Supplemental Digital Content is available in the text
Keywords: cardiac output, circulatory shock, clinical examination, critical illness, diagnostic accuracy, physical examination, shock
Abstract
Purpose of review
In the acute setting of circulatory shock, physicians largely depend on clinical examination and basic laboratory values. The daily use of clinical examination for diagnostic purposes contrasts sharp with the limited number of studies. We aim to provide an overview of the diagnostic accuracy of clinical examination in estimating circulatory shock reflected by an inadequate cardiac output (CO).
Recent findings
Recent studies showed poor correlations between CO and mottling, capillary refill time or central-to-peripheral temperature gradients in univariable analyses. The accuracy of physicians to perform an educated guess of CO based on clinical examination lies around 50% and the accuracy for recognizing a low CO is similar. Studies that used predefined clinical profiles composed of several clinical examination signs show more reliable estimations of CO with accuracies ranging from 81 up to 100%.
Summary
Single variables obtained by clinical examination should not be used when estimating CO. Physician's educated guesses of CO based on unstructured clinical examination are like the ‘flip of a coin’. Structured clinical examination based on combined clinical signs shows the best accuracy. Future studies should focus on using a combination of signs in an unselected population, eventually to educate physicians in estimating CO by using predefined clinical profiles.
INTRODUCTION
Many critically ill patients suffer from circulatory shock, which places them at increased risks of multiorgan failure, long-term morbidity and mortality [1,2]. Combinations of clinical, hemodynamic and biochemical variables are recommended for diagnosing shock [3,4].
Daily use of clinical examination (in any patient) for diagnostic purposes contrasts with the limited number of studies, so that the level of evidence in the critically ill is considered best practice [4]. Much remains unknown about the value of clinical examination in diagnosing shock, reflected by an inadequate cardiac output (CO) or maldistribution of blood flow. More knowledge on this topic could assist physicians in the diagnostic process and guide interventions. Previous overviews have evaluated the value of physical examination in sepsis patients [5], cardiovascular patients [6▪▪] and in hemodynamically unstable patients for predicting fluid responsiveness [7▪]. We aim to provide an overview of the diagnostic test accuracy of clinical examination findings for estimating CO in critically ill patients.
Box 1.
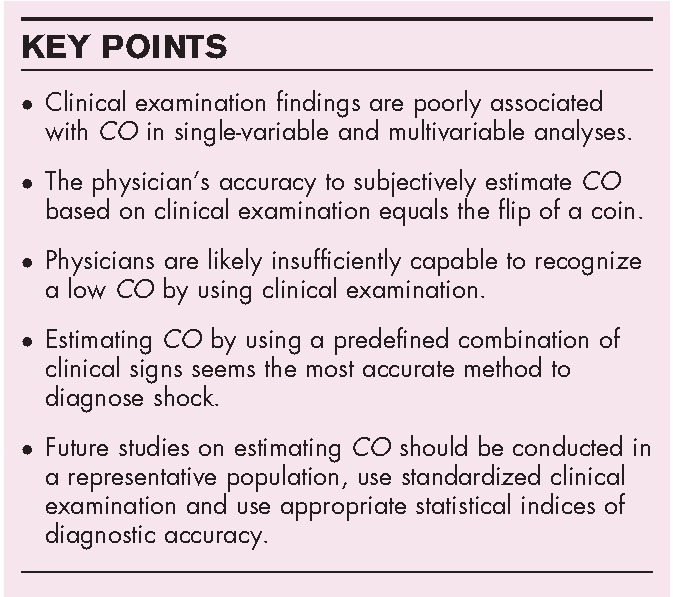
no caption available
BACKGROUND
‘Clinical examination’ of the cardiovascular system has been performed for a long time. The first evaluations of heart rate by palpation of the arterial pulse rate date back as far as approximately 335–280 B.C. [8]. Around the second century A.D., physicians recognized the value of pulse rate in diagnosing diseases. Pulse quality and quantity were extensively evaluated and distinctions were made in pulse fullness, rate, rhythm and size [9]. However, it would still take hundreds of years before the clinical assessment of circulatory shock ‘had evolved’ into the way as it is conducted today. In 1941, Ebert et al.[10] elaborately described the complexity of symptoms seen in systemic and peripheral circulatory failure in septic shock patients. He encountered the same clinical picture that we still face today:
(..) All the patients studied presented a similar clinical picture. They were stuporous or comatose. The rectal temperatures ranged from 36.1 to 41.3 degrees Celsius. The skin was pale and often covered with perspiration. The extremities were cold, and this finding usually preceded the fall in arterial pressure. The skin of the body was usually warm, although in terminal stages it too became cool. The radial pulse was feeble or impalpable. The pulse rate was rapid. (..)
For years, clinical examination was considered the cornerstone for diagnosing shock. Reliance on examination declined when Swan et al.[11] introduced pulmonary artery catheterization (PAC) in 1970. PAC allowed a wide range of pressure and flow-based hemodynamic measurements, including variables such as pulmonary capillary wedge pressure, systemic vascular resistance and CO[12]. Several studies concluded that the use of PAC frequently resulted in change of therapy compared with clinical examination [13–18]. However, PAC remained controversial because of its invasiveness in the absence of any clinical benefit [19–22]. Today, PAC has largely been replaced by less-invasive methods for assessment of CO, ranging from echo to pulse pressure analysis devices [23–26].
