrations interspersed with hygroscopic regions (Figure 1). Consequently, it is thought that these gradual transitions in elemental and biochemical compositions provide resistance to functional demands including fracture (48). The importance of a gradual transition permitted by multiple zones is highlighted specifically when it is contrasted with a single zone, indicated by a sharp increase or decrease in organic to inorganic ratio (54, 55). These single zone regions are observed as elastic discontinuities within load-bearing systems with impaired function (Figure 2) (43).
Figure 2. Physical characteristics of a normal and compromised PDL and the bone-PDL-cementum complex using various high resolution imaging and AFM-based nanoindentation techniques.
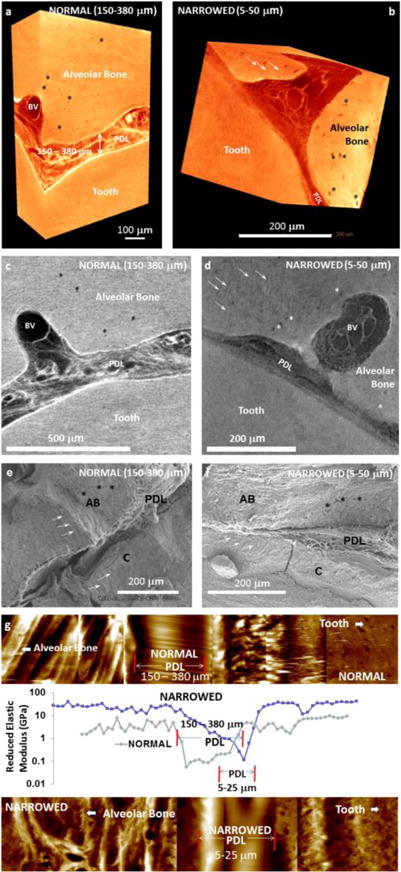
Digitally segmented blocks from human molars illustrate the PDL under seemingly normal (a) and compromised conditions (b). Normal condition is identified as a PDL-space of 150–380 μm (a), and the narrowed PDL-space illustrates 5–50 μm (b). Similar to the 3D digital reconstructions, virtual sections illustrate vascular bundles (blood vessels: BV) within the normal PDL, and similar structures are visible in narrowed PDL (d). Additionally, PDL-inserts can be observed in less X-ray attenuating regions in 3D reconstructed volumes and 2D virtual sections (white arrows in b and d). Similarly osteocytic lacunae are also identified (asterisks in a-d). Scanning electron micrographs of normal (e) and narrowed (f) conditions illustrate PDL between alveolar bone (AB) and cementum (C). Elastic modulus mapping under wet conditions using a nanoindenter illustrate a discontinuity in the narrowed bone-PDL-cementum complex (g).
a. The structural integration in tandem with the gradual variation in physical and chemical characteristics at the PDL-bone and PDL-cementum interfaces exist within a bone-PDL-cementum complex (Figure 1) (32)
Interfaces continue to be characterized as bioengineered templates upon which tissues can be regenerated to replace injured and/or chronically inflamed regions that impair locomotion. Within joints, interfaces are localized regions and act as “functional machines” due to crosstalk between a multitude of cells and cell-extracellular matrix structural components that work in concert to accommodate functional forces and permit joint motion (56). Within musculoskeletal and oral and craniofacial systems, these interfaces are grossly categorized as 1) indirect fibrocartilaginous, in which tendons ligaments insert into bone through and intermediate tissue type such as cartilage, and 2) direct fibrous entheses, in which tendons/ligaments insert into bone with no identifiable cartilaginous tissue (57). Studies guided by principles from biomechanics and mechanobiology illustrated PDL-bone and PDL-cementum interfaces of the bone-tooth fibrous joint to express characteristics analogous to direct fibrous entheses. Regardless, applying principles of mechanics of materials, amplification of strains within the cells at the soft-hard tissue interfaces compared to adjacent bulk tissues per se renders the tethered ends of the softer tissues to be susceptible to increasing shear and flexural moments that are commonly identified in load-bearing joints with short and long-range motions. However, from a mechanics perspective to prevent catastrophic failure of the joint through fatigue, cells interspersed with increased concentrations of matrix molecules including the adhesive proteoglycans (PGs) at these tethered ends (58) can promote cell migration and permit their adhesion and matrix interactions needed for tissue regeneration and remodeling (2).
PGs are hydrophilic macromolecules that are responsible for resisting the compressive forces through their water-retention characteristics, and sustaining functional loads in the periodontium (35). PGs are secreted by various cells in the PDL to sequester biomolecules and directly and indirectly modulate extracellular matrix formation. PGs have varied function in that their concentration and arrangement within the 5–10 μm region adjoining cementum and alveolar bone is distinctly different from the PDL-cementum and PDL-bone attachment sites (Figure 1). These include regulating adhesion of fibroblasts, cementoblasts, and osteoblasts to adjacent tissues to repair both degraded tissues and the attachment mechanism, and acting as potential modulators of biominerals (3, 17, 18). Hence, it is conceivable that the inherent characteristics of the PG-decorated PDL-cementum and PDL-bone interfaces and partial swelling at the adjoining hypomineralized regions consisting of hygroscopic PDL-inserts are vital for tooth attachment and modulating physiological loads (31–33, 35, 40) (Figs. 1c and 1d). These identifiable gradual transitions from softer to harder tissues (and vice versa) are used as a standard “blue print” to engineer functionally graded interfaces (FGI). The guiding principle of an FGI is to alleviate mechanical strains by distributing them over a wider interface thereby decreasing susceptibility to failure through tears and ruptures (32, 59–61) thus increasing resistance to fracture. This signature of nature’s FGI has been consistently demonstrated across diverse species that include the squid beak, bamboo stem, and vascular bundle of the Washingtonia robusta palm (62–64) to name a few. Within each of these species, gradients in mechanical stiffness have developed as an adaptive response in an attempt to reinforce underlying organ structure to resist the external stimulus. As such, interfaces can be then defined as a collection of related tissues at and near the attachment site (enthesis), and develop in response to environmental cues, such as shifts in mechanical strains. Consequently, the stimulated cells attempt to reinforce regions as they react to the biomechanical signals (mechanical strains related to tension, compression, and shear) that stem as function for any organism. The reinforcement can be identified as a multi-zone interface exhibiting a gradual transition, or a single-zone interface exhibiting an abrupt transition from soft to hard tissues and vice versa. These single zone regions are identified as elastic discontinuities within load-bearing systems with impaired function (Figure. 2).
b. Deviation from measured normal PDL-space (150–380 μm) to adapted regions of (5–50 μm) at the original ligament-bone and ligament-cementum attachment are to resist functional demands (Figure 2) (33)
When external stimuli that modulate functional homeostasis are altered and include abnormal regimes, irreversible modeling occurs and physicochemical properties change until a new functional homeostasis within a joint is reached (31, 33). Under these conditions, the question is; what is the functional relevance of these locally measured shifts in physicochemical gradients specifically at the PDL-bone and PDL-cementum and bone-PDL-cementum complex? That is, how does this shift in stiffness gradient affect overall joint function?
Under normal conditions, the graded stiffness from PDL to bone and PDL to cementum could vary due to natural physiological tooth movement with age known as active eruption, as well as by biomechanical function such as; masticatory forces due to normal and malocclusion. This normal adaptive role of the PDL-bone and PDL-cementum interfaces is amplified with the addition of external perturbations such as therapeutic load caused by orthodontic forces or disease as is the case with periodontitis. In support of the mechanics of materials related principle, a higher incidence of inflammation and failure in the form of tears at soft-hard tissue interfaces is commonly observed when PDL to bone and PDL to cementum interfaces are subjected to nonphysiologic loading. Due to the presence of cells, the increasingly strained interfaces become local regulators and form self-governing zones, and are ultimately observed as chronically inflamed sites. These injuries commonly occur with repeated insults/nonphysiologic stimuli or high impact sports, exemplified by development of insertional tendinopathies at the elbow, the Achilles tendon, and the knee (65–67). Such injuries are also known as enthesopathies, gradually formed at the soft-hard tissue entheses organs/interfaces with continuous nonphysiologic cyclic loads which over time can impede joint mobility and cause joint impairment (68).
