Abstract
Background
We aimed to determine the maturational changes in systolic ventricular strain mechanics by two-dimensional speckle tracking echocardiography in extreme preterm neonates from birth to one year of age, and discern the impact of common cardiopulmonary abnormalities on the deformation measures.
Methods
In a prospective multi-center study of 239 extreme preterm infants (< 29 weeks gestation at birth), left ventricle (LV) global longitudinal strain and systolic strain rate (GLS, GLSRs), interventricular septal wall (IVS) GLS and GLSRs, right ventricle free wall longitudinal S and SR (RV FWLS, FWLSRs), and segmental LS (SLS) in the RVFW, LVFW and IVS were serially measured at Days 1, 2, 5–7, 32 weeks and 36 weeks post-menstrual age (PMA), and one year corrected age (CA). Premature infants who developed bronchopulmonary dysplasia (BPD) or had echocardiographic findings of pulmonary hypertension (PH) were analyzed separately.
Results
In uncomplicated preterm infants (n=103, 48%), LV GLS and GLSRs remained unchanged from Day 5–7 to one year CA (p=0.60 and 0.59). RV FWLS, FWLSRs and IVS GLS and GLSR significantly increased over the same time period (p < 0.01 for all measures). A significant base-to-apex (highest to lowest) SLS gradient (p < 0.01) in the RVFW and a reverse apex-to-base gradient (p < 0.01) existed in the LVFW. In infants with BPD and/or PH (n=119, 51%), RV FWLS and IVS GLS were significantly lower (p < 0.01), LV GLS and GLSRs were similar (p=0.56), and IVS SLS persisted as an RV dominant base-to-apex gradient from 32 weeks PMA to one year CA.
Conclusions
This study tracks the maturational patterns of global and regional deformation by 2DSTE in extreme preterm infants from birth to one year CA. The maturational patterns are ventricular specific. BPD and PH leave a negative impact on RV and IVS strain, while LV strain remains stable.
Keywords: Cardiac function, Prematurity, Strain Imaging, Echocardiography
INTRODUCTION
Ventricular performance is an important prognostic determinant of clinical status and long-term outcome in preterm neonates.1–3 Ventricular mechanics begin to undergo maturational changes in the early and late postnatal periods that can have a long-term impact on cardiac function beyond the first year of age.2,3 The exposure of an immature preterm heart to a sustained increase in hemodynamic load of postnatal circulation, at a time in the development when the heart primarily supports a low resistance circulation, induces myoarchitectural adaptation that may lead to ventricular remodeling.4,5 The proper evaluation of ventricular function in preterm infants by echocardiography has been limited by the lack of reliable quantitative parameters.1 Furthermore, there is paucity of longitudinal studies on prematurity-related alterations in the maturation of cardiac function beyond the early neonatal period. The establishment of sensitive indices of cardiac function in birth cohorts affected by prematurity and its common cardiorespiratory complications is a necessary prerequisite for the clinical adoption of a normative references patterns for use in evaluating pathologic changes and progression.
Myocardial strain is a measure of tissue deformation and strain rate is the rate at which deformation occurs. Longitudinal deformation by two-dimensional speckle tracking echocardiography (2DSTE) has been validated as a reproducible measure of ventricular function in premature infants.6–8 Initial data indicate that measuring deformation values in this population could have clinical implication, as they appears to have superior prognostic value for assessing and potentially predicting major adverse cardiopulmonary events when compared with conventional measurements (i.e. shortening and ejection fraction).9,10 Maturational patterns of 2DSTE derived longitudinal strain measures during the transitional period through the first month of age have recently been established in preterm infants.7,8,11–14 However, the evolution of ventricular strain mechanics from birth to one year of age for clinical application has not been comprehensively described in a large longitudinal preterm cohort.13,15 Disturbances in myocardial function may also impact neonatal morbidity and mortality, but there is limited information on how different prematurity associated cardiopulmonary conditions, such as bronchopulmonary dysplasia (BPD), pulmonary hypertension (PH), and a persistent patent ductus arteriosus (PDA), influence the normal changes in longitudinal cardiac function.16
Since the right ventricle (RV) and left ventricle (LV) are embryologically and structurally distinct and their functional roles change in the postnatal period,17 we hypothesized that (1) prematurity related maturational changes in RV and LV deformation measures would have uniquely different trajectories; and (2) prematurity associated cardiopulmonary conditions would influence changes differently in LV and RV mechanics. Accordingly, we aimed to determine the maturational (age- and weight-related) changes in LV, RV, and interventricular septum (IVS) strain mechanics by 2DSTE in healthy uncomplicated preterm infants not affected by significant cardiopulmonary abnormalities, and study the influence of the cardiopulmonary abnormalities on the maturational changes in myocardial deformational indices from birth through one year of corrected age (CA).
METHODS
Study Population
All data were prospectively obtained as part of an observational research study that included patients who were enrolled between August 2011 and January 2016 at hospitals affiliated with two academic institutions (Washington University School of Medicine, Saint Louis Children’s Hospital, and Royal College of Surgeons in Ireland, Rotunda Hospital). Two hundred and thirty nine preterm infants (born 23 0/7 to 28 6/7 weeks gestation) were recruited at birth and longitudinally followed until one year corrected age (CA). The preterm infants enrolled from the Washington University site were among infants participating in the Prematurity and Respiratory Outcomes Program (PROP, Clinical Trials number: NCT01435187).18 Infants with any suspected congenital anomalies of the airways, congenital heart disease (except atrial septal defects), chromosomal anomalies, intrauterine growth restriction (IUGR) or small for gestational age (SGA, birth weight < 10th centile for gestation) were excluded from the healthy uncomplicated cohort arm of the study.
At both centers, reference values and maturational patterns of RV fractional area of change from these cohorts have been recently published, but deformation imaging by 2DSTE has not been reported.19,20 At the Washington University School of Medicine site, a small proportion of the deformation data was previously used to test feasibility and reporducbility.6,21 At the Royal College of Surgeons in Ireland site, deformation imaging by tissue Doppler has been assessed in the transitional period and up to 36 weeks post-menstrual age (PMA).11,22 The institutional review board of Washington University and the ethical committee on human research at Royal College of Surgeons approved the protocol. Written informed consent was obtained from the parents or guardians of all participants.
Inclusion Criteria in Uncomplicated Cohort
Only infants with ‘cardio-respiratory healthiness’ were classified as healthy uncomplicated infants in this study.19,23 In the early neonatal period, a large proportion of premature infants present with acute respiratory failure that often require some sort of respiratory support up to 36 weeks PMA, making it difficult to determine a true definition of ‘respiratory healthiness’.19,23 Respiratory disease syndrome and the need for invasive and non-invasive ventilation are common in extreme preterm birth in the early postnatal period. BPD, defined as the need for persistent supplemental oxygen support at 36 weeks’ PMA, is recognized as the most significant respiratory consequence of premature birth in the late postnatal period.24 If preterm infants still required any respiratory support at or beyond 36 weeks PMA, they were excluded from the uncomplicated cohort.24 We assessed for the contributions of BPD, as defined by a modified definition of the 2001 National Institutes of Health (NIH) BPD workshop24, in a sub-analysis.
Infants with any of the following echocardiographic signs of late onset PH, identified at any time point from 32 weeks PMA through one year CA were excluded: an estimated right ventricular systolic pressure (RVSP) greater than 40 mm Hg, a ratio of RVSP to systemic systolic blood pressure greater than 0.5, any cardiac shunt with bidirectional or right-to-left flow, unusual degree of right ventricular hypertrophy or dilatation, or ventricular septal wall flattening.19,25 They were assessed in a separate analysis. Since the incidence of PH ranges from 12–25% of infants with BPD26, we performed stepwise regression to analyze the influence of PH and BPD on ventricular strain patterns.
The significance of a PDA, and its impact on long term cardiorespiratory health, remain areas of ongoing debate in neonatology.27 Even the persistent patency of the PDA remains an ongoing clinical conundrum, as most premature neonates who fail to close their PDA in the first week of age or even by the time of discharge will undergo spontaneous closure a few weeks later.28 Prolonged patency is associated with numerous adverse outcomes, but the extent to which these adverse outcomes are attributable to the hemodynamic consequences of ductal patency, if at all, has not been established.29 However, infants with moderate to large PDA, based on its size relationship to the left pulmonary artery (LPA), have a 15 times greater likelihood of requiring treatment for clinically and hemodynamically significant PDA than those with small PDA.29,30 We, therefore, excluded any infant with a moderate to large hemodynamically significant PDA (hsPDA) in the first week of age, and any size PDA from 32 weeks and beyond, but a separate sub-analysis was performed for this cohort. For this study a hsPDA was defined as a PDA diameter of > 1.5 mm with the presence of flow reversal in the descending aorta and a left atrial to aortic root ratio (LA:Ao) of > 1.5.27 In addition, we used the relationship of the PDA to LPA to define size and clinical significance (large = PDA/LPA ratio > 1, moderate = PDA/LPA ratio < 1 but > 0.5, and small = PDA/LPA ratio < 0.5).30 Combined, these approaches allowed us to properly assess PDA characteristics, signs of pulmonary over circulation, and left heart-loading condition. Finally, infants that underwent pharmacological or surgical intervention at any time point in their neonatal course to close a PDA were also excluded from this cohort.
Echocardiographic Examination
Echocardiograms were performed at six time points from birth to one year CA, (Figure 1): at Washington University School of Medicine site, the echocardiograms were performed serially at Day 1 (n=30), Day 2, (n=30), 32 weeks PMA (n=117), 36 weeks PMA (n=117), and one year CA (n=80). At the Royal College of Surgeons in Ireland site, echocardiograms were performed at Day 1 (n=102), Day 2, (n=102), Day 5–7 (n=98), and 36 weeks PMA (n=47), (Figure 1). We chose three time points in the first week of age to capture the physiological changes that occur during the transitional period in preterm infants. The timings of the echocardiograms at 32 weeks PMA, 36 weeks PMA and one year CA were carefully selected to avoid the early postnatal period of clinical and cardiopulmonary instability and early mortality associated with extreme preterm birth.6 Choosing to study all infants at a common PMA and CA optimizes the determination of the impact of gestational and chronological age on cardiac function at a specific developmental stage, and allows for the analysis of measures by post-gestational weeks from birth.6 The infants’ antenatal, delivery and demographic characteristic were obtained.
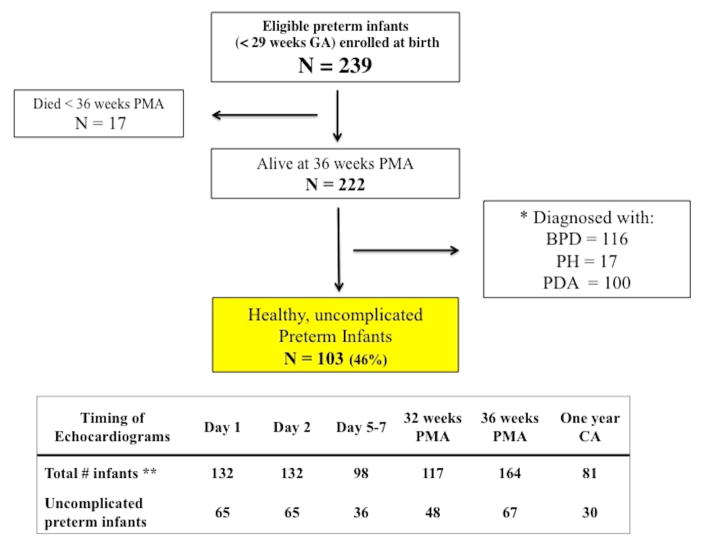
Figure 1. Enrollment and follow-up of study participants.