Despite these technological improvements, clinical examination still holds a prominent position in diagnosing circulatory shock [4,27]. We aimed to provide an overview of the diagnostic accuracy of clinical examination for the assessment of circulatory shock measured by CO or cardiac index (CI). We only included studies that estimated CO using clinical examination based on a one-time snapshot. Physicians mostly use changes in clinical examination findings as proxy for changes in CO to guide their interventions. To evaluate the diagnostic accuracy of changes in clinical examination in relation to changes in CO was beyond the scope of this review. In this review, we were mainly interested which clinical examination findings may accommodate clinical needs, because in daily practice these snapshot measurements guide treatment decisions as triggers for interventions.
METHODS
A sensitive search strategy was used to identify eligible studies (Appendix 1, http://links.lww.com/COCC/A17). In addition, we used the snowball and citation search methods on the selected articles. We attempted to include all studies that provided results on clinical examination findings in relation to CO. We excluded prognostic studies. We separated studies that evaluated univariable associations from studies that used multivariable analyses. Varying statistical indices for describing diagnostic test accuracy as well as a varying prevalence of low CO were encountered, limiting interstudy comparison. Whenever available, we used likelihood ratios as the preferred modality to describe diagnostic accuracy. Likelihood ratios may provide valuable information on disease probability in an individual and do not change with pretest probability (i.e. the prevalence of disease) [28–30]. We calculated sensitivity, specificity, predictive values and likelihood ratios of clinical examination for the detection of low CO whenever possible.
RESULTS
Our search resulted in 8128 hits of which 28 publications were selected. An additional six publications were identified through snowballing. After selection, we included 34 publications in this overview.
UNIVARIABLE STUDIES
Thirteen studies evaluated univariable associations of clinical examination variables with CO, including skin temperature or temperature gradients (n = 8) [31–38], capillary refill time (CRT; n = 1) [39], temperature gradient and CRT (n = 1) [40], mottling (n = 1) [41], heart rate and mean arterial pressure (n = 1) [42] and central venous pressure (n = 1; Table 1) [31–43]. The method used for measuring CO varied, including, for example thermodilution with the PAC or Doppler wave with transesophageal or transthoracic echocardiography.
Table 1.
Prediction of cardiac output using a single variable
| Results | ||||||
| Author, year | Patients | Population | Variables of interest | Measurement method | Nonsignificant | Significant |
| Peripheral temperature | ||||||
| Kaplan et al. 2001 [31] | 264a | Surgical ICU patients | Temp, subjective: foot (‘cool’ or ‘warm’) | PAC, technique not mentioned | – | ’Cool’ : CI = 2.9 ± 1.2 ’Warm’: CI = 4.3 ± 1.2 |
| Schey et al. 2009 [32] | 10a | Post cardiac surgery | Temp, subjective: foot: (‘cool’ or ‘cool-warm’ or ‘warm’)Temp, objective of foot | PAC, thermodilution | Tskin, objective: r = 0.11 | ’Cool’ : CO = 3.71 ’Cool-warm’: CO = 4.83 ’Warm’ : CO = 5.12 |
| Joly et al. 1969 [33] | 100 | Circulatory shock | Temp, objective: toeΔT: toe – ambient (ΔTp-a) | Indicator dilution technique | – | Tskin objective: r = 0.71 ΔTp-a: r = 0.73 |
| Woods et al. 1987 [34] | 26a | Circulatory shock | ΔT: central – toe (ΔTc-p) | PAC, thermodilution | ΔTc-p: no correlation | |
| Vincent et al. 1988 [35] | 15a | Cardiogenic and septic shock | ΔT: toe – ambient (ΔTp-a) | PAC, thermodilution | ΔTp-a in septic shock: no correlation | ΔTp-a in cardiogenic shock: r = 0.63 |
| Bailey et al. 1990b [40] | 40a | Post cardiac surgery | ΔT: central – toe (ΔTc-p) | PAC, thermodilution | ΔTc-p day of operation: no correlation | ΔTc-p postoperative day 1: r = −0.60 |
| Sommers et al. 1995 [36] | 21a | Post cardiac surgery | Tskin, objective: axillary, groin, knee, ankle, toe | PAC, thermodilution | Tskin, objective: no correlation in any site | – |
| Boerma et al. 2008 [37] | 35 | Sepsis and septic shock | ΔT: central – foot (ΔTc-p) | TEE, Doppler wave | ΔTc-p: r = −0.15 | – |
| Bourcier et al. 2016 [38] | 103a | Sepsis and septic shock | ΔT: toe – ambient (ΔTp-a) | TTE, technique not mentioned | ΔTp-a: no correlation | – |
| Capillary refill time | ||||||
| Bailey et al. 1990b [40] | 40a | Post cardiac surgery | CRT: site not mentioned | PAC, thermodilution | CRT: no correlation | – |
| Ait-Oufella et al. 2014 [39] | 59 | Septic shock | CRT: index finger | FloTrac, arterial pressure waveform analysis | CRT: no correlation | – |
| Skin mottling | ||||||
| Ait-Oufella et al. 2011 [41] | 60 | Septic shock | Mottling score: knee | TTE, Doppler wave | Mottling score: no correlation | – |
| Systemic hemodynamic variables | ||||||
| Wo et al. 1993 [42] | 256a | Severe injury and critically ill postoperative | HR, MAP | PAC, thermodilution | HR: r = 0.27, r2 = 0.07, MAP: r = −0.01, r2 = 0.0001, | MAP during severe hypotension: r = 0.50, r2 = 0.25 |
| Kuntscher et al. 2006 [43] | 16a | Major burns | Central venous pressure | Thermal dye double indicator dilution | – | Central venous pressure: r = 0.40 |
a=repeated measurements in each patient.
b=same study population.
