Abstract
Background
Strain and strain rate are sensitive markers of left ventricular (LV) myocardial function. This study sought to assess reference ranges and regional patterns of LV strain and strain rate using two dimensional speckle tracking echocardiography in a large black and white population.
Methods
This study involved a retrospective review of prospectively collected images in 557 CARDIA study participants who remained healthy at the year-25 examination. LV deformation parameters were measured in apical four-chamber, apical two-chamber and parasternal short-axis views in 509, 391 and 521 subjects respectively.
Results
Mean age was 49.6 ± 3.6 years, 61.6% women and 69.5% white. White women showed the highest LV systolic and diastolic deformation values, reflected by more negative reference range for apical four-chamber longitudinal strain [−16.4% (95% prediction interval: −20.8 to −12.0)], and higher positive reference range for early diastolic strain rate [0.93 (1/s) (95% PI: 0.41 to 1.46)] respectively. The lowest LV systolic and diastolic deformation values were found in black men, with apical four-chamber longitudinal strain [14.7% (95% PI: −19.1 to −10.3)] and early diastolic strain rate [0.79 (1/s) (95% PI: 0.42 to 1.16)]. Absolute strain increased from the epicardium toward the endocardium. A base-to-apex gradient of longitudinal strain toward the apex is exhibited in inferior and inferoseptal regions and, in contrast, in the opposite direction in anterior and anterolateral walls. Sex had the strongest influence on LV deformation variability.
Conclusions
Strain and strain rate reference values were sex-and race-related. White women showed the highest reference ranges for LV deformation, while the lowest values were found in black men. Significant layer- and level-specific patterns in regional LV deformation were identified.
Keywords: echocardiography, left ventricular function, myocardial contraction, myocardial strain, speckle tracking echocardiography
INTRODUCTION
Left ventricular (LV) ejection fraction reflects global ventricular function, usually impaired in advanced stages of myocardial damage.[1] Strain and strain rate, measures of tissue deformation, provide quantitative measures of global and regional function, and have been shown to be earlier markers of LV dysfunction, with independent and incremental prognostic value for major adverse cardiac events.[2] Two-dimensional speckle tracking echocardiography (2D-STE) is a non-invasive and portable technique to evaluate LV deformation, with a relatively higher temporal resolution, when compared with other methods, such as cardiac magnetic resonance imaging and cardiac computed tomography.[3] Recently, 2D-STE has expanded the understanding of systolic and diastolic function in many settings, such as myocardial ischemia, valve diseases and cardiomyopathies, and has been introduced in clinical practice.[4] We have recently shown race and gender differences in speckle tracking parameters in middle-aged adults as well as the association of blood pressure and obesity with these measures.[5–7] However, there is a paucity of information regarding reference ranges in those without cardiovascular risk factors or disease for deformation parameters using 2D-STE, especially accounting for race and sex differences in healthy individuals.[8] We aimed to provide race- and sex-specific reference ranges for LV strain and strain rate in a middle-aged biracial group of men and women without cardiovascular risk or disease (healthy subgroup) and to describe regional characteristics and correlates of LV deformation.
METHODS
Study Design and Participants
The CARDIA study design and population characteristics have been previously described.[9] Briefly, the CARDIA study is a prospective cohort study designed to evaluate development and progression of coronary disease risk factors in young adults. Initially, 5115 healthy black and white men and women aged 18 to 30 years (1985–1986) were enrolled and examined at 4 Field Centers in Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California. Of 3498 participants attending the Year-25 (2010–2011) examination, 3474 individuals underwent echocardiography, including 2D-STE. This study involved a retrospective review of prospectively collected images in a total of 557 CARDIA study participants who remained healthy at the year-25 evaluation. The Institutional Review Board at each site has approved the study protocol. Written informed consent was obtained from all participants. The work has been conducted in accordance with Declaration of Helsinki.
Echocardiographic Acquisition
Transthoracic echocardiography was performed utilizing commercially available ultrasound system Artida (Toshiba Medical Systems, Japan) with a 1.8 to 4.2 MHz phased-array transducer. Images were acquired by experienced sonographers, trained centrally and certified for standardized protocols across all four field centers. Two-dimensional images were obtained from apical four-chamber and two-chamber views, as well as from parasternal long-axis and short-axis views. Image acquisition was optimized by adjusting gain, compression and transducer frequency. Sector width and image depth were adjusted to ensure the entire left ventricle could be visualized throughout a complete cardiac cycle. Focus depth was positioned at LV mid-cavity. At frame rate ranging from 40 to 90 frames/second, at least two cardiac cycles were acquired from each view. M-mode and Doppler-derived methods were performed for complete a conventional echocardiographic evaluation. The exams were recorded in DICOM standard, as well as in vendor-specific format, and transmitted to the Year-25 Echocardiographic Reading Center at the Johns Hopkins University.
Echocardiographic Analysis
All analyses were performed off-line by four experienced readers, trained centrally and certified according to predefined analysis protocol. Conventional echocardiographic measures were evaluated according to previously published guidelines[10], utilizing commercially available DigiView software, (Digisonics Inc., USA), version 3.7.7. LV ejection fraction was estimated by Simpson’s biplane method.
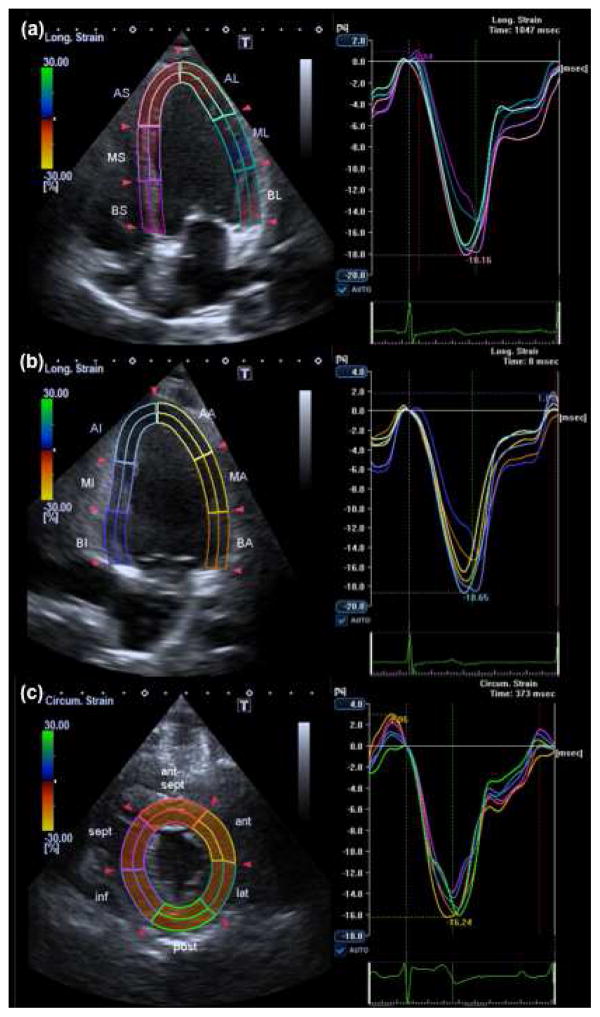
LV 2D-STE was performed using the validated Advanced Cardiology Package 2D Wall Motion Tracking (Toshiba Medical Systems Co., Japan), version 3.0. Longitudinal deformation was assessed from apical four-chamber and two-chamber views; circumferential deformation and radial deformation were assessed from the parasternal short-axis view at mid-ventricular level (figure 1). A region of interest (ROI) was automatically defined after manual selection of landmarks in endocardial border. If necessary, further manual adjustments of ROI thickness and endocardial and epicardial borders were made. Finally, myocardial tracking was obtained automatically. If needed, borders were edited or re-traced. End-diastole was considered as the beginning of the QRS segment, while end-systole was defined as the lowest LV volume.