BPD, bronchopulmonary dysplasia; PH, pulmonary hypertension; PDA, patent ductus arteriosus.
*119/222 infants were excluded from the healthy uncomplicated cohort because they either were: a) diagnosed with BPD, defined as the requirement any respiratory support at 36 weeks PMA, and based on a modified NIH workshop definition24; b) evidence of PH on echocardiogram at 32 and/or 36 weeks PMA, defined as any infant with any conventional echocardiographic signs identified, by an estimated RV systolic pressure > than 40 mm Hg, a ratio of RVSP to systemic blood pressure > than 0.5, any cardiac shunt with right-to-left flow, unusual degree of RV hypertrophy or dilatation, or any degree of ventricular septal wall flattening;25 or c) evidence of a hemodynamically significant PDA by Day 5–7, (defined by PDA characteristics, signs of pulmonary over circulation, and left heart-loading condition),30 or any PDA at 32 and 36 week PMA. There was significant overlap between these four categories.
** There were 239 infants recruited for this study (137 infants from the Washington University School of Medicine site in Saint Louis, USA and 102 infants from the Royal College of Surgeons in Ireland site in Dublin, Ireland). Echocardiograms were performed at Day 1 (n=30), Day 2 (n=30), 32 weeks PMA (n=117), 36 weeks PMA (n=117), and one year CA (n=81) in Saint Louis, USA. Echocardiograms were performed at Day 1 (n=102), Day 2 (n=102), Day 5 (n=98), and 36 weeks PMA (n=47) in Dublin, Ireland.
Echocardiograms were performed using the same commercially available ultrasound imaging system (Vivid 7 and 9; General Electric Medical Systems, Milwaukee, Wisconsin) at each center. One designated trained pediatric cardiac sonographer at each center obtained all the echocardiographic images using a phased array transducer (7.5–12 MHz).6 The echocardiographic images were acquired using a standardized image acquisition protocol in decubitus position during restful period without changing the position of the infant or disturbing the hemodynamic condition to minimize heart rate and respiratory variation during image acquisition.31 The image data were digitally stored in raw DICOM cine-loop format for offline analysis. Heart rate and blood pressure readings were recorded at the time of each echocardiogram.
Strain Analysis
Two-Dimensional Speckle Tracking Imaging
Myocardial mechanics were analyzed by the quantification of LV, RV, and IVS longitudinal strain (LS, %) and systolic strain rate (SRs, %/sec) using previously published image acquisition and data analysis protocols from our laboratories. 6,21 A frame rate to heart rate ratio (FR/HR) between 0.7 and 0.9 frames/sec per bpm was utilized to optimize myocardial speckle tracking and mechanical event timing.21 LV global LS (LV GLS) and SRs (LV GLSRs) were calculated by averaging all values of the regional peak LS and SRs obtained from 17 segments in two-chamber, apical long-axis, and four- chamber apical views.32 RV free wall LS (RV FWLS) and SRs (RV FWLSRs) were calculated as the average of the three segmental longitudinal strain (SLS) measures in the RV FW from the RV focused apical four-chamber view.6 LV SLS at the apex, mid- and basal ventricular levels was calculated by averaging each segment from the two-chamber, apical long-axis, and four- chamber apical views of the LVFW. RV SLS was obtained from the segments at the apical, mid-ventricular, and basal levels of the RVFW from the RV focused apical 4-chamber view. Although IVS strain is incorporated in LV GLS, we decided to also measure IVS global and SLS with a new region of interest that only covered the width of the IVS from the trigonal crux at the basal septal side of the triannular plane to the apical point at the apical junction of the IVS and free walls.6 This separate region of interest would allow us to determine if the IVS strain has its own unique trajectory, distinct from the LV. In this model, IVS GLS is averaged from all the values obtained from 9-segments in the two-chamber, apical long-axis, and four- chamber apical views along the IVS wall only. Peak strain for each index was measured as end-systolic strain at the closure of the aortic valve.33 Two observers, who were blinded to the maternal and infant clinical and cardio-respiratory conditions, analyzed the strain imaging using vendor customized commercially available software (EchoPAC; General Electric Medical Systems, Waukesha, WI, USA, version 112).
Reproducibility of Strain imaging in Preterm Infants
We along with other groups have previously demonstrated that 2DSTE-derived LS imaging of the RV, LV, and IVS is highly feasible and reproducible in premature infants using specific cardiac image acquisition and postprocessing data analysis protocols.6–8,14,34,35 These protocols have improved the image acquisition and reduced variance that has resulted in improved reliability of strain as measure of ventricular function in preterm infants. Reproducibility of SLS has not been comprehensively analyzed in preterm infants.7,14 We assessed SLS reproducibility using intra- and inter-observer variability analysis (Bland Altman plot analysis, intraclass correlation coefficient, and coefficient of variation) in 25% of the images at each time point. Two observers performed offline analysis using the same measurement protocol and were blinded to the patients’ clinical status, and one another.
Statistical Analysis
All data are expressed as mean ± SD or as percentages. Continuous variables of strain imaging were tested for normality using the Kolmogorov-Smirnov test and a histogram illustration of the data. Analysis of variance and student t tests were used to compare the changes in deformation values from birth to one year CA in the preterm infants and to compare the patterns between uncomplicated preterm infants and those with BPD, PH, and/or a persistent PDA, respectively. All outcome variables with non-normal distributions were analyzed in simple comparisons using Wilcoxon rank sum tests or Kruskal-Wallis one-way analysis of variance for tests with more than two independent groups. Chi-square tests (or Fisher Exact test as appropriate) were used to assess the association between categorical variable. Two-way ANOVA with repeated measures was used to compare change over time between infants with and without BPD, PDA, and PH. Percentile charts (mean ± SD) were created using linear regression to assess the independent effect of postnatal age (in weeks) and weight (at time of echocardiogram) on each strain measurement, while adjusting for gestational age at birth and gender. Finally, generalized logistic regression models were developed to identify risk factors for BPD and/or PH at 36 weeks PMA using a stepwise variable selection.25 The statistical analysis was performed using SPSS version 14.0 (SPSS, Inc., Chicago, IL).
RESULTS
Study Population Characteristics
Two–hundred and thirty-nine infants with a median gestation of 27.0 weeks (IQR 26.0 – 28.0) and birthweight of 960 g (IQR 800–1,138) were recruited in this study (137 patients from the Washington University site and 102 patients from the Royal College of Surgeon site). Of the 239 patients, 17 infants (7% with equal distribution amongst centers) died prior to hospital discharge and were excluded from the analysis, leaving 222 infants with data to be analyzed. Ninety-five (43%) were female and 182 (82%) were delivered by caesarean section. Two hundred and thirteen (96%) received at least one course of antenatal steroids and all infants received postnatal surfactant replacement therapy. There were relatively lower rates of chorioamnionitis (n = 20, 9%), pre-eclampsia (n = 56, 25%) and antepartum hemorrhage (n = 38, 17%). Complete studies were available for >95% of infants on Days 1, 2, 5–7, and at 32 weeks PMA. Fifty-eight infants were transferred to peripheral hospitals prior to 36 weeks PMA leaving 164 infants (74%) available for assessment at that time point (Figure 1). Although echocardiographic data was unavailable at 36 weeks for the transferred infants, their respiratory outcome data at 36 weeks PMA was obtained. At one year CA, 81 (69% of the eligible infants from the Washington University cohort) returned for an echocardiogram. The clinical and demographic characteristics of the preterm patients are summarized and compared by time point in Table 1.
Table 1.
Demographic and clinical characteristics of preterm infants based on timing of echocardiogram evaluation
| Timing of Echocardiograms | Day 1 | Day 2 | Day 5–7 | 32 weeks PMA | 36 weeks PMA | One year CA |
|---|---|---|---|---|---|---|
| Total # infants at each time point * | 132 | 132 | 98 | 117 | 154 | 81 |
| Healthy uncomplicated infants** | 65 | 65 | 36 | 48 | 67 | 30 |
| Respiratory | ||||||
| RR (breaths/min) ** | 50 ± 10 | 50 ± 10 | 50 ± 10 | 50 ± 10 | 51 ± 10 | 52 ± 9 |
| Invasive Mechanical ventilation | 66 (49%) | 43 (32%) | 25 (25%) | 6 (5%) | 4 (3%) | 0 |
| Bronchopulmonary dysplasia | 69 (59%) | 69 (59%) | 69 (59%) | 69 (59%) | 69 (59%) | 49 (60%) |
| Cardiovascular | ||||||
| PDA | 126 (95%) | 114 (86%) | 69 (70%) | 19 (16%) | 24 (16%) | 0 |
| hsPDA | NA | NA | 35 (34%) | NA | NA | NA |
| Pulmonary hypertension | NA | NA | 17 (14.5%) | 17 (14.5%) | 17 (14.5%) | 1 (1%) |
| HR (beats/minute) ** | 155 ± 15 | 161 ± 12 | 165 ± 12 | 159 ± 18 | 163 ± 14 | 133 ± 19 |
| SBP (mm Hg) ** | 49 ± 10 | 53 ± 13 | 56 ± 10 | 68 ± 9 | 71 ± 8 | 83 ± 10 |
| DBP (mm Hg) ** | 30 ± 8 | 30 ± 9 | 31 ± 9 | 42 ± 9 | 42 ± 9 | 62 ± 9 |
| MAP (mm Hg) ** | 36 ± 6 | 38 ± 8 | 39 ± 9 | 48 ± 12 | 50 ± 12 | 59 ± 14 |
Data are presented as mean ± SD, or number (Percentage); NA, not applicable
PMA, post-menstrual age; CA, corrected age
RR, respiratory rate; HR, heart rate; SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure; MAP, mean arterial blood pressure
There were 239 infants recruited for this study (137 infants from the Washington University School of Medicine site in Saint Louis, USA and 102 infants from the Royal College of Surgeons in Ireland site in Dublin, Ireland). Echocardiograms were performed at Day 1 (n=30), Day 2 (n=30), 32 weeks PMA (n=117), 36 weeks PMA (n=117), and one year CA (n=81) in Saint Louis. Echocardiograms were performed at Day 1 (n=102), Day 2 (n=102), Day 5 (n=98), and 36 weeks PMA (n=47).
