Abstract
Background
Patients with rheumatoid arthritis (RA) have increased risk of heart failure with preserved ejection fraction. The development and progression of left ventricular dysfunction before onset of clinical heart failure are unknown. The objective of this study was to evaluate longitudinal changes in cardiac structure and function of patients with RA compared with persons in the general population.
Methods
A prospective longitudinal study of a population-based cohort of 160 patients with RA and a population-based cohort of 1,391 persons without RA (non-RA cohort) was performed. Each participant underwent 2-dimensional, pulsed-wave tissue Doppler echocardiography at baseline and after 4 to 5 years of follow-up. Age- and sex-adjusted linear regression models were used to test for differences between the RA and non-RA cohorts in annualized rates of change for echocardiographic parameters.
Results
Mitral A velocity increased more rapidly among the patients with RA than the non-RA cohort (age- and sex-adjusted parameter estimate, 0.030; P<.001). Correspondingly, the mean mitral inflow E/A ratio decreased faster in the RA cohort than the non-RA cohort (adjusted parameter estimate, −0.096; P<.001). The left atrial volume index increased at a higher rate in the RA cohort than the non-RA cohort (adjusted parameter estimate, 0.150; P<.001).
Conclusions
This pattern of echocardiographic findings confirms previous cross-sectional studies and indicates that subclinical changes in diastolic function occur more rapidly over 5 years in RA patients than in the general population. Further research into the mechanisms of myocardial disease in these patients and the relationship with disease activity and treatment is warranted.
Keywords: echocardiography, heart failure, rheumatoid arthritis
Introduction
Heart failure (HF) is a cardiovascular comorbidity associated with rheumatoid arthritis (RA) (1). The incidence of HF in population-based RA cohorts is approximately 2-fold greater than the general population (2–4). This increased HF risk is explained by neither traditional risk factors nor typical coronary artery disease (5). HF with preserved ejection fraction (HFpEF) is more common among patients with RA than patients with HF in the general population (6).
Even without clinical heart disease, patients with RA have a high prevalence of abnormalities in cardiac structure and function. Aslam et al (7) published a systematic review and meta-analysis of 25 cross-sectional echocardiographic studies of left ventricular (LV) structure and function, comparing 1,614 patients with 4,222 control participants (controls). Using data from matched and unmatched studies, the authors found that RA patients had greater mitral inflow A wave velocities, lower E/A ratios, and longer isovolumetric relaxation times than non-RA controls, essentially confirming that RA is associated with alterations in diastolic function. In addition, they observed that RA patients had increases in left atrial (LA) dimension, LV mass index, and estimated pulmonary artery pressure.
However, little is known about the importance and progression of abnormalities in myocardial structure and function in patients with RA. Whether these alterations regress, remain stable, or progress over time and whether they differ for persons with RA compared with the general population is unknown. These knowledge gaps represent barriers to development of screening approaches that identify persons with asymptomatic LV dysfunction who might benefit from early cardiovascular intervention (8).
A population-based cohort study is underway at our institution to understand the pathophysiologic factors of myocardial dysfunction in patients with RA. Previously, we have reported the baseline echocardiographic findings (9). Herein, we report the 5-year prospective longitudinal follow-up of cardiac structure and function assessed with echocardiography of RA patients compared with persons from the general population.
Materials and Methods
A prospective, longitudinal, population-based cohort study was conducted using the resources of the Rochester Epidemiology Project, a distinctive centralized, communitywide medical records linkage system (10). The sampling frame for patients with RA was a previously assembled population of adult (age≥18 years) residents of Olmsted County, Minnesota, who fulfill the American College of Rheumatology 1987 criteria for the classification of RA (11). The study used a previously identified, randomized sample of persons from the general population of this community, assembled to evaluate the burden of LV systolic and diastolic dysfunction as a comparison (non-RA) cohort (12–14). All members of both cohorts were included; there were no exclusion criteria. The institutional review boards (IRBs) of Mayo Clinic (IRB number 06-005445) and Olmsted Medical Center (IRB number 039-omc-06) in Rochester, Minnesota approved this study. All participants provided written informed consent.
Both cohorts were evaluated longitudinally at 2 prespecified time points, hereafter called examination 1 and examination 2. In the non-RA cohort, examination 1 occurred from 1997 through 2000; examination 2 occurred from 2001 through 2004. In the RA cohort, examination 1 occurred from 2007 through 2009 and examination 2 from 2012 through 2014. The interval between examinations was approximately 4 years for the non-RA cohort and 5 years for the RA cohort. At each research appointment, participants completed questionnaires regarding HF symptoms, cardiovascular risk factors, and medications. Patients completed the global assessment (range, 0–100), Health Assessment Questionnaire disability index, and Routine Assessment of Patient Index Data 3 (RAPID3) (15,16). Data were collected from participants’ questionnaires and health records. Cardiovascular risk factors (ie, coronary or ischemic heart disease, diabetes mellitus, hypertension, and obesity) were defined as described previously (9,13). Clinical HF was defined by the Framingham criteria (17).
Comprehensive standardized echocardiography was performed for all participants by registered diagnostic cardiac sonographers and according to the recommendations of the American Society of Echocardiography (9,12,13,18,19). The echocardiographic methods of the more contemporary (RA) cohort were designed to be as similar as possible to the historical (non-RA) cohort, with minor changes in measurements (eg, LA volume index) in accordance with current guidelines. The methods were also similar for each cohort at the baseline and follow-up time-points. Interpretation of the echocardiographic images for the non-RA cohort was performed by a single echocardiologist as previously described (12). All echocardiographic images for the RA cohort were interpreted in the core laboratory.