Figure 1. Two-dimensional speckle tracking echocardiography.
Longitudinal strain analyzed in apical four-chamber view (a), and apical two-chamber view (b), and circumferential strain evaluated in parasternal short-axis view (c).
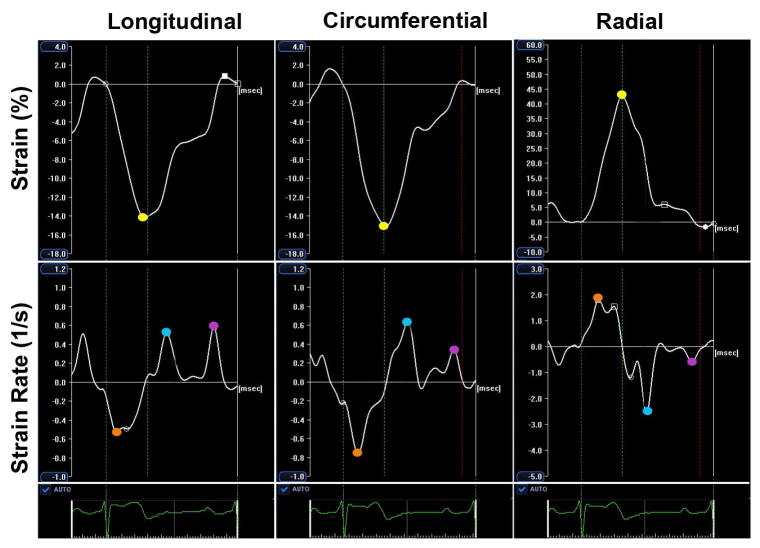
Strain was calculated as the peak systolic change in the segment length relative to the length at end-diastole, and represented as percentage (%). Strain rate is defined as the deformation ratio, estimated as the temporal derivative of strain. Strain rate parameters were analyzed at systolic peak, early-diastolic peak and late-diastolic peak, and represented as deformation per second (1/s). Both strain and strain rate were presented as global and segmental measurements. Each echocardiographic view was divided into a six-segment model; global values were defined as the average of segmental peaks. Peak systolic strain was also analyzed at endocardial, mid-wall and epicardial layers.
Longitudinal and circumferential strain and strain rate parameters reflect shortening, so during systole, more negative values represent enhanced deformation, and during diastole, enhanced deformation rate is represented by more positive values. Radial strain and strain rate parameters reflect thickening, so during the systole, more positive values represent enhanced deformation, and during the diastole, enhanced deformation rate is represented by more negative values (figure 2).
Figure 2. Global LV strain and strain rate curves.
Strain was measured as the maximum systolic deformation (yellow points); strain rate was assessed at systolic peak (orange points), early-diastolic peak (blue points) and late-diastolic peak (purple points).
Echocardiographic quality control and reproducibility
Quality control and reproducibility of echocardiographic measurements in CARDIA Year-25 examination have been published.[11] Briefly, segments unsuitable for myocardial tracking were excluded. Echocardiographic views with more than three segments excluded were not considered for global measurements. Moreover, two-dimensional images were classified as optimal, if scored as good or excellent, or suboptimal, if scored as fair or poor, according to the two-dimensional spatial resolution. Acquisition and reading procedures were highly reproducible. Inter- and intra-reader reproducibility of speckle tracking derived variables were assessed for all four analysts in a subset of 40 and 160 images respectively. Intraclass correlation coefficient (ICC) and residual coefficient of variation (CoV) based on a linear mixed model were used to assess variability. ICC and CoV of longitudinal apical four- and two-chamber, circumferential and radial strains were 0.55 (10.4%), 0.71 (10.7%), 0.67 (12.9%) and 0.84 (15%) respectively for inter-reader reproducibility, and 0.79 (6.6%), 0.87 (5.5%), 0.81 (6.8%) and 0.89 (12.1%).
Healthy subgroup
A healthy subgroup was established from CARDIA participants who underwent the Year-25 follow-up. For this purpose, individuals with a history of cardiovascular disease or those with hypertension, dyslipidemia, diabetes, obesity, former and current smoking, subclinical atherosclerosis, cancer, hyperthyroidism, or HIV infection identified in CARDIA Year-25 examination were excluded. Cardiovascular disease was defined as the presence of one or more of the following: a history of myocardial infarction, coronary revascularization, cardiomyopathy, congestive heart failure, any aortic stenosis or other cardiac valve disease greater than mild, or pulmonary hypertension. Hypertension was defined as blood pressure ≥ 140/90 mmHg or use of antihypertensive medications; diabetes mellitus was defined as combination of one or more of the following: fasting plasma glucose ≥ 126 mg/dL, 2-hour oral glucose tolerance test ≥ 200 mg/dL, glycated hemoglobin ≥ 6.5% or use of anti-diabetes medications; dyslipidemia as triglycerides ≥ 150 mg/dL, high-density lipoprotein (HDL) cholesterol < 40 mg/dL for males or HDL < 50 mg/dL for females; obesity as body mass index (BMI) ≥ 30 m2/Kg. Subclinical atherosclerosis was considered as coronary artery calcium score greater than zero or common carotid intima-media thickness (IMT) mean value > 1.0 mm.[12] Calcium score and IMT were measured in 85% and 99% of the healthy population respectively. Only non-pregnant women were included in the healthy subgroup. Individuals excluded from the apparently healthy subgroup were included in the subgroup with known cardiovascular risk factors/and other diseases. The most frequent exclusion criterion was obesity (43.5%), followed by smoking history (38.3%), hypertension (33.0%), subclinical atherosclerosis (28.0%), dyslipidemia (16.9%), diabetes mellitus (10.4%), cancer (7.5%), cardiovascular disease (5.8%), hyperthyroidism (1.7%), HIV infection (0.9%) and pregnancy (0.1%). After application of exclusion criteria, the entire healthy population was comprised of 557 individuals.
Statistical Analysis
Baseline characteristics comparisons were evaluated by Student’s t-test, Wilcoxon rank-sum test and Chi-square test as appropriate. Reference ranges for deformation parameters were described as mean and 95% confidence interval, and prediction intervals were also provided. Normality of the data was verified by Shapiro-Wilk test and histograms. Due to skewed distribution, radial early diastolic strain rate and all late diastolic strain rate measurements were log-transformed to calculate prediction intervals. One-way analysis of variance was used to verify differences among race and sex categories, with Tukey’s test for post-hoc multiple comparisons. Multivariable linear regression was utilized to assess the association between strain and strain rate (as dependent variable) in the healthy subgroup, and demographics (age, sex and race), educational years, anthropometric and hemodynamic variables (BMI, heart rate and systolic blood pressure), and technical parameters (frame rate and spatial resolution of two-dimensional images). Variance inflation factor was used to evaluate collinearity, with mean value of 1.15 (ranging from 1.01 to 1.29), reflecting absence of significant multi-collinearity. All tests were two-tailed and differences considered statistically significant when p< .05. All the statistical analyses were done using STATA 12.1 (StataCorp, USA).