In a human bone-PDL-tooth fibrous joint, these elastic gradients at entheses were found to be caused by a gradual increase in inorganic to organic ratio as the softer PDL transits into stiffer bone and cementum (32, 40, 49, 69, 70) (Figure 1). Partial swelling at the hypomineralized regions within the attachment sites between PDL and bone or cementum could establish the interface width over which the mechanical properties vary from the lower PDL (10–50 MPa) to higher values observed in alveolar bone (0.2–9.6 GPa) and cementum (1.1–8.3 GPa). As a result, under normal function, such interfaces can protect against excessive shear, torsional, bending, rotational, tensile, and compressive forces accompanied with joint movement (59, 71), ensuring optimum load distribution and transmission of cyclic loads (i.e. chewing forces) that prevent wear and tear due to fatigue. However, the following questions are invoked; what would happen should this gradual change in stiffness undergo abrupt change as observed in Figure 2? What would this shift in strain-adaptive properties (in this case the property in Figure 2 is reduced elastic modulus) mean in the context of joint function? And under what external stimuli including clinical scenarios would such a shift in graded properties arise? These questions will be addressed in the following sections.
The adaptive nature of the PDL and its interfaces is thought to be due to vascular elements (blood vessels – BV, Figure 2) and nerves (33) that are continuous with the extracellular matrix of the PDL that consists of fibrous proteins, including the dominant type I collagen, and trace type III and V collagens, elastin and oxytalan fibers, and globular proteins, including proteoglycans and other noncollagenous proteins (72–74). Progenitor cells and their differentiated lineages of osteoblasts, fibroblasts, and cementoblasts, reside within the matrix, but are localized in strain-specific regions of the complex (2). The surface layer of alveolar bone adjacent to the PDL consists of an underlying osteoid matrix with maturing inorganic minerals in the predominant forms of hydroxyapatite and carboxyapatite (75, 76). Typically, the general structure of an alveolar bone consists of an outer dense shell of compact bone known as the lamina dura to which a heavily vascularized, spongy cancellous bone is attached. The thickness of the compact bone as well as size and number of trabeculae in cancellous bone can increase and/or decrease in density in response to hyperfunction and hypofunction, respectively (77–79), and is a controlled by magnitude and duration of loading. These remodeling aspects of bone occur through an orchestrated effort of osteoblasts of mesenchymal and osteoclasts of hematopoetic origins, creating reversal lines caused by deposition and resorption activities, respectively (80–82). Hence, from a functional perspective, one can ask, do these protrusions in bone (Figure 2g) with a higher elastic modulus cause an elastic discontinuity in bone despite dominance in hygroscopic PDL-inserts? And does this discontinuity within the tissue and in the joint shift joint biomechanics from physiologic to pathologic or maladapted function?
Alveolar bone along with its bundle bone protrusion works in concert with another mineralized tissue, cementum within a bone-PDL-tooth fibrous joint. Opposite to the PDL-bone attachment site of the complex, a mineralizing matrix known as the cementoid also exists at the PDL-cementum interface (2, 5, 32, 83). Cementum is thought to be similar in structure to bone; however, it possesses an acellular zone in the anatomical coronal half to two-thirds of the tooth root (5, 83). Deposition and resorption of mineral also occur in cementum as they do in bone; however, these changes are usually in the form of modeling as opposed to remodeling (5, 84). Most often it is thought that cementum is lamellar, does not adapt, and has minimal adaptive response. While this could be true when compared to vascularized bone, results from our laboratory have indicated that cementum could have a delayed response. If so, could this be the reason why bone grows into the PDL-space (Fig. 2b, 2d and 2f), while cementum could subsequently make way to accommodate bone growth? Both these events need not be seen in the same sectioned two-dimensional (2D) plane, and indeed occur in three-dimensional (3D) volumes of the bone-PDL-cementum complex (37). And could this be the reason why secondary events such as conforming or nonconforming forms between the bony alveolar socket and cementum of the tooth can occur?
1.2. Physiologic and Pathologic Strain Amplifications at the PDL-bone and PDL-cementum Interfaces: Conforming and Nonconforming Alveolar Bone-Tooth Surfaces (Figures 1 and 2) (31, 33)
In musculoskeletal and dental orthopedics, conforming and nonconforming surfaces can define the form-function behavior/adaptation of a joint, and provide insights into the local behavior at a tissue level and resulting cellular responses and subsequent genetic and molecular expression. These hierarchical length-scale events provide a continuum-like effect to address functional homeostasis of a joint. Based on the fundamental seminal concept by Julius Wolff, the long-term effect of functional aberrations at the macroscale could result in pathological deformations to maintain function. Over time pathological deformations of mineralized tissues change the internal architecture, as the joint continues to function. Extending this to bone-PDL-tooth fibrous joint, the relative motions between members through a combination of soft and hard tissues and their interfaces will continue to adapt tissues locally. This adaptation is guided by the principles of joint biomechanics intertwined with mechanobiology of tissues. Adaptation is identified by spatiotemporal changes in physical and chemical properties of soft and mineralized tissues including their interfaces which in turn can result in an overall change in a form-function relationship, commonly termed as functional or biomechanical adaptation.
This section will elucidate the effect of eccentric loads, loads which do not align with the anatomical axis of the joint as opposed to concentric loads. Eccentric loads are those imposed by parafunctional habits with varying magnitudes and frequencies in which the principle vector is converted into a moment, and can exacerbate functional adaptation between two nonconforming surfaces in motion, specifically at the tethered ends of the PDL-bone and PDL-cementum attachment sites. These adaptations over time can be observed as physical features known as constrictions, and when investigated in the context of function can lead to stress concentrations and impending failure of the once biomechanically efficient joint. Other significant load-induced perturbations include traumatic and therapeutic loads from orthodontic braces all of which can change form-function relationship and decreased functional efficacy of the bone-PDL-tooth fibrous joint which will be discussed in section 2.
c. These constriction sites within the PDL-space over time are new load-bearing sites and could eventually impair joint function (Figure 2) (31)
Form modulates biomechanical response of individual structural components of the fibrous joint including its interfaces to facilitate optimal tooth movement (31) under physiologic and nonphysiologic conditions, including therapeautic loads. The contextual information of interfacial biomechanics is best highlighted by performing in situ experiments through visualization of interfaces at no load and under loaded conditions. Following image registration/analyses, the deformation within the PDL as a result of concentric loading (vertical load aligned with the anatomical axis of the bone-PDL-tooth fibrous joint) was mapped as shown in Figure 1. A normal PDL-space of 150–380 μm as observed is mechanically strained under uniformly loaded conditions. However, in situ eccentric (off centered) loading of the joint illustrated that tilting of the root in the bony socket can occur and induce tissue adaptations, not only at the interfaces, but within periodontal tissues per se by converting strain-induced deformation within respective matrices (mechanical energy) into biological processes (chemical energy). This conversion predominantly occurs intracellularly, and is facilitated by matrix-cell, and cell-cell interactions (32, 33, 85–88). Specifically, mechanical strains can be localized in regions where nonconformity between the surfaces of the tooth and the alveolar socket is appreciated (Figure 1a – red patches, 1b–block arrows and white arrows). At these regions it is likely that strains at the tendon/ligament-bone entheses could be up to four times the deformation experienced by the tendon/ligament bulk tissue, with microtears and/or microfractures occurring mainly within the interface (89, 90). As a result, cells within tissues near entheses are subjected to high incidences of strain amplification, subsequently prompting wear and tear at a tissue level and causing degenerative diseases called enthesopathies (57, 89).
