Published online 9 March 2017
Key Words: ulcerative colitis, biologic therapies, psychosocial aspects of IBD, quality of life in IBD
Abstract
Background:
We analyzed in-office communication between patients with ulcerative colitis (UC) and their gastroenterologists.
Methods:
Participating gastroenterologists (United States N = 15; Europe N = 8) identified eligible patients with scheduled clinic visits. Patients (United States N = 40; Europe N = 28; ≥18 yr old; physician-defined moderately-to-severely active ulcerative colitis for approximately ≥1 yr; ≥1 flare in preceding year; prior or current therapy with 5-aminosalicylates and/or corticosteroids) consented to have their visit recorded. Follow-up interviews were conducted separately with gastroenterologists and patients. Transcripts were analyzed using sociolinguistic methods to explore quality of life (QoL) impacts, treatment goals, and attitudes to therapies.
Results:
In the European and U.S. research, the trend was for patients not to discuss ulcerative colitis QoL impacts during their visits. In the U.S. research, complete patient–physician alignment on QoL impacts (patient and physician stating the same impacts) was seen in 40% of cases. Variation in treatment goals was seen between gastroenterologists and patients: 3% of U.S. patients described absence of inflammation as a treatment goal versus 25% of gastroenterologists. This goal was not always conveyed to the patient during visits. Consistent with guidelines, physicians generally framed biologic therapy as suitable for patients refractory to conventional therapies. However, although putative efficacy offered by biologic therapy is generally aligned with patients' stated treatment goals, many considered biologic therapy as more appropriate for more severe disease than theirs.
Conclusions:
Alignment between patients and physicians on ulcerative colitis QoL impact, treatment goals, and requirement of advanced therapies is poor. New tools are needed to cover this gap.
Ulcerative colitis (UC) is a chronic relapsing condition characterized by mucosal inflammation of the colon.1 Patients suffering from UC often require life-long treatment and the associated burden on health-related quality of life (QoL) is significant,2 with clinical features of the disease including rectal bleeding, diarrhea, abdominal pain, and urgency for defecation.1 Conventional therapies included in treatment guidelines for UC include 5-aminosalicylic acid (5-ASA), corticosteroids, and immunomodulatory agents such as azathioprine and 6-mercaptopurine.3,4 Biologic therapies for the treatment of UC include the antagonists to tumor necrosis factor, infliximab, adalimumab, and golimumab,3,4 and the anti-integrin, vedolizumab.5 Even with optimal use of available therapies, many patients do not enter remission and approximately 30% of patients with severe disease require surgery despite availability of biologic therapies.6 Drugs under development, including new biologic therapies and novel small-molecule therapies, may help to address unmet needs.7
Due to the chronic nature of UC and the significant impact on QoL of its symptoms, the relationship between patients and their gastroenterologists is important in understanding and implementing appropriate treatment strategies.8,9 Fostering more effective relationships between patients and their physicians has been shown to enhance understanding of available treatment strategies and, in turn, led to both increased patient satisfaction and improved outcomes.10
Culturally sensitive approaches to the patient–physician relationship are essential. Here, we conducted novel in-office research to analyze the dialog between U.S. and European patients with UC and their gastroenterologists.
MATERIALS AND METHODS
Enrollment of Gastroenterologists and Patients with UC
In the U.S. research, physicians in community-based practices were invited to participate. To participate, physicians had to be board-certified gastroenterologists, in full-time practice for 3 to 30 years, spend at least 75% of their professional time in direct patient care, and primarily see patients in an office or private practice setting. To select practices taking care of patients with moderately-to-severely active disease, physicians also had to see at least 25 patients diagnosed with UC in a typical month and have initiated biologic therapy for at least 15% of their patients with UC. Participation criteria for the European research were similar; the main specialty of participating European physicians had to be gastroenterology, and they had to spend at least 60% of their time in direct patient care and to primarily treat adults.
Participating gastroenterologists were asked to identify eligible participants attending their clinics to take part in this research. Eligible patients were ≥18 years of age with a diagnosis of moderate-to-severe UC for approximately ≥1 year and at least one flare within the past year according to participating gastroenterologists. Patients had previously received or were currently receiving therapy with 5-ASA and/or corticosteroids. Patients had to be fluent in English (not a requirement for the European research) with no cognitive impairment. Physicians in the European research were planning to discuss immunosuppressant or biologic treatment with their patient at their next visit.
For the U.S. research, an ethnographic researcher conducted 1 day of research at each participating practice. Patients with a regularly scheduled appointment who met the enrollment criteria were invited to participate in the study by an office staff member on the day of the research. Interested patients then met the researcher to receive study information and provide consent.
