Abstract
Background and objectives
It is recognized that orthodontic force has an aggravating effect on the progression of destructive periodontitis if periodontitis have not been well controlled. However, the underlying mechanism is not completely clear. This study was to investigate the effect of antibiotic administration on orthodontic force-aggravated, ligature-induced experimental periodontitis in mice.
Methods
C57BL/6 mice (male, 8-week old) were divided into three groups (n=8). Silk ligatures (SL) were tied around the maxillary right (Group 1) or both (Groups 2&3) first molars on Day 0, removed on Day 8, and systemic antibiotics (SA) was administered through drinking water (Group 3) since Day 8. Orthodontic force (OF) was applied on the maxillary right first molars since Day 13 (Groups 2&3). All mice were sacrificed on Day 20.
Results
Total oral bacteria load was significantly higher in Group 2 when compared to Group 1 on Day 20, whereas such count was greatly reduced in Group 3 when antibiotics were administered. Periodontal bone loss (PBL) was significantly increased on SL side vs. control side in Group 1. PBL was significantly increased on OF+SL side vs. SL side in Group 2 (p < 0.05) but not in Group 3 when SA was administered. Gingival mRNA and protein expressions of receptor activator of nuclear factor kappa-B ligand (RANKL) / osteoprotegerin (OPG) were significantly increased on OF+SL side vs. SL side in Group 2 (p < 0.01) but not in Group 3. However, comparable levels of TRAP+ cell formation within periodontal space and tooth movement were observed on OF+SL side in Group 2 and Group 3.
Conclusion
Our results suggest that reduction of oral bacterial load by antibiotic administration alleviate orthodontic force-aggravated periodontitis bone loss.
Keywords: periodontitis, orthodontic force, bone loss, antibiotics
INTRODUCTION
Periodontal disease is an inflammatory disease triggered by host immune response to microorganism(s) associated with periodontal biofilm (1, 2). It is a common chronic disease in humans, which can cause alveolar bone destruction, leading to the pathological tooth migration and occlusal trauma (3, 4). These problems as a vicious circle can accelerate the breakdown of periodontal supporting apparatus (5, 6). Studies have shown that advanced periodontitis patients can benefit from orthodontic treatment to improve their periodontal health (3, 7). Occlusal trauma could be ameliorated by orthodontic correction of adverse occlusion and improper loading distribution (8, 9).
However, when applying orthodontic treatment, special attention must be paid to the periodontal status of the patient who had experienced advanced periodontal breakdown. It was suggested that orthodontic movement has an aggravating effect on the progression of destructive periodontitis if periodontitis have not been well controlled (10). The fixed orthodontic appliances may influence oral microbiota and exacerbate periodontal parameters following placement of fixed appliances (9, 11). For example, plaque formation during orthodontic therapy increased probing depth of periodontally compromised individuals (12). Therefore, even though it is commonly accepted that orthodontic treatment can be applied after treatments of active periodontitis (8, 13), periodontal breakdown may still occur during orthodontic treatment if rigorous periodontal monitoring and maintenance are not achieved.
Conventional treatments of active periodontitis usually include mechanical removal of dental biofilm and local and systemic antibiotic administration (14–16). Given the infectious nature of periodontal diseases and the limitation of conventional mechanical therapies for the treatment of some forms of periodontitis (aggressive and refractory), the use of antibiotics is often indicated in certain cases (17). However, it is not completely clear whether reduction of oral bacteria load by antibiotic administration affects periodontal bone resorption during tooth movement-aggravated periodontitis.
In this study we established a mouse model to investigate the effect of systemic antibiotic administration on ligature-induced experimental periodontitis in conjunction with orthodontic tooth movement.
MATERIALS AND METHODS
Mice
Wild-type C57BL/6 mice (male, 8-week old) from the Jackson Laboratory (Bar Harbor, ME) were used in this study. All the mice used in the study were maintained in specific pathogen-free (SPF) units of the Forsyth Institute Animal Facility. The mice were kept on a 12-hour light/dark cycle. The experimental protocols were approved by the Institutional Animal Care and Use Committee of the Forsyth Institute. Mice were fed powder chow ad libitum for the duration of the experiment.
Animal model
Mice were divided into three groups (n=12 animals per group). For all procedures, the mice were anesthetized with ketamine xylazine mixture (500 mg/kg ketamine, 100 mg/kg xylazine sodium) in sterile phosphate-buffered saline (PBS). On Day 0, silk ligatures (SL) were tied around the maxillary right first molar in Group 1, and around both first molars in Groups 2 and 3. On Day 8, ligatures were removed from all mice and systemic antibiotics (SA) were administered through drinking water (0.85 mg/ml sulfamethoxazole and 0.17 mg/ml trimethoprim, Hi-TECH Pharmacal Co, Amityville, NY) in Group 3 for the remaining period of the study. On Day 13 (5 days after ligature removal), orthodontic force (OF) was applied on the maxillary right first molars in Groups 2 and 3 till the end of the study. Briefly, OF were applied by a 3.5 mm length of closed-coil springs (0.203 mm wire size; 0.762 mm in diameter; Rocky Mountain Orthodontics, Denver, CO) connecting the incisors and the right first molar with silk ligatures (size 5-0) on each end. The silk ligatures were affixed to the incisors and the right first molar (supragingivally around the crown) by bonding agent (Bond Force, Tokuyama, Japan) and flowable resin (Wave, SDI, USA). Occlusal interference by ligatures or resin was avoided to prevent premature contact between the upper and lower molars. Each coiled spring delivered 20 gram of force, measured by a T-50g dynamometer (Tokyo, Japan). The weight of mice was monitored throughout the experimental period on Days 0, 13, and 20. All mice were sacrificed on Day 20 (See Fig. S1 in supplemental data).
Gingival RANKL and OPG mRNA expressions by real-time quantitative PCR
Animals were euthanized by CO2 inhalation and the maxilla was removed from each mice. Gingival tissues around the first molars were isolated under a surgical microscope and were immediately homogenized using Homogenizing Kit (OMMNI). Totally RNA was extracted using PureLink® RNA Mini Kit (Ambion) and were reversed transcribed using the SuperScript II Reversed Transcriptase kit (Invitrogen). Predesigned primers of RANKL, OPG and GAPDH were from Sigma. The respective primer sequences are as follows: RANKL: TGTACTTTCGAGCGCAGATG and AGGCTTGTTTCATCCTCCTG; OPG: AGCAGGAGTGCAACCGCACC and TTCCAGCTTGCACCACGCCG; GAPDH: CCCCAGCAAGGACACTGAGCAA and GTGGGTGCAGCGAACTTTATTGATG. Quantitative PCR was conducted using the LightCycler® SYBR Green I master and LightCycler® 480 Instrument system (Roche).