Enthesopathies can occur as a result of disease and/or load-induced perturbations of higher frequencies and magnitudes of cyclic mechanical forces. The shift in strain within the PDL-space simply due to load (Figure 1a and 1b) and other aberrant inputs, such as bacterial insult, changes in food hardness, and therapeutic loads, can in turn shift the overall displacement of the tooth relative to the socket in response to load, thereby altering the self-regulating biomechanical cycle of the joint and triggering its adaptive nature in perpetuity (Figures 1–3). In other cases, proinflammatory factor expressions, cellular recruitment, and metaplasia at sites of entheses in response to excessive extraneous loads and/or prolonged inflammatory conditions have been shown to propagate the development of bony spurs that discourage joint mobility (56, 57). Hence, prolonged perturbations in the form of excessive extraneous loads often cause chronic inflammatory diseases that could alter or break down tissues involved in constructing stress concentration-relieving entheses organs. The observed bony protrusions could occur due to nonphysiological functional demands at the tethered ends of the PDL-bone and PDL-cementum attachment sites, and strengthen the region to accommodate functional demands. This compromised PDL-space will continue upon further loading, as the constriction sites will become the new “load-bearing sites” that eventually cause direct local fusion of bone with cementum. Similarly, it is speculated that altering function of the fibrous joint, i.e. tooth movement, generates adaptation of tissue composition to address changes in biomechanics (Figure 2 – differences between normal and a compromised human bone-PDL-tooth complex). Clinically, while a narrowed PDL-space can be considered asymptomatic when superimposed with other aberrant loads and clinical interventions it can cause failure of the fibrous joint.
Figure 3. Clinical scenario that exploits manipulating PDL to promote orthodontic tooth movement, and an animal model to link physical perturbations on the crown to biological processes in the bone-PDL-tooth load-bearing fibrous joint.
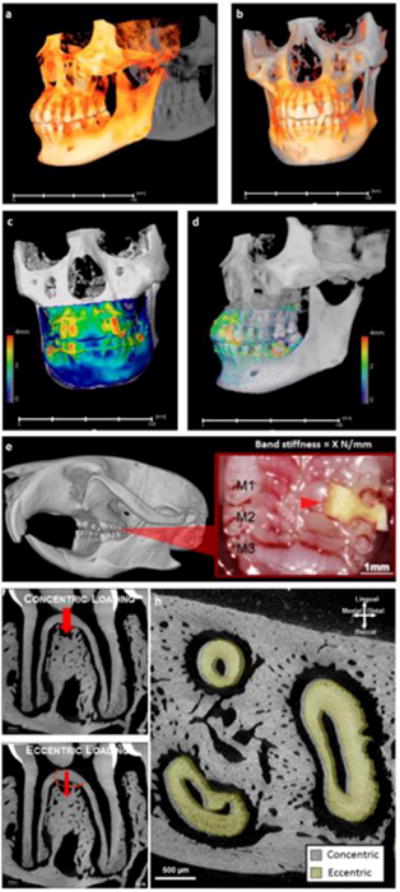
Orthodontic intervention is a clinical scenario that could stimulate plausible discontinuities in physical characteristics of the bone-PDL-tooth complex. Overall tooth displacement by comparing cone-beam computed tomographic reconstructed volumes taken before and after one year of orthodontic intervention is illustrated. Note that observed maximum tooth-displacement is 4 mm and is significantly larger than the 150–380 μm of PDL-space generally identified in humans. Physical changes due to mechanical stimulation resulting from biological processes to prompt tooth translation are programmed in rodents, specifically in mice between the molars 1 and 2 (M1 and M2, red arrow head) with an elastic band of known stiffness (X N/m) (e) to first perform comparative analyses on PDL-space changes (h) under centric (f) and eccentric (g) conditions. Note that in the illustrated scenario the roots of an eccentrically loaded first molar were displaced in the distal direction (h) specifically when compared concentric conditions (g).
2. In vivo models can be used to manipulate and help understand the adaptive capacities of entheses/interfaces. “Natural intelligence” of interfaces can be determined by extracting mechanoresponsive signals from cells within local regions specifically within the context of a clinical setting such as orthodontic tooth movement (OTM) (Figure 3) (9, 37, 38)
In mammalian species, function-related thresholds are most often modulated by food hardness (7, 34, 91–94). Previous studies have shown that the width of the PDL-space changes in response to magnitude and direction of occlusal loads (7). Hyperfunction during which force exceeds physiologic loading (increased magnitude and decreased frequency) has shown to increase PDL-space through an upregulated osteoclastic response, while conditions of hypofunction (decreased magnitude and increased frequency) illustrated modeling of the PDL width to a narrower space than under control conditions (17, 34, 78, 83, 95). Here lies an intriguing aspect of plausible mechanobiological differences between PDL-bone and PDL-cementum interfaces, in that adaptation to magnitude and frequency of loading at bone interface could be significantly different from that which occurs at the cementum interface, and respective tissues alone. This concept will be revisited under the clinical scenario of orthodontics, an extrapolation of fundamentals from structural engineering/orthopedics that states that the anatomical axis and loading axis should coincide (concentrically loaded) for physiological function of a joint. In its absence, the net shift in mechanical strain can prompt increased pullout forces at the PDL-bone and PDL-cementum attachment sites causing cells to differentiate and undergo different rates of durotaxis and haptotaxis. These semiautonomous biological events lead us to propose that the natural plasticity of these tissues continues to guide the organ within physiological limits, but significant shifts in mechanical strain contributed by a change from concentric or eccentric loads or vice versa may result in closing or widening of the PDL-space. These physical transformations resulting from mineral formation and resorption related events at the ligament interface can guide the complex to a nonphysiologic regime. This concept will be illustrated by presenting a clinical scenario, namely orthodontics.
d. The semiautonomous events at interfaces guide the joint within physiological limits, but significant mineral formation and resorption at the PDL-interfaces may result in closing or widening of the PDL-space and can guide the complex to a nonphysiological regime (Figure 4) (9, 37, 38)
Figure 4. In vivo experimental model to program the fundamentals of orthodontic tooth movement to investigate the “intelligence” of the PDL-bone and PDL-cementum interfaces within the bone-PDL-tooth complex.
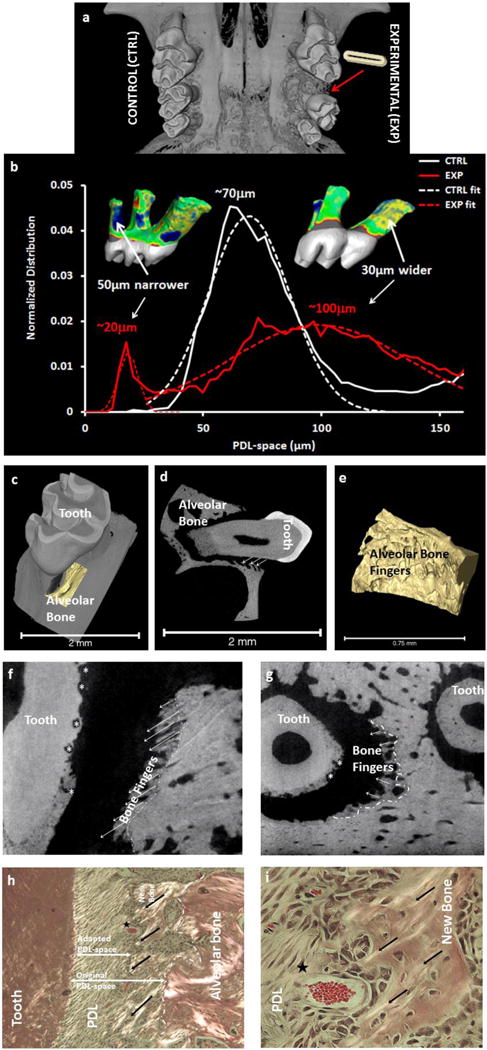
Measured physical changes by comparing the experimental (shows the location and tooth movement due to elastic placement) with control (a) illustrated a homogenous distribution with a peak at 70 μm compared to narrowed (indicated in blue) and widened (indicated in yellow) regions of the PDL-space. The widened PDL-space contributed to bone growth along the strained PDL fibers (b-d, highlighted 3D volume (c, e) and located by arrows in 2D (d) spatial domains). Note the resorption pits in and through cementum invading dentin (asterisks) in longitudinal (f) and transverse (g) directions. Polarized light microscopy on these sections illustrate strained PDL fibers (black arrows) and micro-vessels (star) within regions closer to PDL-bone attachment (g) including hypertrophic cells at the attachment sites (h) compared to polarized cells within PDL perse (g, h). Note the differences between original and adapted PDL-spaces (dashed line demarcates original and newly formed bone. Matrix structure is used as a cue to demarcate original PDL-bone attachment site from adapted) as mineral forms on the strained PDL-fibers.