BPD was diagnosed in 52% of all infants (n=116/222). There were echocardiographic signs of PH in (15%) at 32 and 36 week PMA. On Day 5 – 7, 57 infants (58%) had a PDA of which 33 (34% of total cohort) were classified as hsPDA. None of the infants underwent PDA treatment during the first week of age. However, 66 infants (30%) eventually received pharmacological therapy and 26 (12%) underwent surgical intervention to close the PDA. Of the remaining infants who did not receive any intervention to augment its closure, a PDA was evident in 19 infants (16%) at 32 weeks PMA, 18 infants (16%) at 36 weeks PMA. None of the infants received inotropes or administration of inhaled nitric oxide. Therefore, after meeting all inclusion criteria, 103 patients (47%) infants were classified as “healthy uncomplicated preterm infants” (Figure 1). Table 2 compares the maternal and infant characteristics between those infants classified as healthy uncomplicated and infants classified with cardio-respiratory disease.
Table 2.
Maternal and infant characteristic comparisons between cohorts
| ALL | Uncomplicated Cohort * | Infants with either BPD, PH, and/or PDA | P -Value | |
|---|---|---|---|---|
| N=222 | N=103 | N = 119 | ||
| Birthweight (g) | 895 [767,1010] | 960 [805,1130] | 880 [750,980] | 0.007 |
| Birthweight strata (g) | ||||
| 500 – 749 (n=43) | 650 [595,698] | 690 [650,700] | 640 [578.685] | 0.13 |
| 750 – 999 (n=90) | 880 [820,950] | 853 [809,940] | 890 [830,950] | 0.47 |
| 1000 – 1250 (n=89) | 1125 [903,1255] | 1140 [1052,1265] | 1110 [1045,1217] | 0.71 |
| Gestational age | 27 [26,28] | 27 [26,28] | 26 [25,27] | < 0.01 |
| Gender (female) | 108 (49%) | 60 (58%) | 72 (60%) | 0.22 |
| Race | 0.64 | |||
| White | 156 (70%) | 91 (82%) | 68 (57%) | |
| Black | 63 (54%) | 21 (20%) | 39 (33%) | |
| Asian | 2 (2%) | 1 (1%) | 1 (1%) | |
| Other | 1 (1%) | 0 | 1 (1%) | |
| Ethnicity | 0.36 | |||
| Hispanic or Latino | 3 (3%) | 2 (2%) | 1 (1%) | |
| Not Hispanic or Latino | 219 (97%) | 101 (98%) | 118 (99%) | |
| Maternal Smoking | 28 | 11 (11%) | 17 (14%) | 0.67 |
| Antenatal corticosteroids | 197 (89%) | 99 (96%) | 98 (82%) | 0.76 |
| Surfactant Replacement Therapy | 222 (100%) | 103 (100%) | 119(100%) | 0.84 |
| Multiples | 14 (6%) | 3 (3%) | 11 (9%) | 0.23 |
| Cesarean section | 160 (72%) | 75 (73%) | 85 (71%) | 0.56 |
| Maternal complications | ||||
| Gestational DM | 10 (5%) | 7 (7%) | 3 (3%) | 0.57 |
| Gestational HTN | 35 (16%) | 17 (17%) | 18 (15%) | 0.28 |
| Prolonged rupture of membranes | 38 (17%) | 15 (15%) | 23 (19%) | 0.58 |
| Chorioamnionitis | 20 (9%) | 10 (10%) | 10 (8%) | 0.51 |
| Preeclampsia | 56 (25%) | 26 (26%) | 30 (25%) | 0.62 |
| Placental Abruption | 38 (17%) | 17 (17%) | 21 (18%) | 0.89 |
| Necrotizing enterocolitis | 21 (9%) | 11 (11%) | 10 (8%) | 0.53 |
| ROP threshold (Stage 2 or higher) | 43 (19%) | 8 (9%) | 35 (29%) | 0.004 |
| IVH (Grade 3 or 4) | 28 (13%) | 9 (9%) | 19 (16%) | 0.09 |
| Total oxygen days (NICU) | 84 [40,107] | 34 [19,50] | 92 [82,115] | < 0.001 |
| Length of stay (NICU) | 91 [76,114] | 79 [64,89] | 98 [87,119] | < 0.001 |
Data are presented as median [interquartile range], or number (Percentage)
BPD, bronchopulmonary dysplasia; PH, pulmonary hypertension; PDA, patent ductus arteriosus
ROP, retinopathy of prematurity; IVH, intraventricular hemorrhage
Healthy uncomplicated cohort, defined as preterm infants without bronchopulmonary dysplasia, echocardiographic signs of pulmonary hypertension at 32 or 36 weeks PMA, and/or a hemodynamically significant patent ductus arteriosus at Day 5–7 or any size PDA at 32 or 36 weeks PMA.
Maturational Patterns of Myocardial Strain in Uncomplicated Preterm Infants
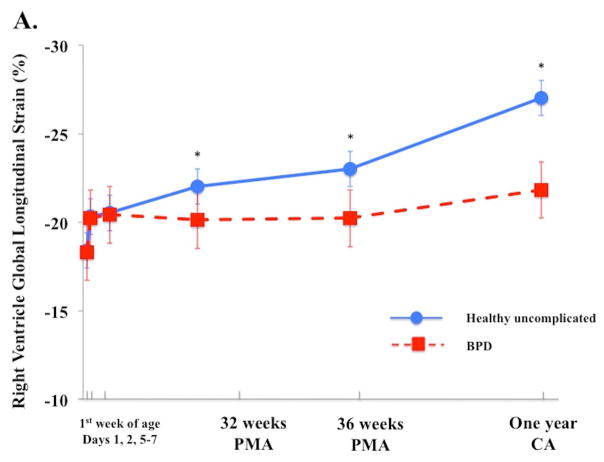
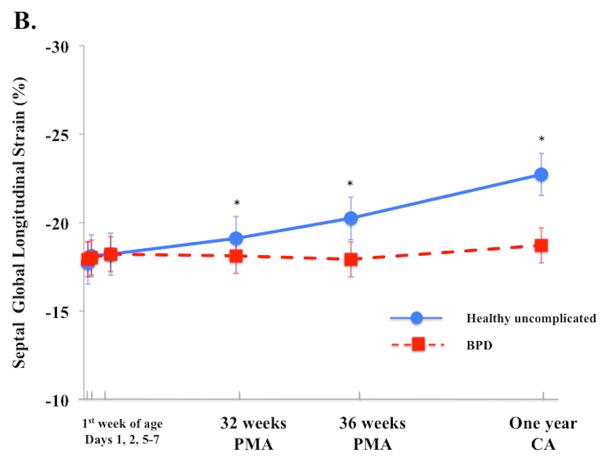
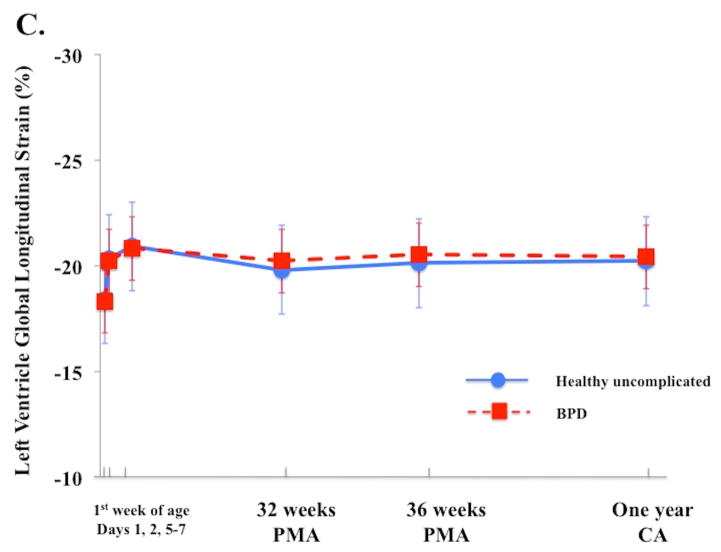
In uncomplicated preterm infants (n=103), a time-specific maturational pattern revealed that RV FWLS and FWLSRs had stable pattern from Day 1 and 2 and their magnitudes increased by Day 5–7, p < 0.05. (Table 3). RV FWLS and FWLSRs continued increasing in magnitude from Day 5–7 to one year CA (−20.5% ± 3.2 to −27.2% ± 2.3, p < 0.01 and −2.7% ± 1.2 to −3.3 ± 0.3 p < 0.01, Figure 2A). IVS GLS and GLSRs followed the same pattern as RV strain mechanics (Figure 2B, Table 3). The magnitudes of LV GLS and LV LSRs increased from Day 1 to Day 5–7 (−18.4% ± 3.5 to −21.8% ± 3.2 and −1.8 1/s ± 0.3 to−2.4 1/s ± 0.4, p < 0.05 for both) and then remained stable through one year CA (−21.8% ± 3.3 to 21.1% ± 0.4, p=0.56 and −1.9 ± 0.2 to −1.9 ± 0.7, Figure 2C).
Table 3.
Deformation measurements over the first week of age.
| Day 1 | Day 2 | Day 5 – 7 | p | |
|---|---|---|---|---|
| Entire cohort | ||||
| Number of infants | 132 | 132 | 98 | |
| LV GLS (%) | −18.4 ± 3.8 | −20.5 ± 3.1* | −21.8 ± 3.3* † | <0.001 |
| LV GLSRs (1/s) | −1.8 ± 0.4 | −2.1 ± 0.4* | −2.4 ± 0.4* † | <0.001 |
| IVS GLS (%) | −17.7 ± 2.1 | −17.9 ± 2.1 | −18.4 ± 2.1*† | <0.001 |
| IVS GLSR (1/s) | −1.7 ± 0.2 | −1.8 ± 0.2 | −1.9 ± 0.2† | 0.16 |
| RV FWLS (%) | −18.8 ± 4.7 | −20.1 ± 5.1 | −21.1 ± 4.7 | 0.05 |
| RV FWLSRs (1/s) | −2.0 ± 0.6 | −2.3 ± 0.7* | −2.8 ± 0.6* † | <0.001 |
| Healthy uncomplicated cohort | ||||
| Number of infants | 65 | 65 | 36 | |
| LV GLS (%) | −18.4 ± 3.5 | −20.3 ± 3.2* | −20.7 ± 3.0 † | <0.001 |
| LV GLSRs (1/s) | −1.8 ± 0.3 | −2.1 ± 0.3* | −2.3 ± 0.4 † | <0.001 |
| IVS GLS (%) | −17.7 ± 2.1 | −18.0 ± 2.1 | −18.4 ± 2.1*† | <0.001 |
| IVS GLSR (1/s) | −1.7 ± 0.2 | −1.8 ± 0.2 | −1.9± 0.2† | 0.12 |
| RV FWLS (%) | −18.1 ± 4.0 | −20.3 ± 3.2 | −20.5 ± 3.2* | 0.02 |
| RV FWLSRs (1/s) | −1.9 ± 0.5 | −2.2 ± 0.6 | −2.7 ± 0.7† | 0.004 |
Values are presented as mean (standard deviation). One way ANOVA with repeated measures was used to assess the change in values over time.
= p < 0.05 compared with previous day measurement;
= p < 0.05 compared with Day 1 measurement (with Bonferroni adjustment).
RV, right ventricle; LV, left ventricle; IVS, interventricular septum
FWLS, free wall longitudinal strain; FWLSRs, free wall longitudinal systolic strain rate
GLS, global longitudinal strain; GLSRs, global systolic longitudinal strain rate
Figure 2. Maturational patterns of Ventricular Deformation over the first year of age.
