Abstract
Background
Genetic factors influence the physical and neurobehavioral manifestations of prenatal alcohol exposure (PAE). Animal models allow the investigation of specific genes that confer vulnerability to, or protection from, birth defects associated with fetal alcohol spectrum disorders (FASDs). The objective of the present experiments was to determine if genetic alterations in the Sonic Hedgehog (Shh) signaling pathways affect the vulnerability to PAE-induced skeletal defects involving the fore- and/or hind-limbs.
Method
Wild-type C57BL/6J female mice were bred with males in which one copy of the Shh or Gli2 genes had been knocked out, to produce litters with both wild-type (+/+) and heterozygous (+/−) embryos. Alcohol doses (two injections of 2.9 g/kg, four hours apart) or vehicle were administered starting at GD-9.25, 9.5 or 9.75, a critical exposure time for inducing limb defects. Limb defects were examined at GD 17 using a dysmorphology scale based on abnormalities ranging from increased interdigital spacing to the deletion of multiple fingers and the ulna.
Results
Alcohol treatment caused a high incidence of forelimb defects, particularly on the right side, that was higher in Shh+/− and Gli2+/− fetuses compared to WT fetuses. Dysmorphology scores were also significantly higher in the Shh+/− and Gli2+/− mice.
Conclusions
These results extend previous findings demonstrating enhanced sensitivity to PAE-induced craniofacial dysmorphology and support the hypothesis that genetic alterations in the Shh signaling pathway influences the vulnerability to alcohol-induced birth defects. Moreover, these results emphasize the importance of understanding the interactions between genes and prenatal exposure to alcohol or other teratogens.
Keywords: Fetal Alcohol Syndrome, Limb Development, Oligodactyly, Ethanol, Skeletal
Introduction
Genetic and environmental interactions are increasingly recognized as modifiers of many birth defects (Eberhart and Parnell, 2016; Krauss and Hong, 2016). In the case of prenatal alcohol exposure (PAE), one of the most common and preventable teratogen exposures, genetic risk factors may help explain the considerable variability in the consequences of PAE (Smith et al., 2014). Not all individuals exposed to alcohol prenatally develop the structural birth defects and/or neurobehavioral disturbances associated with fetal alcohol spectrum disorders (FASD) and those who do often express a range of defects, from relatively minor to severe. Although the amount and timing of PAE are clear factors in this variability, identifying the contribution of critical genes to the sensitivity to PAE is an important research agenda.
Many skeletal and limb defects have been reported in clinical populations with PAE, including radioulnar synostosis, campto- and clino-dactyly, ectrodactyly, polydactyly, and amelia (Herrmann et al., 1980; Pauli and Feldman, 1986; Spiegel et al., 1979). However, a recent large scale analysis showed that light patterns of alcohol exposure were not associated with limb defects, but that heavy patterns might be associated with a subset of severe defects (Caspers Conway et al., 2014). In animal studies, where the amount and timing of PAE can be carefully controlled, limb defects can be readily observed (Chernoff, 1977; Johnson et al., 2007; Kotch et al., 1992; McMechan et al., 2004) and, on the basis of strain comparisons, there appear to be genetic influences (Boehm et al., 1997; Zimmerman et al., 1990). In humans, several gene mutations are associated with limb defects (Zuniga et al., 2012) and it is possible that some of these may interact with PAE to make individuals more vulnerable to alcohol-induced limb defects.
Limb development is a carefully regulated and complex process that begins in embryos at the 20 – 30 somite stage (forelimb and hindlimb, respectively), in the fourth week of human pregnancy (equivalent to gestational days 9–10 in the mouse). Initial limb bud outgrowth from the flank mesenchyme and the formation of the apical ectodermal ridge (AER) is governed by the coordinated actions of retinoic acid (RA), fibroblast growth factors (FGF), bone morphogenetic proteins (BMP), WNT, and Hox genes. As outgrowth continues, AER FGFs and flank RA signals establish the Sonic Hedgehog (Shh) containing zone of polarizing activity (ZPA) in the caudal (posterior) limb bud. The temporal and concentration gradients of Shh as well as its downstream interactions with Gli transcription factors further determine the size of the developing limb and specify the number, and the type, of digits (Zeller et al., 2009). PAE during limb bud induction in the mouse causes a specific post-axial deficit that can resemble those of complete Shh gene deletions and Shh pathway inhibition (Litingtung et al., 2002). Remarkably, even in very severe alcohol-induced limb defects, where the entire ulna and digits 5, 4, and 3 are absent, the thumb and second digit are not affected, as these digits normally develop in the presence of little to no Shh (Johnson et al., 2007; Kotch et al., 1992).