Alveolar bone loss and tooth movement by morphometric analysis
The maxilla were de-fleshed in dermestid beetle colony, bleached and stained with 1% toluidine blue solution. Maxillary images were captured with a digital stereomicroscope system on a holder to enable the visualization of the cementoenamel junction (CEJ) and alveolar bone crest. The polygonal areas palatal and mesial to the first molars were measured using Image J (NIH) for each segment and a standard calibrator was used for calibration at the same magnification. The alveolar bone loss was evaluated by measuring the polygonal area as previously described (18), which was enclosed coronally by the CEJ of the molars, laterally by the exposed mesial and distal root of the first molar, and apically by the alveolar crest. The orthodontic tooth movement was assessed by measuring the closest distance (CD) between the proximal contours of first and second molars and were presented as millimeters (mm).
Detection of serum total IgG, gingival RANKL and OPG by ELISA
To monitor the systemic immune status before and after the treatment, peripheral blood was collected on Day 0 and Day 20 from mice tail vein and the serum total IgG were detected using IgG Mouse ELISA Kit according to manufacturer’s instructions (Abcam). To determine the gingival protein level of RANKL and OPG, gingival tissues were homogenized with a Dounce glass homogenizer in RIPA buffer and proteinase inhibitor cocktail (Sigma). Gingival RANKL and OPG protein level were measured by Mouse RANKL and OPG ELISA MAXTM Standard kit (R&D Systems) following the manufacturer’s protocol. Each sample assay was performed in duplicates and a standard curve was run with each assay. The absorbance at 450 nm was measured in a microplate reader (BioTek) and the RANKL and OPG concentration (pg/mL) was calculated based on the standard curve respectively.
Histological analysis
The maxillae from the mice were fixed in 4% formaldehyde and decalcified in 0.1M TRIS + 10% ethylenediaminetetraacetic acid for 2 weeks at 4°C. Paraffin-embedded samples were sectioned at 5µm. Consecutive vertical sections were obtained from the mesial and distal-buccal roots of each first molar (n=6 animals per group). Sections from the same positions (6–10 sections per animal) of the roots were used for histological analysis. Tartrate-resistant acid phosphatase (TRAP) staining (387A-1KT, Sigma) was used to detect activated osteoclasts. The consecutive fields were selected in the areas from gingiva papilla to root apical between the root and alveolar bone (4–6 fields per section). Each field was acquired under light microscope at objective magnification 40×. The individual osteoclasts were identified at 40× magnification as multi-nucleated TRAP-positive (stained red) cells lining along the alveolar bone surface and was counted along the alveolar bone surface from the pressure side of the mid-third of the distal-buccal root of first molars. Data were presented as mean ± SD. To evaluate the horizontal bone resorption in vivoHaemotoxylin and Eosin (HE) staining was used (6–10 sections per animal, 6 animals per group). To evaluate the relative hight of alveolar crest, the distance between the CEJ to the alveolar crest (D-CA) was measured in micrometers (µm) by Image J software. All measurements were made without prior knowledge of the group designation of the animals and the recordings were verified by a second examiner.
Quantitation of total oral bacteria DNA
On Day 0 and Day 20, oral swabs were taken from around molar teeth and periodontal gingival surface (after the removal of close coil spring appliance on Day 20) from each mouse. Samples were resolved in 200µl of PBS and DNA was extracted from each sample as previously described (19) and was quantitated by real-time PCR (RT-PCR) using the LightCycler® SYBR Green I master and LightCycler® 480 Instrument system (Roche). A universal 16S rRNA gene primer was used to quantitate the total oral bacteria recovered from each animal: 5’-GAGTTTGATYMTGGCTCAG and 5’-AAGGAGGTGWTCCARCC-3’. The quantity of total bacterial DNA from each sample was extrapolated from the DNA standard curve derived from serial dilution of E. coli bacteria with known concentrations. Each experiment was carried out in duplicate and the data were obtained from all animals in each group (n=8).
Statistical analyses
Statistical analyses were performed using a software program (SPSS v.18, IBM, Chicago, IL). Results are presented as means ± SD. Paired and non-paired Student's t-test were used to analyze differences within and among groups respectively. Results with probability values of less than 0.05 were considered statistically significant.
RESULTS
Oral bacterial load, serum total IgG level, and animal weight monitoring
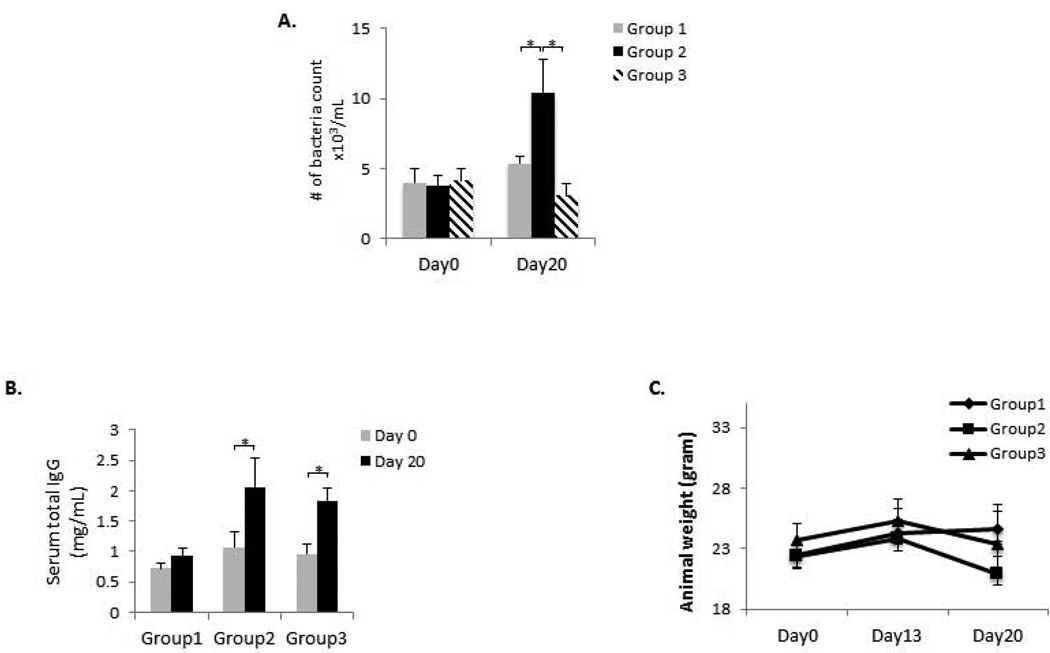
In order to determine the OF and SA administration on oral bacteria load, total bacterial DNA from oral swaps were quantitated. On Day 20, the number of bacteria count was significantly increased (p < 0.05) when OF was applied (Group 2 vs. Group 1), whereas the number of bacteria count was significantly decreased (p < 0.05) when additional SA was administered (Group 3 vs. Group 2) (Fig. 1A). In order to evaluate the overall immune status after the treatments, serum total IgG level was measured on Day 0 and Day 20 in each group of mice. Serum total IgG levels were significantly higher in Groups 2 and 3 as compared to those in Group 1 (p < 0.05). However, no difference was observed between Group 2 and Group 3 (Fig. 1B). Furthermore, in order to ensure that animals are not chronically stressed due to the combined SL+OF+SA treatments, we have monitored the animal weight throughout the 20-Day period (Fig. 1C). There was a consistent weight gain from Day 0 to Day 13 for all three groups of mice. The weight of the mice in Groups 2 and 3 was slightly decreased on Day 20 as compared to the weight on Day 0 (maximum loss less than 7% of initial weight), probably due to the application of orthodontic appliances and minor impediment of food intake. Overall, these data indicated that the treatments (SL, OF and SA) did not generate significant irreversible stress and metabolic variation among treated mice.