RESULTS
Demographics and speckle tracking feasibility
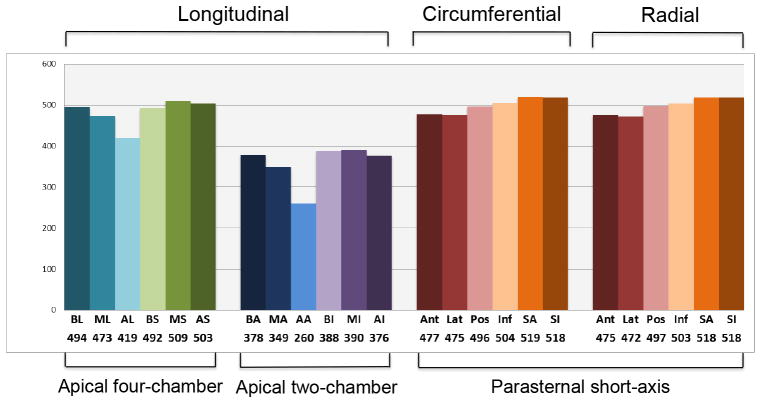
Baseline characteristics of the participants are listed in table 1. Healthy subgroup included 557 individuals with mean age of 49.6 ± 3.6 years, 61.6% women and 69.5% whites. In a multiple comparison, white men and black men presented higher height (178.4 ± 6.4 cm and 177.0 ± 7.2 cm respectively) than white women and black women (165.5 ± 6.3 cm and 165.4 ± 6.8 cm respectively), p< .001. Overweight (BMI ≥ 25 Kg/m2 and < 30 Kg/m2) was identified in 254 (45.6%) individuals. White women showed lower systolic blood pressure compared to black men (p<0.05). Prevalence of exclusion criteria is detailed in the appendix (Supplemental Table 1). Speckle tracking feasibility in the healthy subgroup was high; global parameters for apical four-chamber, apical two-chamber and parasternal short-axis views were defined respectively in 91.4% (n=509), 70.2% (n=391) and 93.5% (n=521) of the echocardiograms. Unsuitable tracking was more frequent in the anterior and anterolateral segments, especially in the mid and apical levels (figure 3). Mean frame rate of apical four-chamber, apical two-chamber and parasternal short-axis views were respectively 46.3 ± 1.6, 46.5 ± 2.3 and 46.8 ± 3.4 frames per second. On the healthy population, strain rate was highly feasible given frame rate < 50 frames per second in apical four-chamber (99.6%) view, apical two-chamber view (99.4%) and short axis view (100%).
Table 1.
Baseline characteristics
| Healthy Subgroup (n = 557)
|
|
|---|---|
| Demographics | |
| Age (years) | 49.6 ± 3.6 |
| Women (%) | 343 (61.6) |
| White (%) | 387 (69.5) |
| Clinical characteristics | |
| Body mass index (Kg/m2) | 24.7 ± 2.9 |
| Body surface area (m2) | 2.6 ± 0 |
| Heart Rate (bpm) | 64 ± 9 |
| Systolic blood pressure (mmHg) | 111 ± 11 |
| Diastolic blood pressure (mmHg) | 67 ± 9 |
| Glucose (mg/dL) | 90 ± 8 |
| Total Cholesterol (mg/dL) | 191 ± 32 |
| HDL cholesterol (mg/dL) | 67 ± 17 |
| Triglycerides (mg/dL) | 76 ± 27 |
| Echocardiographic parameters | |
| Left ventricular mass index (g/m2) | 78 ± 17 |
| Left ventricular ejection fraction (%) | 62 ± 6 |
| Peak systolic strain (%) | |
| Longitudinal (four-chamber) | −15.9 ± 2.2 |
| Longitudinal (two-chamber) | −16.6 ± 2.4 |
| Circumferential | −15.7 ± 2.6 |
| Radial | 36.6 ± 11.0 |
| Systolic strain rate (1/s) | |
| Longitudinal (four-chamber) | −0.68 ± 0.10 |
| Longitudinal (two-chamber) | −0.70 ± 0.11 |
| Circumferential | −0.70 ± 0.13 |
| Radial | 1.69 ± 0.54 |
| Early diastolic strain rate (1/s) | |
| Longitudinal (four-chamber) | 0.88 ± 0.23 |
| Longitudinal (two-chamber) | 0.99 ± 0.26 |
| Circumferential | 0.84 ± 0.30 |
| Radial | −2.30 ± 1.19 |
| Late diastolic strain rate (1/s) | |
| Longitudinal (four-chamber) | 0.54 ± 0.21 |
| Longitudinal (two-chamber) | 0.52 ± 0.20 |
| Circumferential | 0.44 ± 0.25 |
| Radial | −1.24 ± 0.98 |
Continuous variables and categorical variables are expressed as mean ± SD and n (%) respectively. HDL=high-density lipoprotein.
Figure 3. Segmental tracking feasibility.
Number of segments analyzed. AA=apical anterior; AI=apical inferior; AL=apical anterolateral; AS=apical inferoseptal; BA=basal anterior; BI=basal inferior; BL=basal anterolateral; BS=basal anteroseptal; MA=mid anterior; MI=mid inferior; ML=mid anterolateral; MS=mid inferoseptal; SA ant=anterior; ant-sep=anteroseptal; inf=inferior; lat=lateral; post=posterior; sep=septal
Reference ranges and regional patterns of LV deformation
Global strain and strain rate reference ranges for left ventricle are presented in table 2. Mean values for longitudinal strain in apical four- and two-chamber were −15.9% and −16.6%, whereas circumferential and radial peak systolic strains were −15.7% and 36.6% respectively.
Table 2.
Reference values for strain and strain rate in the healthy population
| n | Mean ± SD or Median (IQR) | Limits of Normal* | |
|---|---|---|---|
|
|
|||
| Peak systolic strain (%) | |||
| Longitudinal four-chamber | 509 | −15.9 ± 2.2 | [−20.3, −11.5] |
| Longitudinal two-chamber | 391 | −16.6 ± 2.3 | [−21.3, −12.0] |
| Longitudinal combined four- and two-chamber | 382 | −16.4 ± 2.0 | [−20.4, −12.4] |
| Circumferential | 521 | −15.7 ± 2.6 | [−21.0, −10.5] |
| Radial | 521 | 36.6 ± 11.0 | [14.9, 58.3] |
| Systolic strain rate (1/s) | |||
| Longitudinal four-chamber | 509 | −0.68 ± 0.10 | [−0.90, −0.47] |
| Longitudinal two-chamber | 391 | −0.70 ± 0.11 | [−0.93, −0.48] |
| Longitudinal combined four- and two-chamber | 382 | −0.69 ± 0.10 | [−0.89, −0.49] |
| Circumferential | 521 | −0.70 ± 0.13 | [−0.98, −0.43] |
| Radial | 521 | 1.69 ± 0.54 | [0.61, 2.77] |
| Early diastolic strain rate (1/s) | |||
| Longitudinal four-chamber | 509 | 0.88 ± 0.23 | [0.41, 1.36] |
| Longitudinal two-chamber | 391 | 0.99 ± 0.26 | [0.47, 1.50] |
| Longitudinal combined four- and two-chamber | 382 | 0.93 ± 0.21 | [0.51, 1.35] |
| Circumferential | 521 | 0.84 ± 0.30 | [0.22, 1.45] |
| Radial | 521 | −2.18 (−3.06;−1.42) | [−6.46, −0.60] |
| Late diastolic strain rate (1/s) | |||
| Longitudinal four-chamber | 509 | 0.49 (0.40;0.63) | [0.23, 1.10] |
| Longitudinal two-chamber | 391 | 0.49 (0.39;0.60) | [0.22, 1.07] |
| Longitudinal combined four- and two-chamber | 382 | 0.50 (0.42;0.64) | [0.28, 0.94] |
| Circumferential | 521 | 0.39 (0.28;0.53) | [0.14, 1.08] |
| Radial | 521 | −0.91 (−0.62;−1.44) | [−3.85, −0.24] |
Limits of normal are based on 95% prediction intervals.