Interference with Shh signaling is thought be a pathogenic mechanism for several consequences of PAE, including craniofacial dysmorphology and limb defects (Ahlgren et al., 2002; Aoto et al., 2009; Johnson et al., 2007). Importantly, mice lacking a single copy of the CDON (a Shh co-receptor) gene, the Shh gene, or its downstream activator Gli2, were more sensitive to alcohol-induced midline craniofacial defects (Hong and Krauss, 2012; Kietzman et al., 2014). These results highlight the fact that gene knockdowns that are not by themselves teratogenic, can enhance the vulnerability to PAE. The experiments described herein test the hypothesis that single allele deletions of Shh or Gli2 would also enhance the vulnerability to alcohol-induced limb defects.
Methods
Animals
Dams (n = 75) were C57BL/6J mice (C57, The Jackson Laboratory, Bar Harbor, ME) mated to male Shh+/− or Gli2+/− mice (C57 mice in which one copy of the Shh or Gli2 genes had been knocked out; Kietzman et al., 2014) for one to two hours to generate heterozygous and wild-type littermates. Detecting a copulatory plug established gestational day 0 (GD 0) at the beginning of the breeding period. Except for drug administrations, the dams were not disturbed until fetus collection. Food (Isopro RMH 3000; Purina, St. Louis, MO) and water were freely available and all experiments followed NIH guidelines using methods approved by the IACUC of UNC at Chapel Hill.
Procedures
Alcohol (25% v/v ethanol in lactated Ringer’s solution) was injected intraperitoneally at a 2.9 g/kg dose starting at GD 9.25 (Shh = 13, Gli2 = 12, litters), GD 9.5 (Shh = 10, Gli2 = 8, litters), or GD 9.75 (Shh = 9, Gli2 = 9, litters), and a second alcohol injection was given four hours later. Vehicle injected mice (Shh = 9, Gli2 = 5, litters) received the equivalent volume of Ringer’s. At GD 17, fetuses were collected, examined and scored for limb dysmorphology using the following scale (Figure 1): 0, unaffected; 1, slightly wide space between 4th and 5th digit; 2, wide space between 4th and 5th digit; 3, partial 5th digit deletion; 4, 5th digit deletion; 5, 4th digit deletion; 6, 4th and 5th digit deletion; 7, multiple digit deletion with apparent absence of the ulna. Genotyping was performed from a small sample of the tail (Kietzman et al., 2014). Fetuses were fixed in 95% ethanol for photographing and skeletal staining with an Alcian blue/Alizarin red procedure modified from McLeod (McLeod, 1980). After clearing in 1% KOH, forelimbs were photographed using a MicroPublisher 5.0 camera and QCapture Imaging software. The incidence of any right or left forelimb defect was compared between alcohol-treated heterozygous and wild-type mice using a Chi-square statistic for each alcohol exposure time. Unpaired t-tests compared the severity of limb defects among affected heterozygous and wild-type individuals, using the most severe defect on either the right or left forelimb. Dysmorphology scores (0 – 7) were further analyzed with two-way (Day x Genotype) ANOVAs.
Figure 1.
Dysmorphology scale for quantifying limb defects after alcohol treatment. Top Panels: Right forelimbs from GD 17 fetuses after fixation. Bottom Panels: The same forelimbs following Alcian Blue and Alizarin Red staining for cartilage and ossified bone, respectively. The colored arrow indicates the score for each defect ranging from 0 to 7: 0, unaffected; 1, slightly wide space between 4th and 5th digit; 2, wide space between 4th and 5th digit; 3, partial 5th digit deletion; 4, 5th digit deletion; 5, 4th digit deletion; 6, 4th and 5th digit deletion; 7, multiple digit deletion with apparent absence of the ulna. Note the appearance of an extra cartilaginous outgrowth in panel 7, black arrow.