(A) RV FW longitudinal strain (RV FWLS%): In all infants RV FWLS % increased from Day 5–7 to one year CA (p < 0.01). RV FWLS % was higher and tracked superior in the uncomplicated preterm infants (blue circles with blue solid line) when compared to the preterm infants who developed BPD (red squares, dotted red lines) on echocardiogram at the time of evaluation. (B) Interventricular septal (IVS) global longitudinal strain (GLS, %): In all infants IVS GLS increased from 32 weeks PMA to one year CA (p < 0.01). IVS GLS was higher and tracked superior in the uncomplicated preterm infants when compared to the preterm infants who developed BPD on echocardiogram at the time of evaluation. (C) LV global longitudinal strain (LV GLS): LV GLS was preserved and relatively unchanged from 32 weeks PMA to one year CA (p=0.6), irrespective on neonatal cardiopulmonary disease.
Maturational changes in LV, RV, and IVS strain were further analyzed to produce percentile charts (mean ± 2 SD) related to gestational age at birth and postnatal age- and weight-related changes. RV FWLS and IVS GLS were linearly associated with gestational age (r=0.76 and 0.77, p=0.001), while LV GLS and FWLS were not (r=0.34 and 0.44, p > 0.1). Step-wise regression analysis of the effects of gender, birth weight, change in postnatal weight, total oxygen days, length of stay, and common neonatal morbidities (necrotizing enterocolitis, intraventricular hemorrhage, and retinopathy of prematurity, Table 2) on maturation of LS revealed that at all time points increasing RV FWLS and IVS GLS were associated with both increasing weight (r = 0.76, 0.78. p < 0.01) and postnatal age (r = 0.78, 0.81, p < 0.01), Table 4. LV, RV, and IVS SRs imaging followed similar patterns as strain values, when adjusted for weight and postnatal age (Tables 4 and 5).
Table 4.
Ventricular deformation in healthy uncomplicated preterm infants by weight
| Weight (Kilograms) | Infants * (n) | RV FWLS (%) | RV FWLSRs (1/sec) | LV GLS (%) | LV GLSRs (1/sec) | IVS GLS (%) | IVS GLSRs (1/sec) |
|---|---|---|---|---|---|---|---|
| 0.5 – 0.9 | 66 | −18.8 ± 4.7 | −2.0 ± 0.6 | −19.1 ± 2.0 | −1.8 ± 0.1 | −17.7 ± 2.1 | −1.7 ± 0.2 |
| 1.0 – 1.4 | 82 | −20.7 ± 0.3 | −2.5 ± 0.4 | −19.4 ± 2.1 | −1.9 ± 0.2 | −18.0 ± 2.5 | −1.9 ± 0.4 |
| 1.5 – 1.9 | 62 | −21.5 ± 0.8 | −2.8 ± 0.3 | −20.2 ± 0.5 | −1.9 ± 0.3 | −18.4 ± 0.3 | −2.3 ± 0.3 |
| 2.0 – 2.4 | 50 | −23.3 ± 3.3 | −2.8 ± 0.4 | −20.6 ± 0.6 | −1.8 ± 0.4 | −19.4 ± 1.2 | −2.2 ± 0.4 |
| 2.5 – 2.9 | 21 | −24.0 ± 3.6 | −3.1 ± 0.5 | −20.4 ± 0.6 | −1.9 ± 0.5 | −19.3 ± 1.6 | −2.4 ± 0.5 |
| 8.0 –9.0 | 16 | −27.3 ± 2.3 | −3.2 ± 0.3 | −21.1 ± 0.4 | −1.7 ± 0.6 | −21.7 ± 0.5 | −2.6 ± 0.3 |
| 10.0 –12.0 | 14 | −27.7 ± 2.3 | −3.5 ± 0.7 | −20.1 ± 0.4 | −1.9 ± 0.7 | −22.9 ± 1.3 | −2.6 ± 0.7 |
Data are presented as mean ± SD.
Healthy uncomplicated cohort, defined as preterm infants without bronchopulmonary dysplasia, echocardiographic signs of pulmonary hypertension at 32 or 36 weeks PMA, and/or a hemodynamically significant patent ductus arteriosus at Day 5–7 or any size PDA at 32 or 36 weeks PMA. At Day 1 there were n=65, Day 2, n=65, Day 5–7, n=36, 32 weeks PMA, n=48, 36 weeks PMA, n=67, and One year CA, n=30.
RV, right ventricle; LV, left ventricle; IVS, interventricular septum
FWLS, free wall longitudinal strain; FWLSRs, free wall longitudinal systolic strain rate
GLS, global longitudinal strain; GLSRs, global systolic longitudinal strain rate
Table 5.
Ventricular deformation in uncomplicated preterm infants by age beyond the first week .
| Age (weeks) | Infants* (n) | RV FWLS (%) | RV FWLSRs (1/sec) | LV GLS (%) | LV GLSRs (1/sec) | IVS GLS (%) | IVS GLSRs (1/sec) |
|---|---|---|---|---|---|---|---|
| 4 – 6 | 40 | −21.0 ± 2.5 | −2.3 ± 0.4 | −19.7 ± 2.3 | −1.9 ± 0.3 | −17.9 ± 2.3 | −1.8 ± 0.4 |
| 6 – 8 | 57 | −23.2 ± 2.8 | −2.6 ± 0.5 | −20.4 ± 2.4 | −1.9 ± 0.2 | −18.9 ± 2.0 | −2.1 ± 0.3 |
| 9 – 11 | 18 | −22.7 ± 2.2 | −2.7 ± 0.5 | −20.0 ± 2.3 | −1.8 ± 0.2 | −19.1 ± 1.5 | −2.4 ± 0.3 |
| 60 – 79 | 26 | −27.5 ± 2.4 | −2.9 ± 0.5 | −21.4 ± 2.0 | −1.9 ± 0.2 | −21.4 ± 3.0 | −2.5 ± 0.4 |
| 80 – 99 | 4 | −28.7 ± 2.5 | −3.5 ± 0.4 | −21.1 ± 1.6 | −1.7 ± 0.3 | −22.1 ± 2.3 | −2.6 ± 0.6 |
Data are presented as mean ± SD.
Uncomplicated, defined as infants without bronchopulmonary dysplasia, echocardiographic signs of pulmonary hypertension at 32 or 36 weeks. There were 48 uncomplicated preterm infants at 32 weeks PMA, 67 uncomplicated preterm infants at 36 weeks
PMA, and 30 uncomplicated preterm infants at one year CA. PMA, and/or a hemodynamically significant patent ductus arteriosus at Day 5–7 or any size PDA at 32 or 36 weeks PMA.
RV, right ventricle; LV, left ventricle; IVS, interventricular septum
FWLS, free wall longitudinal strain; FWLSRs, free wall longitudinal systolic strain rate
GLS, global longitudinal strain; GLSRs, global systolic longitudinal strain rate
Maturational Patterns of Regional Myocardial Strain in Uncomplicated Preterm Infants
RV FW had a persistent base-to-apex SLS (highest to lowest) gradient from birth to one year CA (p < 0.001 at all time points). Similar to the RV FWLS, the magnitude of each SLS value at the basal-, mid-, and apical- ventricular regions increased throughout maturation (p < 0.001 for each level). IVS SLS had an initial base-to-apex gradient on Day 1 and Day 2 (p = 0.002 and p = 0.003, respectively) that switched to an apex-to-base gradient (highest to lowest) at Day 5–7 (p < 0.001) that persisted to one year CA (p < 0.001). LV FW had a stable apex-to-base longitudinal strain gradient over the same time period (p < 0.001 for all time points), (Table 6).
Table 6.
Regional ventricular deformation patterns in preterm infants at one year corrected age
| Base | Mid-ventricle | Apex | P -value | |
|---|---|---|---|---|
| Healthy uncomplicated Preterm Infants* | ||||
| RV SLS (%) | −23.1 ± 1.6 | −24.2 ± 2.1 | −26.7 ± 1.9 | < 0.01 |
| LV SLS (%) | −21.2 ± 2.1 | −19.4 ± 2.1 | −18.1 ± 1.3 | < 0.01 |
| IVS SLS (%) | −19.1 ± 1.5 | −18.9 ± 2.0 | −17.9 ± 2.3 | < 0.01 |
| Preterm Infants with BPD and/or PH | ||||
| RV SLS (%) | −20.1 ± 1.4 | −23.2 ± 1.3 | −24.7 ± 2.1 | 0.01 |
| LV SLS (%) | −20.9 ± 2.2 | −19.2 ± 1.1 | −18.5 ± 2.1 | < 0.01 |
| IVS SLS (%)** | −16.1 ± 1.8 | −17.9 ± 2.1 | −18.1 ± 1.9 | < 0.01 |
Data are presented as mean ± SD.
RV, right ventricle; LV, left ventricle; IVS, interventricular septum
SLS, segmental longitudinal strain
BPD, bronchopulmonary dysplasia; PDA, patent ductus arteriosus,
Healthy uncomplicated, defined as infants without bronchopulmonary dysplasia, echocardiographic signs of pulmonary hypertension at 32 or 36 weeks PMA, and/or a hemodynamically significant patent ductus arteriosus at Day 5–7 or any size PDA at 32 or 36 weeks PMA.
IVS SLS with preterm infants with BPD and/or PH remains in an apex-to-base pattern (highest to lowers), reflective of RV regional gradient.
Confounding Cardiopulmonary Factors
BPD. Infants with BPD (n = 116, 52%) had a significantly lower gestational age than those without BPD [median (range); 25.7 (25.1 – 28.2) vs. 27.5 (24.6 – 27.6) weeks, p < 0.001] There were no significant differences in measures of LV, RV, or IVS strain and SRs between groups during the first week of age (all P < 0.19). However, the magnitude of RV FWLS and IVS GLS were significantly lower in preterm infants with BPD compared to uncomplicated preterm infants at 32 weeks (p=0.002 and p < 0.001, respectively), and these patterns persisted to one year CA (p < 0.001 for both, Figure 2A and 2B). The association between BPD and low RV FWLS (p = 0.024) and low IVS GLS (p = 0.015) remained significant when adjusting for gestational age, gender, and postnatal steroid use. Despite the differences between groups, the magnitudes of RV FWLS and IVS GLS increased, but at a slower rate from 32 weeks to one year CA in preterm infants with BPD (slope of change comparison between infants without BPD and with BPD for RV FWLS and IVS, 0.26% vs. 0.07, p< 0.001 and 0.32% vs. 0.11, p< 0.001, respectively), (Figure 2A). RV FW maintained a base-to-apex SLS gradient from 32 weeks to one year CA (p < 0.001 at each time point), but the magnitude of each segment was decreased in preterm infants with BPD compared to uncomplicated preterm infants (p=0.01). On the other hand, LV GLS, GLSr, FWLS, and FWLSRs all remained stable (p=0.56, p=0.62, p=0.59, p=0.45 respectively) with a persistent FW apex-to-base SL gradient in preterm infants with and without BPD at each time point (p < 0.01 for all), (Figure 2C). The individual IVS strain segments were decreased in premature infants with BPD when compared to uncomplicated preterm infants at all time points (p < 0.05 for each segment at each time period), (Table 6). There was also a persistent base-to-apex IVS SLS gradient (reflective of the RV pattern) by Day 5–7 (p < 0.01) that did not reverse to the expected apex-to-base IVS SLS gradient (reflective of the LV pattern) in infants with BPD, even by one year CA (p = 0.002), (Table 6). Gestational age at birth and a persistent IVS SLS base-apex-pattern at Day 5–7 were significantly associated with the development of BPD in this analysis, (RR, 1.15; 95% CI, 1.03–1.33, p=0.03 and RR, 1.23; 95% CI, 1.09–1.40, p < 0.001, respectively).