ΔTc-p, central-to-peripheral temperature gradient (°C); ΔTp-a, peripheral-to-ambient temperature gradient (°C); CI, cardiac index (l/min/m2); CO, cardiac output (l/min); CRT, capillary refill time (s); HR, heart rate (beats/min); MAP, mean arterial pressure (mmHg); PAC, pulmonary artery catheter; TEE, transoesophageal echocardiography; Temp, temperature (°C); TTE, transthoracic echocardiography.
Circulatory shock may lead to compensatory vasoconstriction of nonvital, peripheral tissues such as the skin. Peripheral perfusion can easily be evaluated by measurement of skin temperature, CRT and degree of skin mottling. Two studies demonstrated that a subjectively cool skin temperature was associated with a lower CO[31,32]. Studies evaluating the correlation between objective temperature measurements and CO showed conflicting results; some observed moderate correlations [33,35,40], whereas most observed no correlation [34–38]. Skin temperature measurement methods differ widely and are likely influenced by several factors: age, ambient temperature, hypothermia, peripheral vascular disease, vasopressors, pain and anxiety have all been proposed as influencing circumstances [44,45]. This may explain the conflicting results and may limit its usefulness for estimating CO in clinical practice. Several studies have emphasized the prognostic value of prolonged CRT and mottling of the skin [39,41,46–49], but only three studies have evaluated their associations with CO and found no relevant correlations [39–41].
Prospective studies on systemic hemodynamic variables showed that heart rate, mean arterial pressure and central venous pressure were not directly correlated to CO[42,43,50]. Only during episodes of deep hypotension, one study observed a moderate correlation between mean arterial pressure and CO[42]. These systemic hemodynamic variables seem to be poor indicators of CO, which supports the common conception that low blood pressure is a late sign of circulatory shock and should not be relied on for early diagnosis [4,51].
MULTIVARIABLE STUDIES
Twenty-one studies evaluated multivariable associations of clinical variables with CO. Because of the differing methods of estimating CO, we subdivided our results into studies that evaluated the capacity of physicians to estimate CO (n = 17; Table 2) [13–18,52–61,62▪▪] and studies that constructed clinical profiles based on multiple variables (n = 3) or a multivariable model (n = 1) to correlate clinical examination findings with CO (Table 3) [63–66]. Furthermore, we could calculate the diagnostic test accuracy for physician's estimation of low CO in nine studies (Table 2).
Table 2.
Physician's capacity to estimate cardiac output based on clinical examination
| Variables of interest | Results | ||||||
| Author, year | Patients | Setting | Classification | Estimation based on | Measurement method | Estimation | Diagnostic accuracy for low CO (95% CI) |
| Connors et al. 1983 [13] | 62a | ICU | CI categorical: < 2.5; 2.5–3.5; > 3.5 | Clinical assessment, laboratory and X-ray | PAC, thermodilution | 44% correct estimation | Sens 58% (45–68%); Spec 60% (48–71%)PPV 58% (49–65%); NPV 60% (52–67%)LR+ 1.43 (1.02–2.00); LR– 0.71 (0.51–0.98) |
| Eisenberg et al. 1984 [14] | 97 | ICU | CO categorical: < 4.5; 4.5–7.5; > 7.5 | Not described | PAC, thermodilution | 51% correct estimation | Sens 71% (54–85%); Spec 56% (43–69%)PPV 48% (39–57%); NPV 78% (66–86%)LR+ 1.64 (1.15–2.33); LR– 0.51 (0.29–0.89) |
| Tuchschmidt et al. 1987 [15] | 35 | ICU | CO continuous | Clinical assessment and X-ray | PAC, thermodilution | r = 0.72 | – |
| Connors et al. 1990 [17] | 461 | ICU | CI dichotomous: < 2.2; ≥2.2CI continuous | Clinical assessment, laboratory, X-ray and ECG | PAC, thermodilution | 64% correct estimationMean CI-difference in CI = 1.0 ± 0.9 | Sens 49% (40–57%); Spec 70% (65–75%)PPV 43% (38–49%); NPV 74% (71–77%)LR+ 1.62 (1.28–2.05); LR– 0.73 (0.62–0.87) |
| Celoria et al. 1990b [16] | 114 | Surgical ICU | CO categorical: < 4; 4–8; > 8 | Clinical assessment, laboratory and X-ray | PAC, thermodilution | 51% correct estimationr = 0.47 | Sens 67% (30–93%); Spec 80% (71–87%)PPV 22% (14–34%); NPV 97% (92–99%)LR+ 3.33 (1.83–6.07); LR– 0.42 (0.16–1.05) |
| Steingrub et al. 1991b [53] | 152 | Surgical and medical ICU | CO categorical: < 4; 4–8; > 8 | Clinical assessment, laboratory and X-ray | PAC, thermodilution | 51% correct estimation | Sens 54% (37–70%); Spec 73% (63–81%)PPV 40% (31–51%); NPV 82% (76–87%)LR+ 1.96 (1.29–2.98); LR– 0.64 (0.44–0.91) |
| Mimoz et al. 1994 [18] | 112 | ICU | Combinations of CI, PAOP and SVRI | Clinical assessment, laboratory, X-ray and echocardiography | PAC, thermodilution | 56% correct estimation | – |