Results
A total of 108 Shh+/−, 130 Shh+/+, 130 Gli2+/−, 95 Gli2+/+ alcohol-treated and 28 Shh+/−, 25 Shh+/+, 21 Gli2+/−, 17 Gli2+/+ vehicle-treated embryos were examined. No limb defects were observed in any of the vehicle-treated control or heterozygous mice (data not shown). However, post-axial, but not pre-axial, limb reduction defects (Figure 1) were observed following each of the alcohol exposure times; these tended to be more frequent and severe after the GD 9.25 or 9.5 exposures, rather than the 9.75 exposures (Figure 2). Most defects were on the right forelimb, and to a lesser degree, the left forelimb; three hindlimb oligodactylies were observed in Shh+/− mice after the GD 9.5 or GD 9.75 exposure. As previously reported (Kotch et al., 1992), there were occasional polydactylies; three hindlimb (one post-axial, two pre-axial) and three forelimb post-axial polydactylies were observed in Shh+/+ mice after the GD 9.5 exposure and one forelimb post-axial polydactyly was observed in a Gli2+/+ mouse after GD 9.25 exposure. Additionally, three GD 9.25-treated, one GD 9.75-treated Gli2+/− mice, and one GD 9.75-treated Gli+/+ mouse had an extra cartilaginous nub attached to a digit or in one case, the presumed elbow (Figure 1, Defect 7). Polydactylies were not included in the analysis because of their low incidence.
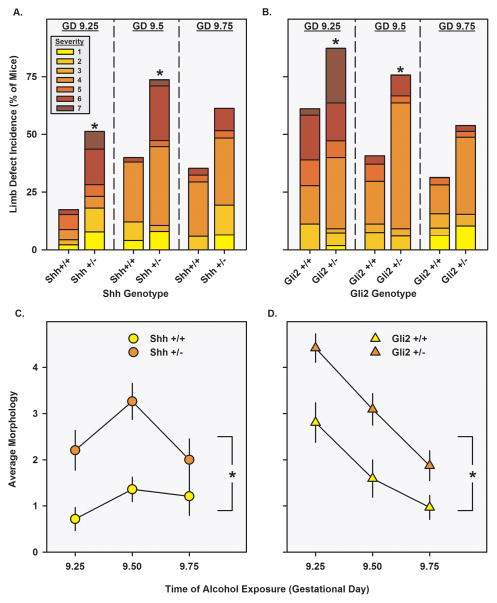
Figure 2.
Incidence and severity of alcohol-induced limb defects. Panels A. and B. portray the incidence of either a left or a right forelimb defect (filled bars) following alcohol exposure on GD 9.25, GD 9.5, or GD 9.75 in Shh+/+ or Shh+/− mice (panel A.) and in Gli2+/+ or Gli2+/− mice (panel B.) The incidence of the most severe left or right forelimb defect is portrayed in colors graded from yellow (least affected, 1) to dark red (most affected, 7). Asterisks denote significant incidence differences between +/+ and +/− mice following Chi square tests (p<0.05). Panels C. and D. portray the average morphology scores (± SEM, vertical lines) for each genotype following alcohol exposure (Panel C.: Shh+/+, yellow circles; Shh+/−, orange circles. Panel D.: Gli2+/+, yellow triangles; Gli2+/−, orange triangles). Asterisks denote significant morphology differences between +/+ and +/− mice following a main effect of genotype (p<0.05).