Infants who received antenatal steroids had a higher LV GLS [−18.6 ± 3.5) vs. −16.4 ± 3.9) %, p=0.04] at Day 1. This relationship remained significant when adjusting for gestation on linear regression (β = 2.1, p=0.04), but disappeared after Day 1. Postnatal steroids were administered in 35 infants (16%) at least one time during their hospital course. The majority (n=31/35, 88%) of the infants received postnatal steroids to treat lung disease beyond the first month of age, and these infants were also diagnosed with BPD. The four infants who did not develop BPD received steroids in the first month of age to facilitate a trial of extubation. There was no statistical difference in the maturational patterns of strain between those infants that did and did not receive postnatal steroids (p=0.45), although the study was not properly powered to answer this question.
PH
Based on echocardiographic evidence, we found an overall incidence of PH of 15% (n=17) at 32 weeks PMA, 9% (n=17) at 36 weeks PMA, and 1% (n=1) at one year CA. Overall, 71% (n=12) of the preterm infants with PH at 36 weeks PMA returned for follow up at one year CA, of whom 25% (n=3) did not carry a diagnosis of BPD. None of the infants with PH at 36 weeks PMA required oxygen or respiratory support at one year CA. LV GLS, GLSRs and FW SLS patterns were unchanged between the infants with and without the evidence of PH at 32 and 36 weeks PMA (p > 0.35 for all). All the infants with PH at 32 and 36 weeks PMA continued to have persistently decreased RV FWLS and IVS GLS (p=0.01 and p=0.002), with an altered IVS base-to-apex SLS gradient at one year CA (reflective of the RV pattern) when compared to those infants without PH, even after adjusting for the presence of BPD (β=2.9, p<0.001), (Table 6). The Day 5–7 risk factor of a persistent base-to-apex gradient (RR, 2.15; 95% CI, 1.18–4.33, p=0.02) was associated with late PH at 36 weeks PMA. There were three infants at 32 weeks PMA and 5 infants at 36 weeks PMA that were not diagnosed with BPD, but had echocardiographic signs of PH. These infants also had lower values of RV FWLS and IVS GLS (p=0.02 and p=0.002), an IVS base-to-apex (reflective of the RV pattern) SLS gradient (p < 0.01), and preserved LV GLS (p=0.62) with an apex-to-base SLS gradient that persisted to one year CA (p < 0.001), when compared to uncomplicated infants..
PDA
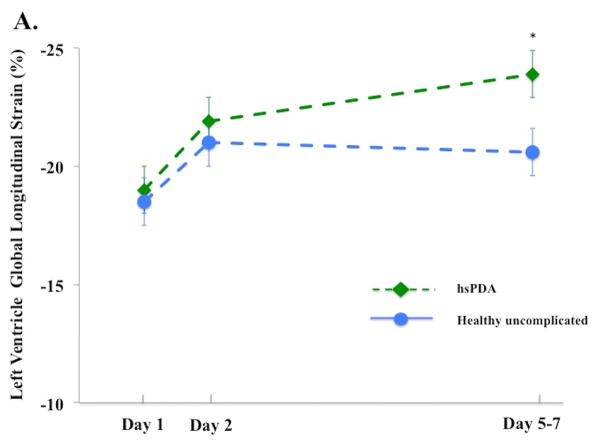
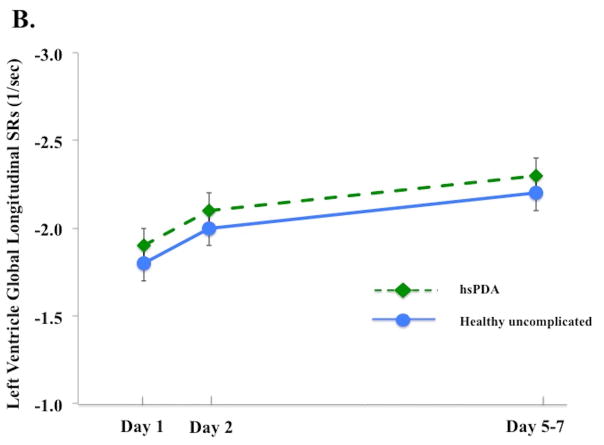
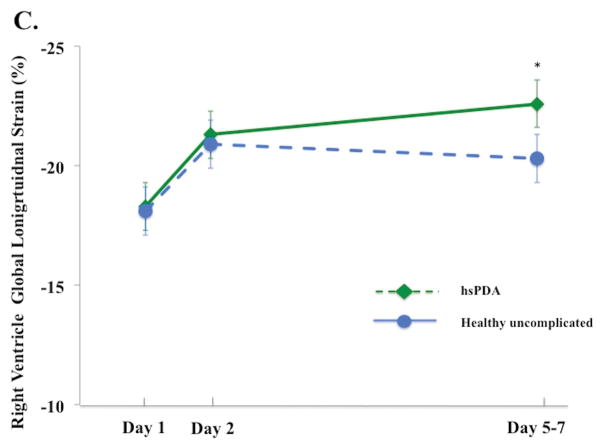
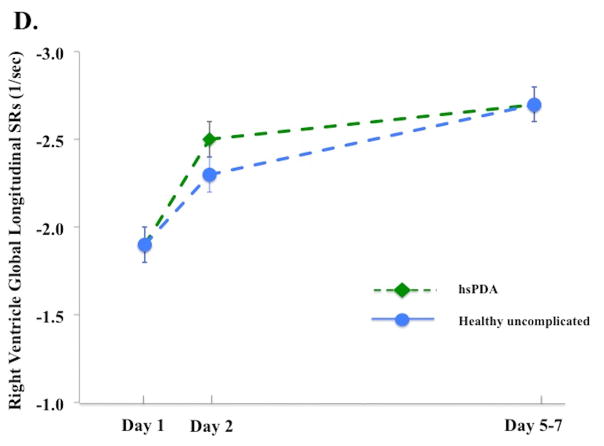
The influence of a hsPDA present at Day 5 – 7 on the deformation measurements was assessed. Thirty-three infants (out of 98, 34%) were classified as having a hsPDA: their PDA diameter (3.1 [2.7 – 3.6] vs 0 [0 – 2.2] mm, p<0.001), and LA:Ao ratio [1.8 ± 0.3 vs. 1.4 ± 0.3, p<0.001] were all higher than infants without a hsPDA (and all hsPDA infants had flow reversal in the abdominal aorta). On Day 5 – 7, infants with a hsPDA had higher LV GLS [−23.9% ± 2.4) vs. −20.6% ± 3.3), p<0.001] and higher RV FWLS [−22.6 ± 5.2) vs. −20.3 ± 4.3), p=0.04], Figure 3A and 3C). There was no difference in LV or RV SRs between the two groups (p > 0.52 for all measures) at any time points in the first week of age (Figure 3B and 3D). A hsPDA maintained its independent effect on LV GLS when adjusting for LV SRs (hsPDA β=2.7, p<0.001 and LV SRs β=5.0, p<0.001). However, the association between a hsPDA and RV FWLS was lost when adjusting for RV SRs (hsPDA β=2.1, p=0.1 and RV SRs β=2.1, p=0.04). There was no difference in the maturational patterns of RV, LV, or IVS GLS or SLS between infants who still had PDA at 32 weeks PMA (n=25, 21%), 36 weeks PMA (n=14, 12%), or one year CA (n=2, 2%), (adjusted for age, gender, and PDA size) and those who did not have PDA at these time points (p = 0.26, p= 0.32, p=0.29, respectively).
Figure 3. Left and right ventricle strain and systolic strain rate in infants with and without a hemodynamically significant patent ductus arteriosus on Day 5 – 7.
(A) LV GLS. On Day 5 – 7, infants with a hsPDA30 (Green diamond, dotted green lines) had higher LV GLS than the healthy uncomplicated cohort (blue circle, solid blue line); (B) LV GLSRs. There was no difference in the magnitude of LV GLSRs between cohorts at any time point in the first week of age; (C) RV GLS. On Day 5 – 7, infants with a hsPDA (Green diamond, dotted green lines) had higher RV GLS than the healthy uncomplicated cohort (blue circle, solid blue line); (D) RV GLSr. There was no difference in the magnitude of RV GLSRs between cohorts at any time point in the first week of age.
*= p < 0.05. LV: Left ventricle; RV: Right ventricle; SRs: systolic strain rate; FWLS, free wall longitudinal strain; FWLSRs, free wall longitudinal systolic strain rate; GLS, global longitudinal strain; GLSRs, global systolic longitudinal strain rate; hsPDA: hemodynamically significant patent ductus arteriosus.
Feasibility and Reproducibility
The reproducibility analysis for RV, LV, and IVS SLS measures is summarized in Appendix 1. The measurements were feasible in 89% of the images using the methods previously described by our group for image acquisition.6 For all measurements (intra- and inter-observer variability), the bias ranged between 8% and 14%, coefficients of variations ranged between 9% and 16%, and intraclass correlation coefficient ranged between 0.81and 0.93.
DISCUSSION
In a prospective multi-center longitudinal study of a large cohort of premature infants (≤ 29 weeks at birth), we evaluated ventricular mechanics with 2DSTE derived deformational indices from birth to one year CA to determine the maturational patterns of postnatal cardiac adaptation. The main findings of this study are that: (1) RV longitudinal strain has a distinct regional magnitude distribution with a base-to-apex gradient and an incremental progression, while LV strain has an apex-to-base magnitude gradient with relatively unchanged progression through the neonatal period and infancy in uncomplicated preterm infants; and (2) preterm infants who develop BPD and/or PH have similar LV strain patterns but with decreased magnitudes of RV and IVS strain that track lower to those in uncomplicated preterm infants through the late neonatal period and infancy, even after clinical resolution of BPD and PH.
The functional evaluation of each ventricle by echocardiography in preterm infants is an area of ongoing research, but has been limited by the lack of reliable quantitative parameters that can be used for the assessment of both RV and LV function.1 The complex three-dimensional structure of the RV, with thin wall and high compliance, is evolutionally and embryologically different from the thick-walled and highly contractile LV. Despite these morphological differences, deformation imaging by 2DSTE has emerged as a reliable technique to assess global and regional myocardial function in each ventricle and permits a more direct assessment of myocardial function in preterm infants during this transitional period and through one year CA that could not be previously obtained with conventional modalities.9,10 In addition, deformation imaging has been mostly limited to the early postnatal period in preterm infants.1,6–8,10,12–14,16,21,35 We leveraged the large cohort of preterm infants in a multi-center format to study the impact of prematurity on the maturation of cardiac function in the preterm infants.18 This is the largest study to longitudinally assess strain imaging with 2DSTE in preterm infants up to one year CA. We showed that myocardial mechanics in both ventricles undergo unique longitudinal maturational changes in preterm infants, and that this maturation trajectory is influenced by body weight and age. We also studied the impact of different cardiopulmonary diseases’ influence on these adaptive processes.