| Staudinger et al. 1998 [54] | 149 | ICU | CI categorical: < 2.0; 2.0–4.0; > 4.0 | Clinical assessment, medical history, laboratory and X-ray | PAC, thermodilution | 62% correct estimation | – |
| Rodriguez et al. 2000 [55] | 33 | ED + respiratory distress or hypotension | CI categorical: < 2.6; 2.6–4.0; > 4.0. | Clinical assessment, medical history, laboratory, X-ray and ECG | TEE, Doppler wave | κ1 = −0.04 (95% CI–0.31–0.24)κ2 = 0.07 (95% CI −0.17–0.31) | – |
| Linton et al. 2002 [56] | 50 | Post cardiac surgery | CI categorical: < 1.9; 1.9–3.5; > 3.5 | Not described | LiDCO, indicator-dilution | 54% correct estimation | Sens 42% (15–72%); Spec 74% (57–87%)PPV 33% (18–54%); NPV 80% (71–87%)LR+ 1.58 (0.67–3.72); LR– 0.79 (0.47–1.32) |
| Iregui et al. 2003 [57] | 105 | ICU | CI categorical: < 2.5; 2.5–4.5; > 4.5 | Clinical assessment, laboratory and X-ray | TEE, Doppler wave | 44% correct estimation | – |
| Veale et al. 2005 [58] | 68 | ICU | CI categorical: < 2.5; 2.5–4.2; > 4.5 | Not described | BioZ CO monitor, Impedance cardiography | 42% correct estimation | Sens 22% (6–48%); Spec 66% (51–79%)PPV 19% (8–38%); NPV 70% (63–76%)LR+ 0.65 (0.25–1.68); LR– 1.18 (0.86–1.62) |
| Rodriguez et al. 2006 [59] | 31 | ED + endotracheal intubation | CI categorical:ranges not specified | Clinical assessment, medical history, laboratory and X-ray | TEE, Doppler wave | κ = 0.57 (95% CI 0.36–0.77) | – |
| Nowak et al. 2011 [60] | 38 | ED + respiratory distress | CO categorical < 4.0; 4.0–8.0; > 8.0 | Clinical assessment and medical history | Nexfin, ABP waveform analysis | 50% correct estimation | |
| κ = −0.02 (95% CI −0.25–0.20) | Sens 33% (4–78%); Spec 63% (44–79%) | ||||||
| PPV 14% (5–36%); NPV 83% (73–90%) | |||||||
| LR+ 0.89 (0.26–3.00); LR– 1.07 (0.57–2.00) | |||||||
| Duan et al. 2014 [61] | 132 | ICU | CI categorical:<3; 3–5; >5 | Not described | PiCCO, thermodilution | 50% correct estimation | – |
| Perel et al. 2016 [62▪▪] | 206a | ICU | CO continuous | Clinical assessment | PiCCO, thermodilution | Percentage error = 66% | |
| Absolute mean difference in CO = −1.5 ± 2.2 | – | ||||||
a=repeated measurements in each patient.
b=overlapping study populations.
95% CIs, 95% confidence intervals; CI, cardiac index (l/minute/m2); CO, cardiac output (l/min); ECG, electrocardiography; ICU, intensive care unit; LiDCO, lithium dilution cardiac output; LR–, negative likelihood ratio; LR+, positive likelihood ratio; NPV, negative predictive value; PAC, pulmonary artery catheter; PAOP, pulmonary artery occlusion pressure (mmHg); PiCCO, pulse contour cardiac output; PPV, positive predictive value; Sens, sensitivity; Spec, specificity; SVRI, systemic vascular resistance index (dynes · s/cm5 · min2); TEE, transesophageal echocardiography.
Table 3.
Combined signs of clinical examination for estimation of CO
| Variables of interest | ||||||
| Author, year | Patients | Population | Clinical profile | Clinical profile based on | CO-measurement | Results |
| Combined clinical profiles | ||||||
| Ramo et al. 1970 [63] | 98 | AMI | I (normal CI): no signs of HFII (normal CI): mild-to-moderate HFIII (low CI): overt pulmonary edemaIV (low CI): cardiogenic shock | Mean arterial pressure, cool extremities, urine output, mental status, third heart sound gallop rhythm and rales | PAC, indicator-dilution technique | I (normal CI): 23 of 45 (51%)II (normal CI): 19 of 30 (63%)III (low CI): 10 of 10 (100%)IV (low CI): 13 of 13 (100%) |
| Forrester et al. 1977 [64] | 200 | AMI | I (normal CI): no pulmonary congestion or peripheral hypoperfusionII (normal CI): pulmonary congestion onlyIII (low CI): hypoperfusion onlyIV (low CI): both | Heart rate, blood pressure, cool extremities, urine output and mental status | PAC, thermodilution | Overall: 81% correct estimations of CII & II (normal CI): 84 of 95 (88%)III & IV (low CI): 76 of 105 (72%) |
| Grissom et al. 2009 [65] | 405 | ALI | I: All three clinical signs aberrantII: Any one clinical sign aberrant | Capillary refill time, knee mottling and cool extremities | PAC, thermodilution | 92% correct estimations of CI in class I:Sens 12% (3–28%); Spec 98% (97–99%)PPV 40% (17–69%); NPV 93% (92–93%)LR+ 7.52 (2.23–25.3); LR– 0.89 (0.79–1.01)75% correct estimations of CI in class II:Sens 52% (34–69%); Spec 78% (73–82%)PPV 17% (12–23%); NPV 95% (93–96%)LR+ 2.31 (1.58–3.38); LR– 0.62 (0.44–0.89) |
| Multivariable analysis | ||||||
| Sasse et al. 1996 [66] | 23a | ICU patients | CO continuous | Heart rate, respiratory rate, mean arterial pressure and temperature | PAC, thermodilution | Heart rate: R2 = 0.05Respiratory rate: R2 = 0.14Mean arterial pressure: R2 = 0.03 |
a=repeated measurements in each patient.