Variables normally distributed are reported as mean ± standard deviation; variables not normally distributed are expressed as median (interquartile range).
IQR = interquartile range; n = number of exams analyzed; SD = standard deviation.
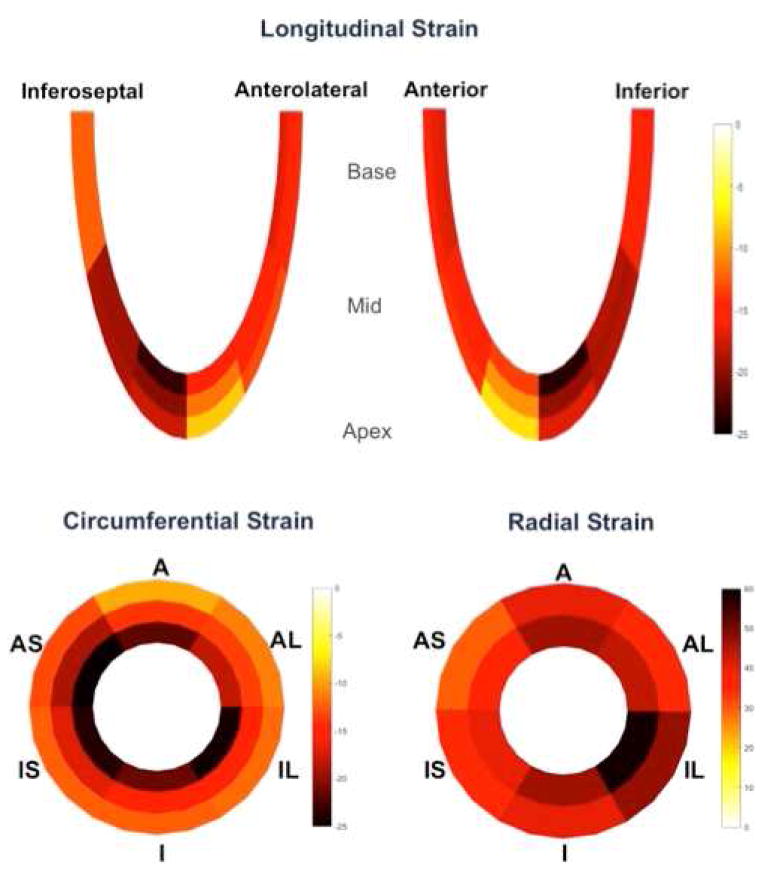
Circumferential peak systolic shortening significantly increased from the epicardium (−11.7 ± 2.2%) toward the endocardium (−23.2 ± 3.6%), and a similar pattern was found for radial thickening, increasing from the epicardium (31.1 ± 9.7%) toward the endocardium (43.8 ± 14.3%), p< .001 for all. For longitudinal shortening in apical four- and two-chamber views, the increasing gradient from epicardium to endocardium was significant in the apex (−13.8 ± 3.7% to −19.5 ± 4.8% in four-chamber; −13.7 ± 3.6% to −19.5 ± 5.2% in two-chamber), p< .001 for all, but not significant in mid and basal LV regions, as illustrated in figure 4.
Figure 4. Analysis of strain according to myocardial layers.
Outer, mid and inner segments correspond respectively to epicardial, mid-ventricular and endocardial layers. A=anterior; AL=anterolateral; IL=inferolateral; I=inferior; IS=inferoseptal; AS=anteroseptal
Longitudinal shortening increased significantly from the base toward the apex in inferior (−16.0 ± 4.4% to −20.0 ± 5.3%) and inferoseptal (−12.3 ± 2.9% to −20.3 ± 4.9%) regions, p< .001 for all, and in contrast, decreased from the base toward the apex in anterior (−16.4 ± 4.6% to −10.3 ± 4.1%) and anterolateral (−15.5 ± 5.1% to −11.7 ± 4.2%) walls, p< .001 for all.
Reference values for regional strain in the healthy are presented in the appendix (Supplemental Table 2).
Race and sex differences
Race and sex differences are reported in table 3. White women showed significantly better systolic deformation than both white and black men, as demonstrated by longitudinal and circumferential systolic strain and strain rate, p< .05 for all. The lowest values of systolic deformation were found in black men, especially for longitudinal systolic strain, with lower values than all the other race and sex categories, p< .05. Regarding diastolic deformation, race and sex differences were statistically significant for early diastolic strain rate parameters, but not for late diastolic strain rate. Women showed significant better early diastolic deformation than men, and black men exhibited the lowest values of early diastolic deformation, as evaluated by longitudinal early diastolic strain rate, p< .05. For systolic and diastolic deformation, black women and white men showed intermediate values, usually reflecting lower deformation than white women and higher deformation than black men.
Table 3.
Reference values for strain and strain rate in the healthy population according to race and sex groups
| Peak systolic strain (%) | 1. White Women n = 243 |
2. African American Women n = 98 |
3. White Men n = 142 |
4. African American Men n = 69 |
P-value < 0.05 |
|---|---|---|---|---|---|
|
| |||||
| Mean ± SD or Median (interquartile range) and [limits of normal] | |||||
|
|
|||||
| Longitudinal | |||||
| 4-chamber | −16.4±2.2 [−20.8,−12.0] | −15.9±1.9 [−19.9,−12.0] | −15.6±2.0 [−19.7,−11.5] | −14.7±2.2 [−19.1,−10.3] | 1–3, 1–4, 2–4, 3–4 |
| 2-chamber | −17.3±2.3 [−22.0,−12.6] | −16.7±2.1 [−21.0,−12.5] | −16.5±2.2 [−21.0,−11.9] | −14.9±1.8 [−18.7,−11.1] | 1–3, 1–4, 2–4, 3–4 |
| 4ch and 2ch combined | −17.0±2.0 [−21.0,−15.0] | −16.5±1.8 [−20.1,−12.9] | −16.1±1.8 [−19.7,−12.5] | −14.8±1.9 [18.6,11.0] | 1–3, 1–4, 2–4, 3–4 |
| Circumferential | −16.2±2.6 [−21.4,−10.9] | −15.9±2.7 [−21.4,−10.4] | −15.4±2.3 [−20.0,−10.7] | −14.7±2.6 [−20.0,−9.5] | 1–3, 1–4, 2–4 |
| Radial | 35.5±11.3 [12.8,58.2] | 38.4±10.5 [17.2,59.6] | 37.6±10.3 [16.8,58.3] | 36.0±10.4 [15.1,56.8] | - |
| Systolic SR (1/s) | |||||
| Longitudinal | |||||
| 4-chamber | −0.70±0.11 [−0.92,−0.48] | −0.70±0.10 [−0.90,−0.49] | −0.66±0.09 [−0.86,−0.47] | −0.64±0.10 [−0.85,−0.42] | 1–3, 1–4, 2–4 |
| 2-chamber | −0.72±0.11 [−0.95,−0.50] | −0.72±0.12 [−0.97,−0.47] | −0.70±0.10 [−0.90,−0.49] | −0.64±0.09 [−0.82,−0.45] | 1–4, 2–4, 3–4 |
| 4ch and 2ch combined | −0.72±0.10 [−0.52,−0.92] | −0.71±0.10 [−0.51,−0.91] | −0.69±0.09 [−0.51,−0.87] | −0.64±0.09 [−0.46,0.82] | 1–4, 2–4, 3–4 |
| Circumferential | −0.70±0.13 [−0.97,−0.44] | −0.72±0.15 [−1.03,−0.41] | −0.68±0.12 [−0.94,−0.43] | −0.69±0.14 [−0.98,−0.41] | - |
| Radial | 1.64±0.54 [0.55,2.73] | 1.76±0.52 [0.71,2.81] | 1.75±0.55 [0.64,2.85] | 1.68±0.51 [0.65,2.71] | - |
| SRe (1/s) | |||||
| Longitudinal | |||||
| 4-chamber | 0.93±0.26 [0.41,1.46] | 0.91±0.23 [0.45,1.37] | 0.83±0.18 [0.45,1.20] | 0.79±0.18 [0.42,1.16] | 1–3, 1–4, 2–3, 2–4 |
| 2-chamber | 1.04±0.25 [0.52,1.56] | 1.09±0.25 [0.57,1.60] | 0.92±0.23 [0.46,1.38] | 0.85±0.24 [0.37,1.34] | 1–3, 1–4, 2–3, 2–4 |
| 4ch and 2ch combined | 0.99±0.21 [0.57,1.41] | 1.01±0.22 [0.57,1.45] | 0.88±0.18 [0.52,1.24] | 0.82±0.18 [0.46,1.18] | 1–3, 1–4, 2–3, 2–4 |
| Circumferential | 0.88±0.30 [0.27,1.49] | 0.86±0.32 [0.20,1.52] | 0.90±0.29 [0.21,1.39] | 0.73±0.27 [0.19,1.27] | 1–4 |
| Radial | −2.19(−3.0,−0.75) [−6.33,−0.55] | −2.7(−3.51,−1.76) [−6.61,−0.87] | −2.0(−2.9,−0.85) [−6.26,−0.53] | −2.3(−3.3,−1.4) [−6.48,−0.65] | 1–2, 2–3 |
| SRa (1/s) | |||||
| Longitudinal | |||||