Maturational Patterns of Ventricular Strain in Uncomplicated Preterm Infants
RV FWLS and IVS GLS
Myocardial function undergoes unique longitudinal changes in preterm infants that are specific to each ventricle. RV FWLS, and IVS GLS remained relatively stable from Day 1 to Day 2, but then physiologically increased from Day 5–7 to one-year CA. Nasu et al. found no change in RV strain patterns over the first 72 hours of age (ranged between -19.0% and −22.0%).8 Schubert et al observed similar values in the first week of age (−20.5% ± 5.5).16 Interestingly, deformation indices were also higher in the RV than the LV beginning at 32 weeks PMA and persisting to one year CA, which is in concordance with previous studies in children and neonates.22,35,36 In this study, the increase of RV and IVS strain in uncomplicated preterm infants was influenced by both postnatal increase in weight (in grams) and post-gestational age (in weeks), Figure 3. The relative higher values of RV strain and its progressive increase throughout the late neonatal period and infancy (when compared to LV strain) is probably related to the more longitudinal orientation of RV fibers with a predominant longitudinal contraction pattern in the RV compared to the LV.6,32
The higher RV strain is also reflected in the increased metabolic demand of RV myocardium that leads to a larger percentage ratio of collagen matrix to myofibers when compared to the LV myocardium.37 Despite the overall reduction in cardiomyocyte endowment in the preterm neonate, the increased extracellular matrix (ECM) deposition in the RV and the changing postnatal loading conditions result in different properties of deformation between the LV and RV.5,17 The decreasing afterload that results from a postnatal drop in pulmonary vascular resistance also leads to an increase in RV deformation over time.19 In a small comparative study of preterm and term infants from birth to six months of age, Schubert et al. observed an increasing upward trend in RV and IVS strain values.16 In larger studies, both Hefler and Cerznick38 and James et al11,22 observed that tissue Doppler- derived RV and IVS strain values rose significantly from birth to 28 days of age and 36 weeks PMA, respectively. With 2DSTE-derived strain, Czernik et al. also observed a rise in IVS GLS over the first 28 days of age.14 Our findings are consistent with these recent reports that had shorter follow-up time.
LV GLS
Compared to RV strain patterns, LV GLS remained relatively unchanged from Day 5–7 through one year CA. We did observe an increase in LV GLS from Day 1 to Day 5–7. LV strain values do not change markedly with age or heart rate in uncomplicated children, despite the alteration in loading conditions and changes in myocardial properties that occur during early childhood.39 There are a few studies in neonates that have demonstrated similar stable maturational pattern of global LV strain from birth through six months of age,8,11–14,16 however, none of these studies followed the infants to one year CA. Nasu et al. performed eight serial echocardiograms over the first 72 hours of age and demonstrated no change in LV strain.8 Czernik et al.14 and de Waal et al.12 each serially observed stable 2DSTE-derived LV GLS in preterm infants from birth to 28 days of age. Hirose et al. demonstrated that clinically healthy preterm infants (delivered at < 30 weeks gestation) also have constant 2DSTE-derived LV strain measures from 28 days of age to near term equivalent age (3 months CA).13 Schubert et al. observed that in a small heterogeneous cohort of 25 preterm infants (16% had BPD, and 40% had septicemia), and 30 term infants that there was relatively uniform LV strain values from birth to six month of age.16 These results coincide with the findings of Helfer and Czernik38 and James et al11,22 whose groups used tissue Doppler-derived strain imaging to demonstrate relative stability in LV strain imaging in preterm infants from birth through 28 days and 36 weeks PMA, respectively. Two recent meta-analyses defined reference values of 2DSTE-derived LV GLS in health term-born neonates that were also stable from birth to one year of age. 32,40 Although we observed a slight increase in LV GLS from Day 1 to Day 5–7, the impact of this early transitional period on LV strain patterns remains unclear from individual studies because of the variable methodologies and timings of scans employed in all those studies described above.
All of the previous studies in preterm infants only measured LV deformation from the apical four-chamber view.7,8,12–14,16 This is the first study to calculate the true “global” strain as an average from the three apical views.32 We further speculate that this difference is one reason for the disparity in reported values between studies, as well as the observed increase in LV GLS from Day 1 to Day 5–7 in our study. LV GLS is defined by different LV segmentation models, and it is recommended that each segment should be evaluated in multiple views to assess complete wall motion.31–33,41 Despite these recommendations, deformation imaging is still reported only from the apical 4- chamber view.32 However, despite that lack of uniform consensus on “which approach is more accurate or correlates more efficiently with health and disease,” it appears that by the end of the first week of age there is relative stability in LV strain patterns that persists throughout maturation, irrespective of which approach is utilized.32
Segmental LS
In the RVFW there is a base-to-apex SLS gradient that reflects the base-to-apex alignment of the dominant deep longitudinal layers of the RV, and allows for greater longitudinal shortening.42 In this study and the report by Schubert et al.16 there was a base-to-apex SLS gradient in the RVFW that was preserved throughout maturation. In the LVFW there exists an apex-to-base SLS gradient in children that occurs because of two primary reasons: (1) Torsional mechanisms of LV deformation is greatest toward the apex, as the right-handed helix in the subendocardium and the left-handed helix in the subepicardium converge toward the apex to form the “vortex of the double helical loop;” and (2) the electric excitation of cardiac motion begins in the apex and travels to the base.32,43 Coinciding with previous reports by Czernik et al.14 and de Waal et al.7, there existed a stable apex-to-base LV dominant SLS gradient in the LVFW that reflects the relatively constant geometry of the normal heart with maturation. In the IVS, there was also a persistent apex-to-base gradient (LV-dominant pattern32) in uncomplicated preterm infants that increased from Day 5–7 to one year CA. A series of meta-analysis defined similar reference ranges and physiological developmental patterns of SLS at the apical-, mid-, and basal- ventricular levels of the LV (apex-to-base) and RV (base-to-apex) FWs in healthy term-born children less than one year of age.32,42 Alteration of these “physiological” gradients have the potential to discern clinical changes in regional myocardial function in patients with different disease processes.32,42
Clinical Implications of Deformation Imaging in Neonates
Children with BPD and PH are much more likely than their gestational age counterparts to develop long-term respiratory morbidity that may affect the cardiovascular system.44 The extent to which prematurity-related alterations in cardiopulmonary structures affect their function has remained unclear due to the lack of early detection by a reliable biomarker of the disease, making the clinical management, intervention planning, and outcomes prediction for these patients challenging.1 We believe evaluation of cardiac function in preterm infants by speckle-tracking deformation imaging may now provide a marker (that was not previously available with conventional imaging) to longitudinally track cardiopulmonary disease over the first year of age. With an accepted physiological maturation patterns of ventricular strain in uncomplicated preterm infants based on postnatal weight and age over the first year age, we feel that these myocardial deformation parameters can provide a valid basis that allows comparison among studies and between health and disease.1
BPD and Respiratory Support
Improved survival of extremely premature babies has led to increased recognition of RV dysfunction and IVS abnormalities in infants with BPD.19,25,26 In this study we found a significant decrease in RV FWLS and IVS GLS in infants with BPD compared to infants without BPD (uncomplicated preterm infant), which remained significant after adjusting for the gestational age at birth, gender, and postnatal steroid use. In preterm infants with BPD, the magnitude of RV FWLS increased from 32 weeks to one year CA, but remained lower and tracked inferior when compared to infants without BPD by one-year CA, even when they no longer displayed clinical evidence of lung disease with oxygen requirement or respiratory support. Helfer and Czernik observed that tissue Doppler-derived RV global strain measures increased over the first month of life, but the rate of increase was also slower in infants who developed BPD.38 These findings suggest that RV function, as characterized by deformation values, remains subnormal in infants with BPD.
In contrast to RV strain measure, LV GLS was preserved from Day 5–7 through one year CA, irrespective of neonatal lung status. Czernik et al. also demonstrated that LV GLS by 2DSTE remained relatively constant from birth to 28 days of life in infants with and without BPD.14 Subtle differences in LV SLS values were seen at the mid-ventricular level in the early neonatal period that disappeared by one month of age.14 Similarly, James et al. demonstrated that BPD negatively impacted RV FWLS, but that LV tissue Doppler deformation values were unaffected by BPD.22 We also demonstrated that there was a preserved physiological segmental apex-to-base strain gradient in infants with and without BPD, suggesting that both global and regional longitudinal LV function remained preserved in the setting of lung disease. However, infants who developed BPD not only had decreased IVS GLS from 32 weeks PMA to one year CA, but a persistent base-to-apex (reflective of an RV dominant pattern) IVS strain gradient that never reversed, even by one year of age. Infants without BPD demonstrated an IVS apex-to-base SLS gradient, reflective of an LVFW pattern.
Mechanical ventilation can alter the pre- and after-load conditions and affect deformation values.14 In this study, 5% (n=12) of infants were on mechanical ventilation at 32 weeks PMA and 2% (n=4) of the infants at 36 weeks PMA. There were no infants on respiratory support at one year CA. We were unable to properly investigate the effect of mechanical ventilation on deformation changes because nearly all of the mechanically ventilated infants (85%, n=10) at 32 weeks and all infants at 36 weeks PMA developed BPD. We found an interesting association between the use of antenatal steroids and LV function on the first day of age. Infants whose mothers received antenatal steroids had higher LV GLS and SRs, which was independent of gestation. No effect was observed on RV function. Preterm infants may suffer from a combination of adrenocortical insufficiency and down regulation of cardiovascular adrenergic receptors.45 Therefore, it is possible that the administration of antenatal steroids can reverse this phenomenon leading to improved function.