There exist many clinical interventions in skeletal (distraction osteogenesis), and oral and craniofacial orthopedics (cranial grafts, orthodontics) that involve the use of mechanical forces to “mold” and/or regenerate bone and its adjacent tissues. However, very little is known about the influence of mechanical stimulus on the biomineralization of tissue per se within the bone-PDL-tooth complex or functional interfaces between ligament-bone and ligament-cementum. The current doctrine regarding the role of PDL in response to applied loads has been summarized as: 1) the PDL distributes applied loads to the alveolar bone, 2) the direction, frequency, duration and magnitude of loading determine both the extent, rate of bone remodeling and “quality’ of modeled bone, and 3) the absence of PDL severely limits the extent of bone remodeling (96) and tooth movement.
The importance of functional adaptations of the bone, in particular at the interfaces, is not limited to orthodontics, but extends into the orthopedics. Within orthopedics, it has been suggested that functional adaptation of the bone results from cellular response to strain density within the softer matrix (97). The effects of strain density within the PDL and PDL-interfaces on biomineralization within the bone-PDL-tooth joint can be correlated to the cellular and tissue adaptations in a mouse model. The specific sites that are mechanically stimulated include the PDL-bone and PDL-cementum functional attachment sites. Due to the nature of the ligament-bone interfaces in the complex, i.e. disparate tissues interfacing over a distance of 10–50 μm, these sites are mechanically strained (45, 61) and the rate of adaptation at the interfaces is higher compared to other modeling sites. For this reason, the attachment sites present themselves as excellent model systems where mechano-responsiveness, i.e. the response of mechanical strain amplification on mineral formation and resorption can be investigated and better understood.
In humans, fundamental components for OTM include force, moment arm, and frequency of loading. The effect of these components when observed from cone beam computed tomography scans before and after OTM of the same patient, registered about the center of rotation of the skull, revealed vectors both translation and rotation of teeth (Figure 3). Fundamentally, from a structural engineering perspective, the principle vector under normal conditions is converted to a moment under eccentrically loaded conditions, and as a result is thought to accentuate strains, specifically at regions where dissimilar materials are attached. This concept is constantly leveraged in OTM or experimental tooth movement (ETM) in rodents using various methods of eccentric load placement including the use of an elastic spacer (Figure 3e) to amplify strains at the functional interfaces and attachment sites of the PDL-bone and PDL-cementum. While applied force does lead to gross tooth movement, and perhaps shifts in rates of biomineralization at the ligament interfaces, it is important to consider that bone modeling and drifts that prompted the overall form of the tooth-socket are an attempt to maintain optimum biomechanics needed for continued chewing. Worthy of note is the growth of bony fingers along the strained fibers of the ligament (Figures 4c–4i); a growth not observed at the ligament-cementum interface within the same 2D spatial field (Figures 4d, 4f–4i). The intriguing aspect of plausible mechanobiological differences and resulting adaptation to shifts in magnitudes and frequencies at the PDL-bone and PDL-cementum interfaces can be answered by investigating the genetic expressions of varied cell populations (note the varied cell morphologies commonly identified at strained ligament-bone and ligament-cementum attachment sites, Figures 4h and 4i) at these site-specific volumes within the bone-PDL-cementum complex. Based on this experimental model, several questions follow. Which volumes within the three dimensional (3D) complex should be examined to investigate the mechanobiological effect on cells at these localized regions that act as “functional machines”? That is, where are the semiautonomous regions within the complex located? Additionally, what is the association of genetics with matrix molecular expressions within the same complex? And more from an experimental approach perspective is a 2D field an accurate representation of mechanobiological effects acting on a 3D complex?
Leveraging recent developments in technologies has enabled imaging intact specimens and thereby the 3D bone-PDL-tooth fibrous joint has been subjected to ETM to subsequently provide changes in physical disturbances of the PDL-space otherwise omitted by 2D micrographs. By comparing experimental tooth movement to controls, data provided insights into tooth displacement and strains under ETM, including observations that ETM can exceed distances far greater than PDL-space (Figures 4a and 4b). Undoubtedly, the significantly strained tethered ends of the ligament and its interfaces termed as “functional machines” will elicit cell responses locally. Outcomes of these mechanobiological cell responses are observed as regenerated matrices with physicochemical characteristics adequate of the felt shifts in magnitudes and frequencies at these site-specific regions within a 3D spatial field of the complex. These biological processes are an attempt to restore physiologic PDL-space via active mineral formation and resorption at the PDL interfaces, and other regions of alveolar bone and cementum of the periodontium. The rapid shifts prompted by experimental tooth movement can favor rapid deposition of mineral by cells interacting with the naïve yet mechanically strained organic fabric at the attachments sites and interfaces, and can question the “quality” of bone.
Bone quality assumes several flavors. According to American Society for Bone Mineral Research (ASBMR) (98), bone quality is identified as the “turnover, damage accumulation (.e.g. microfractures) and mineralization of the organic matrix, the chemical composition and extent of crosslinking of the organic matrix, bone architecture, fatigue from repeated loading of bone, the rate and direction of deformation of the bone during trauma and is determined by a complex interplay between the amount of mineralized tissue present in the bone, and the extent of biomineralization” (98). For these reasons, it is clear that this dynamic tissue cannot be simply described by a sole parameter, but functional insight if adaptations in bone maintain optimum function or shift the structure to a maladapted state to address functional demands above or below physiologic threshold range should be gathered. Regardless, upon prolonged stimulus (above or below physiologic threshold range), rates of adaptation are increased at the richly differentiating semiautonomous zones of the PDL-entheses and therefore are susceptible to controlled modulation or modulation toward pathology under these exacerbated conditions. Given that the load-bearing complex also contains cementum and its interface with the ligament, different reaction rates (shifts in magnitude and frequency), where resorption and bony finger formations can be thought of as need biological processes in an attempt to ‘correct’ the orthodontically widened PDL-space. These biological processes at the PDL-bone interface can occur at a significantly higher rate than those at PDL-cementum that would prompt external root resorption of cementum.
It can be argued that the pathologic extension of new bone (Figures 4f–4i) in response to ETM creates a more fragile periodontal complex. Concepts extrapolated from physical measurements are in concurrence with existing (99)thoughts ascertaining that any ETM that prompts tooth-shift by a distance greater than its PDL-space will elicit resorption. Conceptually, in ETM, any region that narrows by more than PDL-space requires resorption of hard tissue, in order to maintain a viable PDL-space for tooth function. In humans, the average PDL-space ranges from 150μm to 38Oμm (32), and any OTM shift greater than the physiologic PDL-space will create similar hard tissue resorption not limited to bone, as it can also occur in cementum. This concept should be acknowledged in the clinic when prompting substantial OTM in patients with short or developing roots, as they may experience dramatic root resorption and subsequent tooth mobility/loss. From fundamental science and engineering perspectives, given such excessive strains in the PDL, it is plausible that the physicochemical properties could be significantly different, which would question the functional quality of bone and the load-bearing dentoalveolar complex. From a clinical perspective, regenerated weaker bone due to aseptic inflammation caused by orthodontics could leave patients at a higher risk of bone loss or root resorption if they later develop periodontitis, a form of septic inflammation (Figure 5).
Figure 5. Adaptation cycle of a bone-PDL-tooth fibrous joint.