| 4-chamber | 0.50 (0.40,0.67) [0.23,1.15] | 0.49(0.41,0.63) [0.26,1.04] | 0.49(0.41,0.61) [0.24,1.01] | 0.48(0.37,0.60) [0.19,1.16] | - |
| 2-chamber | 0.50 (0.41,0.62) [0.23,1.07) | 0.44(0.36,0.54) [0.18,1.04] | 0.50(0.39,0.60) [0.22,1.10] | 0.49(0.37,0.68) [0.24,1.06] | - |
| 4ch and 2ch combined | 0.52 (0.43,0.67) [0.28,1.00] | 0.47(0.42,0.61) [0.28,0.86] | 0.50(0.40,0.62) [0.29,0.90] | 0.50(0.37,0.65) [0.25,0.95] | - |
| Circumferential | 0.39 (0.28,0.55) [0.14,1.13] | 0.40(0.30,0.51) [0.16,1.04] | 0.37(0.25,0.49) [0.12,1.05] | 0.41(0.29,0.54) [0.14,1.08] | - |
| Radial | −0.93 (−1.51,−0.59) [−4.09,−0.24] | −0.84(−1.21,−0.41) [−3.33,−0.23] | −1.01(−1.68,−0.64) [−4.29,−0.26] | −0.87(−1.27,−0.53) [−3.05,−0.27] | - |
Comparisons between subgroups were evaluated by one-way ANOVA and Tukey’s test post hoc analysis.
4ch = four-chamber; 2ch = two-chamber; n = exams analyzed; SD = standard deviation; SR = strain rate; SRa = late diastolic strain rate; SRe = early diastolic strain rate.
Correlates of strain and strain rate in the healthy subgroup
Sex demonstrated the highest correlation with longitudinal strain according to standardized β-coefficient (women was associated with better systolic deformation than men, p< .001), table 4. Race was also independently related to longitudinal strain (whites were associated with better deformation than blacks, p< .05). Advancing age was related to more negative longitudinal early diastolic strain rate, indicative of lower diastolic function. BMI within the non-obese range was associated with more negative longitudinal and circumferential early diastolic strain rate, so lower diastolic function. Higher HR was related to more positive longitudinal strain and more negative radial strain, indicating lower systolic function. Higher SBP, within the normal and pre-hypertensive range was associated with more positive longitudinal strain as well as more negative radial strain, reflecting lower longitudinal deformation and higher thickening.
Table 4.
Correlates of strain and early diastolic strain rate in the healthy population
| Peak Systolic Strain | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Longitudinal Four-chamber | Longitudinal Two-chamber | Circumferential | Radial | |||||||||
|
| ||||||||||||
| R2 | 0.13 | 0.19 | 0.05 | 0.04 | ||||||||
|
| ||||||||||||
| Constant term* | −22 | −22 | −16 | 18 | ||||||||
|
| ||||||||||||
| β | SE | S-β | β | SE | S-β | β | SE | S-β | β | SE | S-β | |
|
|
||||||||||||
| Frame rate (fps) | −0.002 | 0.060 | −0.001 | −0.034 | 0.049 | −0.032 | 0.001 | 0.034 | 0.001 | −0.281 | 0.140 | −0.088† |
| Sub-optimal image (vs. Optimal) | 0.624 | 0.222 | 0.121† | 0.669 | 0.285 | 0.113† | 0.597 | 0.286 | 0.091† | −1.042 | 1.192 | −0.038 |
| Age (years) | 0.028 | 0.027 | 0.046 | 0.025 | 0.033 | 0.039 | −0.015 | 0.033 | −0.021 | 0.056 | 0.138 | 0.019 |
| Female (vs. Male) | −0.948 | 0.207 | −0.209‡ | −1.200 | 0.245 | −0.250‡ | −0.812 | 0.252 | −0.150† | 0.274 | 1.049 | 0.012 |
| White (vs. Black) | −0.593 | 0.233 | −0.122† | −0.978 | 0.276 | −0.188‡ | −0.261 | 0.283 | −0.045 | −1.377 | 1.180 | −0.058 |
| Educational years | 0.002 | 0.041 | 0.002 | −0.012 | 0.049 | −0.013 | −0.003 | 0.050 | −0.002 | 0.363 | 0.209 | 0.082 |
| Body mass index (Kg/m2) | 0.021 | 0.036 | 0.028 | 0.054 | 0.045 | 0.063 | 0.007 | 0.043 | 0.008 | 0.056 | 0.180 | 0.015 |
| Heart rate (bpm) | 0.037 | 0.010 | 0.153‡ | 0.058 | 0.012 | 0.226‡ | 0.009 | 0.013 | 0.033 | −0.129 | 0.052 | −0.109† |
| Systolic blood pressure (mmHg) | 0.023 | 0.009 | 0.120† | 0.015 | 0.010 | 0.073 | 0.014 | 0.011 | 0.060 | 0.107 | 0.046 | 0.112† |
| Early-Diastolic Strain Rate | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Longitudinal Four-chamber | Longitudinal Two-chamber | Circumferential | Radial | |||||||||
|
| ||||||||||||
| R2 | 0.10 | 0.14 | 0.06 | 0.02 | ||||||||
|
| ||||||||||||
| Constant term* | 2.10 | 1.7 | 1.0 | −1.4 | ||||||||
|
| ||||||||||||
| β | SE | S-β | β | SE | S-β | β | SE | S-β | β | SE | S-β | |
|
|
||||||||||||
| Frame rate (fps) | −0.012 | 0.007 | −0.077 | 0.005 | 0.006 | 0.039 | −0.007 | 0.004 | −0.074 | 0.017 | 0.015 | 0.049 |
| Sub-optimal image (vs. Optimal) | −0.020 | 0.024 | −0.036 | 0.015 | 0.032 | 0.022 | −0.024 | 0.033 | −0.031 | −0.034 | 0.128 | −0.012 |
| Age (years) | −0.011 | 0.003 | −0.162‡ | −0.015 | 0.004 | −0.200‡ | 0.000 | 0.004 | −0.004 | 0.006 | 0.015 | 0.017 |
| Female (vs. Male) | 0.101 | 0.023 | 0.206‡ | 0.134 | 0.028 | 0.253‡ | 0.056 | 0.029 | 0.088 | −0.172 | 0.113 | −0.071 |
| White (vs. Black) | 0.043 | 0.025 | 0.082 | 0.012 | 0.031 | 0.021 | −0.010 | 0.033 | −0.014 | 0.450 | 0.127 | 0.175‡ |
| Educational years | −0.002 | 0.004 | −0.019 | 0.004 | 0.006 | 0.037 | 0.011 | 0.006 | 0.088 | −0.067 | 0.022 | −0.141† |
| Body mass index (Kg/m2) | −0.004 | 0.004 | −0.054 | −0.012 | 0.005 | −0.124† | −0.011 | 0.005 | −0.107† | −0.005 | 0.019 | −0.012 |
| Heart rate (bpm) | 0.001 | 0.001 | 0.028 | −0.002 | 0.001 | −0.057 | 0.003 | 0.001 | 0.081 | 0.000 | 0.006 | −0.003 |
| Systolic blood pressure (mmHg) | −0.002 | 0.001 | −0.077 | 0.000 | 0.001 | −0.008 | −0.002 | 0.001 | −0.065 | −0.004 | 0.005 | −0.038 |
Multivariate linear regressions to evaluate association of strain and strain rate with demographics, anthropometrics, hemodynamic variables and technical parameters.