Interviews and Analysis
The study designs of the U.S. and European research are summarized in Figure 1. Without the researcher present in the examination room, a brief patient “selfie” testimonial and a visit between the gastroenterologist, patient, and any office staff present were audio-recorded and video-recorded. After the visit, each patient participated in a semi-structured interview with the researcher lasting approximately 20 minutes and completed a brief questionnaire. In the U.S. research, each gastroenterologist participated in an interview to discuss their general approach to treating patients with UC (approximately 10–15 min) and to discuss each specific patient (approximately 20 min per patient). In the European research, patient and physician follow-up interviews were conducted by telephone (45 min in duration for patient interviews; 60 min for physician interviews). U.S. gastroenterologists also completed a written questionnaire about each patient. This included categorizing each patient's severity of disease as one of the following: (1) only ever moderate; (2) only ever severe; (3) currently moderate, severe in the past; (4) currently severe, moderate in the past. In the European research, the gastroenterologists were asked to rate the severity of each patient's symptoms and impact on QoL on a scale of 1 to 5, with 1 to 2 meaning “mild,” 3 “moderate,” 4 “moderate to severe,” and 5 “severe.”
FIGURE 1.
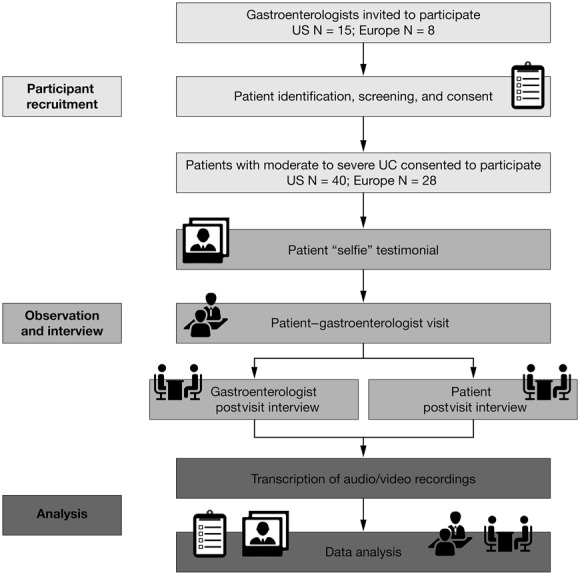
U.S. and European study designs. UC, ulcerative colitis.
The gastroenterologists were allowed to refer to each patient's medical records; these were not shared with the researcher. Patients and gastroenterologists were interviewed following standard discussion guides and were asked similar questions to gauge alignment. Patients, physicians, and research coordinators received monetary compensation for their participation in the research.
Data Analyses
All visits and interviews were transcribed using the audio recordings. Where available, video recordings were used for transcript quality control and to assess nonverbal cues (e.g., nodding). Analysis was performed following interactional sociolinguistic methods, which included categorization of discourse at the level of words and topics, conversational flow, and speaker roles.11,12 A subset of transcripts was reviewed to generate hypotheses, which were then further explored in the full data-set to test their validity. Patient–physician visit dialog was compared with the corresponding postvisit interviews to discover gaps in communication.
In the U.S. research, specific semiquantitative analyses performed included quantifying the percentage of words spoken by the participants and the length of each visit; determining alignment between patients and physicians on QoL impacts (complete alignment was defined as patients and physicians providing all of the same QoL impacts; partial alignment was defined as providing a portion of the same impacts; no alignment was defined as not providing any of the same impacts); and comparing patient and physician descriptions of treatment success and cataloging visits in which these goals were discussed.
In addition, the following were cataloged:
In which visits the physician asked patients about QoL
Which patients were experiencing QoL and emotional impacts due to their disease and, of these, which patients discussed these impacts during the visit
Which patients demonstrated evidence of resigning themselves to active symptoms as acceptable (i.e., “a new normal” or “learned helplessness”)
In which visits the physician interrupted the patient and the number of interruptions
In which visits the physician asked primarily closed-ended questions to assess symptoms
In which visits physicians conveyed a significant gap in their perception of conventional therapies for UC (e.g., 5-ASA and corticosteroids) and advanced therapies (e.g., immunomodulators and biologic therapies)
In which visits advanced therapies were framed as a last resort and thus considered only if absolutely necessary
In which visits advanced therapies were discussed and where they were positioned in the treatment sequence
Which patients considered advanced therapies as more appropriate for more severe stages of disease than they believed they were experiencing.
Descriptive statistics were used to explore the above themes.