Linear measurements of LV dimensions and wall thicknesses were obtained from the parasternal long-axis view to calculate LV mass. LV chamber volumes were derived from apical 4- and 2-chamber views and calculated with the modified Simpson biplane method (18). LV diastolic function was assessed with pulsed-wave Doppler examination of mitral inflow (E and A velocities and E/A ratio) and tissue Doppler examination of septal and lateral mitral annular velocities (e′ velocity) (19). LA volume index was determined in the non-RA cohort with the formula π/6(SA1 · SA2 · LA), where SA1 is the M-mode LA dimension and SA2 and LA are measurements of short and long axis in the apical 4-chamber view at ventricular end systole, indexed to body surface area (m2) (20). For the RA cohort, LA volume index was calculated using the area-length method, according to current recommendations (19). (For further background on the echocardiographic methods, please see references 12 and 19.)
Of the 244 patients with RA in our original study (9), 160 returned for examination 2, yielding a return participation rate of 66%. Of the 1,402 participants in the non-RA population (14), 9 were excluded because of enrollment in the RA cohort and 2 were excluded because of missing echocardiographic measurements at both time points. Therefore, 1,391 participants comprised the non-RA cohort.
Descriptive statistics were used to summarize participant characteristics at baseline and follow-up. Age- and sex-adjusted linear or logistic regression models were used to compare the RA and non-RA cohorts for baseline characteristics and annualized rates of change in echocardiographic parameters. Changes in these parameters between baseline and follow-up were tested using paired t tests. Standardized regression coefficients–computed by dividing a parameter estimate by the ratio of the sample standard deviation of the echocardiographic variable to the sample standard deviation of cohort variable–were used to facilitate comparison of annualized rates of change across the parameters. Interactions between age and sex were used to determine whether rates of change differed between women and men with RA. Smoothing splines displayed trends in these figures. Analyses were performed with SAS statistical software version 9.4 (SAS Institute, Inc) and R software version 3.0.2 (R Foundation for Statistical Computing).
Results
The RA cohort consisted of 160 patients; the non-RA cohort had 1,391 participants (Table 1). At examination 1, mean age was slightly less in the RA cohort than the non-RA cohort (58.5 vs 61.1 years, P=.001). As expected, the proportion of female participants was greater in the RA cohort (76.3% vs 50.6%, P<.001). Patients with RA had greater prevalence of hypertension, obesity, and HF than non-RA participants. With regard to CV medications, baseline use of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and β-blockers was significantly greater in the RA cohort.
Table 1.
Characteristics of Patients With RA and non-RA Comparator Participants
| Non-RA (n=1,391) | RA (n=160) | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Characteristica | Exam 1b | Exam 2b | Exam 1b | Exam 2b | P Valuec |
| Age, mean (SD), y | 61.1 (9.5) | 65.2 (9.5) | 58.5 (11.8) | 63.5 (11.8) | .001 |
| RA disease duration, mean (SD), y | NA | NA | 11.2 (8.7) | 16.2 (8.7) | |
| Female sex | 704 (50.6) | NA | 122 (76.3) | NA | <.001 |
| Body mass index, mean (SD), kg/m2 | 28.2 (5.0) | 28.4 (5.0) | 28.9 (5.9) | 29.2 (5.8) | .04 |
| Systolic blood pressure, mean (SD)d | 130.7 (19.8) | 125.9 (19.1) | 126.6 (16.4) | 125.2 (17.8) | .28 |
| Diastolic blood pressure, mean (SD)d | 73.4 (10.0) | 69.4 (10.4) | 71.0 (9.0) | 69.6 (10.6) | .26 |
| Risk factor | |||||
| Hypertension | 339 (24.4) | 588 (42.3) | 91 (56.9) | 99 (61.9) | <.001 |
| Obesity | 432 (31.1) | 442 (31.8) | 63 (39.4) | 72 (45.0)d | .03 |
| Diabetes mellitus | 86 (6.2) | 143 (10.3) | 11 (6.9) | 16 (10.0) | .35 |
| Coronary disease | 143 (10.3) | 211 (15.2) | 17 (10.6) | 26 (16.3) | .15 |
| Heart failure | 16 (1.2) | 31 (2.2) | 6 (3.8) | 9 (5.6) | .003 |
| Smoking | .55 | ||||
| Missing, No. | 3 | 43 | 1 | 1 | |
| Current | 99 (7.1) | 82 (6.1) | 9 (5.7) | 9 (5.7) | |
| Former | 577 (41.6) | 575 (42.7) | 57 (35.8) | 59 (37.1) | |
| Medication Use | |||||
| DMARD | |||||
| Prednisone | NA | NA | 38 (23.8) | 36 (22.5) | |
| Dose, mean (SD), mg | NA | NA | 4.5 (2.3) | 6.5 (4.2) | |
| Methotrexate | NA | NA | 89 (55.6) | 88 (55.0) | |
| Hydroxychloroquine | NA | NA | 53 (33.1) | 45 (28.1) | |
| Other csDMARDf | NA | NA | 16 (10.0) | 19 (11.9) | |
| TNF biologicsg | NA | NA | 27 (16.9) | 29 (18.1) | |
| Non-TNF biologicsh | NA | NA | 2 (1.3) | 6 (3.8) | |
| NSAIDs | NA | NA | 103 (64.4) | 123 (76.9) | |
| Cardiovascular | |||||
| ACEI or ARB | 120 (8.6) | 248 (17.8) | 26 (16.3) | 39 (24.4) | <.001 |
| β-Blocker | 188 (13.5) | 302 (21.7) | 32 (20.0) | 36 (22.5) | .006 |
| Calcium channel blocker | 80 (5.8) | 103 (7.4) | 12 (7.5) | 16 (10.0) | .17 |
| Diuretice | 197 (15.3) | 310 (23.5) | 28 (17.5) | 27 (16.9) | .35 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; exam, examination; csDMARD, conventional synthetic disease-modifying antirheumatic drug; DMARD, disease-modifying antirheumatic drug; exam, examination; NA, not available; RA, rheumatoid arthritis; TNF, tumor necrosis factor.
Values are presented as number (percentage) of patients unless specified otherwise.