R2 = coefficient of determination. S-β=standardized beta-coefficient. SE=standard error.
Constant term = Y intercept derived from multivariable linear regression.
p<.05
p<.001
DISCUSSION
This study provided reference ranges for global and segmental LV deformation parameters derived from 2D-STE, considering both systolic and diastolic parameters in all three linear components. This is the largest study of speckle tracking echocardiography reference ranges using Toshiba’s ultrasound system and software. We also identified race and sex specific reference ranges as well as regional patterns of LV deformation.
In this cohort, Kishi et al. have shown the association of race with LV strain and strain rate in the overall population.[5] In the larger cohort, some of the race and sex differences are attributed to the differential presence of cardiovascular risk between men and women, and between blacks and whites. Similarly, the trends observed in the healthy subgroup paralleled those in the larger study that included those with cardiovascular disease, with white women having the highest deformation, while black men, the lowest.
Overall, the association of biological factors with strain and early diastolic strain rate variances in the healthy subgroup was low, especially for circumferential and radial measurements. Sex showed the strongest relationship with longitudinal strain and early diastolic strain rate, in accordance with prior studies demonstrating higher myocardial deformation in women than in men.[13, 14] Beyond sex, higher heart rate was associated with poorer longitudinal strain, even after accounting for echocardiographic frame rate; the higher the heart rate, the lower the systolic function. Our findings are in agreement with prior study that demonstrated association between heart rate and systolic function assessed by strain, using tagged magnetic resonance imaging.[15] For longitudinal early diastolic strain rate, the second most important correlate was age. Higher BMI, within a non-obese range, was related to more negative values of longitudinal and circumferential early diastolic strain rate, reflecting worse diastolic function. Although present for longitudinal early diastolic strain rate measured at two-chamber view, this relationship was not significant for four-chamber measurements. Regional abnormalities can explain this difference, but it may also be a consequence of residual effect of the image quality, irrespective of adequate quality control measures.
Even after strict inclusion criteria for a healthy population, residual effect of environmental exposures might, at least in part, explain the race and sex differences on sensitive parameters of myocardial function. However, in the multivariable analysis, including those factors as covariates, race and sex demonstrated independent association with strain and strain rate, suggesting that other factors, such as genetic differences, could also be a determinant on those differences.
Variability in a population study is a result of the combined biological variability and measurement variability. Comparing the inter-reader coefficient of variation with the relative standard deviation (ratio of the standard deviation to the mean) of the healthy population, measurement variability for apical four- and two-chamber, circumferential and radial strains were respectively 71.4%, 73.3%, 76.5% and 50.0%.
In our study, mean values of longitudinal strain in apical four- and two-chamber views, circumferential strain and radial strain were −15.9 ± 2.2%, −16.7 ± 2.4%, −15.7 ± 2.6% and 36.7 ± 11.0% respectively. In a meta-analysis including 24 eligible studies, the reported mean values of longitudinal, circumferential and radial strains varied from −15.9 to −22.1%, from −20.9 to −27.8% and from 35.1% to 59.0% respectively.[8] Our study presents the first report of reference values in a bi-racial population of blacks and whites. As discussed previously, black race was independently associated with lower absolute values of longitudinal and circumferential strain, thus partly explaining the lower absolute values for strain and strain rate in our study in comparison with prior investigations. The lowest mean value for longitudinal strain in the meta-analysis was demonstrated for men in the HUNT study in Norway.[16] Interestingly, the HUNT study was the largest cohort (673 females and 623 males) in the meta-analysis, whereas only other three studies included more than one hundred participants to determine normal values. Thus, sample size might also contribute to observed differences in reference values between studies.
Reference values were described in our study by means and prediction intervals. Prediction intervals do not need assumptions about the population means and allow for random error associated with future observations, and are thus more robust than confidence intervals. [17] Therefore, prediction intervals might provide thresholds for strain and strain rate that more accurately define LV function, exhibiting intervals usually wider than confidence intervals commonly reported in studies for normal ranges.
Furthermore, analysis of LV deformation by 2D-STE can be vendor dependent. Post-processing variations among different software packages have been reduced after a task force to standardize echocardiographic deformation imaging, but they are still statistically significant.[18] In the meta-analysis, 23 datasets were evaluated using EchoPAC (GE Healthcare, Milwaukee, WI), while only two studies utilized Advanced Cardiology Package. In the EACVI/ASE inter-vendor comparison study, Farsalinos et al. showed a bias (limits of agreement) of 2.4 (−1.7 to 6.5)% for longitudinal strain measurements using software from GE (EchoPAC) and Toshiba (Advanced Cardiology Package).[19] Takigiku et al. also found lower values for LV deformation using Toshiba ultrasound system and post processing software in an Asian population, compared to measurements from GE.[20] This prior single center investigation of normal ranges of 2D-STE in Asian individuals used the same ultrasound system and software platform as we utilized in our study and demonstrated higher normal values in comparison with this present study. Of note, in a prior multi-center study of myocardial function assessed by tagged magnetic resonance imaging in an adult population free of cardiovascular disease from the Multi-Ethnic Study of Atherosclerosis (MESA) study, Chinese-Americans had higher circumferential strain, especially compared to African-Americans.[21]
Regional patterns
Although the overall 2D-STE feasibility has been high in this study, the predominance of the deformation curves considered as suboptimal in the anterior and anterolateral walls was noteworthy, mainly at apex in both apical four-chamber and two-chamber views. Usually, these LV walls are more likely to be affected by artifacts, such as shadowing and reverberation from the ribs. Moreover, the lateral motion of the anterior and anterolateral walls along the cardiac cycle, combined with the translational movement of the heart, frequently drive the mid and apical segments of these walls out of the imaging sector. Similar results have been shown by Marwick et al., using a different ultrasound system, and analysis software from a different vendor to define normal values for longitudinal LV systolic strain.[22] In that study, anterior and posterolateral walls exhibited the worst tracking quality score.