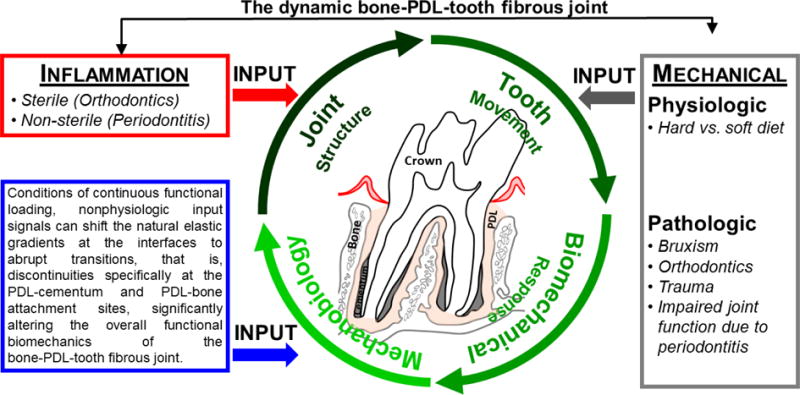
Encircling green arrows represent the processes involved in joint maintenance and adaptation. The red arrow represents inflammatory perturbations as induced by sterile (mechanical stimulation) and/or non-sterile (bacteria) and other systemic diseases related to metabolic syndromes (diabetes/hypertension) that invariably induce acerbated mechanobiological adaptation by altering the joint structure. The gray arrow represents physiologic and pathologic perturbations that induce strain-mediated adaptation through altered tooth movement in the alveolar socket and plausible impaired joint function. PDL: Periodontal ligament.
II. CONCLUSIONS
a. Elastic discontinuities in tissues and adaptive nature of joints can impair function (Figures 1 and 2)
Several naturally graded interfaces exist in the dynamic joint of the bone-PDL-tooth complex (32, 48–50, 52, 58). A gradual gradient in stiffness between a soft and hard tissue can adapt to an abrupt gradient to accommodate physiologic or non-physiologic demands on joints. It is this philosophy that was observed to highlight adaptation/regeneration in regions farther away from the site of injury/insult (which normally occurs closer or on the crown) within the context of joint function (33, 43).
b. Form and function can explain the “plastic” nature of a fibrous joint in humans (Figures 2 and 3)
The alveolar bone of a human jaw is minimally interrogated from a functional perspective. Studies presented to date on tissue mechanics are seldom evaluated within the context of joint function. We sought a holistic approach with the use of state-of-the-art in situ mechanical testing coupled to a micro X-ray computed tomography unit; and identified that bony protrusions within a 150–380 μm functional space of a biomechanically active interface can cause joint malfunction (33, 37).
c. A holistic overview of joint- and tissue-level biomechanics (Figure 3)
With the use of advanced technology, the effect of overall form, and the physicochemical properties of dynamic joint/tissues can be investigated. Through this validated technology, it is possible to establish that joint function is governed by form-mediated strain felt by bone, PDL, and cementum tissues that predominantly makeup the load-bearing complex. The coupled effect of overall form at a joint-level and strain-adaptive properties at a tissue-level illustrates the importance of functional continuation of the bone-PDL-tooth fibrous joint, and that load-mediated joint adaptation is best understood through a multiscale biomechanics approach (6–9).
d. Differentiating zones and regenerative potential at the soft-hard functional attachment sites and interfaces (Figure 4)
In load-bearing joints, strain amplification occurs at the attachment sites where disparate tissues attach (12, 100). Shifts in mechanical strains due to physiological or nonphysiological (including therapeutic loads) (37) function at the attachment sites and interfaces mediate self-governing zones (2, 37). The spatiotemporally observed biochemical signals (matrix and intracellular proteins) at the attachment sites provide insights to postulate that biophysical signals can modulate mineral formation and resorption related events in the periodontal complex (2). The shift in specificity and localization of biomolecules with development, function, and therapeutic loading is critical for developing “functional regeneration” strategies, and should be investigated with an emphasis on joint function.
e. Multiscale biomechanics approach can provide insights into joint biomechanics, malfunction, and functional regeneration of tissues and interfaces (Figure 5)
A multiscale biomechanics approach (6–8) is important for correlating tissue-level strain-adaptive properties with overall effects of joint form on function and pathology. Elucidating the shift in localization of biomolecules with development, function, and therapeutic loading of the joint is critical for developing “functional regeneration” strategies with an emphasis on restoring physiological joint function.
Acknowledgments
The authors acknowledge funding support NIH/NIDCR R00DE018212 (SPH), NIH/NIDCR-R01DE022032 (SPH), NIH/NIDCR T32 DE07306, NIH/NCRR S10RR026645, (SPH) and Departments of Preventive and Restorative Dental Sciences and Orofacial Sciences, UCSF; Faculty of Engineering, McMaster University (Hamilton, Canada) (KG). In addition, assistance from national facilities through user based program was provided by Stanford Synchrotron Radiation Lightsource (SSRL), SLAC National Accelerator Laboratory, Stanford University, CA and The Molecular Foundry, Lawrence Berkeley National Laboratory, Berkeley, CA. The TXM work performed at SSRL, SLAC was done on TXM purchased through a grant from National Institutes of Health (NIH)/National Institute of Biomedical Imaging and Bioengineering grant number R01-EB004321 (PP). Work at the SSRL was supported by the U.S. Department of Energy under contract number DE-AC02–76SF00515. Work at the Molecular Foundry was supported by the Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under Contract No. DE-AC02–05CH11231.
References
- 1.Cate T, Nanci A. Ten Cate’s – Oral Histology: Development, Structure, and Function. St. Louis, MO: Elsevier; 2013. [Google Scholar]
- 2.Lee JH, Pryce BA, Schweitzer R, Ryder MI, Ho SP. Differentiating zones at periodontal ligament-bone and periodontal ligament-cementum entheses. Journal of periodontal research. 2015 Dec;50(6):870–80. doi: 10.1111/jre.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCulloch CA, Lekic P, McKee MD. Role of physical forces in regulating the form and function of the periodontal ligament. Periodontology 2000. 2000 Oct;24:56–72. doi: 10.1034/j.1600-0757.2000.2240104.x. [DOI] [PubMed] [Google Scholar]
- 4.Newman MG, KP R, CF A. Craranza’s Clinical Periodontology. 2014;12 editor. [Google Scholar]
- 5.Bosshardt DD, Selvig KA. Dental cementum: the dynamic tissue covering of the root. Periodontology 2000. 1997 Feb;13:41–75. doi: 10.1111/j.1600-0757.1997.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 6.Jang AT, Lin JD, Seo Y, Etchin S, Merkle A, Fahey K, et al. In situ compressive loading and correlative noninvasive imaging of the bone-periodontal ligament-tooth fibrous joint. Journal of visualized experiments : JoVE. 2014 Mar 07;(85) doi: 10.3791/51147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jang AT, Merkle A, Fahey K, Gansky SA, Ho SP. Multiscale biomechanical responses of adapted bone-periodontal ligament-tooth fibrous joints. Bone. 2015 Jul 4; doi: 10.1016/j.bone.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin JD, Ozcoban H, Greene JP, Jang AT, Djomehri SI, Fahey KP, et al. Biomechanics of a bone-periodontal ligament-tooth fibrous joint. Journal of biomechanics. 2013 Feb 1;46(3):443–9. doi: 10.1016/j.jbiomech.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin JD, Lee J, Ozcoban H, Schneider GA, Ho SP. Biomechanical adaptation of the bone-periodontal ligament (PDL)-tooth fibrous joint as a consequence of disease. Journal of biomechanics. 2014 Jun 27;47(9):2102–14. doi: 10.1016/j.jbiomech.2013.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naveh GR, Lev-Tov Chattah N, Zaslansky P, Shahar R, Weiner S. Tooth-PDL-bone complex: response to compressive loads encountered during mastication – a review. Archives of oral biology. 