ETHICAL CONSIDERATIONS
The U.S. research was an ethnographic observational study of naturally occurring office visit discussions between physicians and patients. It was conducted with the oversight of the New England Institutional Review Board and designed to comply with the Health Insurance Portability and Accountability Act of 1996. The sponsor was not identified to participants and the identities of participants were not shared with the sponsor. The European research was carried out in accordance with the European Pharmaceutical Market Research Association Code of Conduct. All patients in both arms of the research provided written informed consent.
RESULTS
Sample Profile
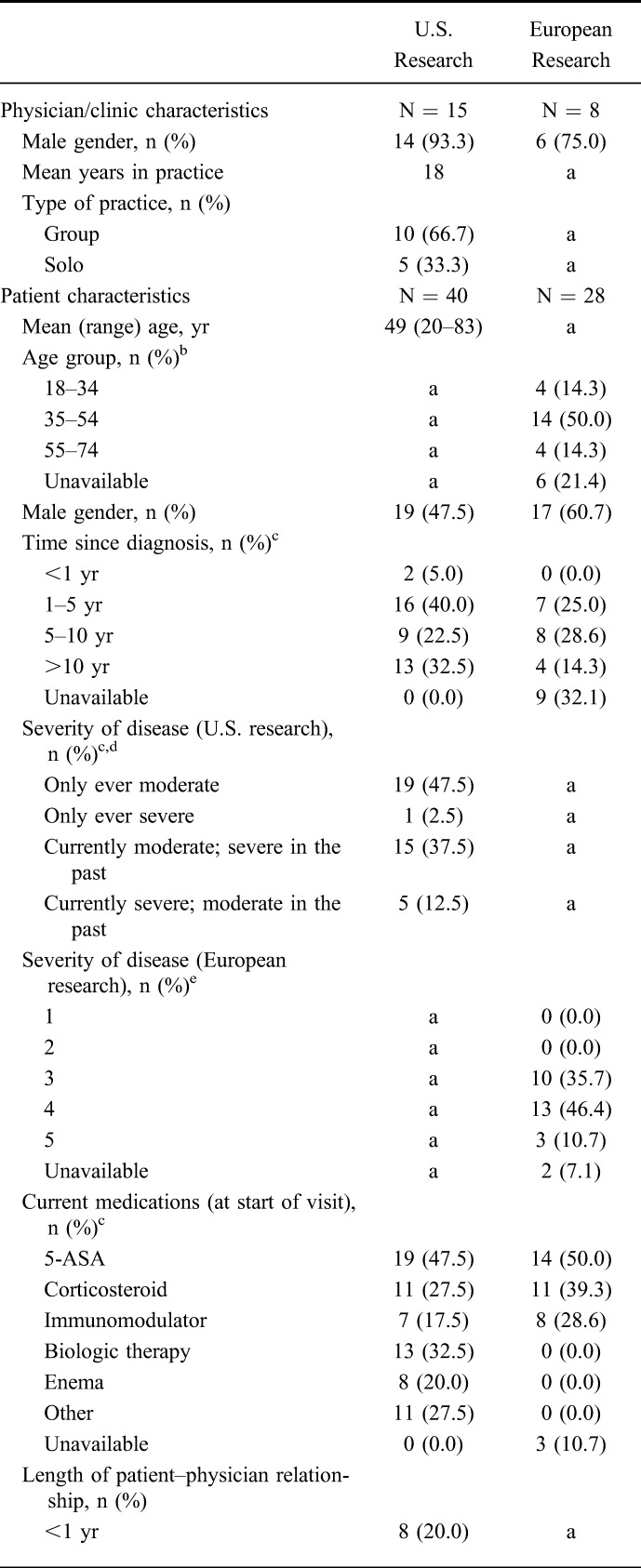
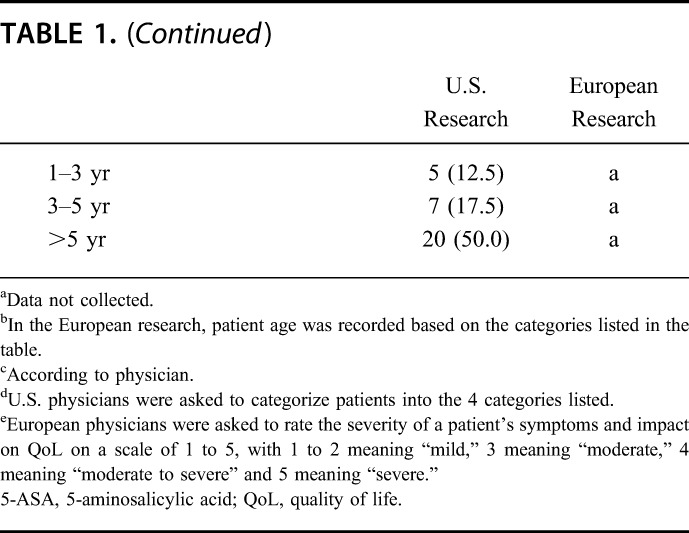
The U.S. sample comprised a total of 40 patient visits with 15 gastroenterologists from around the United States. The European sample comprised 28 patients recruited by 8 gastroenterologists (Italy [n = 5], Germany [n = 2], and France [n = 1]). The characteristics of the participating physicians/clinics and patients are summarized in Table 1.