ALI, acute lung injury; AMI, acute myocardial infarction; CI, cardiac index (l/min/m2); CO, cardiac output (l/min); HF, heart failure; LR–, negative likelihood ratio; LR+, positive likelihood ratio; NPV, negative predictive value; PAC, pulmonary artery catheter; PPV, positive predictive value; Sens, sensitivity; Spec, specificity.
PHYSICIAN'S CAPACITY TO ESTIMATE CO BASED ON CLINICAL EXAMINATION
Seventeen studies evaluated the accuracy of physician's estimates or ‘educated guesses’ of CO as compared to objectively measured CO. Estimates were based on clinical examination, with or without knowledge of medical history, biochemical values and/or radiological imaging (Table 2). Some studies used a categorical variable for CO estimates (e.g. ‘low’, ‘normal’ or ‘high’), whereas others used a continuous scale (e.g. 1–12 l per min) [15,17,62▪▪]. Physician's estimates were correct in 42–62% of the time [13–18,52–61]. Moderate-to-reasonable correlations and a high percentage error were found when physician's estimates of continuous CO were compared to objectively measured CO[15,16,62▪▪]. Moderate-to-very poor agreements were found in studies that used weighted κ statistics to address agreement occurring by chance [55,59,60,67]. In addition, two studies reported that 21 and 26% of the CO estimations were completely disparate (an estimated high CO when the objective CO was low or vice versa) [55,59].
Nine studies provided enough data for calculation of the diagnostic accuracy of physician's estimates for detecting low CO. The overall results appeared disappointing [13,14,16,17,53,54,56,58,60] (Table 2). Furthermore, two studies concluded that physicians more frequently overestimated (31–33%) rather than underestimated (18–23%) CO[14,57], implicating that physicians were more prone to miss an insufficient CO. Perel et al.[62▪▪] found the opposite when physicians were asked to estimate CO on a continuous scale.
These results suggest that physicians are not very capable to subjectively estimate CO based on clinical examination. The widely varying diagnostic accuracies are probably the result of different populations or cutoffs for a low CO, but overall it seems that physician's estimates are ‘an inaccurate diagnostic test’. This is in accordance with two studies of Saugel et al.[67,68], which both demonstrate the incapability of physicians to reliably assess volume status using simple clinical signs. Furthermore, five out of six studies concluded that predictions of senior staff members were equally bad as those of residents or fellows [13,18,54,61,62▪▪,69]. Finally, one study found that the accuracy of estimates was unrelated to the level of confidence physicians had in their assessment [69].
Several important limitations apply. Many studies did not elaborate their methods of clinical examination in terms of variables used and definitions employed, leaving variability at the physician's discretion so that these studies cannot be reproduced. PAC was used in most studies, but only in selected patients who failed to respond to initial therapy or in whom clinical examination alone was deemed insufficient, so that evaluation of the accuracy of clinically estimated CO will be biased by definition. Likewise, many other studies also used convenience samples, which hampers generalizability of their results. Clinical examination should be performed in a standardized fashion, according to a protocol, to maximize interobserver agreement and generalizability.
COMBINED SIGNS OF CLINICAL EXAMINATION FOR ESTIMATION OF CO
Three studies have compared predefined clinical profiles based upon clinical examination with objectively measured CI (Table 3). Forrester et al.[64] found a good agreement in patients with acute myocardial infarction (AMI). In their study, 75% of patients with low CI and 96% of patients with very low CI had clinical signs of peripheral hypoperfusion, such as decreased skin temperature, confusion or oliguria in conjunction with either arterial hypotension or tachycardia. Ramo et al.[63] observed 100% correct estimation of low CI when patients with AMI had overt signs of pulmonary edema or signs of cardiogenic shock. In their study, clinical signs of overt pulmonary edema were defined by rales or a third heart sound gallop rhythm and cardiogenic shock was diagnosed by the presence of a systolic blood pressure below 90 mmHg, oliguria, cold extremities and disorientation. These findings suggest that physicians can diagnose cardiogenic shock in patients with AMI using clinical examination. Accurate estimation of CO for diagnosing shock in all critically ill patients based on clinical examination might appear much more difficult because of large interindividual differences. Grissom et al.[65] combined CRT, mottling and skin temperature to predict CI in an unselected cohort of patients with acute lung injury. The presence of all three physical signs had a high specificity (98%) but a low sensitivity (12%) for diagnosing shock, suggesting that these three signs accurately rule in, but inaccurately rule out circulatory shock. Varying types of shock are probably associated with varying clinical signs [70], so that a ‘one size fits all’ approach seems inappropriate. Roughly, one-third of all patients with circulatory shock suffer from a low CO, whereas two-thirds have distributive shock with associated high CO[1,71]. Especially in the latter, clinical examination may indicate inadequate circulation regardless of the height of CO and it is difficult to establish how much CO is sufficient for each individual patient.
PREDICTING CO USING A MULTIVARIABLE MODEL
One study used multivariable regression analyses to estimate CO based on heart rate, respiratory rate, mean arterial pressure and central temperature (Table 3) [66]. These multivariable results confirm that systemic hemodynamic variables do not correspond well with CO. Future diagnostic studies of CO should therefore incorporate all clinical and hemodynamic variables in a multivariable model.