Relative to Shh+/+ and Gli2+/+ mice, Shh+/− and Gli2+/− mice had a significantly greater incidence of limb defects following the GD 9.25 (Shh χ=9.5; p=0.002, Gli2 χ=7.0; p=0.008) and 9.5 (Shh χ=8.6; p=0.003, Gli2 χ=6.2; p=0.01) exposures (Figures 2A and 2B). Among affected individuals, Shh+/− mice more severe defects following the GD 9.5 exposure (t46= 2.3; p=0.03), but the severity of affected mice was not significantly different between Gli2+/− and Gli2+/+ mice. Analyzing the data using individual dysmorphology scores, indexing a combination of incidence and severity, there were significant main effects of Shh and Gli2 genotype (F1,232=25.3; p<0.001 and F1,216=20.8; p<0.001, respectively), such that heterozygotes were more sensitive to alcohol-induced limb defects than were their wildtype littermates. Similar results were found when dysmorphology scores were analyzed using the genotype means from each litter. Surprisingly, the Gli2+/+ mice had a higher incidence of limb defects after GD 9.25 treatment, compared to the Shh+/+ mice and prior C57 studies. Since the Shh+/+ mice were most sensitive to GD 9.5 and GD 9.75 exposures, we hypothesized that Gli2+/− -mated litters may have been developmentally advanced, as compared to the Shh+/−-mated litters. However, a somite staging experiment revealed that Gli2+/− -mated litters were only one somite pair older (2 hours) than were Shh+/−-mated litters, indicating that embryo age does not entirely account for the difference. Further studies of alcohol sensitivity in Gli2+/− -mated litters may uncover important paternally-derived contributions to PAE sensitivity.
Discussion
Many PAE effects on the brain, face, and limbs resemble those of genetic syndromes involving defects in Shh signaling, and alterations in this pathway may be critical pathophysiological mechanisms of PAE. The current study demonstrates forelimb defects in a mouse model following discrete PAE on the ninth gestational day (20–30 somite pairs, approximately the fourth gestational week in humans), confirming that limb bud induction is a sensitive exposure period for severe limb defects. Most importantly, the current study identifies a genetic vulnerability to alcohol-induced limb defects, as mice containing a single Shh or Gli2 allele were more likely to develop limb defects than were their littermates with both copies of the genes. These results extend previous findings of Shh pathway genetic vulnerabilities to alcohol-induced craniofacial defects (Hong and Krauss, 2012; Kietzman et al., 2014) and reiterate the importance of understanding the complex interactions between alcohol and Shh.
Previous mouse studies have revealed that PAE during GD 9 induces excessive cell death in the AER and downregulates Shh expression in the ZPA, an effect that may be secondary to alcohol’s hypothesized inhibition of RA synthesis, FGFs, or cholesterol modification (Chrisman et al., 2004; Duester, 1991; Johnson et al., 2007; Kotch et al., 1992; Li et al., 2007). Excessive cell death has not been noted in the ZPA, which suggests that, at least in the forelimb, PAE does not kill Shh expressing cells. In fact, Shh is not fully expressed in the C57 mouse limb bud until GD 10, a time when severe limb defects are very rare following PAE and likely occur only in the youngest stage embryos (Johnson et al., 2007). Interestingly, the latest exposure period (GD 9.75), which would be expected to overlap with high levels of Shh expression, was the least sensitive to genotype influences, further suggesting that direct Shh inhibition is not the primary pathogenic mechanism of PAE. Rather, Shh pathway genetic vulnerabilities may reflect intermediate mechanisms between the initial PAE insult and the subsequent structural development of the limb. Alcohol undoubtedly affects multiple mechanisms in limb development, but the current results indicate that allelic variations in the Shh signaling pathway can alter genetic vulnerabilities to alcohol pathogenesis.
The presence of limb defects in clinical PAE populations may represent an important gene by environment interaction that should be studied further (May et al., 2010). Given the number of genes directly linked to limb defects (Zuniga et al., 2012), it is likely that many limb specific genes will interact with PAE. However, genes that affect Shh signaling, or mutations of the FGFs, BMPs, and WNTs, as well as their respective transcription factors, are strong potential candidate genes relevant for PAE-induced limb defects. There is significant conservation between the roles of Shh signaling in the development of the limb and craniofacies and brain; and converging evidence from both craniofacial and limb defects suggests that Shh signaling is a major modifier of PAE. Larger gene sequencing studies have so far focused on understanding the genetic contributions to craniofacial defects (Eberhart and Parnell, 2016). However, given the well-defined role of Shh in the limb, studying limb defects will be useful for understanding how PAE interacts with the Shh pathway. Furthermore, a genetic vulnerability involved in several PAE effects, such as craniofacial, limb dysmorphology, and neurobehavioral alterations, is likely to be one with a strong mechanistic role in FASD. Continued investigation of the interactions between Shh genotypes and PAE will help determine the full extent of the role of Shh signaling in FASD.