2012 Dec;57(12):1575–84. doi: 10.1016/j.archoralbio.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Popowics TE, Herring SW. Load transmission in the nasofrontal suture of the pig, Sus scrofa. Journal of biomechanics. 2007;40(4):837–44. doi: 10.1016/j.jbiomech.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian L, Todo M, Morita Y, Matsushita Y, Koyano K. Deformation analysis of the periodontium considering the viscoelasticity of the periodontal ligament. Dental materials : official publication of the Academy of Dental Materials. 2009 Oct;25(10):1285–92. doi: 10.1016/j.dental.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 13.Naveh GR, Shahar R, Brumfeld V, Weiner S. Tooth movements are guided by specific contact areas between the tooth root and the jaw bone: A dynamic 3D microCT study of the rat molar. Journal of structural biology. 2012 Feb;177(2):477–83. doi: 10.1016/j.jsb.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Dorow C, Krstin N, Sander FG. Experiments to determine the material properties of the periodontal ligament. Journal of orofacial orthopedics = Fortschritte der Kieferorthopadie : Organ/official journal Deutsche Gesellschaft fur Kieferorthopadie. 2002 Mar;63(2):94–104. doi: 10.1007/s00056-002-0107-4. [DOI] [PubMed] [Google Scholar]
- 15.Krstin N, Dorow C, Franke RP, Sander FG. Experiments to determine the time dependent material properties of the periodontal ligament. Biomedizinische Technik Biomedical engineering. 2002 Jul-Aug;47(7–8):202–8. doi: 10.1515/bmte.2002.47.7-8.202. Experimente zur Bestimmung der zeitabhangigen Materialeigenschaften des Parodontalligaments. [DOI] [PubMed] [Google Scholar]
- 16.Dorow C, Krstin N, Sander FG. Experimental model of tooth mobility in the human “in vivo”. Biomedizinische Technik Biomedical engineering. 2002 Jan-Feb;47(1–2):20–5. doi: 10.1515/bmte.2002.47.1-2.20. Experimentelle Untersuchung der Zahnbeweglichkeit am Menschen “in vivo”. [DOI] [PubMed] [Google Scholar]
- 17.Walker CG, Ito Y, Dangaria S, Luan X, Diekwisch TG. RANKL, osteopontin, and osteoclast homeostasis in a hyperocclusion mouse model. European journal of oral sciences. 2008 Aug;116(4):312–8. doi: 10.1111/j.1600-0722.2008.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiu R, Li W, Herber RP, Marshall SJ, Young M, Ho SP. Effects of biglycan on physico-chemical properties of ligament-mineralized tissue attachment sites. Archives of oral biology. 2012 Feb;57(2):177–87. doi: 10.1016/j.archoralbio.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Dangaria SJ, Ito Y, Luan X, Diekwisch TG. Successful periodontal ligament regeneration by periodontal progenitor preseeding on natural tooth root surfaces. Stem cells and development. 2011 Oct;20(10):1659–68. doi: 10.1089/scd.2010.0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004 Jul 10–16;364(9429):149–55. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 21.Shi S, Bartold PM, Miura M, Seo BM, Robey PG, Gronthos S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthodontics & craniofacial research. 2005 Aug;8(3):191–9. doi: 10.1111/j.1601-6343.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- 22.Chen SC, Marino V, Gronthos S, Bartold PM. Location of putative stem cells in human periodontal ligament. Journal of periodontal research. 2006 Dec;41(6):547–53. doi: 10.1111/j.1600-0765.2006.00904.x. [DOI] [PubMed] [Google Scholar]
- 23.Ivanovski S, Gronthos S, Shi S, Bartold PM. Stem cells in the periodontal ligament. Oral diseases. 2006 Jul;12(4):358–63. doi: 10.1111/j.1601-0825.2006.01253.x. [DOI] [PubMed] [Google Scholar]
- 24.Bartold PM, Shi S, Gronthos S. Stem cells and periodontal regeneration. Periodontology 2000. 2006;40:164–72. doi: 10.1111/j.1600-0757.2005.00139.x. [DOI] [PubMed] [Google Scholar]
- 25.Menicanin D, Mrozik KM, Wada N, Marino V, Shi S, Bartold PM, et al. Periodontal-ligament-derived stem cells exhibit the capacity for long-term survival, self-renewal, and regeneration of multiple tissue types in vivo. Stem cells and development. 2014 May 1;23(9):1001–11. doi: 10.1089/scd.2013.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pavlin D, Gluhak-Heinrich J. Effect of mechanical loading on periodontal cells. Critical reviews in oral biology and medicine : an official publication of the American Association of Oral Biologists. 2001;12(5):414–24. doi: 10.1177/10454411010120050401. [DOI] [PubMed] [Google Scholar]
- 27.Purvis JA, Embery G, Oliver WM. Molecular size distribution of proteoglycans in human inflamed gingival tissue. Archives of oral biology. 1984;29(7):513–9. doi: 10.1016/0003-9969(84)90072-4. [DOI] [PubMed] [Google Scholar]
- 28.Yu H, Ren Y, Sandham A, Ren A, Huang L, Bai D. Mechanical tensile stress effects on the expression of bone sialoprotein in bovine cementoblasts. The Angle orthodontist. 2009 Mar;79(2):346–52. doi: 10.2319/011508-20.1. [DOI] [PubMed] [Google Scholar]
- 29.Yamaguchi M, Aihara N, Kojima T, Kasai K. RANKL increase in compressed periodontal ligament cells from root resorption. Journal of dental research. 2006 Aug;85(8):751–6. doi: 10.1177/154405910608500812. [DOI] [PubMed] [Google Scholar]
- 30.Roberts WE. Bone dynamics of osseointegration, ankylosis, and tooth movement. Journal. 1999 Fall;78(3):24–32. [PubMed] [Google Scholar]
- 31.Ho SP, Kurylo MP, Grandfield K, Hurng J, Herber RP, Ryder MI, et al. The plastic nature of the human bone-periodontal ligament-tooth fibrous joint. Bone. 2013 Dec;57(2):455–67. doi: 10.1016/j.bone.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ho SP, Kurylo MP, Fong TK, Lee SS, Wagner HD, Ryder MI, et al. The biomechanical characteristics of the bone-periodontal ligament-cementum complex. Biomaterials. 2010 Sep;31(25):6635–46. doi: 10.1016/j.biomaterials.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hurng JM, Kurylo MP, Marshall GW, Webb SM, Ryder MI, Ho SP. Discontinuities in the human bone-PDL-cementum complex. Biomaterials. 2011 Oct;32(29):7106–17. doi: 10.1016/j.biomaterials.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niver EL, Leong N, Greene J, Curtis D, Ryder MI, Ho SP. Reduced functional loads alter the physical characteristics of the bone-periodontal ligament-cementum complex. Journal of periodontal research. 2011 Dec;46(6):730–41. doi: 10.1111/j.1600-0765.2011.01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leong NL, Hurng JM, Djomehri SI, Gansky SA, Ryder MI, Ho SP. Age-related adaptation of bone-PDL-tooth complex: Rattus-Norvegicus as a model system. PloS one. 2012;7(4):e35980. doi: 10.1371/journal.pone.0035980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takano-Yamamoto T, Takemura T, Kitamura Y, Nomura S. Site-specific expression of mRNAs for osteonectin, osteocalcin, and osteopontin revealed by in situ hybridization in rat periodontal ligament during physiological tooth movement. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1994 Jul;42(7):885–96. doi: 10.1177/42.7.8014472. [DOI] [PubMed] [Google Scholar]
- 37.Grandfield K, Herber RP, Chen L, Djomehri S, Tam C, Lee JH, et al. Strain-guided mineralization in the bone-PDL-cementum complex of a rat periodontium. Bone reports. 2015 Dec 1;3:20–31. doi: 10.1016/j.bonr.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pal A, Chen L, Yang L, Yang F, Meng B, Jheon AH, et al. Micro-anatomical responses in the periodontal complex to calibrated orthodontics forces on the crown. Orthodontics and Craniofacial Research. 2016 doi: 10.1111/ocr.12172. accepted for publication. [DOI] [PubMed] [Google Scholar]