CONCLUSION
Clinical examination findings are poorly associated with CO in single-variable and multivariable analyses. Physicians seem to be insufficiently capable to estimate CO or recognize a low CO using their clinical examination. The most promising results were found when CO was estimated by using predefined profiles composed of combined clinical examination signs. However, most studies were conducted in highly selected populations and the details of estimations were not specified. On the basis of current evidence, using clinical examination to diagnose CO can, to our opinion, not be considered best practice. Future studies on this topic should be conducted in a representative population, use standardized clinical examination and use appropriate statistical indices of diagnostic accuracy. Ultimately, these results should guide education of physicians to estimate CO using predefined clinical profiles.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.co-criticalcare.com).
REFERENCES
- 1.De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010; 362:779–789. [DOI] [PubMed] [Google Scholar]
- 2.Vincent JL, Marshall JC, Namendys-Silva SA, et al. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med 2014; 2:380–386. [DOI] [PubMed] [Google Scholar]
- 3.De Backer D, Donadello K, Sakr Y, et al. Microcirculatory alterations in patients with severe sepsis: impact of time of assessment and relationship with outcome. Crit Care Med 2013; 41:791–799. [DOI] [PubMed] [Google Scholar]
- 4.Cecconi M, De Backer D, Antonelli M, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med 2014; 40:1795–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Postelnicu R, Evans L. Monitoring of the physical exam in sepsis. Curr Opin Crit Care 2017; 23:232–236. [DOI] [PubMed] [Google Scholar]
- 6▪▪.Elder A, Japp A, Verghese A. How valuable is physical examination of the cardiovascular system? BMJ 2016; 354:i3309. [DOI] [PubMed] [Google Scholar]; This review extensively elaborates the diagnostic accuracy of physical examination of the cardiovascular system.
- 7▪.Bentzer P, Griesdale DE, Boyd J, et al. Will this hemodynamically unstable patient respond to a bolus of intravenous fluids? JAMA 2016; 316:1298–1309. [DOI] [PubMed] [Google Scholar]; This review provides a comprehensive overview of studies that measure hypovolemia.
- 8.Billman GE. Heart rate variability: a historical perspective. Front Physiol 2011; 2:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bedford DE. The ancient art of feeling the pulse. Br Heart J 1951; 13:423–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebert RV, Stead EA. Circulatory failure in acute infections. J Clin Invest 1941; 20:671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swan HJ, Ganz W, Forrester J, et al. Catheterization of the heart in man with use of a flow-directed balloon-tipped catheter. N Engl J Med 1970; 283:447–451. [DOI] [PubMed] [Google Scholar]
- 12.Ganz W, Donoso R, Marcus HS, et al. A new technique for measurement of cardiac output by thermodilution in man. Am J Cardiol 1971; 27:392–396. [DOI] [PubMed] [Google Scholar]
- 13.Connors AF, Jr, McCaffree DR, Gray BA. Evaluation of right-heart catheterization in the critically ill patient without acute myocardial infarction. N Engl J Med 1983; 308:263–267. [DOI] [PubMed] [Google Scholar]
- 14.Eisenberg PR, Jaffe AS, Schuster DP. Clinical evaluation compared to pulmonary artery catheterization in the hemodynamic assessment of critically ill patients. Crit Care Med 1984; 12:549–553. [DOI] [PubMed] [Google Scholar]
- 15.Tuchschmidt J, Sharma OP. Impact of hemodynamic monitoring in a medical intensive care unit. Crit Care Med 1987; 15:840–843. [DOI] [PubMed] [Google Scholar]
- 16.Celoria G, Steingrub JS, Vickers-Lahti M, et al. Clinical assessment of hemodynamic values in two surgical intensive care units. Effects on therapy. Arch Surg 1990; 125:1036–1039. [DOI] [PubMed] [Google Scholar]
- 17.Connors AF, Jr, Dawson NV, Shaw PK, et al. Hemodynamic status in critically ill patients with and without acute heart disease. Chest 1990; 98:1200–1206. [DOI] [PubMed] [Google Scholar]
- 18.Mimoz O, Rauss A, Rekik N, et al. Pulmonary artery catheterization in critically ill patients: a prospective analysis of outcome changes associated with catheter-prompted changes in therapy. Crit Care Med 1994; 22:573–579. [DOI] [PubMed] [Google Scholar]
- 19.Robin ED. Death by pulmonary artery flow-directed catheter. Time for a moratorium? Chest 1987; 92:727–731. [DOI] [PubMed] [Google Scholar]
- 20.Shah MR, Hasselblad V, Stevenson LW, et al. Impact of the pulmonary artery catheter in critically ill patients: meta-analysis of randomized clinical trials. JAMA 2005; 294:1664–1670. [DOI] [PubMed] [Google Scholar]
- 21.Marik PE. Obituary: pulmonary artery catheter 1970 to 2013. Ann Intensive Care 2013; 3:38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajaram SS, Desai NK, Kalra A, et al. Pulmonary artery catheters for adult patients in intensive care. Cochrane Database Syst Rev 2013; 2:CD003408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vincent JL, Rhodes A, Perel A, et al. Clinical review: update on hemodynamic monitoring – a consensus of 16. Crit Care 2011; 15:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alhashemi JA, Cecconi M, Hofer CK. Cardiac output monitoring: an integrative perspective. Crit Care 2011; 15:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schloglhofer T, Gilly H, Schima H. Semi-invasive measurement of cardiac output based on pulse contour: a review and analysis. Can J Anaesth 2014; 61:452–479. [DOI] [PubMed] [Google Scholar]