The median intervals between echocardiographic exams 1 and 2 were 4 and 5 years for non-RA and RA cohorts, respectively (P<.001).
P values are for the differences between the baseline characteristics for the non-RA and RA groups. Comparisons are adjusted for age and sex.
For blood pressure, 4 and 3 participants in the non-RA cohort had missing data at exams 1 and 2; 6 and 1 patients in the RA cohort had missing data at exams 1 and 2.
For diuretic use, 105 and 70 participants in the non-RA cohort had missing data at exams 1 and 2.
Other csDMARDs include sulfasalazine, leflunomide, and azathioprine.
TNF biologics include adalimumab, certolizumab, etanercept, and golimumab (all within 1 month) and infliximab (within 3 months).
Non-TNF biologics include abatacept (within 1 month), tocilizumab (within 1 month), and rituximab (within 6 months).
The interval between echocardiographic examinations 1 and 2 was a median (range) of 4.0 (2.1–5.7) years for the non-RA cohort and 5.0 (4.8–5.5) years for the RA cohort (P<.001). The trends in differences between the cohorts for these characteristics at examination 1 appeared to persist at examination 2; however, these trends were not tested formally because of the different follow-up intervals between the 2 cohorts. Age- and sex-adjusted annualized rates of change in echocardiographic parameters differed between the cohorts (Supplementary Table, Table 2, and Figure 1).
Table 2.
Adjusted Rates of Change in LV Structural and Functional Parametersa
| Patients, No. | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Dependent Variable | Non-RA (n=1,391) | RA (n=160) | Parameter Estimateb | Standard Error | Standardized Estimate | P Value | P Valuec |
|
|
|||||||
| Cardiac structure | |||||||
| LV septal thickness, diastole | 1,045 | 113 | −0.015 | 0.004 | −0.112 | <.001 | .001 |
| Posterior wall thickness, diastole | 1,038 | 115 | −0.010 | 0.004 | −0.082 | .006 | .01 |
| LV end-diastolic dimension | 949 | 102 | 0.029 | 0.009 | 0.095 | .002 | .008 |
| LV mass index | 919 | 116 | −0.005 | 0.460 | −0.0004 | .99 | .97 |
| Left atrial volume index | 1,264 | 102 | 1.506 | 0.160 | 0.251 | <.001 | <.001 |
| Cardiac function | |||||||
| LV ejection fraction | 942 | 159 | −0.568 | 0.144 | −0.120 | <.001 | <.001 |
| E velocity | 1,370 | 158 | −0.012 | 0.003 | −0.100 | <.001 | <.001 |
| A velocity | 1,354 | 149 | 0.030 | 0.003 | 0.233 | <.001 | <.001 |
| E/A ratio | 1,348 | 149 | −0.096 | 0.008 | −0.307 | <.001 | <.001 |
| Tissue Doppler e′ | 1,163 | 156 | −0.00006 | 0.001 | −0.002 | .96 | .86 |
| E/e′ ratio | 1,156 | 155 | −0.124 | 0.092 | −0.038 | .18 | .16 |
| Pulmonary artery pressure | 721 | 64 | −0.538 | 0.180 | −0.106 | .003 | .002 |
| Deceleration time | 1,329 | 153 | −0.326 | 0.930 | −0.009 | .73 | .90 |
Abbreviations: exam, examination; LV, left ventricular; RA, rheumatoid arthritis.
Results are shown for age- and sex-adjusted linear regression models, testing for differences in rates of change (difference per time) for echocardiographic parameters from exam 1 to exam 2 between the non-RA and RA cohorts. The numbers are listed of patients in each group with observations for each dependent variable.
The parameter estimate is for the difference in the annualized rates of change between exams associated with the RA cohort compared with the non-RA cohort.
P values for linear regression models adjusted for age, sex, hypertension, obesity, diabetes, coronary disease, and smoking status.
Figure 1.
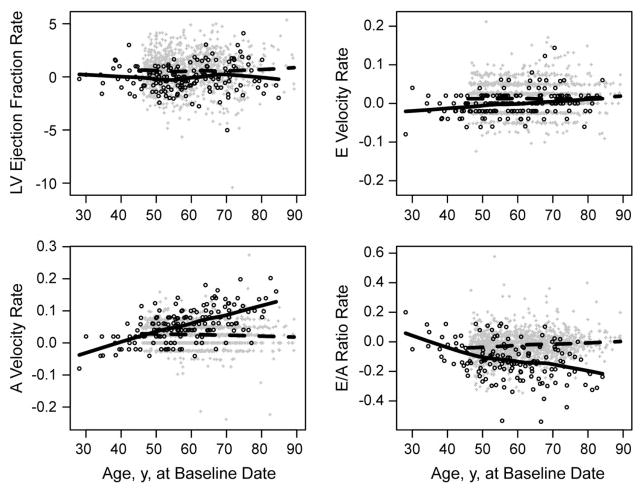
Accelerated Progression of Early Diastolic Dysfunction in Patients With RA Compared With Non-RA Participants Over 5 Years of Follow-up. Annualized rates of change in key echocardiographic LV structural and functional parameters of study participants are shown according to age at baseline examination. Solid and dashed lines represent smoothed trends for RA and non-RA cohorts, respectively. A, Rate of change in LV ejection fraction. B, Rate of change in E velocity. C, Rate of change in A velocity. D, Rate of change in E/A ratio. The data show that compared with non-RA participants, RA patients had accelerated reduction in left atrial compliance, indicated by faster increases of A velocity (P<.001) and faster decreases of E/A ratio (P<.001). LV indicates left ventricular; RA, rheumatoid arthritis.
In cardiac structure, both LV septal thickness and posterior wall thickness decreased in both cohorts (Supplementary Table). After adjustment for age and sex, the LV septal thickness (parameter estimate, −0.015; P<.001) and the posterior wall thickness (parameter estimate, −0.010; P=.006) declined at a faster rate among patients with RA. These dimensional changes persisted after further adjustment for hypertension, obesity, diabetes, coronary artery disease, and smoking status (Table 2). In contrast, the LV end-diastolic dimension increased more rapidly in the RA cohort than the non-RA cohort (parameter estimate, 0.029; P=.002).