Acknowledgments
The authors thank Deborah Dehart for her consultation on optimizing the skeletal staining. This work was supported by NIH U54-AA019756, K99/R00-AA018697, and U01-AA021651. All or part of this work was done in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which is funded by grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). Additional information about CIFASD can be found at www.cifasd.org. Additional funding was from Golden LEAF Foundation and the BIOIMPACT Initiative of the State of North Carolina through then Biomanufacturing Research Institute & Technology Enterprise (BRITE) Center for Excellence at North Carolina Central University. The authors report no conflict of interests.
References
- Ahlgren SC, Thakur V, Bronner-Fraser M. Sonic hedgehog rescues cranial neural crest from cell death induced by ethanol exposure. Proc Natl Acad Sci U S A. 2002;99(16):10476–10481. doi: 10.1073/pnas.162356199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoto K, Shikata Y, Imai H, Matsumaru D, Tokunaga T, Shioda S, Yamada G, Motoyama J. Mouse Shh is required for prechordal plate maintenance during brain and craniofacial morphogenesis. Dev Biol. 2009;327(1):106–120. doi: 10.1016/j.ydbio.2008.11.022. [DOI] [PubMed] [Google Scholar]
- Boehm SL, 2nd, Lundahl KR, Caldwell J, Gilliam DM. Ethanol teratogenesis in the C57BL/6J, DBA/2J, and A/J inbred mouse strains. Alcohol. 1997;14(4):389–395. doi: 10.1016/s0741-8329(97)87950-5. [DOI] [PubMed] [Google Scholar]
- Caspers Conway KM, Romitti PA, Holmes L, Olney RS, Richardson SD National Birth Defects Prevention S. Maternal periconceptional alcohol consumption and congenital limb deficiencies. Birth Defects Res A Clin Mol Teratol. 2014;100(11):863–876. doi: 10.1002/bdra.23292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernoff GF. The fetal alcohol syndrome in mice: an animal model. Teratology. 1977;15(3):223–229. doi: 10.1002/tera.1420150303. [DOI] [PubMed] [Google Scholar]
- Chrisman K, Kenney R, Comin J, Thal T, Suchocki L, Yueh YG, Gardner DP. Gestational ethanol exposure disrupts the expression of FGF8 and Sonic hedgehog during limb patterning. Birth Defects Res A Clin Mol Teratol. 2004;70(4):163–171. doi: 10.1002/bdra.20019. [DOI] [PubMed] [Google Scholar]
- Duester G. A hypothetical mechanism for fetal alcohol syndrome involving ethanol inhibition of retinoic acid synthesis at the alcohol dehydrogenase step. Alcohol Clin Exp Res. 1991;15(3):568–572. doi: 10.1111/j.1530-0277.1991.tb00562.x. [DOI] [PubMed] [Google Scholar]
- Eberhart JK, Parnell SE. The Genetics of Fetal Alcohol Spectrum Disorders. Alcohol Clin Exp Res. 2016;40(6):1154–1165. doi: 10.1111/acer.13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann J, Pallister PD, Opitz JM. Tetraectrodactyly and other skeletal manifestations in the fetal alcohol syndrome. Eur J Pediatr. 1980;133(3):221–226. doi: 10.1007/BF00496080. [DOI] [PubMed] [Google Scholar]
- Hong M, Krauss RS. Cdon mutation and fetal ethanol exposure synergize to produce midline signaling defects and holoprosencephaly spectrum disorders in mice. PLoS Genet. 2012;8(10):e1002999. doi: 10.1371/journal.pgen.1002999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CS, Zucker RM, Hunter ES, 3rd, Sulik KK. Perturbation of retinoic acid (RA)-mediated limb development suggests a role for diminished RA signaling in the teratogenesis of ethanol. Birth Defects Res A Clin Mol Teratol. 2007;79(9):631–641. doi: 10.1002/bdra.20385. [DOI] [PubMed] [Google Scholar]