TABLE 1.
Characteristics of Gastroenterologists and Patients Participating in the Research
Patient Experience and Visit Interaction
In the U.S. research, gastroenterologists typically used closed-ended questions when assessing patients' symptoms and disease progression. Completion of electronic medical records often guided the dialog and thus eye contact and elaboration of symptoms by the patient were limited.
Physician: “So, bowel movements…tell me again. Quantitate that for me.”
—visit with a 44-year-old male patient from the United States
In the patient–physician visits observed in the European research, the gastroenterologists often focused on a checklist of test results.
Physician: “Red blood cells 4,500,000; hemoglobin 14.8; MCV, that is Mean Corpuscular Volume, which is 95. White blood cells have risen again, that is, 4500…C-reactive protein has increased a lot…”
—visit with an Italian patient
During visits, gastroenterologists in the U.S. research spoke, on average, 61% of the words spoken during the visit and asked primarily closed-ended questions to assess symptoms in 73% (n = 29) of the visits. Patients contributed 36% of the words spoken during visits, and other participants such as assisting health professionals and visit companions contributed the remaining 3% of words spoken. Gastroenterologists asked patients about QoL (e.g., impacts on daily life, work, social, family life) or emotional impacts of their disease in 23% (n = 9) of visits. Gastroenterologists interrupted patients at least once in 63% (n = 25) of visits. The average number of interruptions per visit was 1.5. Average visit length increased with the number of interruptions (Fig. 2).
FIGURE 2.
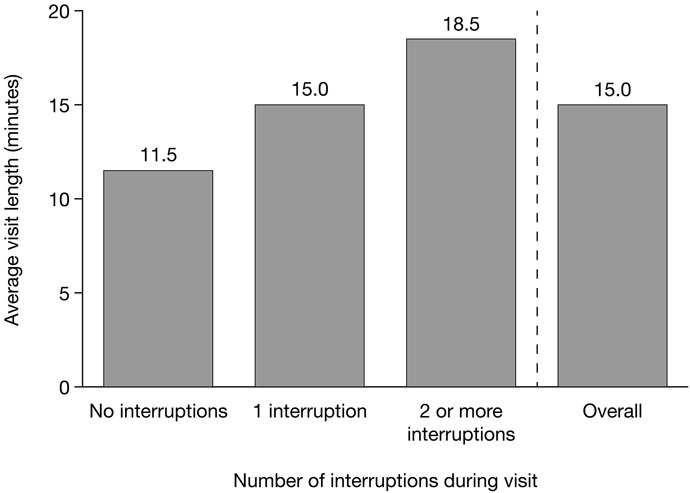
Average visit length overall and by the number of gastroenterologist interruptions during the visit. Data shown are for the U.S. research.
QoL and Emotional Impacts of UC
Qualitative observations from both the U.S. and European research followed a similar trend, where patients rarely spoke of the impact of UC on their lives to their physicians unless directly asked.
Patient: “The consultations are not thorough enough to allow one to really talk about one's discomfort, one's life. No, we just talk about my symptoms…we only talk about the treatment.”
—postvisit interview with a French patient
During the follow-up interviews, U.S. patients frequently conceptualized the emotional impact of their disease as being trapped or confined, irrespective of whether they were experiencing any symptoms at the time.
Patient: “It controls me. It really does. It's embarrassing. You can't…you feel like you have to stay home. You can't go anywhere. You live around the restroom.”