The mean LV mass index declined between examinations from 94.3 g/m2 to 90.9 g/m2 in the non-RA cohort (P<.001) and from 80.9 g/m2 to 77.3 g/m2 in the RA cohort (P=.03). Annualized rates of decline in the LV mass index were similar between cohorts (Table 2).
The LA volume index increased from 24.4 mL/m2 to 24.7 mL/m2 (P=.06) in the non-RA cohort and from 26.9 mL/m2 to 34.4 mL/m2 (P<.001) in the RA cohort (Supplementary Table). During follow-up, the LA volume index increased significantly faster in the RA than the non-RA cohort (parameter estimate, 0.150; P<.001), after adjusting for age, sex, and cardiovascular risk factors (Table 2). Notwithstanding the differences between cohorts in the calculation of this parameter, the changes in LA volume index were substantial and significant.
With respect to systolic function, a statistically significant increase occurred in the ejection fraction over time among non-RA participants. No change was seen in ejection fraction among RA patients, resulting in a significant difference in the rates of change between the groups, albeit not clinically meaningful (Table 2 and Figure 1A).
With respect to diastolic function through assessment of mitral inflow velocities, the mean E velocity increased between examinations among the non-RA participants (from 0.67 to 0.73, P<.001) but did not change significantly among the RA patients (from 0.70 to 0.70, P=.80) (Supplementary Table). Although the adjusted rates differed between the groups (Table 2), the plots of the E velocities over time appeared similar (Figure 1B).
The principal findings concern longitudinal changes in the mitral inflow A wave velocity and E/A ratio. The mean A velocity increased significantly in both groups (Supplementary Table), but the increase was larger in the RA cohort than the non-RA cohort (from 0.42 to 0.70 vs from 0.63 to 0.74). Comparison of rates of change showed that RA is associated with significant acceleration in A velocity (parameter estimate, 0.030; P<.001) compared with non-RA, with adjustment for age and sex (Table 2 and Figure 1C). Correspondingly, the mean E/A ratio decreased more in the RA cohort (from 1.70 to 1.08 for RA vs 1.14 to 1.05 for non-RA). The rate of E/A ratio decline was significantly faster for patients with RA than non-RA participants (parameter estimate, −0.096; P<.001) (Table 2 and Figure 1D). These RA-associated differences became more prominent at older age (Figure 1C and 1D). Additional adjustment for cardiovascular risk factors did not change either of these results (P<.001 for both).
Tissue Doppler analysis of mitral annular motion at both time points showed a decrease in e′ velocity and an increase in E/e′ ratios in the cohorts, consistent with modest increases in LV filling pressures (Supplementary Table). However, the rates of change over time were nearly identical between the cohorts (Table 2). Pulmonary arterial systolic pressures tended to increase slightly among the non-RA participants and to decrease slightly among the RA patients.
In evaluation for sex differences, all analyses reported in Table 2 were repeated separately among female and male participants. The results showed no evidence of statistically significant differences between them in the annualized rates of change of A velocity (P for interaction, .30), E/A ratio (P for interaction, .26), or any other diastolic parameters (data not shown). The small numbers of men limited meaningful comparisons (data not shown).
Within the RA population, modest but statistically significant correlations were observed between several measures of RA disease activity and severity and 5-year changes in diastolic parameters (Table 3). The patient global assessment, RAPID3, Health Assessment Questionnaire, and C-reactive protein level, as well as current use of glucocorticoids, were associated with the 5-year changes in A velocity. The positive direction of these correlations indicated that greater disease activity was associated with increasing A velocity during follow-up. Higher levels of C-reactive protein and interleukin 6 were associated with increasing e′ velocity. The E/e′ ratio was associated with the patient global assessment and RAPID3 score.
Table 3.
Age- and Sex-Adjusted Correlations of RA Disease Characteristics With 5-Year Progression in Echocardiographic Parameters (Examination 2 –Examination 1) in the RA Population
| E Velocityb | A Velocityb | E/A Ratiob | e′b | E/e′ Ratiob | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||
| Parameter | Baselinea | r | P Value | r | P Value | r | P Value | r | P Value | r | P Value |
| RF positivity | 0.69 (0.46) | −0.07 | .40 | 0.05 | .56 | −0.09 | .26 | 0.13 | .10 | −0.14 | .09 |
| Anti-CCP positivity | 0.48 (0.50) | −0.03 | .68 | 0.04 | .67 | 0.04 | .65 | 0.03 | .76 | −0.05 | .55 |
| Glucocorticoid, current use | 38 (23.8) | 0.07 | .36 | 0.17 | .04 | −0.09 | .27 | −0.04 | .62 | 0.08 | .34 |
| Methotrexate, current use | 89 (55.6) | 0.00 | .99 | −0.07 | .41 | 0.10 | .22 | −0.00 | .97 | −0.06 | .45 |
| Biologic, use at any time in 5 y | 42 (26.2) | 0.10 | .21 | 0.00 | .97 | 0.05 | .55 | 0.04 | .64 | 0.10 | .23 |
| Patient global | 22.2 (23.6) | 0.19 | .02 | 0.21 | .01 | −0.13 | .11 | −0.08 | .30 | 0.23 | .005 |
| RAPID3 | 6.42 (5.8) | 0.15 | .06 | 0.24 | .003 | −0.17 | .04 | −0.04 | .61 | 0.19 | .02 |
| HAQ | 0.47 (0.55) | 0.05 | .54 | 0.21 | .01 | −0.19 | .02 | 0.03 | .69 | 0.06 | .43 |
| CRP | 4.21 (6.29) | −0.01 | .91 | 0.21 | .01 | −0.16 | .047 | 0.19 | .02 | −0.08 | .30 |
| IL-6 | 5.63 (11.3) | 0.01 | .86 | 0.15 | .07 | −0.13 | .12 | 0.19 | .02 | −0.11 | .16 |
Abbreviations: anti-CCP, anticyclic citrullinated peptide antibodies; CRP, C-reactive protein; HAQ, Health Assessment Questionnaire; IL-6, interleukin 6; r, age- and sex-adjusted Spearman correlation coefficient; RA, rheumatoid arthritis; RAPID3, Rapid Assessment of Patient Index Data 3; RF, rheumatoid factor.