- 26.Teboul JL, Saugel B, Cecconi M, et al. Less invasive hemodynamic monitoring in critically ill patients. Intensive Care Med 2016; 42:1350–1359. [DOI] [PubMed] [Google Scholar]
- 27.Sevransky J. Clinical assessment of hemodynamically unstable patients. Curr Opin Crit Care 2009; 15:234–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sackett DL. The rational clinical examination. A primer on the precision and accuracy of the clinical examination. JAMA 1992; 267:2638–2644. [PubMed] [Google Scholar]
- 29.McGee S. Simplifying likelihood ratios. J Gen Intern Med 2002; 17:646–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sackett D, Simel D. Keitz S. A primer on the precision and accuracy of the clinical examination & an updated summary. The rational clinical examination: evidence-based clinical diagnosis. New York: McGraw-Hill Professional Jama & Archives Journals; 2009. 1–16. [Google Scholar]
- 31.Kaplan LJ, McPartland K, Santora TA, Trooskin SZ. Start with a subjective assessment of skin temperature to identify hypoperfusion in intensive care unit patients. J Trauma 2001; 50:620–627. discussion 627-8. [DOI] [PubMed] [Google Scholar]
- 32.Schey BM, Williams DY, Bucknall T. Skin temperature as a noninvasive marker of haemodynamic and perfusion status in adult cardiac surgical patients: an observational study. Intensive Crit Care Nurs 2009; 25:31–37. [DOI] [PubMed] [Google Scholar]
- 33.Joly HR, Weil MH. Temperature of the great toe as an indication of the severity of shock. Circulation 1969; 39:131–138. [DOI] [PubMed] [Google Scholar]
- 34.Woods I, Wilkins RG, Edwards JD, et al. Danger of using core/peripheral temperature gradient as a guide to therapy in shock. Crit Care Med 1987; 15:850–852. [DOI] [PubMed] [Google Scholar]
- 35.Vincent JL, Moraine JJ, van der Linden P. Toe temperature versus transcutaneous oxygen tension monitoring during acute circulatory failure. Intensive Care Med 1988; 14:64–68. [DOI] [PubMed] [Google Scholar]
- 36.Sommers MS, Stevenson JS, Hamlin RL, Ivey TD. Skin temperature and limb blood flow as predictors of cardiac index. Clin Nurs Res 1995; 4:22–37. [DOI] [PubMed] [Google Scholar]
- 37.Boerma EC, Kuiper MA, Kingma WP, et al. Disparity between skin perfusion and sublingual microcirculatory alterations in severe sepsis and septic shock: a prospective observational study. Intensive Care Med 2008; 34:1294–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bourcier S, Pichereau C, Boelle PY, et al. Toe-to-room temperature gradient correlates with tissue perfusion and predicts outcome in selected critically ill patients with severe infections. Ann Intensive Care 2016; 6:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ait-Oufella H, Bige N, Boelle PY, et al. Capillary refill time exploration during septic shock. Intensive Care Med 2014; 40:958–964. [DOI] [PubMed] [Google Scholar]
- 40.Bailey JM, Levy JH, Kopel MA, et al. Relationship between clinical evaluation of peripheral perfusion and global hemodynamics in adults after cardiac surgery. Crit Care Med 1990; 18:1353–1356. [DOI] [PubMed] [Google Scholar]
- 41.Ait-Oufella H, Lemoinne S, Boelle PY, et al. Mottling score predicts survival in septic shock. Intensive Care Med 2011; 37:801–807. [DOI] [PubMed] [Google Scholar]
- 42.Wo CC, Shoemaker WC, Appel PL, et al. Unreliability of blood pressure and heart rate to evaluate cardiac output in emergency resuscitation and critical illness. Crit Care Med 1993; 21:218–223. [DOI] [PubMed] [Google Scholar]
- 43.Kuntscher MV, Germann G, Hartmann B. Correlations between cardiac output, stroke volume, central venous pressure, intra-abdominal pressure and total circulating blood volume in resuscitation of major burns. Resuscitation 2006; 70:37–43. [DOI] [PubMed] [Google Scholar]
- 44.Kholoussy AM, Sufian S, Pavlides C, Matsumoto T. Central peripheral temperature gradient. Its value and limitations in the management of critically ill surgical patients. Am J Surg 1980; 140:609–612. [DOI] [PubMed] [Google Scholar]
- 45.Schey BM, Williams DY, Bucknall T. Skin temperature and core-peripheral temperature gradient as markers of hemodynamic status in critically ill patients: a review. Heart Lung 2010; 39:27–40. [DOI] [PubMed] [Google Scholar]
- 46.Champion HR, Sacco WJ, Hannan DS, et al. Assessment of injury severity: the triage index. Crit Care Med 1980; 8:201–208. [DOI] [PubMed] [Google Scholar]
- 47.van Genderen ME, Lima A, Akkerhuis M, et al. Persistent peripheral and microcirculatory perfusion alterations after out-of-hospital cardiac arrest are associated with poor survival. Crit Care Med 2012; 40:2287–2294. [DOI] [PubMed] [Google Scholar]
- 48.Coudroy R, Jamet A, Frat JP, et al. Incidence and impact of skin mottling over the knee and its duration on outcome in critically ill patients. Intensive Care Med 2015; 41:452–459. [DOI] [PubMed] [Google Scholar]