—postvisit interview with a 58-year-old female patient from the United States
During postvisit interviews with U.S. patients and gastroenterologists, 78% (n = 31) of patients stated that they were experiencing QoL impacts (including emotional impacts) from their disease. Fifty-five percent of the 31 patients who were experiencing QoL impacts either did not discuss these impacts during their visit (n = 10; 32%) or underplayed the impacts (n = 7; 23%). Of patients experiencing symptoms, most demonstrated evidence of resigning themselves to a “new normal” of suboptimal treatment. In postvisit interviews with gastroenterologists, complete alignment with their patients' assessment of QoL impacts was demonstrated in 40% (n = 16) of cases, partial alignment in 43% (n = 17) of cases, and no alignment in 18% (n = 7) of cases (Fig. 3). Of the 17 cases where partial alignment was demonstrated, 12 were due to the gastroenterologist underestimating the QoL impacts; 3 were due to gastroenterologist overestimation; and 2 were due to the gastroenterologist and patient mentioning different QoL impacts.
FIGURE 3.
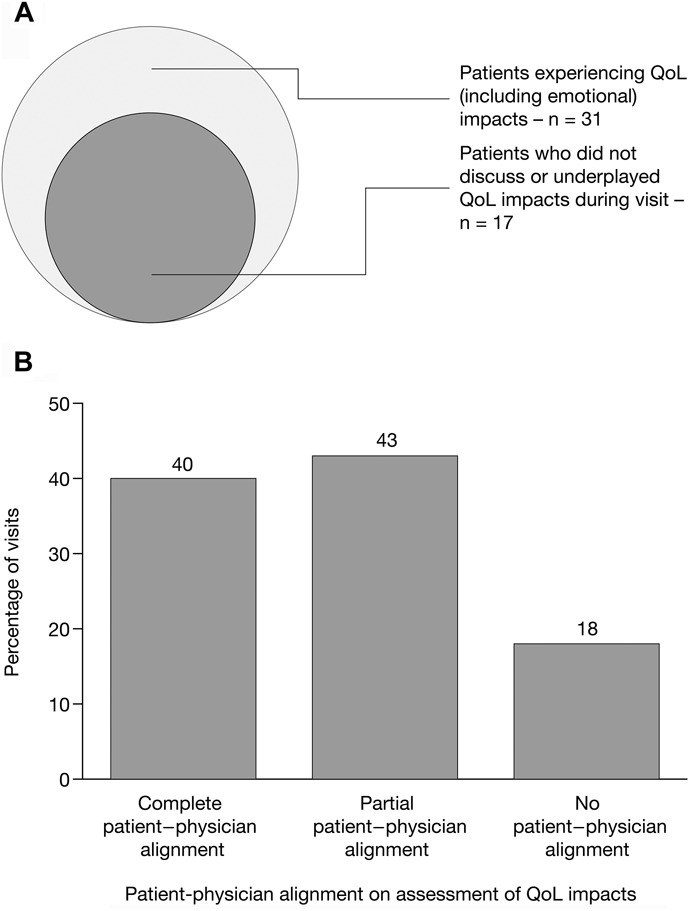
A, Patients experiencing QoL impacts of UC, emotional impacts of UC, and both QoL and emotional impacts of UC. B, Patient–physician alignment on assessment of QoL impacts of UC. Data shown are for the U.S. research; patient–physician alignment was based on the postvisit interviews conducted with physicians and patients. QoL, quality of life; UC, ulcerative colitis.
Patient Understanding of Inflammation
In both the U.S. and European research, the term “inflammation” was used in a range of contexts by gastroenterologists.
Physician: “And so from the way you're feeling, you certainly don't need any more medicine, but if you wanted to get the inflammation down, you would need more medicine.”
—visit with a 67-year-old male patient from the United States
Physician: “If we eliminate the inflammation of the bowel, we won't have these issues anymore.”
—visit with an Italian patient
Physician: “But with what I saw on the colonoscopy, you have pretty bad inflammation. Alright?”
—visit with a 50-year-old female patient from the United States
In the United States, the term “inflammation” (or a variation thereof) was used by gastroenterologists in 60% (n = 24) of visits and an average of 4 times per visit. Most frequently (n = 20), the term was used during the assessment of the patient's disease. Gastroenterologists also used the term during treatment justification (n = 9) and disease education (n = 8), among other contexts. Gastroenterologists described what they meant by the term in only one (2.5%) of the visits and explained the relevance of the term in 3 (7.5%). Patients used the term “inflammation” (or variations) in 13% (n = 5) of visits. During postvisit interviews, patients voluntarily used the term in 48% (n = 19) of cases, but none described what they meant by the term and none demonstrated clinical understanding of it. Patients' use of the term “inflammation” was most frequently to justify treatment (30% of all visits; n = 12) or in the assessment of their disease (15%; n = 6).
Physician Goals of Treatment Versus Patient Goals of Treatment
Patients in the U.S. research described treatment success as an absence of clinical symptoms, i.e., clinical remission.