Baseline values represent number (percentage) of patients or mean (SD).
Bold indicates statistically significant values.
Discussion
Previous cross-sectional studies (21–26) have consistently reported findings of subclinical LV diastolic dysfunction in patients with RA (9,21–26) (for a systematic review, see reference 7). One small prospective longitudinal study of 25 patients with RA by Yazici et al (27) reported no major deterioration in LV diastolic dysfunction over 5 years. The present study confirms the importance of the previous findings and further demonstrates that subclinical changes in diastolic function occur more rapidly in patients with RA than non-RA persons over 5 years of follow-up. In particular, the mitral A velocity increases faster for RA patients than non-RA persons whereas the E velocity stays relatively unchanged. In addition, the LA volume index increases significantly for RA patients, suggestive that LV compliance is reduced. Together, the findings suggest that among patients with RA, greater dependence on atrial contraction may be an early manifestation of diastolic dysfunction. This manifestation would occur before any clinically meaningful impairment in LV relaxation indicated by declining mitral E velocity and prolonged deceleration time.
In this study, the typical patient with RA had an E/A ratio approaching 1.0, which according to 1 grading system for diastolic function (12) suggests the pseudonormalization pattern, or grade II diastolic dysfunction. However, the mean e′ velocity stays well preserved over time, and the mean E/e′ ratio of 10.7, although a modest increase, is not consistent with a pseudonormal pattern. Rather, the findings suggest that the typical patient with RA in this study had an earlier, intermediate stage between normal and grade I diastolic dysfunction.
The finding of increasing A velocity as an early sign of LV diastolic dysfunction contrasts the paradigm in the non-RA population, in which changing E velocity (ie, impaired relaxation) is the first sign of diastolic dysfunction (14). Because dependence on the atrial contraction is associated with aging, it is noteworthy that patients with RA were significantly younger than the non-RA participants, fitting with the concept of accelerated aging in the pathophysiologic factors of cardiovascular disease in RA (28). Whether increased A velocity without concurrent decreased E velocity is a marker of even earlier diastolic dysfunction than grade I, or simply a different pattern of progression to overt diastolic dysfunction in RA, is unclear.
In the present study, RA patients had a higher prevalence of 2 cardiovascular risk factors, hypertension and obesity, as well as HF. Previous studies have reported greater prevalence of hypertension and obesity in patients with RA. Although these 2 factors could have contributed to early diastolic function changes in this study, the reported association between RA and increasing A velocity persisted after adjustment for hypertension and obesity, suggesting these factors alone cannot explain the findings.
The RA patients also had greater use of 3 important classes of medications – angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and β-blockers—that are effective for treatment of hypertension. No differences between the cohorts were found in the blood pressure measurements at the time of the echocardiographic examinations. Randomized clinical trials have not shown a definite benefit of these medications on cardiac structure or function for patients with diastolic dysfunction or HFpEF (29).
Studies of LV mass in patients with RA have come to differing conclusions, with some reporting increased LV mass (30) and others, including a study by our group, reporting decreased LV mass (9,31). A recent meta-analysis of pooled data from 16 studies suggested that RA characteristically correlates with increased absolute or indexed LV mass (32). In the present study, we report that both the patients with RA and the non-RA participants have declining LV mass index over 5 years of follow-up. In our non-RA population, this change was shown previously and was postulated to result from effects of antihypertensive therapy (13). However, in this study, blood pressures were not significantly different between the echocardiographic examinations. Diastolic dysfunction is generally associated with hypertrophy, so the observation of progressive diastolic dysfunction in the clinical setting of declining LV mass index seems counterintuitive. In this regard, a limitation of the present study is that analysis of mean changes in echocardiographic parameters was done without consideration for patterns of LV remodeling.
Historically, diastolic dysfunction and HFpEF have been attributed to concentric remodeling, where LV mass increases because of concentric wall thickening without LV dilatation—for example, in hypertensive heart disease (33). In contrast, eccentric remodeling with normal or thin LV walls amid chamber dilatation has been more typically associated with HF with reduced ejection fraction, as in ischemic cardiomyopathy. Previously, we reported that RA is correlated with LV concentric remodeling (34). The present longitudinal analysis suggests that RA-associated myocardial disease may also involve eccentric remodeling, resulting from thinning of LV septal and posterior walls. The finding is important in view of the recently recognized heterogeneity of HFpEF, which can be associated with either concentric or eccentric remodeling (35–37).
By performing strain imaging using 2-dimensional speckle-tracking echocardiography, Kraigher-Krainer et al (35) showed that patients with HFpEF have impairments in both longitudinal and circumferential strain. We previously reported that patients with RA have impaired LV longitudinal strain compared with age- and sex-matched control participants and that strain is associated with markers of disease severity (38). The implication of these findings is that eccentric remodeling related to subclinical systolic dysfunction has a role in the pathophysiologic development of RA-associated myocardial disease. Further research is warranted into the trajectories of LV remodeling in RA compared with patients who have other comorbidities.