- 39.Jang AT, Prevost R, Ho SP. Strain mapping and correlative microscopy of the alveolar bone in a bone-PDL-tooth fibrous joint. 2016 doi: 10.1177/0954411916655183. under review. [DOI] [PubMed] [Google Scholar]
- 40.Ho SP, Marshall SJ, Ryder MI, Marshall GW. The tooth attachment mechanism defined by structure, chemical composition and mechanical properties of collagen fibers in the periodontium. Biomaterials. 2007 Dec;28(35):5238–45. doi: 10.1016/j.biomaterials.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luan X, Walker C, Dangaria S, Ito Y, Druzinsky R, Jarosius K, et al. The mosasaur tooth attachment apparatus as paradigm for the evolution of the gnathostome periodontium. Evolution & development. 2009 May-Jun;11(3):247–59. doi: 10.1111/j.1525-142X.2009.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herring SW. Mineralized tissues in oral and craniofacial science: biological principles and clinical correlates. 1st. John Wiley & Sons, I; 2012. editor. [Google Scholar]
- 43.Lin JD, Aloni S, Altoe V, Webb SM, Ryder MI, Ho SP. Elastic discontinuity due to ectopic calcification in a human fibrous joint. Acta biomaterialia. 2013 Jan;9(1):4787–95. doi: 10.1016/j.actbio.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson DW. On Growth and Form. Cambridge University Press; 1961. On form and mechanical efficiency. [Google Scholar]
- 45.Liu YX, Thomopoulos S, Birman V, Li JS, Genin GM. Bi-material attachment through a compliant interfacial system at the tendon-to-bone insertion site. Mechanics of materials : an international journal. 2012 Jan;44 doi: 10.1016/j.mechmat.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shahab-Osterloh S, Witte F, Hoffmann A, Winkel A, Laggies S, Neumann B, et al. Mesenchymal stem cell-dependent formation of heterotopic tendon-bone insertions (osteotendinous junctions) Stem cells. 2010 Sep;28(9):1590–601. doi: 10.1002/stem.487. [DOI] [PubMed] [Google Scholar]
- 47.Marshall GW, Jr, Balooch M, Gallagher RR, Gansky SA, Marshall SJ. Mechanical properties of the dentinoenamel junction: AFM studies of nanohardness, elastic modulus, and fracture. Journal of biomedical materials research. 2001 Jan;54(1):87–95. doi: 10.1002/1097-4636(200101)54:1<87::aid-jbm10>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 48.Imbeni V, Kruzic JJ, Marshall GW, Marshall SJ, Ritchie RO. The dentin-enamel junction and the fracture of human teeth. Nature materials. 2005 Mar;4(3):229–32. doi: 10.1038/nmat1323. [DOI] [PubMed] [Google Scholar]
- 49.Ho SP, Balooch M, Marshall SJ, Marshall GW. Local properties of a functionally graded interphase between cementum and dentin. Journal of biomedical materials research Part A. 2004 Sep 1;70(3):480–9. doi: 10.1002/jbm.a.30105. [DOI] [PubMed] [Google Scholar]
- 50.Ho SP, Balooch M, Goodis HE, Marshall GW, Marshall SJ. Ultrastructure and nanomechanical properties of cementum dentin junction. Journal of biomedical materials research Part A. 2004 Feb 1;68(2):343–51. doi: 10.1002/jbm.a.20061. [DOI] [PubMed] [Google Scholar]
- 51.Ho SP, Sulyanto RM, Marshall SJ, Marshall GW. The cementum-dentin junction also contains glycosaminoglycans and collagen fibrils. Journal of structural biology. 2005 Jul;151(1):69–78. doi: 10.1016/j.jsb.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 52.Ho SP, Senkyrikova P, Marshall GW, Yun W, Wang Y, Karan K, et al. Structure, chemical composition and mechanical properties of coronal cementum in human deciduous molars. Dental materials : official publication of the Academy of Dental Materials. 2009 Oct;25(10):1195–204. doi: 10.1016/j.dental.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Habelitz S, Marshall SJ, Marshall GW, Jr, Balooch M. The functional width of the dentino-enamel junction determined by AFM-based nanoscratching. Journal of structural biology. 2001 Sep;135(3):294–301. doi: 10.1006/jsbi.2001.4409. [DOI] [PubMed] [Google Scholar]
- 54.Benjamin M, Evans EJ, Copp L. The histology of tendon attachments to bone in man. Journal of anatomy. 1986 Dec;149:89–100. [PMC free article] [PubMed] [Google Scholar]
- 55.Evans EJ, Benjamin M, Pemberton DJ. Fibrocartilage in the attachment zones of the quadriceps tendon and patellar ligament of man. Journal of anatomy. 1990 Aug;171:155–62. [PMC free article] [PubMed] [Google Scholar]
- 56.Benjamin M, Toumi H, Ralphs JR, Bydder G, Best TM, Milz S. Where tendons and ligaments meet bone: attachment sites (‘entheses’) in relation to exercise and/or mechanical load. Journal of anatomy. 2006 Apr;208(4):471–90. doi: 10.1111/j.1469-7580.2006.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shaw HM, Benjamin M. Structure-function relationships of entheses in relation to mechanical load and exercise. Scandinavian journal of medicine & science in sports. 2007 Aug;17(4):303–15. doi: 10.1111/j.1600-0838.2007.00689.x. [DOI] [PubMed] [Google Scholar]
- 58.Kurylo MP, Grandfield K, Marshall GW, Altoe V, Aloni S, Ho SP. Effect of proteoglycans at interfaces as related to location, architecture, and mechanical cues. Archives of oral biology. 2016 Mar;63:82–92. doi: 10.1016/j.archoralbio.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Claudepierre P, Voisin MC. The entheses: histology, pathology, and pathophysiology. Joint, bone, spine : revue du rhumatisme. 2005 Jan;72(1):32–7. doi: 10.1016/j.jbspin.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 60.Benjamin M, Kumai T, Milz S, Boszczyk BM, Boszczyk AA, Ralphs JR. The skeletal attachment of tendons–tendon “entheses”. Comparative biochemistry and physiology Part A, Molecular & integrative physiology. 2002 Dec;133(4):931–45. doi: 10.1016/s1095-6433(02)00138-1. [DOI] [PubMed] [Google Scholar]
- 61.Genin GM, Kent A, Birman V, Wopenka B, Pasteris JD, Marquez PJ, et al. Functional grading of mineral and collagen in the attachment of tendon to bone. Biophysical journal. 2009 Aug 19;97(4):976–85. doi: 10.1016/j.bpj.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ruggeberg M, Speck T, Paris O, Lapierre C, Pollet B, Koch G, et al. Stiffness gradients in vascular bundles of the palm Washingtonia robusta. Proceedings Biological sciences / The Royal Society. 2008 Oct 7;275(1648):2221–9. doi: 10.1098/rspb.2008.0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tan T, Rahbar N, Allameh SM, Kwofie S, Dissmore D, Ghavami K, et al. Mechanical properties of functionally graded hierarchical bamboo structures. Acta biomaterialia. 2011 Oct;7(10):3796–803. doi: 10.1016/j.actbio.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 64.Miserez A, Schneberk T, Sun C, Zok FW, Waite JH. The transition from stiff to compliant materials in squid beaks. Science. 2008 Mar 28;319(5871):1816–9. doi: 10.1126/science.1154117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Benjamin M, McGonagle D. The enthesis organ concept and its relevance to the spondyloarthropathies. Advances in experimental medicine and biology. 2009;649:57–70. doi: 10.1007/978-1-4419-0298-6_4. [DOI] [PubMed] [Google Scholar]
- 66.Benjamin M, Toumi H, Suzuki D, Hayashi K, McGonagle D. Evidence for a distinctive pattern of bone formation in enthesophytes. Annals of the rheumatic diseases. 2009 Jun;68(6):1003–10. doi: 10.1136/ard.2008.091074. [DOI] [PubMed] [Google Scholar]