Patient: “I really just don't want to have diarrhea anymore and/or bleeding, so that's the biggest thing.”
—postvisit interview with a 23-year-old patient from the United States
From the physicians' perspective, treatment success was defined as clinical remission. Some physicians also described the additional goal of endoscopic remission to their patients; however, the relevance and importance of the additional goal of endoscopic remission was rarely conveyed to the patient.
Physician: “My goal is endoscopic remission.”
—postvisit interview with the physician of the same 23-year-old patient from the United States above
Gastroenterologists in the United States described treatment success for most patients as an absence of clinical symptoms, i.e., clinical remission (73% of cases; n = 29). In 25% of cases (n = 10), gastroenterologists described treatment success as an absence of inflammation, i.e., endoscopic remission; in half of these 10 cases, endoscopic remission was an additional goal and in the other half, endoscopic remission was the only goal. Most patients (78%; n = 31) described treatment success as an absence of clinical symptoms, i.e., clinical remission. In one visit (2.5%), the patient described treatment success as absence of inflammation. During visits with patients for whom the gastroenterologist viewed treatment success as endoscopic remission (10 visits), the gastroenterologist clearly communicated this goal in 40% of cases (n = 4).
Patient and Physician Attitudes Toward Immunomodulatory and Biologic Therapies
Biologic therapies were framed as a last resort by physicians; a treatment option to be considered only if absolutely necessary. U.S. physicians conveyed a lack of confidence in biologic agents when positioning them for their patients owing to their associated side effects.
Physician: “You know, [in] patients that have more severe disease than you have, [biologics] can be very beneficial. That whole class of medications, though, carries with it certain side effects: high risk of infection and things like that.”
—visit with a 41-year-old male patient from the United States
Patients tended to consider advanced therapies as appropriate for more severe stages of disease and were conscious of the risks of serious infections and malignancies.
Patient: “There are downsides [to biologics] and my condition is not severe enough to warrant them.”
—postvisit interview with a 57-year-old female patient from the United States
In the European research, immunomodulatory therapies were generally framed as an intermediate therapy for patients who had failed treatment with conventional therapies.
Physician: “When a patient can't take only [5-ASA] and you need these cycles of cortisone, the advice is to switch to the second-level therapy.”
—during a visit with an Italian patient
Findings from the European research showed that biologic therapy was positioned at the end of the treatment sequence, to be considered once other options had been considered and/or exhausted. Physicians positioned biologic agents as potent, effective therapies with known risks.
Physician: “These are drugs to be respected, monitored, and followed-up, but they allow us to face extreme conditions.”
—during a visit with an Italian patient
Triggers for considering a switch to a biologic therapy included QoL considerations (i.e., to achieve remission), lack of efficacy or tolerability with existing treatment, and contraindication to intermediate therapies. Physicians in Europe endorsed biologic therapies in appropriate cases and focused on their potential to induce remission of symptoms. European physicians tended to pre-empt patients' concerns regarding side effects of biologic therapies, but were transparent about the potential risks. Patients in the European research often shared their physician's optimism that biologic therapy would lead to improvements in QoL and some kind of normality.
Observations from the U.S. research revealed that some U.S. physicians were reticent with respect to making significant changes in a patient's treatment regime. The conversation regarding a switch to advanced therapy evolved over a series of visits with the physician initially making the patient aware of potential treatments, before recommending a course of action during a later visit.
Physician: “I don't think you need this therapy right now. But I want you to know that in the future we can offer you a new class of drugs called biologics.”
—visit with a 23-year-old patient from the United States
In contrast, European gastroenterologists in this research led the decision to move to advanced therapies.
Physician: “Now we got to the last colonoscopy where you can see a significant activity we will start a biologic. How do you feel about the fact that we must change to a new drug?”
—visit with a patient from Germany
In the U.S. research, gastroenterologists conveyed a significant gap between conventional therapies for UC and advanced therapies in 58% of visits (n = 23). These therapies were not compared or were not discussed in the majority of the remaining visits (38% of all visits; n = 15). In 53% of visits (n = 21), gastroenterologists framed biologic therapy as a last resort, to be considered only if absolutely necessary. Biologic therapies were not discussed in 40% of visits (n = 16). Immunomodulatory therapy was discussed in 40% of visits (n = 16) and its position in the treatment sequence was always undifferentiated. In 60% of visits (n = 24), it was clear that gastroenterologists had previously discussed advanced options. Of the patients who were not currently or previously prescribed advanced therapies and who discussed advanced therapy options during the postvisit interview, all (n = 17) considered advanced therapies as more appropriate for stages of disease more severe than their own.