In apical views, a basal-to-apical gradient toward the apex can be clearly identified in anteroseptal and inferoseptal segments. In contrast, in anterior and anterolateral walls, the gradient is in the opposite direction. Dalen et al. in a study of segmental strain and strain rate in healthy population have previously demonstrated a trend for this pattern of gradients [16], while Levy et al. recently showed in a systematic review and meta-analysis of 2D-STE in children an overall stable apex-to-base gradient that is preserved throughout maturation.[23] A more complex motion of the anterior and anterolateral walls, compared with septal and inferior motion during the cardiac cycle, difficult to track in a bi-dimensional plane, might have been the main reason for those opposed patterns and also for the lowest feasibility of apical and mid segments of anterior and anterolateral walls, as discussed above. Using tagged magnetic resonance imaging, this limitation is not present, and the absence of them could explain a more homogeneous basal-to-apex gradient toward the apex for longitudinal strain, regardless of an opposite gradient direction for longitudinal displacement.[21] Vendor differences should be accounted for when applying reference ranges in future studies and in the clinical routine.
Myocardial layer-specific analysis
Evaluation of the three conventional LV wall components - endocardial, mid-ventricular and epicardial layers by 2D-STE has been validated.[24] This technique can be useful in specific scenarios, such as myocardial ischemia, that sometimes can predominantly affect the endocardium.[25] Therefore, understanding normal strain patterns according to LV wall layers is essential before applying this technique in clinical practice. Our results exhibited an epicardium-to-endocardium gradient that increased toward the endocardial layer, similar to what has been observed using tagged magnetic resonance imaging [21]. This gradient is expected due to geometric reasons. During the systole, the inner layer has less circumferential room to expand, and thus has to thicken more, according to the principle of mass conservation. This tendency increases as the outer layer also expands. Circumferential strain is a function of the inwards displacement of the mean circumference of the layer, so the inner circumference is displaced more.
For longitudinal strain, the epicardium-to-endocardium gradient is related to the different lengths of the layers as shown in figures 1 and 4. The inner layer is shorter, thus has a smaller denominator (myocardial length at end-diastole), and consequently the relative shortening (strain) is higher. However, differences in layer-specific longitudinal strain was more pronounced at the apex, than at the basal or mid-level, in agreement with previous study by Leitman et al. using echocardiography.[26] It is also subject to geometrical assumptions. As the mitral ring is part of the fibrous atrial-ventricular plane, the longitudinal contraction does not cause torsion of the ring. Therefore, the myocardium shortening is even across the layers in the basal levels. Moreover, this echocardiographic pattern may be explained by the lateral resolution along the length of the ultrasound beam. Structures closer to the transducer, i.e., in the near field, such as the LV apex, present better spatial resolution than those less proximal, such as the mid and basal LV regions, as the scan lines diverge in the far field of the ultrasound beam, decreasing the line density in this region. [27] Better spatial resolution at the far field can be achieved by decreasing the sector width and image depth as much as possible, as defined in our methods.
Importantly, the myocardial layer-specific patterns found in this study might not be directly applied to measures made using different software methods. Also, other vendor’s analysis systems may not be able to make equivalent three-layer strain measures.
LIMITATIONS
This study involved a retrospective review of prospectively collected images in CARDIA study participants who remained healthy at the year-25 evaluation, so sample size of each race and sex categories are unequal. In the healthy subgroup, the numbers of blacks were lower in comparison to whites, mainly in the black men subgroup. This was a consequence of higher prevalence of certain cardiovascular risk factors/diseases in black participants, especially hypertension, obesity and cardiovascular disease (Supplemental Table 1). Participants were aged from 43 to 55 years. As the whole of the study sample is within the middle age population interval, age related normal values were not provided. Also, this relative narrow range of age might explain why a significant decrease in systolic strain with age was not observed, as previously described.[16] However, middle age is a period that usually precedes an increase in heart failure incidence [28], with potential subclinical LV impairment, which might be earlier revealed by the 2D-STE. The CARDIA year-25 echocardiography protocol included a wide spectrum of echocardiographic evaluation, including M-mode, two-dimensional images and Doppler techniques (spectral, tissue and color Doppler), designed to be consistent with the year-5 examination performed 20 years before. In addition to the conventional echocardiography, the year-25 protocol introduced the speckle tracking echocardiography in this large population-based cohort of black and white individuals. The protocol design included apical four- and two-chamber views acquisitions for offline speckle tracking analysis, a similar approach of using two orthogonal planes for assessment of left ventricular ejection fraction by Simpson’s method. Although apical three-chamber view has been evaluated for conventional echocardiography, images dedicated for further speckle tracking analysis are not available in our database. By the time the year-25 protocol was designed (2009), standardization for echocardiographic assessment of myocardial deformation was not well established. The first ASE/EAE expert consensus statement on techniques for the quantitative evaluation of cardiac mechanics was published in 2011. We acknowledge that not including three-chamber apical view in the estimation of global longitudinal strain can hamper comparisons with other investigations. Circumferential strain was measured at LV mid level. This might be taken as representative for the two other levels (basal and apical), but only in ventricles without regional dysfunction. Repeated echocardiographic acquisitions were not performed, and could potentially show more variability than repeated measurements in the same acquisition.
CONCLUSIONS
In a healthy population, the present study provides reference ranges for LV strain and strain rate using 2D-STE, accounting for sex and race differences. White women show the highest reference ranges for systolic and diastolic function, and the lowest values are found in black men. Circumferential and radial strains increase from epicardium to endocardium, and for longitudinal strain, this pattern is more pronounced at the apex. Longitudinal strain increases from base to apex in inferior and inferoseptal regions and, in contrast, decreases from base to apex in anterior and anterolateral walls. Sex showed the strongest correlation with LV deformation variance in a healthy population, especially with regard to longitudinal strain.
Supplementary Material
Acknowledgments
The Coronary Artery Risk Development in Young Adults Study (CARDIA) is supported by contracts HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HHSN268201300029C, and HHSN268200900041C from the National Heart, Lung, and Blood Institute (NHLBI), the Intramural Research Program of the National Institute on Aging (NIA), and an intra-agency agreement between NIA and NHLBI (AG0005).