PH
We utilized a broad echocardiogram-based definition of PH described by Mourani et al.25 and found an overall incidence of PH of 15% at 32 weeks PMA and 9% at 36 weeks PMA. Infants with BPD had a higher incidence of PH at 32 weeks PMA (12% vs. 3%) and at 36 weeks PMA (10% vs. 5%) when compared to those infants without BPD. Infants with PH at 32 and 36 weeks PMA had lower values of RV FWLS and IVS GLS, with preserved LV GLS. IVS SLS displayed a persistent base-to-apex gradient (RV dominant) in infants with PH that did not reverse by 32 weeks PMA. The base-to-apex pattern that was present at Day 5–7 was associated with a higher risk of late PH. Mourani et al. observed that ventricular septal wall flattening at 7 days of age (as an early sign of pulmonary vascular disease) was also associated with increased risk of late PH.25
Similar to Mourani et al., we also found that 10% of infants without BPD had detectable pulmonary hypertension at 36-week corrected gestational age.25 The patterns of decreased RV FWLS and IVS GLS persisted in the infants without BPD, but with PH from 32 weeks to one year CA. As highlighted by Farrow et al., this observation suggests that a primary vascular injury coupled with RV dysfunction may occur in some extreme preterm infants, independent of the lung disease.26 Although we only observed that one infant had echocardiographic signs of PH at one year CA, the infants with PH on their 36 week PMA echocardiograms that returned for a one-year CA follow-up (n=12 of 17, none of which required oxygen or respiratory support at one year CA ) still had decreased RV FWLS and IVS GLS and altered SLS base-to-apex pattern. Interestingly 25% (n=3) of the infants with PH at 36 weeks PMA that returned at one year CA did not carry a diagnosis of BPD and the patterns of altered strain mechanics persisted in this small sub-cohort as well. These findings might suggest that while some of the mechanisms of BPD and PH overlap, impairments of the developing pulmonary circulation in the extreme preterm infant without BPD may not be severe enough to be clinically recognized as pulmonary hypertension, but may still lead to cardio-respiratory morbidity.46 Whether this PH is clinically significant alone or predicts long-term morbidity in infants with BPD will require further study.26
PDA
The true influence of persistent PDA on ventricular strain patterns beyond the early neonatal period is an area of ongoing research.14,16,22,35,38,47 Infants with a PDA accompanied by evidence of systemic hypoperfusion (diastolic flow reversal in the descending aorta, and pulmonary over-circulation (increased LA:Ao and increased ejection fraction) had a higher LV GLS by Day 5 – 7. However, LV SRs remained similar in the two groups. de Waal et al. also found higher LV GLS and SRs in infants with a PDA.12 It is well documented that a hsPDA significantly increases LV preload, and leads to an increase in LV output.48 The increase in preload is accompanied by an increase in S but not SRs. This adds further support to the relative lack of load dependency of SRs illustrated in animal models.49 In our regression model, both hsPDA presence (and the accompanying increased preload), and LV SRs (representative of inherent contractility) had an independent effect on LV GLS. Therefore, LV longitudinal strain measurements must be interpreted taking into consideration loading conditions.
In this study, the infants who did not undergo pharmacological or surgical intervention to augment closure of the PDA, but still had a persistent PDA beyond 32 weeks, did not show any differences in their strain values when compared to infants without a PDA. Furthermore, infants who received pharmacological or surgical intervention had relatively stable values of RV, LV, and IVS FWS and FWSRs values in the late postnatal period when compared to infants without a PDA. Schubert et al. also found no significant associations between the speckle-tracking outcome variables at three months of corrected age and the diagnosis of PDA.16 We suspect that the effects of a PDA on LV strain imaging during the transition period and early neonatal period that has been observed in previous studies, may either dissipate over time,35,38 may be more of a true reflection in myocardial diastolic performance,13 or are unlikely to be due solely to the presence of PDA.14 The importance of these findings is that they support the notion that most premature neonates who fail to close their PDA after the first week of age may likely not experience significant cardiac morbidity as evidence by similar LV and RV strain patterns.
Study Limitations and Future Directions
In this study we only evaluated longitudinal strain imaging, as the feasibility and reproducibility of radial and circumferential strain has not been fully described in preterm infants beyond the transitional period.7,22 Although longitudinal strain remains the most reproducible quantitative tool of the three to assess LV function in preterm infants during the transitional period and later neonatal period,6,7 future work is now needed to assess the feasibility and reproducibility of circumferential and radial deformation at multiple time points in the first year of age and understand their maturational patterns in the context of uncomplicated preterm infants and those exposed to a PDA, at risk for BPD and or PH.
Both systolic and diastolic strain rate imaging provide unique insight into myocardial contractility and loading conditions,22 but we did not assess early and late diastolic strain rate with 2DSTE, because they too lack reproducibility in preterm infants.6 Finally, we demonstrated reduced inter- and intra- variability of segmental strain data in the LVFW, RVFW and IVS when compared to the reproducibility of global strain imaging in preterm infants.6 The difference in variability between global and regional strain may be due to the notion that in the small heart of a preterm infant each individual segment may not comprise a lot of speckles, and coupled with the elevated baseline heart rates in neonates, will lead to smoothing over of segments. To some extent, this may be mitigated by keeping the frame rate to heart rate ratio (FR/HR) between 0.7 and 0.9 frame/sec per bpm to optimize myocardial speckle tracking and mechanical event timing.21 However, until further validation of regional strain values is performed amongst different vendor platforms, its clinical use may be limited to the research arena.
CONCLUSIONS
This study tracks the maturational patterns of ventricular mechanics with global and regional deformation imaging by 2DSTE in extreme preterm infants during the first year of age. The maturational patterns are ventricular specific. BPD and PH appear to leave a negative impact on RV and IVS strain, while LV strain remains stable through the first year of age, independent of birth weight, postnatal age, and clinical status. This study suggests that 2DSTE derived strain can be used as a complementary modality to assess systolic ventricular function in neonates.
Highlights.
Two-dimensional speckle tracking echocardiography (2DSTE) derived myocardial strain is a feasible and reproducible imaging modality that can be used to characterize systolic ventricular function in premature infants.
This study establishes ventricular specific systolic strain maturational patterns by 2DSTE in a large cohort of extreme preterm infants from birth through one year corrected age (CA).
Common cardiopulmonary morbidities, such as bronchopulmonary dysplasia and pulmonary hypertension appear to leave a negative impact on RV strain, while LV strain remains stable through the first year of age.
With the establishment of the range of maturational patterns of strain mechanics and associated variations up to one year CA, deformation imaging by 2DSTE may now be implemented in preterm infants as a means to identify cardiovascular compromise earlier, guide therapeutic intervention, monitor treatment response, and improve overall outcome.
Acknowledgments
This study was supported by grants from Premature and Respiratory Outcomes Program (NIH 1U01 HL1014650, U01 HL101794), NIH R21 HL106417, Pediatric Physician Scientist Training Grant (NIH 5 T32 HD043010-09), and Postdoctoral Mentored Training Program in Clinical Investigation (NIH UL1 TR000448). Afif EL-Khuffash is funded by multiple sources: EU FP7/2007-2013 grant (agreement no. 260777, The HIP Trial); the Friends of the Rotunda Research Grant (Reference: FoR/EQUIPMENT/101572); Health Research Board Mother and Baby Clinical Trials Network Ireland (CTN-2014-10).
Abbreviations
- 2DSTE
two-dimensional speckle tracking echocardiography
- BPD
bronchopulmonary dysplasia
- CA
corrected age
- FWLS
free wall longitudinal strain
- GLS
global longitudinal strain
- IVS
interventricular septum
- LS
longitudinal strain
- LV
left ventricle
- PDA
patent ductus arteriosus
- PMA
post-menstrual age
- PH
pulmonary hypertension
- RV
right ventricle
- SRs
systolic strain rate
Appendix 1. Reproducibility Analysis for Segmental Longitudinal Strain
| Bland Altman | Intraclass Correlation | CV | |||
|---|---|---|---|---|---|
| Absolute Bias (%) | LOA | (95% CI) | P | (%) | |
| Left ventricle | |||||
| Apex | 11% | −3.3 – 3.2 | 0.81 (0.69–0.88) | 0.03 | 16.4% |
| Mid-ventricle | 9% | −2.1 − 2.3 | 0.89 (0.85–0.91) | 0.03 | 9.7% |
| Base | 8% | −2.3 – 2.6 | 0.91 (0.86–0.93) | 0.04 | 10.6% |
| Right Ventricle | |||||
| Apex | 14% | −3.5 – 3.0 | 0.83 (0.75–0.91) | 0.03 | 15.4% |
| Mid-ventricle | 11% | −2.1 – 2.5 | 0.87 (0.83–0.94) | 0.04 | 8.7% |
| Base | 9% | −2.3 – 2.6 | 0.93 (0.89–0.94) | 0.03 | 8.6% |
| Interventricular Septum | |||||
| Apex | 12% | −3.1 – 3.2 | 0.84 (0.79–0.93) | 0.04 | 13.4% |
| Mid-ventricle | 9% | −2.6 − 2.2 | 0.89 (0.85–0.96) | 0.03 | 9.7% |
| Base | 8% | −2.3 – 2.6 | 0.92 (0.88–0.94) | 0.01 | 8.6% |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Breatnach CR, Levy PT, James AT, Franklin O, El-Khuffash A. Novel echocardiography methods in the functional assessment of the newborn heart. Neonatology. 2016;110:248–60. doi: 10.1159/000445779. [DOI] [PubMed] [Google Scholar]
- 2.Lewandowski AJ, Bradlow WM, Augustine D, Davis EF, Francis J, Singhal A, et al. Right ventricular systolic dysfunction in young adults born preterm. Circulation. 2013;128:713–20. doi: 10.1161/CIRCULATIONAHA.113.002583. [DOI] [PubMed] [Google Scholar]
- 3.Lewandowski AJ, Augustine D, Lamata P, Davis EF, Lazdam M, Francis J, et al. Preterm heart in adult life: cardiovascular magnetic resonance reveals distinct differences in left ventricular mass, geometry, and function. Circulation. 2013;127:197–206. doi: 10.1161/CIRCULATIONAHA.112.126920. [DOI] [PubMed] [Google Scholar]
- 4.Kluckow M. Low systemic blood flow and pathophysiology of the preterm transitional circulation. Early Hum Dev. 2005;81:429–37. doi: 10.1016/j.earlhumdev.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 5.LeGRICE IJ. Laminar structure of the heart: ventricular myocyte arrangement and connective tissue architecture in the dog. Am J Physiol. 1995;269:H571–82. doi: 10.1152/ajpheart.1995.269.2.H571. [DOI] [PubMed] [Google Scholar]