DISCUSSION
In this qualitative study of in-office patient–physician visits in the United States and Europe, we assessed the dialog that took place between patients with moderate-to-severe UC and their gastroenterologists. We cataloged themes relating to the patient–physician interaction during the visit which encompassed: patient and physician assessment of symptoms, including QoL and emotional impacts of UC; patient and physician goals of treatment; and patient and physician attitudes toward biologic therapies for UC.
Emphasis during the patient–physician visits was placed on quantification of symptoms. Patients rarely offered or were asked to describe the emotional impact of their disease. Although these physicians had the goal of endoscopic remission in mind, this was rarely articulated to the patient. Although endoscopic demonstration of complete mucosal healing may not be a therapeutic endpoint for all patients who achieve clinical remission,3 there is an unmet need for tools to encourage discussion between patients and physicians to align treatment goals.
In the context of UC, the term “inflammation” had a specific meaning and associated implications and it was not always clear that these were understood by the patient. In this context, there is a need for further education (e.g., information leaflets, flash cards) to ensure greater patient understanding of the term “inflammation” and how this relates to treatment goals for UC. Although efficacy offered by advanced therapies aligns closely with the goals of therapy described by physicians in this research, the stigma of associated risks and side effects created a gap between conventional and biologic therapies, and biologic treatments were framed as most suitable for patients with advanced-stage disease. This is consistent with current treatment guidelines,3,4,13 where advanced therapies are typically recommended where conventional therapies have failed. Adopting a shared decision-making treat-to-target approach may empower physicians and patients to assess the risks of treatment in partnership and within the context of the goals of therapy.9 Accordingly, it may be beneficial to discuss the relative benefits and risks of advanced therapies, including immunomodulators, biologic therapies, and novel small-molecule therapies early on in the patient–physician conversation. Earlier positioning of these advanced therapies in patient–physician discussions and in treatment algorithms should also be considered with the aim of achieving improved short-term and longer-term outcomes.
Although the themes identified in the U.S. and European research were broadly similar, we noted some differences in the two settings. Notably, the framing of immunomodulatory agents in the sequence of treatments between U.S. and European physicians was different. In the European research, immunomodulatory agents were clearly positioned in between conventional therapies and biologic therapies (consistent with the position in treatment guidelines for steroid-dependent or refractory patients3,4,13). In contrast, in the U.S. research, the position of immunomodulatory therapies was often undifferentiated. A difference in attitudes toward biologic agents themselves was also observed between the U.S. and European settings. European patients with UC seemed less aware of, and less concerned with, the side effects of biologic agents and focused more on their potential efficacy compared with patients in the U.S. research.
A limitation of this research is that the study designs of the U.S. and European research were developed independently of each other and there were some differences in data collection and analyses performed between the 2 arms of the research. Comparisons between the U.S. and European research should therefore be interpreted cautiously. Due to the small sample size and qualitative nature of the research, conclusions resulting from this research cannot be generalized. However, the sample populations in the U.S. and European research were typical for discourse analysis of this type,14–18 and the demographic characteristics of the patient sample were broadly in line with expectations.19 In addition, all physicians participating in the research were community gastroenterologists. As such, the themes identified here may not be generally applicable to all practice settings and opinions may vary between generalist and specialist physicians. Studies evaluating patient–physician dialog in additional practice settings, among more diverse physician and patient types, and over a longer duration, would allow a greater understanding of the wider relevance of the identified themes. Importantly, this research provides guidance in the design of such studies. Finally, it is recognized that this research described patient–physician interactions where the participants knew they were being recorded and patient and physician behaviors may have been affected by this. The likely effect, if any, of this knowledge may be that patients and physicians would communicate more effectively than in an unobserved consultation.
This research generated a number of hypotheses. We noted that average visit length increased with the number of times physicians interrupted their patients, but did not determine the directionality of this relationship with respect to cause and effect. We also identified that there is a need for better education focused on aligning patients and physicians with respect to recognizing the QoL and emotional impact of patients' disease and determining appropriate and realistic goals of UC therapy. Further education is necessary to bridge the perceived gap between conventional and advanced therapies for UC treatment.
ACKNOWLEDGMENTS
These analyses were sponsored by Pfizer Inc. The authors would like to acknowledge the contribution of Amy Marren of Pfizer Inc in the preparation of this manuscript. Medical writing support under the guidance of the authors was provided by Daniel Binks, PhD, of Complete Medical Communications and funded by Pfizer Inc.