Figure 1. Oral bacteria load, serum total IgG measurement and animal weight monitoring.
(A) On Day 0 and Day 20, DNA was extracted from oral swabs taken from each mouse and was quantitated by real-time PCR. A universal 16S rRNA gene primer was used to quantitate the total oral bacteria recovered from each animal. The number of bacteria count was significantly increased when OF was applied (Group 2 vs. Group 1), whereas the number of bacteria count was significantly decreased when additional SA was administered (Group 3 vs. Group 2). (B) Total serum IgG levels on Day 0 and Day 20 in all three groups of mice were quantitated by ELISA. Serum total IgG levels were significantly higher in Groups 2 and 3 as compared to those in Group 1. However, no difference was observed between Group 2 and Group 3. (C) The weight of mice was monitored throughout the experimental period on Days 0, 13, and 20. A stable body weight was maintained by all the three groups of mice. *p < 0.05. n = 8.
Evaluation of PBL by 2-dimentional bone morphometry
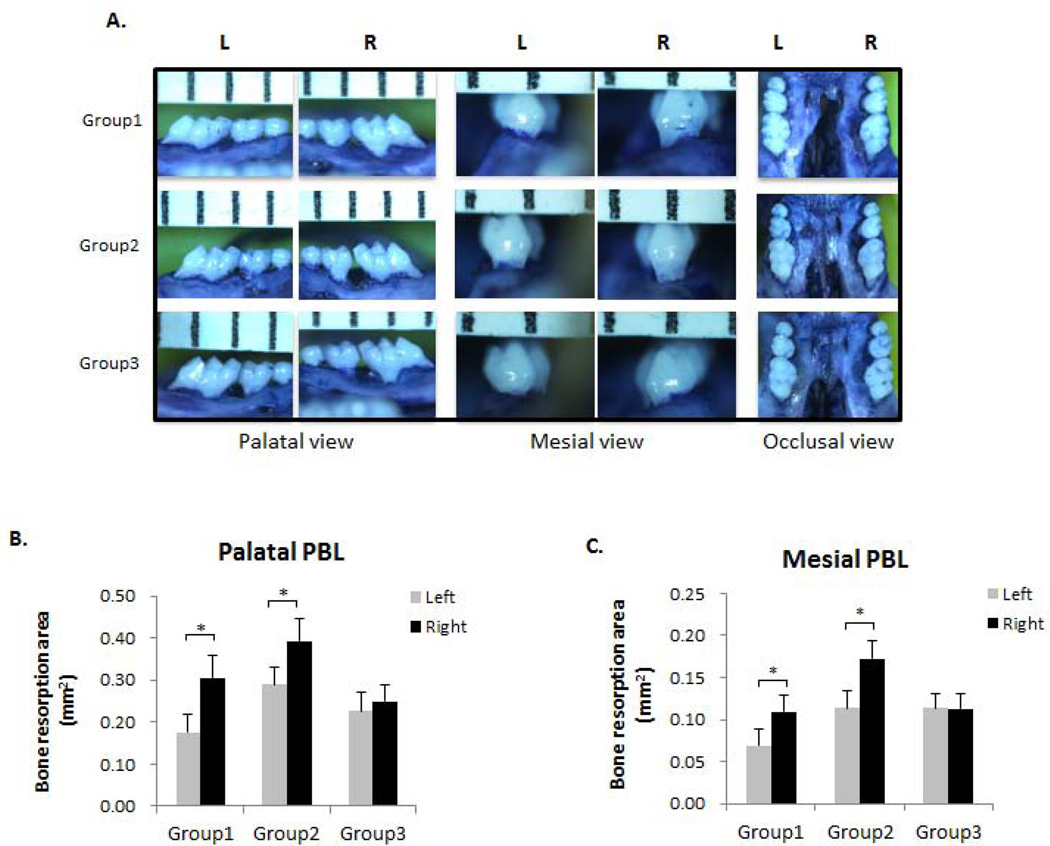
In order to evaluate the effects of different treatments on PBL, the images of polygonal resorption areas palatal and mesial to the first molars were taken and were quantitatively measured (Fig. 2A). The palatal resorption area was significantly larger on the SL side (right) compared to the control side (left) (p < 0.05) in Group 1 (Fig. 2B). The palatal resorption area was significantly larger on the SL+OF side (right) compared to the SL side (left) (p < 0.05) in Group 2, whereas no such difference was observed between SL+OF side (right) and SL side (left) in Group 3 when SA was administered (Fig. 2B). Similar results were observed when resorption areas mesial to the first molars were measured, showing that the resorption area was significantly increased on the SL+OF side compared to the SL side (p < 0.05) in Group 2, whereas SA administration abolished such difference between the two sides in Group 3 (Fig. 2C).