Associations between RA disease characteristics at baseline and the subsequent progression of early changes in diastolic function contribute to the knowledge that RA is an independent risk factor for myocardial disease (9,30,31,39). Particularly, the associations between the RAPID3 score and the 5-year changes in the A velocity, as well as the E/e′ ratio, suggest that inflammatory disease activity associated with RA contributes to subclinical progression of diastolic dysfunction. A limitation of this analysis is that tender and swollen joint counts were not available at the time of the echocardiography, therefore changes in the DAS28 could not be correlated with the diastolic function parameters. However, the RAPID3 score has been shown to correlate well with the DAS28 (15,40–42), underscoring the validity the RAPID3 as a measure of clinical disease activity. The findings of this study suggest that RA should be added to the growing list of comorbidities associated with HFpEF—including obesity, hypertension, and diabetes mellitus—in which systemic inflammation drives the development of myocardial dysfunction and remodeling through coronary microvascular endothelial activation, oxidative stress, cardiomyocyte hypertrophy, and ultimately, myocardial fibrosis (43,44). Further research is necessary to evaluate the possibility that RA drives the progression of LV diastolic dysfunction through coronary endothelial inflammation.
The strength of this study is the comparison of the longitudinal changes in cardiac structure and function between a population-based cohort of patients with RA and a non-RA cohort from the same community over 4 to 5 years. The study design strongly supports the generalizability of the findings. The fact that the echocardiography for both cohorts was done in the same laboratory, using state-of-the-art technology and methods, reinforces the validity of this study.
However, this study has limitations. First, the comparability of the RA and non-RA cohorts needs to be discussed. Cost considerations precluded enrollment of age- and sex-matched controls in this study. Yet, the availability of a comparison cohort of patients from the same underlying community and using the resources of the Rochester Epidemiology Project, as well as the age- and sex-adjusted analyses, strengthens the validity of our conclusions. The time periods of enrollment and follow-up intervals were different between the RA and non-RA cohorts. Second, grading of diastolic function was not possible because strict interpretation according to an accepted algorithm resulted in a large number of —indeterminate grades, a limitation of diastolic function grading (45). Third, hemodynamic observations through echocardiography were not confirmed with direct hemodynamic catheterization. However, hemodynamic and diastolic function assessment with echocardiography is well validated and correlates with direct pressure measurements by hemodynamic catheterization.
Fourth, myocardial tissue characterization by 2-dimensional echocardiography is inferior to cardiac magnetic resonance imaging with gadolinium enhancement or endomyocardial biopsy. Fifth, return participation rates in the RA and non-RA cohorts were suboptimal at 66% and 58%. However, participation in this study was similar to another landmark epidemiologic study, the Multi-Ethnic Study of Atherosclerosis, which had a participation rate of 60% (46). Patients with RA who did not participate at examination 2 were older and more likely to have coronary artery disease, diabetes, diuretic use, and greater wall thicknesses than patients with RA who did participate. Although this finding suggests the potential of participation bias, inclusion of these patients at examination 2 would likely have resulted in even greater changes in diastolic function. Sixth, although the diversity of Olmsted County, Minnesota, is increasing, the predominance of white persons (86%) still limits generalizability to other populations.
In conclusion, the findings of this study suggest that 1) RA is associated with early changes in LV diastolic function over 5 years and 2) the rates of change are significantly higher than those observed among persons who do not have RA. These changes in diastolic function, if progressive over time, could contribute to the known increased risk of HF in these patients. The results of this study will inform future studies assessing long-term myocardial outcomes. Continued longitudinal follow-up of these cohorts will be valuable in further understanding the pathophysiology of HF in RA.
Supplementary Material
Acknowledgments
Funding Support: This work was supported by a grant from the National Institutes of Health (NIH), National Institute of Arthritis and Musculoskeletal and Skin Diseases (Award Number R01AR46849), and was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the NIH under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funding sources had no involvement in the study design or in the collection, analysis, or interpretation of the data.
Abbreviations
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- IRB
institutional review board
- LA
left atrial
- LV
left ventricular
- non-RA
without rheumatoid arthritis
- RA
rheumatoid arthritis
- RAPID3
Routine Assessment of Patient Index Data 3
Footnotes
Presented in abstract form at the American College of Rheumatology Annual Scientific Meeting, Boston, Massachusetts, November 14-19, 2014, and at the European League Against Rheumatism Annual Congress, Rome, Italy, June 10-13, 2015.
Conflict of interest: The authors have no relationships with industry to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wright K, Crowson CS, Gabriel SE. Cardiovascular comorbidity in rheumatic diseases: a focus on heart failure. Heart Fail Clin. 2014 Apr;10(2):339–52. doi: 10.1016/j.hfc.2013.10.003. Epub 2014 Jan 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolfe F, Freundlich B, Straus WL. Increase in cardiovascular and cerebrovascular disease prevalence in rheumatoid arthritis. J Rheumatol. 2003 Jan;30(1):36–40. [PubMed] [Google Scholar]