D. T. Rubin has received consulting fees from AbbVie, Amgen, Janssen, Pfizer Inc, Takeda, and UCB and research grants from AbbVie, Genentech, Janssen, Takeda, and UCB. M. C. Dubinsky has been a consultant for AbbVie, Boehringer Ingelheim, Celgene, Genentech, Janssen, Pfizer Inc, Takeda, and UCB and received support for research from AbbVie and Janssen. S. Martino is a principal of M Health and thus was a paid consultant to Pfizer Inc in connection with performing this research. K. A. Hewett is an employee of Ogilvy CommonHealth Behavioral Insights and thus was a paid consultant to Pfizer Inc in connection with performing this research and with the development of this manuscript. J. Panés has received consulting fees from AbbVie, Boehringer Ingelheim, Genentech-Roche, Janssen, Pfizer Inc, Takeda, and TiGenix; research grants from AbbVie and MSD; and speaker fees from AbbVie, Janssen, MSD, and Pfizer Inc.
Footnotes
Author disclosures are available in the Acknowledgments.
REFERENCES
- 1.Ordàs I, Eckmann L, Talamini M, et al. Ulcerative colitis. Lancet. 2012;380:1606–1619. [DOI] [PubMed] [Google Scholar]
- 2.Husain A, Triadafilopoulos G. Communicating with patients with inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:444–450. [DOI] [PubMed] [Google Scholar]
- 3.Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology Practice Parameters Committee. Am J Gastroenterol. 2010;105:501–523. [DOI] [PubMed] [Google Scholar]
- 4.Mowat C, Cole A, Windsor A, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60:571–607. [DOI] [PubMed] [Google Scholar]
- 5.Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 6.Park SC, Jeen YT. Current and emerging biologics for ulcerative colitis. Gut Liver. 2015;9:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danese S, Grisham MB, Hodge J, et al. JAK inhibition using tofacitinib for inflammatory bowel disease treatment: a hub for multiple inflammatory cytokines. Am J Physiol Gastrointest Liver Physiol. 2016;310:G155–G162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baars JE, Siegel CA, Kuipers EJ, et al. Patient's perspectives important for early anti-tumor necrosis factor treatment in inflammatory bowel disease. Digestion. 2009;79:30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubin DT, Cleveland NK. Using a treat-to-target management strategy to improve the doctor-patient relationship in inflammatory bowel disease. Am J Gastroenterol. 2015;110:1252–1256. [DOI] [PubMed] [Google Scholar]
- 10.Glass KE, Wills CE, Holloman C, et al. Shared decision making and other variables as correlates of satisfaction with health care decisions in a United States national survey. Patient Educ Couns. 2012;88:100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gumperz JJ. On interactional sociolinguistic method. In: Talk, Work and Institutional Order: Discourse in Medical, Mediation and Management Settings. Berlin, Germany: Mouton de Gruyter; 1999:453–471. [Google Scholar]
- 12.Hamilton HE. Symptoms and signs in particular: the influence of the medical concern on the shape of physician-patient talk. Commun Med. 2004;1:59–70. [DOI] [PubMed] [Google Scholar]
- 13.Dignass A, Lindsay JO, Sturm A, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis. 2012;6:991–1030. [DOI] [PubMed] [Google Scholar]
- 14.Hahn SR, Friedman DS, Quigley HA, et al. Effect of patient-centered communication training on discussion and detection of nonadherence in glaucoma. Ophthalmology. 2010;117:1339–1347. [DOI] [PubMed] [Google Scholar]
- 15.Lipton RB, Hahn SR, Cady RK, et al. In-office discussions of migraine: results from the American migraine communication study. J Gen Intern Med. 2008;23:1145–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davidson B, Vogel V, Wickerham L. Oncologist-patient discussion of adjuvant hormonal therapy in breast cancer: results of a linguistic study focusing on adherence and persistence to therapy. J Support Oncol. 2007;5:139–143. [PubMed] [Google Scholar]
- 17.Davidson B, Blum D, Cella D, et al. Communicating about chemotherapy-induced anemia. J Support Oncol. 2007;5:36–40, 46. [PubMed] [Google Scholar]
- 18.Friedman DS, Hahn SR, Quigley HA, et al. Doctor-patient communication in glaucoma care: analysis of videotaped encounters in community-based office practice. Ophthalmology. 2009;116:2277–2285. [DOI] [PubMed] [Google Scholar]
- 19.da Silva BC, Lyra AC, Rocha R, et al. Epidemiology, demographic characteristics and prognostic predictors of ulcerative colitis. World J Gastroenterol. 2014;20:9458–9467. [DOI] [PMC free article] [PubMed] [Google Scholar]