Figure 2. OF increased PBL of ligature-induced experimental periodontitis.
(A) Palatal, mesial and occlusal view of the de-fleshed maxilla stained with 1% toluidine blue solution. Maxillary images were captured with a digital stereomicroscope system on a holder to enable the visualization of the cementoenamel junction (CEJ) and alveolar bone crest. The polygonal areas palatal and mesial to the first molars were measured using Image J (NIH) software. L, left side (represents control side in Group 1 and SL side in Groups 2&3); R, right side (represents SL side in Group 1 and SL+OF side in Groups 2&3). n = 6–8. (B) Morphometric analysis of bone resorption area palatal to the maxillary first molars. (C) Morphometric analysis of bone resorption area mesial to the maxillary first molars. Bone resorption areas were significantly increased on the right side compared to the left side. *p < 0.05. n = 6–8.
Gingival RANKL/OPG expressions
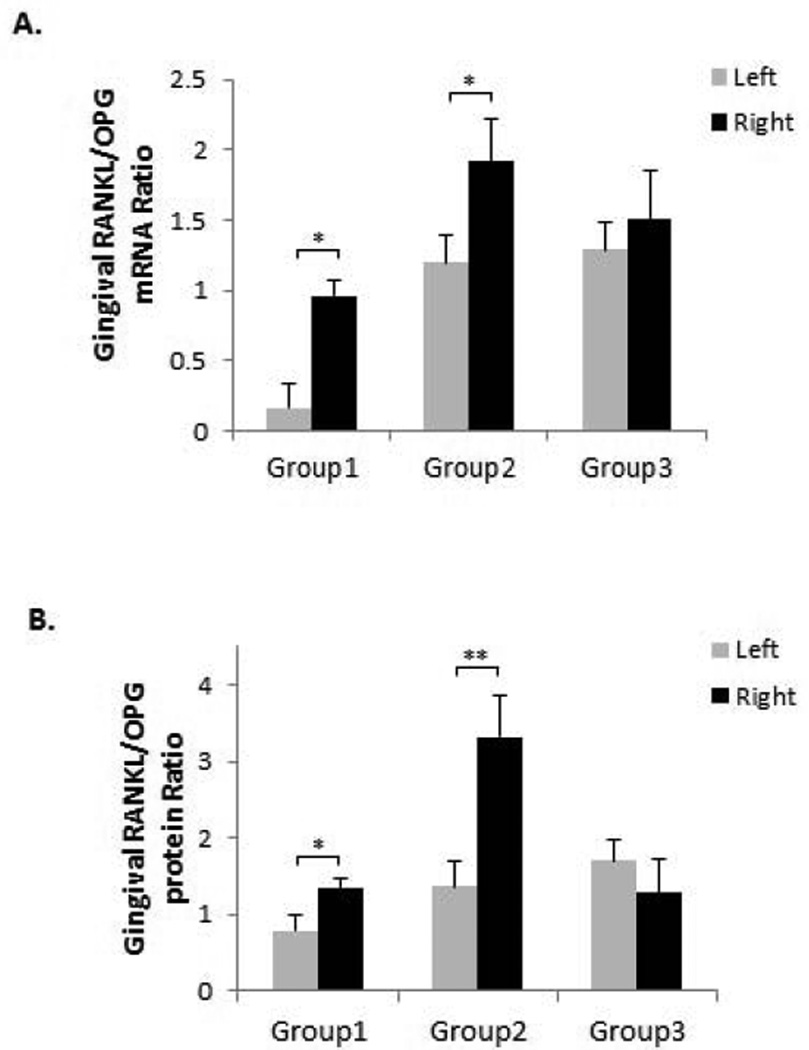
RANKL/OPG ratio has been used to assess the severity of osteoclastogenesis (20–22) and RANKL and OPG levels were oppositely regulated in periodontitis, resulting in an enhanced RANKL/OPG ratio (23, 24). To evaluate these responses when OF was applied during experimental periodontitis, gingival tissues around first molars were isolated and the mRNA and protein expressions were analyzed by real-time PCR and ELISA respectively. Our results showed that gingival RANKL/OPG mRNA expression ratio was significantly increased on the SL side compared to the control side (p < 0.05) in Group 1 (Fig. 3A). The ratio was also significantly greater on the SL+OF side compared to the SL side (p < 0.01) in Group 2, whereas no such difference was observed between SL+OF side and SL side in Group 3 when SA was administered (Fig. 3A). Similarly, the gingival RANKL/OPG protein ratio was also significantly increased on the SL side compared to the control side (p < 0.05) in Group 1, and on the SL+OF side compared to the SL side (p < 0.01) in Group 2 (Fig. 3B).
Figure 3. OF increased gingival RANKL/OPG expression ratio.
(A) Gingival mRNA expressions of RANKL and OPG were detected by real-time PCR and their ratios were calculated. RANKL/OPG ratios were increased on the right side compared to the left side in Groups 1 and 2. For all the experiments, GAPDH gene was used as internal control. (B) Gingival protein levels of RANKL and OPG were detected by ELISA and their ratios were calculated. RANKL/OPG protein ratios were also increased on the right side compared to the left side in Groups 1 and 2. *p < 0.05, **p < 0.01. n = 6–8.