- 3.Wolfe F, Michaud K. Heart failure in rheumatoid arthritis: rates, predictors, and the effect of anti-tumor necrosis factor therapy. Am J Med. 2004 Mar 1;116(5):305–11. doi: 10.1016/j.amjmed.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 4.Nicola PJ, Maradit-Kremers H, Roger VL, Jacobsen SJ, Crowson CS, Ballman KV, et al. The risk of congestive heart failure in rheumatoid arthritis: a population-based study over 46 years. Arthritis Rheum. 2005 Feb;52(2):412–20. doi: 10.1002/art.20855. [DOI] [PubMed] [Google Scholar]
- 5.Crowson CS, Nicola PJ, Kremers HM, O’Fallon WM, Therneau TM, Jacobsen SJ, et al. How much of the increased incidence of heart failure in rheumatoid arthritis is attributable to traditional cardiovascular risk factors and ischemic heart disease? Arthritis Rheum. 2005 Oct;52(10):3039–44. doi: 10.1002/art.21349. [DOI] [PubMed] [Google Scholar]
- 6.Davis JM, 3rd, Roger VL, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. The presentation and outcome of heart failure in patients with rheumatoid arthritis differs from that in the general population. Arthritis Rheum. 2008 Sep;58(9):2603–11. doi: 10.1002/art.23798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aslam F, Bandeali SJ, Khan NA, Alam M. Diastolic dysfunction in rheumatoid arthritis: a meta-analysis and systematic review. Arthritis Care Res (Hoboken) 2013 Apr;65(4):534–43. doi: 10.1002/acr.21861. [DOI] [PubMed] [Google Scholar]
- 8.Mavrogeni S, Dimitroulas T, Sfikakis PP, Kitas GD. Heart involvement in rheumatoid arthritis: multimodality imaging and the emerging role of cardiac magnetic resonance. Semin Arthritis Rheum. 2013 Dec;43(3):314–24. doi: 10.1016/j.semarthrit.2013.05.001. Epub 2013 Jun 17. [DOI] [PubMed] [Google Scholar]
- 9.Liang KP, Myasoedova E, Crowson CS, Davis JM, Roger VL, Karon BL, et al. Increased prevalence of diastolic dysfunction in rheumatoid arthritis. Ann Rheum Dis. 2010 Sep;69(9):1665–70. doi: 10.1136/ard.2009.124362. Epub 2010 May 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kremers HM, Myasoedova E, Crowson CS, Savova G, Gabriel SE, Matteson EL. The Rochester Epidemiology Project: exploiting the capabilities for population-based research in rheumatic diseases. Rheumatology (Oxford) 2011 Jan;50(1):6–15. doi: 10.1093/rheumatology/keq199. Epub 2010 Jul 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 12.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003 Jan 8;289(2):194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 13.Borlaug BA, Redfield MM, Melenovsky V, Kane GC, Karon BL, Jacobsen SJ, et al. Longitudinal changes in left ventricular stiffness: a community-based study. Circ Heart Fail. 2013 Sep 1;6(5):944–52. doi: 10.1161/CIRCHEARTFAILURE.113.000383. Epub 2013 Jun 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC, Jr, et al. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011 Aug 24;306(8):856–63. doi: 10.1001/jama.2011.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pincus T, Swearingen CJ, Bergman M, Yazici Y. RAPID3 (Routine Assessment of Patient Index Data 3), a rheumatoid arthritis index without formal joint counts for routine care: proposed severity categories compared to disease activity score and clinical disease activity index categories. J Rheumatol. 2008 Nov;35(11):2136–47. doi: 10.3899/jrheum.080182. Epub 2008 Sep 15. [DOI] [PubMed] [Google Scholar]
- 16.Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980 Feb;23(2):137–45. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- 17.Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993 Oct;22(4 Suppl A):6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 18.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015 Jan;28(1):1–39. e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009 Feb;22(2):107–33. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 20.Pritchett AM, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM. Left atrial volume as an index of left atrial size: a population-based study. J Am Coll Cardiol. 2003 Mar 19;41(6):1036–43. doi: 10.1016/s0735-1097(02)02981-9. [DOI] [PubMed] [Google Scholar]
- 21.Mustonen J, Laakso M, Hirvonen T, Mutru O, Pirnes M, Vainio P, et al. Abnormalities in left ventricular diastolic function in male patients with rheumatoid arthritis without clinically evident cardiovascular disease. Eur J Clin Invest. 1993 Apr;23(4):246–53. doi: 10.1111/j.1365-2362.1993.tb00769.x. [DOI] [PubMed] [Google Scholar]
- 22.Corrao S, Salli L, Arnone S, Scaglione R, Pinto A, Licata G. Echo-Doppler left ventricular filling abnormalities in patients with rheumatoid arthritis without clinically evident cardiovascular disease. Eur J Clin Invest. 1996 Apr;26(4):293–7. doi: 10.1046/j.1365-2362.1996.133284.x. [DOI] [PubMed] [Google Scholar]
- 23.Di Franco M, Paradiso M, Mammarella A, Paoletti V, Labbadia G, Coppotelli L, et al. Diastolic function abnormalities in rheumatoid arthritis: evaluation by echo Doppler transmitral flow and pulmonary venous flow: relation with duration of disease. Ann Rheum Dis. 2000 Mar;59(3):227–9. doi: 10.1136/ard.59.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montecucco C, Gobbi G, Perlini S, Rossi S, Grandi AM, Caporali R, et al. Impaired diastolic function in active rheumatoid arthritis: relationship with disease duration. Clin Exp Rheumatol. 1999 Jul-Aug;17(4):407–12. [PubMed] [Google Scholar]
- 25.Arslan S, Bozkurt E, Sari RA, Erol MK. Diastolic function abnormalities in active rheumatoid arthritis evaluation by conventional Doppler and tissue Doppler: relation with duration of disease. Clin Rheumatol. 2006 May;25(3):294–9. doi: 10.1007/s10067-005-0014-3. [DOI] [PubMed] [Google Scholar]
- 26.Alpaslan M, Onrat E, Evcik D. Doppler echocardiographic evaluation of ventricular function in patients with rheumatoid arthritis. Clin Rheumatol. 2003 May;22(2):84–8. doi: 10.1007/s10067-002-0677-y. [DOI] [PubMed] [Google Scholar]
- 27.Yazici D, Tokay S, Aydin S, Toprak A, Inanc N, Khan SR, et al. Echocardiographic evaluation of cardiac diastolic function in patients with rheumatoid arthritis: 5 years of follow-up. Clin Rheumatol. 2008 May;27(5):647–50. doi: 10.1007/s10067-007-0820-x. [DOI] [PubMed] [Google Scholar]