ABBREVIATIONS AND ACRONYMS
- 2D-STE
two-dimensional speckle tracking echocardiography
- BMI
body mass index
- CARDIA
Coronary Artery Risk Development in Young Adults
- CoV
coefficient of variation
- DICOM
digital imaging and communications in medicine
- HDL
high-density lipoprotein
- ICC
intraclass correlation coefficient
- IMT
carotid intima-media thickness
- LV
left ventricular
- ROI
region of interest
Footnotes
Conflicts of interest: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Feigenbaum H, Mastouri R, Sawada S. A practical approach to using strain echocardiography to evaluate the left ventricle. Circ J. 2012;76:1550–5. doi: 10.1253/circj.cj-12-0665. [DOI] [PubMed] [Google Scholar]
- 2.Mordi I, Bezerra H, Carrick D, Tzemos N. The Combined Incremental Prognostic Value of LVEF, Late Gadolinium Enhancement, and Global Circumferential Strain Assessed by CMR. JACC Cardiovasc Imaging. 2015;8:540–9. doi: 10.1016/j.jcmg.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Pavlopoulos H, Nihoyannopoulos P. Strain and strain rate deformation parameters: from tissue Doppler to 2D speckle tracking. Int J Cardiovasc Imaging. 2008;24:479–91. doi: 10.1007/s10554-007-9286-9. [DOI] [PubMed] [Google Scholar]
- 4.Blessberger H, Binder T. Two dimensional speckle tracking echocardiography: clinical applications. Heart. 2010;96:2032–40. doi: 10.1136/hrt.2010.199885. [DOI] [PubMed] [Google Scholar]
- 5.Kishi S, Reis JP, Venkatesh BA, Gidding SS, Armstrong AC, Jacobs DR, Jr, et al. Race-ethnic and sex differences in left ventricular structure and function: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. J Am Heart Assoc. 2015;4:e001264. doi: 10.1161/JAHA.114.001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kishi S, Teixido-Tura G, Ning H, Venkatesh BA, Wu C, Almeida A, et al. Cumulative Blood Pressure in Early Adulthood and Cardiac Dysfunction in Middle Age: The CARDIA Study. J Am Coll Cardiol. 2015;65:2679–87. doi: 10.1016/j.jacc.2015.04.042. [DOI] [PubMed] [Google Scholar]
- 7.Kishi S, Armstrong AC, Gidding SS, Colangelo LA, Venkatesh BA, Jacobs DR, Jr, et al. Association of obesity in early adulthood and middle age with incipient left ventricular dysfunction and structural remodeling: the CARDIA study (Coronary Artery Risk Development in Young Adults) JACC Heart Fail. 2014;2:500–8. doi: 10.1016/j.jchf.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yingchoncharoen T, Agarwal S, Popovic ZB, Marwick TH. Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr. 2013;26:185–91. doi: 10.1016/j.echo.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–16. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 10.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the american society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. 2015;28:1–39. e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong AC, Ricketts EP, Cox C, Adler P, Arynchyn A, Liu K, et al. Quality Control and Reproducibility in M-Mode, Two-Dimensional, and Speckle Tracking Echocardiography Acquisition and Analysis: The CARDIA Study, Year 25 Examination Experience. Echocardiography. 2015;32:1233–40. doi: 10.1111/echo.12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50–103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Cheng S, Larson MG, McCabe EL, Osypiuk E, Lehman BT, Stanchev P, et al. Age- and sex-based reference limits and clinical correlates of myocardial strain and synchrony: the Framingham Heart Study. Circ Cardiovasc Imaging. 2013;6:692–9. doi: 10.1161/CIRCIMAGING.112.000627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muraru D, Cucchini U, Mihaila S, Miglioranza MH, Aruta P, Cavalli G, et al. Left ventricular myocardial strain by three-dimensional speckle-tracking echocardiography in healthy subjects: reference values and analysis of their physiologic and technical determinants. J Am Soc Echocardiogr. 2014;27:858–71. e1. doi: 10.1016/j.echo.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Opdahl A, Ambale Venkatesh B, Fernandes VR, Wu CO, Nasir K, Choi EY, et al. Resting heart rate as predictor for left ventricular dysfunction and heart failure: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2014;63:1182–9. doi: 10.1016/j.jacc.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalen H, Thorstensen A, Aase SA, Ingul CB, Torp H, Vatten LJ, et al. Segmental and global longitudinal strain and strain rate based on echocardiography of 1266 healthy individuals: the HUNT study in Norway. Eur J Echocardiogr. 2010;11:176–83. doi: 10.1093/ejechocard/jep194. [DOI] [PubMed] [Google Scholar]
- 17.Kutner MH. Applied linear statistical models. 5. Boston: McGraw-Hill Irwin; 2005. [Google Scholar]
- 18.Farsalinos KE, Daraban AM, Unlu S, Thomas JD, Badano LP, Voigt JU. Head-to-Head Comparison of Global Longitudinal Strain Measurements among Nine Different Vendors: The EACVI/ASE Inter-Vendor Comparison Study. J Am Soc Echocardiogr. 2015;28:1171–81. e2. doi: 10.1016/j.echo.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Farsalinos KE, Daraban AM, Unlu S, Thomas JD, Badano LP, Voigt JU. Head-to-Head Comparison of Global Longitudinal Strain Measurements among Nine Different Vendors: The EACVI/ASE Inter-Vendor Comparison Study. J Am Soc Echocardiogr. 2015 doi: 10.1016/j.echo.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Takigiku K, Takeuchi M, Izumi C, Yuda S, Sakata K, Ohte N, et al. Normal range of left ventricular 2-dimensional strain: Japanese Ultrasound Speckle Tracking of the Left Ventricle (JUSTICE) study. Circ J. 2012;76:2623–32. doi: 10.1253/circj.cj-12-0264. [DOI] [PubMed] [Google Scholar]
- 21.Venkatesh BA, Donekal S, Yoneyama K, Wu C, Fernandes VR, Rosen BD, et al. Regional myocardial functional patterns: Quantitative tagged magnetic resonance imaging in an adult population free of cardiovascular risk factors: The multi-ethnic study of atherosclerosis (MESA) J Magn Reson Imaging. 2015;42:153–9. doi: 10.1002/jmri.24749. [DOI] [PubMed] [Google Scholar]
- 22.Marwick TH, Leano RL, Brown J, Sun JP, Hoffmann R, Lysyansky P, et al. Myocardial strain measurement with 2-dimensional speckle-tracking echocardiography: definition of normal range. JACC Cardiovasc Imaging. 2009;2:80–4. doi: 10.1016/j.jcmg.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Levy PT, Machefsky A, Sanchez AA, Patel MD, Rogal S, Fowler S, et al. Reference Ranges of Left Ventricular Strain Measures by Two-Dimensional Speckle-Tracking Echocardiography in Children: A Systematic Review and Meta-Analysis. J Am Soc Echocardiogr. 2016;29:209–25. e6. doi: 10.1016/j.echo.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishizu T, Seo Y, Enomoto Y, Sugimori H, Yamamoto M, Machino T, et al. Experimental validation of left ventricular transmural strain gradient with echocardiographic two-dimensional speckle tracking imaging. Eur J Echocardiogr. 2010;11:377–85. doi: 10.1093/ejechocard/jep221. [DOI] [PubMed] [Google Scholar]
- 25.Altiok E, Neizel M, Tiemann S, Krass V, Kuhr K, Becker M, et al. Quantitative analysis of endocardial and epicardial left ventricular myocardial deformation-comparison of strain-encoded cardiac magnetic resonance imaging with two-dimensional speckle-tracking echocardiography. J Am Soc Echocardiogr. 2012;25:1179–88. doi: 10.1016/j.echo.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 26.Leitman M, Lysiansky M, Lysyansky P, Friedman Z, Tyomkin V, Fuchs T, et al. Circumferential and longitudinal strain in 3 myocardial layers in normal subjects and in patients with regional left ventricular dysfunction. J Am Soc Echocardiogr. 2010;23:64–70. doi: 10.1016/j.echo.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Lawrence JP. Physics and instrumentation of ultrasound. Crit Care Med. 2007;35:S314–22. doi: 10.1097/01.CCM.0000270241.33075.60. [DOI] [PubMed] [Google Scholar]
- 28.Cowie MR, Wood DA, Coats AJ, Thompson SG, Poole-Wilson PA, Suresh V, et al. Incidence and aetiology of heart failure; a population-based study. Eur Heart J. 1999;20:421–8. doi: 10.1053/euhj.1998.1280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.