Histological evaluation of horizontal bone resorption
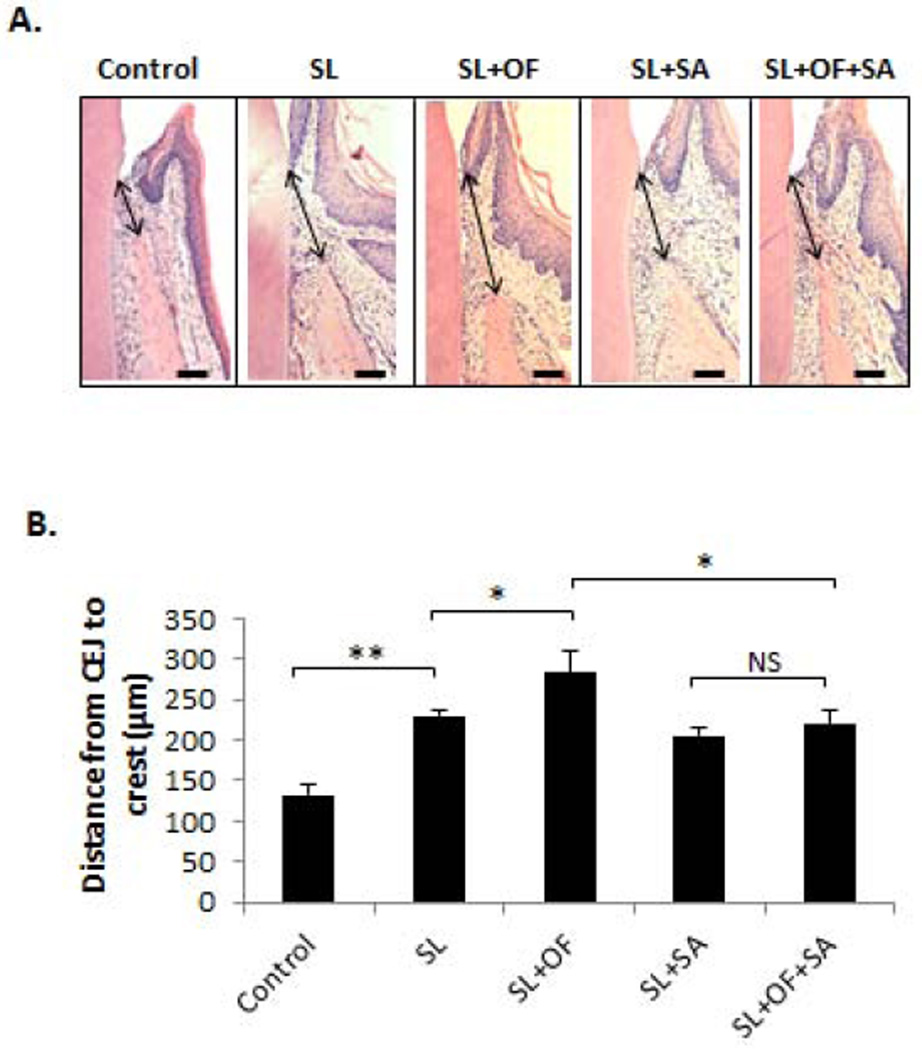
In order to determine the effect of OF and SA on experimental periodontitis histologically, the distance from CEJ to alveolar bone crest (D-CA) was measured on HE sections in order to assess horizontal bone resorption (Fig. 4A). The D-CA was significantly increased in mice treated with SL compared to control (p < 0.01), whereas animals treated with SL+OF further augmented the D-CA when compared to those treated with SL alone (Fig. 4B). However, the D-CA was not changed between animals treated with SL+OF and those treated with SL alone when SA was administered (Fig. 4B).
Figure 4. SA administration inhibited OF-induced horizontal bone loss.
Haemotoxylin and Eosin (HE) staining was used to evaluate the horizontal bone resorption after each treatment. (A) The distance between the CEJ to the alveolar crest (D-CA) were measured by Image J software (indicated by arrows). Bar =100 µm. (B) Quantitative measurement and statistical analysis of the D-CA in each treatment group. The D-CA was significantly increased by OF, but such increase was reduced by SA administration. Moreover, OF did not increased the D-CA when SA was administered. SL, silk ligature; OF, orthodontic force; SA, systemic antibiotics. *p < 0.05, **p < 0.01, NS, not significant. n = 3–5.
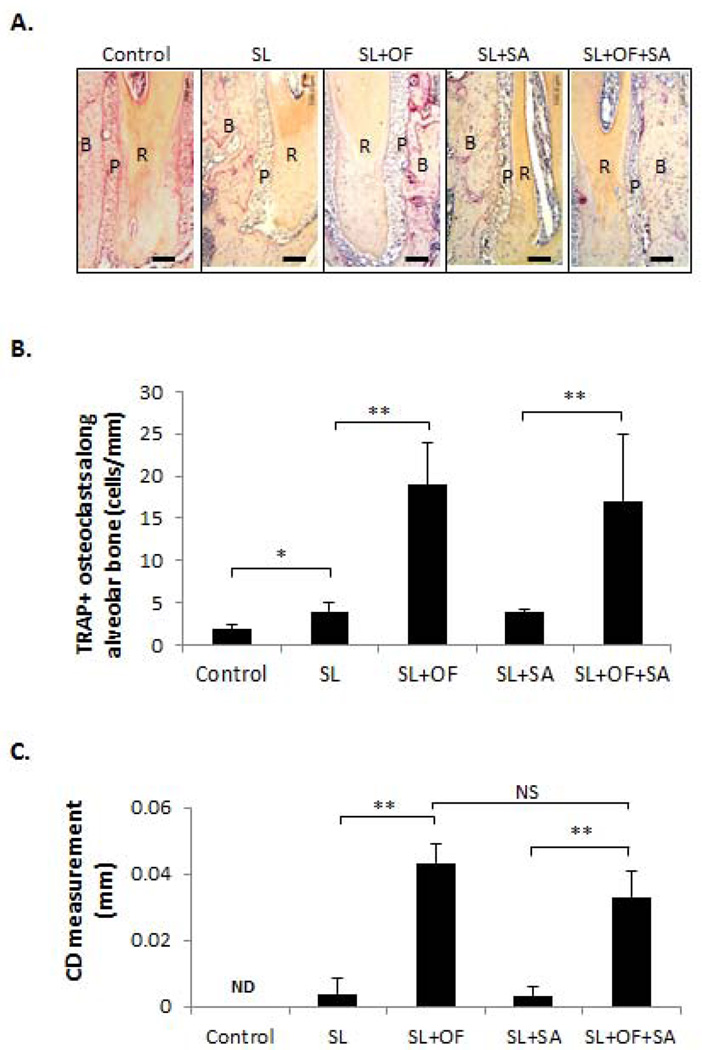
Evaluation of OF-induced osteoclastogenesis and tooth movement
To determine the OF-induced osteoclast activity, TRAP staining were performed to evaluate multi-nucleated TRAP-positive cell formation from the pressure side of the mid-third of the distal-buccal root of first molars (Fig. 5A). Our results demonstrated that the number of TRAP+ cells along the alveolar bone surface were increased on the SL side compared to control in Group 1 (p < 0.05), while much greater number of TRAP+ cell formation (p < 0.01) was detected on the SL+OF side compared to the SL side in both Group 2 and Group 3, regardless of the SA administration (Fig. 5B). To assess the extent of orthodontic tooth movement after different treatments, the closest distance (CD) between the proximal contours of first and second molars was measured in each group. There was a slight CD detected between first and second molars when SL was applied, likely due to the impaction of SL and tooth shifting. As expected, significant increase in CD could be noted between first and second molars when OF was applied in Group 2 (p < 0.01). Comparable level of CD increase was also observed when OF was applied in the presence of SA in Group 3 (p < 0.01) (Fig. 5C).