- 28.Crowson CS, Therneau TM, Davis JM, 3rd, Roger VL, Matteson EL, Gabriel SE. Brief report: accelerated aging influences cardiovascular disease risk in rheumatoid arthritis. Arthritis Rheum. 2013 Oct;65(10):2562–6. doi: 10.1002/art.38071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Senni M, Paulus WJ, Gavazzi A, Fraser AG, Diez J, Solomon SD, et al. New strategies for heart failure with preserved ejection fraction: the importance of targeted therapies for heart failure phenotypes. Eur Heart J. 2014 Oct 21;35(40):2797–815. doi: 10.1093/eurheartj/ehu204. Epub 2014 Aug 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rudominer RL, Roman MJ, Devereux RB, Paget SA, Schwartz JE, Lockshin MD, et al. Independent association of rheumatoid arthritis with increased left ventricular mass but not with reduced ejection fraction. Arthritis Rheum. 2009 Jan;60(1):22–9. doi: 10.1002/art.24148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giles JT, Malayeri AA, Fernandes V, Post W, Blumenthal RS, Bluemke D, et al. Left ventricular structure and function in patients with rheumatoid arthritis, as assessed by cardiac magnetic resonance imaging. Arthritis Rheum. 2010 Apr;62(4):940–51. doi: 10.1002/art.27349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corrao S, Argano C, Pistone G, Messina S, Calvo L, Perticone F. Rheumatoid arthritis affects left ventricular mass: systematic review and meta-analysis. Eur J Intern Med. 2015 May;26(4):259–67. doi: 10.1016/j.ejim.2015.02.008. Epub 2015 Mar 5. [DOI] [PubMed] [Google Scholar]
- 33.Maeder MT, Kaye DM. Heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol. 2009 Mar 17;53(11):905–18. doi: 10.1016/j.jacc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Myasoedova E, Davis JM, 3rd, Crowson CS, Roger VL, Karon BL, Borgeson DD, et al. Brief report: rheumatoid arthritis is associated with left ventricular concentric remodeling: results of a population-based cross-sectional study. Arthritis Rheum. 2013 Jul;65(7):1713–8. doi: 10.1002/art.37949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kraigher-Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, Pieske B, et al. PARAMOUNT Investigators. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014 Feb 11;63(5):447–56. doi: 10.1016/j.jacc.2013.09.052. Epub 2013 Oct 30. Erratum in: J Am Coll Cardiol. 2014 Jul 22;64(3):335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitzman DW, Upadhya B. Heart failure with preserved ejection fraction: a heterogenous disorder with multifactorial pathophysiology. J Am Coll Cardiol. 2014 Feb 11;63(5):457–9. doi: 10.1016/j.jacc.2013.10.007. Epub 2013 Oct 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katz DH, Beussink L, Sauer AJ, Freed BH, Burke MA, Shah SJ. Prevalence, clinical characteristics, and outcomes associated with eccentric versus concentric left ventricular hypertrophy in heart failure with preserved ejection fraction. Am J Cardiol. 2013 Oct 15;112(8):1158–64. doi: 10.1016/j.amjcard.2013.05.061. Epub 2013 Jun 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fine NM, Crowson CS, Lin G, Oh JK, Villarraga HR, Gabriel SE. Evaluation of myocardial function in patients with rheumatoid arthritis using strain imaging by speckle-tracking echocardiography. Ann Rheum Dis. 2014 Oct;73(10):1833–9. doi: 10.1136/annrheumdis-2013-203314. Epub 2013 Jul 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giles JT, Fernandes V, Lima JA, Bathon JM. Myocardial dysfunction in rheumatoid arthritis: epidemiology and pathogenesis. Arthritis Res Ther. 2005;7(5):195–207. doi: 10.1186/ar1814. Epub 2005 Aug 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pincus T, Bergman MJ, Yazici Y, Hines P, Raghupathi K, Maclean R. An index of only patient-reported outcome measures, routine assessment of patient index data 3 (RAPID3), in two abatacept clinical trials: similar results to disease activity score (DAS28) and other RAPID indices that include physician-reported measures. Rheumatology (Oxford) 2008 Mar;47(3):345–9. doi: 10.1093/rheumatology/kem364. [DOI] [PubMed] [Google Scholar]
- 41.Pincus T, Furer V, Keystone E, Yazici Y, Bergman MJ, Luijtens K. RAPID3 (Routine Assessment of Patient Index Data 3) severity categories and response criteria: Similar results to DAS28 (Disease Activity Score) and CDAI (Clinical Disease Activity Index) in the RAPID 1 (Rheumatoid Arthritis Prevention of Structural Damage) clinical trial of certolizumab pegol. Arthritis Care Res (Hoboken) 2011 Aug;63(8):1142–9. doi: 10.1002/acr.20481. [DOI] [PubMed] [Google Scholar]
- 42.Pincus T, Swearingen CJ, Bergman MJ, Colglazier CL, Kaell AT, Kunath AM, et al. RAPID3 (Routine Assessment of Patient Index Data) on an MDHAQ (Multidimensional Health Assessment Questionnaire): agreement with DAS28 (Disease Activity Score) and CDAI (Clinical Disease Activity Index) activity categories, scored in five versus more than ninety seconds. Arthritis Care Res (Hoboken) 2010 Feb;62(2):181–9. doi: 10.1002/acr.20066. [DOI] [PubMed] [Google Scholar]
- 43.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013 Jul 23;62(4):263–71. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 44.van Empel V, Brunner-La Rocca HP. Inflammation in HFpEF: key or circumstantial? Int J Cardiol. 2015;189:259–63. doi: 10.1016/j.ijcard.2015.04.110. [DOI] [PubMed] [Google Scholar]
- 45.Desai CS, Colangelo LA, Liu K, Jacobs DR, Jr, Cook NL, Lloyd-Jones DM, et al. Prevalence, prospective risk markers, and prognosis associated with the presence of left ventricular diastolic dysfunction in young adults: the coronary artery risk development in young adults study. Am J Epidemiol. 2013 Jan 1;177(1):20–32. doi: 10.1093/aje/kws224. Epub 2012 Dec 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galea S, Tracy M. Participation rates in epidemiologic studies. Ann Epidemiol. 2007 Sep;17(9):643–53. doi: 10.1016/j.annepidem.2007.03.013. Epub 2007 Jun 6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