- Kietzman HW, Everson JL, Sulik KK, Lipinski RJ. The teratogenic effects of prenatal ethanol exposure are exacerbated by Sonic Hedgehog or GLI2 haploinsufficiency in the mouse. PLoS One. 2014;9(2):e89448. doi: 10.1371/journal.pone.0089448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotch LE, Dehart DB, Alles AJ, Chernoff N, Sulik KK. Pathogenesis of ethanol-induced limb reduction defects in mice. Teratology. 1992;46(4):323–332. doi: 10.1002/tera.1420460403. [DOI] [PubMed] [Google Scholar]
- Krauss RS, Hong M. Gene-Environment Interactions and the Etiology of Birth Defects. Curr Top Dev Biol. 2016;116:569–580. doi: 10.1016/bs.ctdb.2015.12.010. [DOI] [PubMed] [Google Scholar]
- Li YX, Yang HT, Zdanowicz M, Sicklick JK, Qi Y, Camp TJ, Diehl AM. Fetal alcohol exposure impairs Hedgehog cholesterol modification and signaling. Lab Invest. 2007;87(3):231–240. doi: 10.1038/labinvest.3700516. [DOI] [PubMed] [Google Scholar]
- Litingtung Y, Dahn RD, Li Y, Fallon JF, Chiang C. Shh and Gli3 are dispensable for limb skeleton formation but regulate digit number and identity. Nature. 2002;418(6901):979–983. doi: 10.1038/nature01033. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP, Smith M, Tabachnick BG, Robinson LK, Manning M, Cecanti M, Jones KL, Khaole N, Buckley D, Kalberg WO, Trujillo PM, Hoyme HE. Population differences in dysmorphic features among children with fetal alcohol spectrum disorders. J Dev Behav Pediatr. 2010;31(4):304–316. doi: 10.1097/DBP.0b013e3181dae243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod MJ. Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. Teratology. 1980;22(3):299–301. doi: 10.1002/tera.1420220306. [DOI] [PubMed] [Google Scholar]
- McMechan AP, O’Leary-Moore SK, Morrison SD, Hannigan JH. Effects of prenatal alcohol exposure on forepaw digit length and digit ratios in rats. Dev Psychobiol. 2004;45(4):251–258. doi: 10.1002/dev.20035. [DOI] [PubMed] [Google Scholar]
- Pauli RM, Feldman PF. Major limb malformations following intrauterine exposure to ethanol: two additional cases and literature review. Teratology. 1986;33(3):273–280. doi: 10.1002/tera.1420330304. [DOI] [PubMed] [Google Scholar]
- Smith SM, Garic A, Berres ME, Flentke GR. Genomic factors that shape craniofacial outcome and neural crest vulnerability in FASD. Front Genet. 2014;5:224. doi: 10.3389/fgene.2014.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel PG, Pekman WM, Rich BH, Versteeg CN, Nelson V, Dudnikov M. The orthopedic aspects of the fetal alcohol syndrome. Clin Orthop Relat Res. 1979;(139):58–63. [PubMed] [Google Scholar]
- Zeller R, Lopez-Rios J, Zuniga A. Vertebrate limb bud development: moving towards integrative analysis of organogenesis. Nat Rev Genet. 2009;10(12):845–858. doi: 10.1038/nrg2681. [DOI] [PubMed] [Google Scholar]
- Zimmerman EF, Scott WJ, Jr, Collins MD. Ethanol-induced limb defects in mice: effect of strain and Ro15-4513. Teratology. 1990;41(4):453–462. doi: 10.1002/tera.1420410410. [DOI] [PubMed] [Google Scholar]
- Zuniga A, Zeller R, Probst S. The molecular basis of human congenital limb malformations. Wiley Interdiscip Rev Dev Biol. 2012;1(6):803–822. doi: 10.1002/wdev.59. [DOI] [PubMed] [Google Scholar]