- 6.Levy PT, Holland MR, Sekarski TJ, Hamvas A, Singh GK. Feasibility and reproducibility of systolic right ventricular strain measurement by speckle-tracking echocardiography in premature infants. J Am Soc Echocardiogr. 2013;26:1201–13. doi: 10.1016/j.echo.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Waal K, Lakkundi A, Othman F. Speckle tracking echocardiography in very preterm infants: feasibility and reference values. Early Hum Dev. 2014;90:275–9. doi: 10.1016/j.earlhumdev.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Nasu Y, Oyama K, Nakano S, Matsumoto A, Soda W, Takahashi S, et al. Longitudinal systolic strain of the bilayered ventricular septum during the first 72 hours of life in preterm infants. J Echocardiogr. 2015;13:90–9. doi: 10.1007/s12574-015-0250-8. [DOI] [PubMed] [Google Scholar]
- 9.El-Khuffash AF, Jain A, Weisz D, Mertens L, McNamara PJ. Assessment and treatment of post patent ductus arteriosus ligation syndrome. J Pediatr. 2014;165:46–52. doi: 10.1016/j.jpeds.2014.03.048. [DOI] [PubMed] [Google Scholar]
- 10.James AT, Corcoran JD, Hayes B, Franklin O, El-Khuffash A. The effect of antenatal magnesium sulfate on left ventricular afterload and myocardial function measured using deformation and rotational mechanics imaging. J Perinatol. 2015;35:913–8. doi: 10.1038/jp.2015.104. [DOI] [PubMed] [Google Scholar]
- 11.James AT, Corcoran JD, Jain A, McNamara PJ, Mertens L, Franklin O, et al. Assessment of myocardial performance in preterm infants less than 29 weeks gestation during the transitional period. Early Hum Dev. 2014;90:829–35. doi: 10.1016/j.earlhumdev.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 12.de Waal K, Phad N, Lakkundi A, Tan P. Cardiac Function After the Immediate Transitional Period in Very Preterm Infants Using Speckle Tracking Analysis. Pediatr Cardiol. 2016;37:295–303. doi: 10.1007/s00246-015-1277-3. [DOI] [PubMed] [Google Scholar]
- 13.Hirose A, Khoo NS, Aziz K, Al-Rajaa N, van den Boom J, Savard W, et al. Evolution of Left Ventricular Function in the Preterm Infant. J Am Soc Echocardiogr. 2015;28:302–8. doi: 10.1016/j.echo.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 14.Czernik C, Rhode S, Helfer S, Schmalisch G, Buhrer C, Schmitz L. Development of left ventricular longitudinal speckle tracking echocardiography in very low birth weight infants with and without bronchopulmonary dysplasia during the neonatal period. PLoS One. 2014;9:e106504. doi: 10.1371/journal.pone.0106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy PT, Singh GK. Challenges in Interpreting Deformation Values by Two-Dimensional Speckle-Tracking Echocardiography in Preterm and Term Infants. J Am Soc Echocardiogr. 2017;30:97–8. doi: 10.1016/j.echo.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Schubert U, Muller M, Abdul-Khaliq H, Norman M. Preterm Birth Is Associated with Altered Myocardial Function in Infancy. J Am Soc Echocardiogr. 2016;29:670–8. doi: 10.1016/j.echo.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Spotnitz HM. Macro design, structure, and mechanics of the left ventricle. J Thorac Cardiovasc Surg. 2000;119:1053–77. doi: 10.1016/S0022-5223(00)70106-1. [DOI] [PubMed] [Google Scholar]
- 18.Pryhuber GS, Maitre NL, Ballard RA, Cifelli D, Davis SD, Ellenberg JH, et al. Prematurity and respiratory outcomes program (PROP): study protocol of a prospective multicenter study of respiratory outcomes of preterm infants in the United States. BMC Pediatr. 2016;15:37. doi: 10.1186/s12887-015-0346-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy PT, Dioneda B, Holland MR, Sekarski TJ, Lee CK, Mathur A, et al. Right Ventricular Function in Preterm and Term Neonates: Reference Values for Right Ventricle Areas and Fractional Area of Change. J Am Soc Echocardiogr. 2015;28:559–69. doi: 10.1016/j.echo.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.James AT, Corcoran JD, Franklin O, EL-Khuffash AF. Clinical utility of right ventricular fractional area change in preterm infants. Early Hum Dev. 2016;92:19–23. doi: 10.1016/j.earlhumdev.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez AA, Levy PT, Sekarski TJ, Hamvas A, Holland MR, Singh GK. Effects of frame rate on two-dimensional speckle tracking-derived measurements of myocardial deformation in premature infants. Echocardiography. 2015;32:839–47. doi: 10.1111/echo.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.James AT, Corcoran JD, Breatnach CR, Franklin O, Mertens L, El-Khuffash A. Longitudinal Assessment of Left and Right Myocardial Function in Preterm Infants Using Strain and Strain Rate Imaging. Neonatology. 2015;109:69–75. doi: 10.1159/000440940. [DOI] [PubMed] [Google Scholar]
- 23.Koestenberger M, Nagel B, Ravekes W, Urlesberger B, Raith W, Avian A, et al. Systolic right ventricular function in preterm and term neonates: reference values of the tricuspid annular plane systolic excursion (TAPSE) in 258 patients and calculation of Z-score values. Neonatology. 2011;100:85–92. doi: 10.1159/000322006. [DOI] [PubMed] [Google Scholar]
- 24.Poindexter BB, Feng R, Schmidt B, Aschner JL, Ballard RA, Hamvas A, et al. Comparisons and Limitations of Current Definitions of Bronchopulmonary Dysplasia for the Prematurity and Respiratory Outcomes Program. Ann Am Thorac Soc. 2016;12:1822–30. doi: 10.1513/AnnalsATS.201504-218OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mourani PM, Sontag MK, Younoszai A, Miller JI, Kinsella JP, Baker CD, et al. Early Pulmonary Vascular Disease in Preterm Infants at Risk for Bronchopulmonary Dysplasia. Am J Respir Crit Care Med. 2015;191:87–95. doi: 10.1164/rccm.201409-1594OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farrow KN, Steinhorn RH. Pulmonary Hypertension in Premature Infants. Sharpening the Tools of Detection. Am J Respir Crit Care Med. 2015;191:12–4. doi: 10.1164/rccm.201411-2112ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weber SC, Weiss K, Bührer C, Hansmann G, Koehne P, Sallmon H. Natural History of Patent Ductus Arteriosus in Very Low Birth Weight Infants after Discharge. J Pediatr. 2015;167:1149–51. doi: 10.1016/j.jpeds.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 28.Benitz WE. Patent Ductus Arteriosus in Preterm Infants. Pediatrics. 2016;137:1–6. doi: 10.1542/peds.2015-3730. [DOI] [PubMed] [Google Scholar]
- 29.Ramos FG, Rosenfeld CR, Roy L, Koch J, Ramaciotti C. Echocardiographic predictors of symptomatic patent ductus arteriosus in extremely-low- birth-weight preterm neonates. J Perinatol. 2010;30:535–9. doi: 10.1038/jp.2010.14. [DOI] [PubMed] [Google Scholar]
- 30.Jain A, Shah PS. Diagnosis, Evaluation, and Management of Patent Ductus Arteriosus in Preterm Neonates. JAMA Pediatr. 2015;169:863–72. doi: 10.1001/jamapediatrics.2015.0987. [DOI] [PubMed] [Google Scholar]
- 31.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for chamber quantification. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Levy PT, Machefsky A, Sanchez AA, Patel MD, Rogal S, Fowler S, et al. Reference Ranges of Left Ventricular Strain Measures by Two-Dimensional Speckle-Tracking Echocardiography in Children: A Systematic Review and Meta-Analysis. J Am Soc Echocardiogr. 2016;29:209–25. doi: 10.1016/j.echo.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to Standardize Deformation Imaging. J Am Soc Echocardiogr. 2015;28:183–93. doi: 10.1016/j.echo.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 34.James A, Corcoran JD, Mertens L, Franklin O, El-Khuffash A. Left Ventricular Rotational Mechanics in Preterm Infants Less Than 29 Weeks' Gestation over the First Week after Birth. J Am Soc Echocardiogr. 2015;28(7):808–817. doi: 10.1016/j.echo.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 35.El-Khuffash AF, Jain A, Dragulescu A, McNamara PJ, Mertens L. Acute changes in myocardial systolic function in preterm infants undergoing patent ductus arteriosus ligation: a tissue Doppler and myocardial deformation study. J Am Soc Echocardiogr. 2012;25:1058–67. doi: 10.1016/j.echo.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 36.Lorch SM, Ludomirsky A, Singh GK. Maturational and growth-related changes in left ventricular longitudinal strain and strain rate measured by two-dimensional speckle tracking echocardiography in healthy pediatric population. J Am Soc Echocardiogr. 2008;21:1207–15. doi: 10.1016/j.echo.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 37.Salih C, McCarthy KP, Ho SY. The fibrous matrix of ventricular myocardium in hypoplastic left heart syndrome: a quantitative and qualitative analysis. Ann Thorac Surg. 2004;77:36–40. doi: 10.1016/s0003-4975(03)01472-3. [DOI] [PubMed] [Google Scholar]
- 38.Helfer S, Schmitz L, Bührer C, Czernik C. Tissue Doppler-Derived Strain and Strain Rate during the First 28 Days of Life in Very Low Birth Weight Infants. Echocardiography. 2014;31:765–72. doi: 10.1111/echo.12463. [DOI] [PubMed] [Google Scholar]
- 39.Colan SD, Parness IA, Spevak PJ, Sanders SP. Developmental modulation of myocardial mechanics: age- and growth-related alterations in afterload and contractility. J Am Coll Cardiol. 1992;19:619–29. doi: 10.1016/s0735-1097(10)80282-7. [DOI] [PubMed] [Google Scholar]
- 40.Jashari H, Rydberg A, Ibrahimi P, Bajraktari G, Kryeziu L, Jashari F, et al. Normal ranges of left ventricular strain in children: a meta-analysis. Cardiovascular Ultrasound. 2015;13:37. doi: 10.1186/s12947-015-0029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farsalinos KE, Daraban AM, Unlu S, Thomas JD, Badano LP, Voigt JU. Head-to-head comparison of global longitudinal strain measurements among nine different vendors. J Am Soc Echocardiogr. 2015;28:1171–81. doi: 10.1016/j.echo.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 42.Levy PT, Sanchez A, Machefsky A, Fowler S, Holland MR, Singh GK. Normal ranges of right ventricular systolic and diastolic strain measures in children: a systematic review and meta-analysis. J Am Soc Echocardiogr. 2014;27:549–60. doi: 10.1016/j.echo.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buckberg G, Hoffman JIE, Mahajan A, Saleh S, Coghlan C. Cardiac mechanics revisited: the relationship of cardiac architecture to ventricular function. Circulation. 2008;118:2571–87. doi: 10.1161/CIRCULATIONAHA.107.754424. [DOI] [PubMed] [Google Scholar]
- 44.Islam JY, Keller RL, Aschner JL, Hartert TV, Moore PE. Understanding the Short- and Long-Term Respiratory Outcomes of Prematurity and Bronchopulmonary Dysplasia. Am J Respir Crit Care Med. 2015;19:134–56. doi: 10.1164/rccm.201412-2142PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quintos JB, Boney CM. Transient adrenal insufficiency in the premature newborn. Curr Opin Endocrinol Diabetes Obes. 2010;17:8–12. doi: 10.1097/MED.0b013e32833363cc. [DOI] [PubMed] [Google Scholar]
- 46.Mourani PM, Abman SH. Pulmonary Hypertension and Vascular Abnormalities in Bronchopulmonary Dysplasia. Clin Perinatol. 2015;42:839–55. doi: 10.1016/j.clp.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sehgal A, Doctor T, Menahem S. Cyclooxygenase Inhibitors in Preterm Infants With Patent Ductus Arteriosus: Effects on Cardiac and Vascular Indices. Pediatr Cardiol. 2014;35:1429–36. doi: 10.1007/s00246-014-0947-x. [DOI] [PubMed] [Google Scholar]
- 48.El-Khuffash A, Weisz D, McNamara P. Reflections of the changes in patent ductus arteriosus management during the last 10 years. Arch Dis Child Fetal Neonatal Ed. 2016;101:F474–8. doi: 10.1136/archdischild-2014-306214. [DOI] [PubMed] [Google Scholar]
- 49.Ferferieva V, Van den Bergh A, Claus P, Jasaityte R, Veulemans P, Pellens M, et al. The relative value of strain and strain rate for defining intrinsic myocardial function. Am J Physiol Heart Circ Physiol. 2012;302:H188–95. doi: 10.1152/ajpheart.00429.2011. [DOI] [PubMed] [Google Scholar]