Figure 5. SA administration did not affect OF-induced osteoclastogenesis along alveolar bone surface adjacent to periodontal space.
(A) Periodontal tartrate-resistant acid phosphatase (TRAP) staining under different treatment conditions. R: root. B: alveolar bone. P: periodontal ligament. Bar =100 µm. The direction of force is always from R to B. The individual osteoclasts were identified at 40× magnification as multi-nucleated TRAP-positive (stained red) cells lining along the alveolar bone surface and was counted along the alveolar bone surface from the pressure side of the mid-third of the distal-buccal root of first molars. (B) Quantitative measurement of TRAP positive cells along the alveolar bone surface adjacent to periodontal ligament. TRAP-positive osteoclasts were significantly increased after orthodontic force application but such increase was not affected by the antibiotics administration. (C) The orthodontic tooth movement was assessed by measuring the closest distance (CD) between the proximal contours of first and second molars. CD measurement was increased by OF but was not changed with administration of SA. SL, silk ligature; OF, orthodontic force; SA, systemic antibiotics. *p < 0.05, **p < 0.01, ND, not detectable. n = 3–6.
DISCUSSION
In this study, we established a mouse model of orthodontic movement/ligature-induced periodontitis to determine the effect of antibiotics on PBL during OF-aggravated experimental periodontitis. Our results suggest that OF-aggravated PBL in experimental periodontitis can be abrogated by SA administration. Advantages of using mice as a model include the considerable background information on their biological system, a wide range of genetically engineered strains (e.g., gene knockouts for key receptors or signaling molecules) and availability of high-quality chemical reagents (25). Although numerous animal studies have been carried out to investigate OF on periodontal cellular responses and bone loss under physiological conditions (26–30), only a few animal studies involving orthodontic treatment in the context of periodontal disease were performed, and most of them were performed in rats but not mice (31–33), presumably due to the technical challenges having to do with the relatively small size of the teeth and oral cavity of the mouse. To our knowledge, this is the first time a mouse model was established to investigate the potential mechanism of OF-exacerbated PBL in experimental periodontitis. Using a wide range of genetically engineered mouse strains, this model can be a useful tool for the future mechanistic investigations of orthodontic movement/periodontal diseases that are associated with specific signaling pathways.
Our data demonstrated that OF worsened the PBL when periodontitis was initiated (Fig. 2, Group 2). However, administration of SA ameliorated the OF-associated PBL (Fig. 2, Group 3), likely through the control of oral bacterial load (Fig. 1A) and the subsequent bacteria-initiated inflammatory responses (34). Indeed, recent evidences suggested that the use of topical as well as systemic antibiotics is an effective adjunct to the nonsurgical periodontal treatment to suppress periodontopathogens and promote anti-inflammatory activity (35–37). Recent studies have indicated that orthodontic force induce systemic immune responses related to monocytes and T cells (38, 39). Our finding is consistent with these studies demonstrating the elevated levels of serum total IgG when OF was applied in Group 2 and Group 3 (Fig. 1B). However, such elevation of serum IgG level was not affected by the administration of SA (Fig. 1B, Group 2 vs. Group 3). This may suggest that the observed suppression of OF-associated PBL by SA is not through direct alteration of systemic immune responses, but mostly via the reduction of oral bacteria load. So far there is no report on the systemic effects of this antibiotic on monocytes/osteoclasts activities or bone metabolism. However, it has long been suggested that this antibiotic administration had no adverse effect on the immune responses, such as neutrophil and lymphocyte functions (40, 41). More extensive panel of serum markers of systemic inflammation is required for the definitive answer whether SA administration do not cause systemic immunological changes.
Our results showed that OF-induced increase of TRAP+ cells within periodontal ligament space (Fig. 5A-B) and CD measurement (Fig. 5C), two parameters associated with orthodontic tooth movement, were not affected by the administration of SA. These are in contrast to the results showing the reduced PBL (Fig. 2), gingival RANKL/OPG ratio (Fig. 3) and horizontal bone loss (Fig. 4) after SA administration. While the induction of RANKL/OPG ratio by bacterial load and orthodontic force were clearly demonstrated in this study, further studies are warranted to determine if other mechanisms, such as macrophage-elicited osteoclastogenesis through TNFα production (42, 43), are also contributed to the observed periodontal bone loss.
These findings suggest that SA administration may reduce OF-induced pathological bone loss on alveolar bone crest in experimental periodontitis. However, it should be noted that use of antibiotics in this study was intended for mechanistic exploration of OF-induced bone loss and the clinical implication of such use should be extremely cautioned. Orthodontic treatment may not be conducted in patients with active periodontal disease, which needs to be treated by mechanical therapies (10). It was indicated that active periodontitis could affect the velocity of orthodontic tooth movement in a ligature-induced experimental periodontitis model in mice (44). In addition, antibiotic therapy may have effect during its administration phase but may not prevent alveolar bone loss during the whole course of the orthodontic treatment when antibiotic treatment is ended. Furthermore, it is suggested that the slight additional benefits of adjunctive antimicrobials have to be balanced against their side effects and their prescription should be limited as much as possible (45).
It is recognized that, in adult patients with compromised periodontal conditions, an interdisciplinary approach often offers the best option for achieving a predictable outcome to solve complex clinical problems (46). Improvement of dental function and esthetics may be accomplished by periodontal treatment and maintenance followed by appropriate orthodontic treatment for patients who had experienced advanced periodontitis. Knowledge of the impacts of orthodontic treatment on periodontal status of the patient and the underlying mechanisms that contribute to these effects are useful to prevent and ameliorate periodontal breakdown that might occur during the course of orthodontic treatment. Our results suggest that reduction of oral bacterial load by antibiotic administration alleviate OF-aggravated PBL. Further studies are warranted to determine if additional factors, such as host immune responses or environmental elements, also contribute to the OF-induced PBL.
In conclusion, we established a mouse model of orthodontic movement/ligature-induced experimental periodontitis to investigate the effect of orthodontic tooth movement on periodontal tissue integrity in the context of periodontitis. It may provide teamed periodontists/orthodontists an experimental platform to evaluate different regimens and procedures proposed for periodontally compromised individuals and delineate underlying mechanisms of the observed changes in response to these treatments.