- 67.Francois RJ, Braun J, Khan MA. Entheses and enthesitis: a histopathologic review and relevance to spondyloarthritides. Current opinion in rheumatology. 2001 Jul;13(4):255–64. doi: 10.1097/00002281-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 68.Osteophytes: relevance and biology. Osteoarthritis Cartilage. 2007 doi: 10.1016/j.joca.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 69.Ho SP, Yu B, Yun W, Marshall GW, Ryder MI, Marshall SJ. Structure, chemical composition and mechanical properties of human and rat cementum and its interface with root dentin. Acta biomaterialia. 2009 Feb;5(2):707–18. doi: 10.1016/j.actbio.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Djomehri SI, Candell S, Case T, Browning A, Marshall GW, Yun W, et al. Mineral density volume gradients in normal and diseased human tissues. PloS one. 2015;10(4):e0121611. doi: 10.1371/journal.pone.0121611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Benjamin M, Ralphs JR. Fibrocartilage in tendons and ligaments–an adaptation to compressive load. Journal of anatomy. 1998 Nov;193(Pt 4):481–94. doi: 10.1046/j.1469-7580.1998.19340481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nanci A, Bosshardt DD. Structure of periodontal tissues in health and disease. Periodontology 2000. 2006;40:11–28. doi: 10.1111/j.1600-0757.2005.00141.x. [DOI] [PubMed] [Google Scholar]
- 73.Berkovitz BK. The structure of the periodontal ligament: an update. European journal of orthodontics. 1990 Feb;12(1):51–76. doi: 10.1093/ejo/12.1.51. [DOI] [PubMed] [Google Scholar]
- 74.Berkovitz BK. Periodontal ligament: structural and clinical correlates. Dental update. 2004 Jan-Feb;31(1):46–50. 2, 4. doi: 10.12968/denu.2004.31.1.46. [DOI] [PubMed] [Google Scholar]
- 75.Posner AS, Harper RA, Muller SA, Menczel J. Age changes in the crystal chemistry of bone apatite. Annals of the New York Academy of Sciences. 1965 Sep 30;131(2):737–42. doi: 10.1111/j.1749-6632.1965.tb34837.x. [DOI] [PubMed] [Google Scholar]
- 76.Posner AS. The structure of bone apatite surfaces. Journal of biomedical materials research. 1985 Mar;19(3):241–50. doi: 10.1002/jbm.820190307. [DOI] [PubMed] [Google Scholar]
- 77.Wolff J. Das Gesetz der Transformation der knochen. A. Hirschwald; Berlin: 1892. This monograph was translated to English and was published by Springer-Verlag in 1986. [Google Scholar]
- 78.Shimomoto Y, Chung CJ, Iwasaki-Hayashi Y, Muromoto T, Soma K. Effects of occlusal stimuli on alveolar/jaw bone formation. Journal of dental research. 2007;86:47–51. doi: 10.1177/154405910708600107. [DOI] [PubMed] [Google Scholar]
- 79.Kunii R, Yamaguchi M, Aoki Y, Watanabe A, Kasai K. Effects of experimental occlusal hypofunction, and its recovery, on mandibular bone mineral density in rats. European journal of orthodontics. 2008;2008(30):52–6. doi: 10.1093/ejo/cjm057. [DOI] [PubMed] [Google Scholar]
- 80.Roberts WE, Epker BN, Burr DB, Hartsfield JK, Roberts JA. Remodeling of mineralize tissues, Part II: Control and Pathophysiology. Seminars in orthodontics. 2006;12:238–53. [Google Scholar]
- 81.Roberts WE, Roberts JA, Epker BN, Burr DB, Hartsfiled JK., Jr Remodeling of mineralized tissues, Part I: The Frost Legacy. Seminars in orthodontics. 2006;12:216–37. [Google Scholar]
- 82.Roberts WE, Huja S, Roberts JA. Bone modeling: biomechanics, molecular mechanisms, and clinical perspectives. Seminars in orthodontics. 2004;10:123–61. [Google Scholar]
- 83.Orban B, Bhaskar S. Orban’s oral histoogy and embryology. (11) 1991 [Google Scholar]
- 84.Hassell TM. Tissues and cells of the periodontium. Periodontology 2000. 1993 Oct;3:9–38. doi: 10.1111/j.1600-0757.1993.tb00230.x. [DOI] [PubMed] [Google Scholar]
- 85.Henneman S, Von den Hoff JW, Maltha JC. Mechanobiology of tooth movement. European journal of orthodontics. 2008 Jun;30(3):299–306. doi: 10.1093/ejo/cjn020. [DOI] [PubMed] [Google Scholar]
- 86.Krishnan V, Davidovitch Z. Cellular, molecular, and tissue-level reactions to orthodontic force. American journal of orthodontics and dentofacial orthopedics : official publication of the American Association of Orthodontists, its constituent societies, and the American Board of Orthodontics. 2006 Apr;129(4):469e1–32. doi: 10.1016/j.ajodo.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 87.Ingber DE. Tissue adaptation to mechanical forces in healthy, injured and aging tissues. Scandinavian journal of medicine & science in sports. 2005 Aug;15(4):199–201. doi: 10.1111/j.1600-0838.2005.00481.x. [DOI] [PubMed] [Google Scholar]
- 88.Ingber DE. Cellular basis of mechanotransduction. The Biological bulletin. 1998 Jun;194(3):323–5. doi: 10.2307/1543102. discussion 5–7. [DOI] [PubMed] [Google Scholar]
- 89.Benjamin M, McGonagle D. The anatomical basis for disease localisation in seronegative spondyloarthropathy at entheses and related sites. Journal of anatomy. 2001 Nov;199(Pt 5):503–26. doi: 10.1046/j.1469-7580.2001.19950503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Doschak MR, Zernicke RF. Structure, function and adaptation of bone-tendon and bone-ligament complexes. Journal of musculoskeletal & neuronal interactions. 2005 Mar;5(1):35–40. [PubMed] [Google Scholar]
- 91.Kiliaridis S, Engstrom C, Thilander B. Histochemical analysis of masticatory muscle in the growing rat after prolonged alteration in the consistency of the diet. Archives of oral biology. 1988;33(3):187–93. doi: 10.1016/0003-9969(88)90044-1. [DOI] [PubMed] [Google Scholar]
- 92.Hiiemae KM. Masticatory function in the mammals. Journal of dental research. 1967 Sep-Oct;46(5):883–93. doi: 10.1177/00220345670460054601. [DOI] [PubMed] [Google Scholar]
- 93.Hiiemae K, Heath MR, Heath G, Kazazoglu E, Murray J, Sapper D, et al. Natural bites, food consistency and feeding behaviour in man. Archives of oral biology. 1996 Feb;41(2):175–89. doi: 10.1016/0003-9969(95)00112-3. [DOI] [PubMed] [Google Scholar]
- 94.Thexton A, Hiiemae KM. The effect of food consistency upon jaw movement in the macaque: a cineradiographic study. Journal of dental research. 1997 Jan;76(1):552–60. doi: 10.1177/00220345970760010501. [DOI] [PubMed] [Google Scholar]
- 95.Watarai H, Warita H, Soma K. Effect of nitric oxide on the recovery of the hypofunctional periodontal ligament. Journal of dental research. 2004 Apr;83(4):338–42. doi: 10.1177/154405910408300413. [DOI] [PubMed] [Google Scholar]
- 96.Beertsen W, McCulloch CA, Sodek J. The periodontal ligament: a unique, multifunctional connective tissue. Periodontology 2000. 1997 Feb;13:20–40. doi: 10.1111/j.1600-0757.1997.tb00094.x. [DOI] [PubMed] [Google Scholar]
- 97.Carter DR, Beaupre GS. Skeletal function and form: mechanobiology of skeletal development, aging, and regeneration. Press CU, editor; Cambridge, U. K.: 2001. [Google Scholar]
- 98.Parfitt AM, Goldstein S, Compston J. The American Society for Bone and Mineral Research. 2005. Bone quality: what is it and can we measure it? [Google Scholar]
- 99.Feller L, Khammissa RA, Thomadakis G, Fourie J, Lemmer J. Apical External Root Resorption and Repair in Orthodontic Tooth Movement: Biological Events. BioMed research international. 2016;2016:4864195. doi: 10.1155/2016/4864195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Qian Y, Fan Y, Liu Z, Zhang M. Numerical simulation of tooth movement in a therapy period. Clinical biomechanics. 2008;23(Suppl 1):S48–52. doi: 10.1016/j.clinbiomech.2007.08.023. [DOI] [PubMed] [Google Scholar]