- 49.de Moura EB, Amorim FF, da Cruz Santana AN, et al. Skin mottling score as a predictor of 28-day mortality in patients with septic shock. Intensive Care Med 2016; 42:479–480. [DOI] [PubMed] [Google Scholar]
- 50.Lattik R, Couture P, Denault AY, et al. Mitral Doppler indices are superior to two-dimensional echocardiographic and hemodynamic variables in predicting responsiveness of cardiac output to a rapid intravenous infusion of colloid. Anesth Analg 2002; 94:1092–1099. table of contents. [DOI] [PubMed] [Google Scholar]
- 51.van Genderen ME, Bartels SA, Lima A, et al. Peripheral perfusion index as an early predictor for central hypovolemia in awake healthy volunteers. Anesth Analg 2013; 116:351–356. [DOI] [PubMed] [Google Scholar]
- 52.Connors AF, Jr, Dawson NV, McCaffree R, et al. Assessing hemodynamic status in critically ill patients: do physicians use clinical information optimally? J Crit Care 1987; 2:174–180. [Google Scholar]
- 53.Steingrub JS, Celoria G, Vickers-Lahti M, et al. Therapeutic impact of pulmonary artery catheterization in a medical/surgical ICU. Chest 1991; 99:1451–1455. [DOI] [PubMed] [Google Scholar]
- 54.Staudinger T, Locker GJ, Laczika K, et al. Diagnostic validity of pulmonary artery catheterization for residents at an intensive care unit. J Trauma 1998; 44:902–906. [DOI] [PubMed] [Google Scholar]
- 55.Rodriguez RM, Berumen KA. Cardiac output measurement with an esophageal Doppler in critically ill emergency department patients. J Emerg Med 2000; 18:159–164. [DOI] [PubMed] [Google Scholar]
- 56.Linton RA, Linton NW, Kelly F. Is clinical assessment of the circulation reliable in postoperative cardiac surgical patients? J Cardiothorac Vasc Anesth 2002; 16:4–7. [DOI] [PubMed] [Google Scholar]
- 57.Iregui MG, Prentice D, Sherman G, et al. Physicians’ estimates of cardiac index and intravascular volume based on clinical assessment versus transesophageal Doppler measurements obtained by critical care nurses. Am J Crit Care 2003; 12:336–342. [PubMed] [Google Scholar]
- 58.Veale WN, Jr, Morgan JH, Beatty JS, et al. Hemodynamic and pulmonary fluid status in the trauma patient: are we slipping? Am Surg 2005; 71:621–625. discussion 625-6. [PubMed] [Google Scholar]
- 59.Rodriguez RM, Lum-Lung M, Dixon K, Nothmann A. A prospective study on esophageal Doppler hemodynamic assessment in the ED. Am J Emerg Med 2006; 24:658–663. [DOI] [PubMed] [Google Scholar]
- 60.Nowak RM, Sen A, Garcia AJ, et al. The inability of emergency physicians to adequately clinically estimate the underlying hemodynamic profiles of acutely ill patients. Am J Emerg Med 2012; 30:954–960. [DOI] [PubMed] [Google Scholar]
- 61.Duan J, Cong LH, Wang H, et al. Clinical evaluation compared to the pulse indicator continuous cardiac output system in the hemodynamic assessment of critically ill patients. Am J Emerg Med 2014; 32:629–633. [DOI] [PubMed] [Google Scholar]
- 62▪▪.Perel A, Saugel B, Teboul JL, et al. The effects of advanced monitoring on hemodynamic management in critically ill patients: a pre and post questionnaire study. J Clin Monit Comput 2016; 30:511–518. [DOI] [PubMed] [Google Scholar]; This large multicenter study included a broad ICU cohort and used sophisticated statistical measures to correlate CO to clinical examination.
- 63.Ramo BW, Myers N, Wallace AG, et al. Hemodynamic findings in 123 patients with acute myocardial infarction on admission. Circulation 1970; 42:567–577. [DOI] [PubMed] [Google Scholar]
- 64.Forrester JS, Diamond GA, Swan HJ. Correlative classification of clinical and hemodynamic function after acute myocardial infarction. Am J Cardiol 1977; 39:137–145. [DOI] [PubMed] [Google Scholar]
- 65.Grissom CK, Morris AH, Lanken PN, et al. Association of physical examination with pulmonary artery catheter parameters in acute lung injury. Crit Care Med 2009; 37:2720–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sasse SA, Chen PA, Mahutte CK. Relationship of changes in cardiac output to changes in heart rate in medical ICU patients. Intensive Care Med 1996; 22:409–414. [DOI] [PubMed] [Google Scholar]
- 67.Saugel B, Ringmaier S, Holzapfel K, et al. Physical examination, central venous pressure, and chest radiography for the prediction of transpulmonary thermodilution-derived hemodynamic parameters in critically ill patients: a prospective trial. J Crit Care 2011; 26:402–410. [DOI] [PubMed] [Google Scholar]
- 68.Saugel B, Wagner JY, Wendon J, Perel A. Getting the full diagnostic picture in intensive care medicine: a plea for ‘physiological examination’. Ann Am Thorac Soc 2015; 12:1738–1739. [DOI] [PubMed] [Google Scholar]
- 69.Dawson NV, Connors AF, Jr, Speroff T, et al. Hemodynamic assessment in managing the critically ill: is physician confidence warranted? Med Decis Making 1993; 13:258–266. [DOI] [PubMed] [Google Scholar]
- 70.Vazquez R, Gheorghe C, Kaufman D, Manthous CA. Accuracy of bedside physical examination in distinguishing categories of shock: a pilot study. J Hosp Med 2010; 5:471–474. [DOI] [PubMed] [Google Scholar]
- 71.Sakr Y, Reinhart K, Vincent JL, et al. Does dopamine administration in shock influence outcome? Results of the Sepsis Occurrence in Acutely Ill Patients (SOAP) Study. Crit Care Med 2006; 34:589–597. [DOI] [PubMed] [Google Scholar]