Supplementary Material
Acknowledgments
This work was supported by NIH Grant DE-023807 and DE-025255 from National Institute of Dental and Craniofacial Research.
Footnotes
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
REFERENCES
- 1.Taubman MA, Kawai T, Han X. The new concept of periodontal disease pathogenesis requires new and novel therapeutic strategies. J Clin Periodontol. 2007;34:367–369. doi: 10.1111/j.1600-051X.2007.01065.x. [DOI] [PubMed] [Google Scholar]
- 2.Cekici A, Kantarci A, Hasturk H, Van Dyke TE. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000. 2014;64:57–80. doi: 10.1111/prd.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim YI, Kim MJ, Choi JI, Park SB. A multidisciplinary approach for the management of pathologic tooth migration in a patient with moderately advanced periodontal disease. Int J Periodontics Restorative Dent. 2012;32:225–230. [PubMed] [Google Scholar]
- 4.Pinho T, Coutinho-Alves C, Neves M. Management of pathological tooth migration in patients with advanced periodontal disease. J Clin Orthod. 2013;47:520–528. quiz 559. [PubMed] [Google Scholar]
- 5.Schuback P, Vogel R, Deasy M. Occlusal trauma and inflammatory periodontal disease. Ann Dent. 1976;35:69–74. [PubMed] [Google Scholar]
- 6.Rathod SR, Kolte AP, Chintawar S. The dynamic relationship between pathological migrating teeth and periodontal disease. J Indian Soc Periodontol. 2013;17:762–764. doi: 10.4103/0972-124X.124498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinho T, Neves M, Alves C. Multidisciplinary management including periodontics, orthodontics, implants, and prosthetics for an adult. Am J Orthod Dentofacial Orthop. 2012;142:235–245. doi: 10.1016/j.ajodo.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 8.Gkantidis N, Christou P, Topouzelis N. The orthodontic-periodontic interrelationship in integrated treatment challenges: a systematic review. J Oral Rehabil. 2010;37:377–390. doi: 10.1111/j.1365-2842.2010.02068.x. [DOI] [PubMed] [Google Scholar]
- 9.Ghijselings E, Coucke W, Verdonck A, et al. Long-term changes in microbiology and clinical periodontal variables after completion of fixed orthodontic appliances. Orthod Craniofac Res. 2014;17:49–59. doi: 10.1111/ocr.12031. [DOI] [PubMed] [Google Scholar]
- 10.Wennstrom JL, Stokland BL, Nyman S, Thilander B. Periodontal tissue response to orthodontic movement of teeth with infrabony pockets. Am J Orthod Dentofacial Orthop. 1993;103:313–319. doi: 10.1016/0889-5406(93)70011-C. [DOI] [PubMed] [Google Scholar]
- 11.Freitas AO, Marquezan M, Nojima Mda C, Alviano DS, Maia LC. The influence of orthodontic fixed appliances on the oral microbiota: a systematic review. Dental Press J Orthod. 2014;19:46–55. doi: 10.1590/2176-9451.19.2.046-055.oar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andjelic J, Matijevic S. Condition of periodontium in patients with fixed orthodontic appliances. Vojnosanit Pregl. 2014;71:915–919. [PubMed] [Google Scholar]
- 13.Bollen AM, Cunha-Cruz J, Bakko DW, Huang GJ, Hujoel PP. The effects of orthodontic therapy on periodontal health: a systematic review of controlled evidence. J Am Dent Assoc. 2008;139:413–422. doi: 10.14219/jada.archive.2008.0184. [DOI] [PubMed] [Google Scholar]
- 14.Calderini A, Pantaleo G, Rossi A, Gazzolo D, Polizzi E. Adjunctive effect of chlorhexidine antiseptics in mechanical periodontal treatment: first results of a preliminary case series. Int J Dent Hyg. 2013;11:180–185. doi: 10.1111/idh.12009. [DOI] [PubMed] [Google Scholar]
- 15.Fritoli A, Goncalves C, Faveri M, et al. The effect of systemic antibiotics administered during the active phase of non-surgical periodontal therapy or after the healing phase: a systematic review. J Appl Oral Sci. 2015;23:249–254. doi: 10.1590/1678-775720140453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Apatzidou DA, Kinane DF. Nonsurgical mechanical treatment strategies for periodontal disease. Dent Clin North Am. 2010;54:1–12. doi: 10.1016/j.cden.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Bidault P, Chandad F, Grenier D. Systemic antibiotic therapy in the treatment of periodontitis. J Can Dent Assoc. 2007;73:515–520. [PubMed] [Google Scholar]
- 18.Lin J, Bi L, Yu X, et al. Porphyromonas gingivalis exacerbates ligature-induced, RANKL-dependent alveolar bone resorption via differential regulation of Toll-like receptor 2 (TLR2) and TLR4. Infect Immun. 2014;82:4127–4134. doi: 10.1128/IAI.02084-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han X, Lin X, Yu X, et al. Porphyromonas gingivalis infection-associated periodontal bone resorption is dependent on receptor activator of NF-kappaB ligand. Infect Immun. 2013;81:1502–1509. doi: 10.1128/IAI.00043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Z, Song C, Fu X, et al. High-dose diosgenin reduces bone loss in ovariectomized rats via attenuation of the RANKL/OPG ratio. Int J Mol Sci. 2014;15:17130–17147. doi: 10.3390/ijms150917130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moschen AR, Kaser A, Enrich B, et al. The RANKL/OPG system is activated in inflammatory bowel disease and relates to the state of bone loss. Gut. 2005;54:479–487. doi: 10.1136/gut.2004.044370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grimaud E, Soubigou L, Couillaud S, et al. Receptor activator of nuclear factor kappaB ligand (RANKL)/osteoprotegerin (OPG) ratio is increased in severe osteolysis. Am J Pathol. 2003;163:2021–2031. doi: 10.1016/s0002-9440(10)63560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bostanci N, Ilgenli T, Emingil G, et al. Gingival crevicular fluid levels of RANKL and OPG in periodontal diseases: implications of their relative ratio. J Clin Periodontol. 2007;34:370–376. doi: 10.1111/j.1600-051X.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- 24.Han X, Kawai T, Eastcott JW, Taubman MA. Bacterial-responsive B lymphocytes induce periodontal bone resorption. J Immunol. 2006;176:625–631. doi: 10.4049/jimmunol.176.1.625. [DOI] [PubMed] [Google Scholar]
- 25.Abe T, Hajishengallis G. Optimization of the ligature-induced periodontitis model in mice. J Immunol Methods. 2013;394:49–54. doi: 10.1016/j.jim.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujimura Y, Kitaura H, Yoshimatsu M, et al. Influence of bisphosphonates on orthodontic tooth movement in mice. Eur J Orthod. 2009;31:572–577. doi: 10.1093/ejo/cjp068. [DOI] [PubMed] [Google Scholar]
- 27.Liang Y, Zhou Y, Jiang T, Zhang Z, Wang S, Wang Y. Expression of LIF and LIFR in periodontal tissue during orthodontic tooth movement. Angle Orthod. 2011;81:600–608. doi: 10.2319/102510-622.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JY, Kim BI, Jue SS, Park JH, Shin JW. Localization of osteopontin and osterix in periodontal tissue during orthodontic tooth movement in rats. Angle Orthod. 2012;82:107–114. doi: 10.2319/030911-173.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olson C, Uribe F, Kalajzic Z, et al. Orthodontic tooth movement causes decreased promoter expression of collagen type 1, bone sialoprotein and alpha-smooth muscle actin in the periodontal ligament. Orthod Craniofac Res. 2012;15:52–61. doi: 10.1111/j.1601-6343.2011.01536.x. [DOI] [PubMed] [Google Scholar]
- 30.He D, Kou X, Yang R, et al. M1-like Macrophage Polarization Promotes Orthodontic Tooth Movement. J Dent Res. 2015;94:1286–1294. doi: 10.1177/0022034515589714. [DOI] [PubMed] [Google Scholar]
- 31.Boas Nogueira AV, Chaves de Souza JA, Kim YJ, Damiao de Sousa-Neto M, Chan Cirelli C, Cirelli JA. Orthodontic force increases interleukin-1beta and tumor necrosis factor-alpha expression and alveolar bone loss in periodontitis. J Periodontol. 2013;84:1319–1326. doi: 10.1902/jop.2012.120510. [DOI] [PubMed] [Google Scholar]
- 32.Kirschneck C, Proff P, Maurer M, Reicheneder C, Romer P. Orthodontic forces add to nicotine-induced loss of periodontal bone : An in vivo and in vitro study. J Orofac Orthop. 2015;76:195–212. doi: 10.1007/s00056-015-0283-7. [DOI] [PubMed] [Google Scholar]
- 33.Kawazoe A, Inubushi T, Miyauchi M, et al. Orally administered liposomal lactoferrin inhibits inflammation-related bone breakdown without interrupting orthodontic tooth movement. J Periodontol. 2013;84:1454–1462. doi: 10.1902/jop.2012.120508. [DOI] [PubMed] [Google Scholar]
- 34.Miyata Y, Takeda H, Kitano S, Hanazawa S. Porphyromonas gingivalis lipopolysaccharide-stimulated bone resorption via CD14 is inhibited by broad-spectrum antibiotics. Infect Immun. 1997;65:3513–3519. doi: 10.1128/iai.65.9.3513-3519.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Southward K, Bosy A. Treatment of oral malodor and periodontal disease using an antibiotic rinse. Gen Dent. 2013;61:41–45. [PubMed] [Google Scholar]
- 36.Moreno Villagrana AP, Gomez Clavel JF. Antimicrobial or subantimicrobial antibiotic therapy as an adjunct to the nonsurgical periodontal treatment: a meta-analysis. ISRN Dent. 2012;2012:581207. doi: 10.5402/2012/581207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirsch R, Deng H, Laohachai MN. Azithromycin in periodontal treatment: more than an antibiotic. J Periodontal Res. 2012;47:137–148. doi: 10.1111/j.1600-0765.2011.01418.x. [DOI] [PubMed] [Google Scholar]
- 38.Yan Y, Liu F, Kou X, et al. T Cells Are Required for Orthodontic Tooth Movement. J Dent Res. 2015;94:1463–1470. doi: 10.1177/0022034515595003. [DOI] [PubMed] [Google Scholar]
- 39.Zeng M, Kou X, Yang R, et al. Orthodontic Force Induces Systemic Inflammatory Monocyte Responses. J Dent Res. 2015;94:1295–1302. doi: 10.1177/0022034515592868. [DOI] [PubMed] [Google Scholar]
- 40.Anderson R, Grabow G, Oosthuizen R, Theron A, Van Rensburg AJ. Effects of sulfamethoxazole and trimethoprim on human neutrophil and lymphocyte functions in vitro: in vivo effects of co-trimoxazole. Antimicrob Agents Chemother. 1980;17:322–326. doi: 10.1128/aac.17.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Domer JE, Hector RF. Enhanced immune responses in mice treated with penicillin-tetracycline or trimethoprim-sulfamethoxazole when colonized intragastrically with Candida albicans. Antimicrob Agents Chemother. 1987;31:691–697. doi: 10.1128/aac.31.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papadopoulos G, Weinberg EO, Massari P, et al. Macrophage-specific TLR2 signaling mediates pathogen-induced TNF-dependent inflammatory oral bone loss. J Immunol. 2013;190:1148–1157. doi: 10.4049/jimmunol.1202511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ukai T, Yumoto H, Gibson FC, 3rd, Genco CA. Macrophage-elicited osteoclastogenesis in response to bacterial stimulation requires Toll-like receptor 2-dependent tumor necrosis factor-alpha production. Infect Immun. 2008;76:812–819. doi: 10.1128/IAI.01241-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okamoto A, Ohnishi T, Bandow K, et al. Reduction of orthodontic tooth movement by experimentally induced periodontal inflammation in mice. Eur J Oral Sci. 2009;117:238–247. doi: 10.1111/j.1600-0722.2009.00625.x. [DOI] [PubMed] [Google Scholar]
- 45.Jepsen K, Jepsen S. Antibiotics/antimicrobials: systemic and local administration in the therapy of mild to moderately advanced periodontitis. Periodontol 2000. 2016;71:82–112. doi: 10.1111/prd.12121. [DOI] [PubMed] [Google Scholar]
- 46.Panwar M, Jayan B, Arora V, Singh S. Orthodontic management of dentition in patients with periodontally compromised dentition. J Indian Soc Periodontol. 2014;18:200–204. doi: 10.4103/0972-124X.131325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.