Abstract
In the intestines, the nuclear receptors Fxr and Pxr regulate the transcription of metabolizing enzymes and transporters that dictate the absorption of nutrients and xenobiotics.
Here, we sought to determine whether Fxr and Pxr signaling pathways are disrupted in response to high circulating concentrations of steroid hormones late in pregnancy leading to altered transporter expression. To test this, ileum were collected from virgin and pregnant C57BL/6 mice on gestation days 14, 17 and 19.
Ileum from pregnant mice exhibited suppression of Fgf15 and Cyp3a11 mRNAs, which are the prototypical target genes for Fxr and Pxr, respectively. An overall reduction in the expression of apical efflux transporters, including Mdr1, Mrp2 and Bcrp, was observed in pregnant mice. To assess the ability of steroid hormones to alter intestinal nuclear receptor signaling, transporter mRNA expression was quantified in intestinal LS174T adenocarcinoma cells. In vitro data demonstrated that progestins reduced CYP3A4, MDR1 and MRP2 mRNA expression by 30 to 40%.
These data suggest that progesterone may act as a mediator to negatively regulate efflux transporter expression in the mouse ileum during pregnancy possibly by reducing Pxr/PXR signaling. This may affect drug absorption and disposition during pregnancy.
Keywords: Pxr, efflux transporters, intestine, pregnancy, progestins
Introduction
The small intestine represents the interface where nutrients are absorbed into the body. Importantly, intestinal absorption can be a rate-limiting step for the uptake of orally-ingested chemicals and drugs. Several efflux transporters are located on the apical surface of enterocytes and can decrease xenobiotic bioavailability. These transporters include the multidrug resistance transporter, human MDR1/mouse Mdr1a (Rost et al. 2002; Ujhazy et al. 2001), the multidrug resistance-associated protein, MRP2/Mrp2 (Mottino et al. 2000; Rost et al. 2002), and the breast cancer resistance protein, BCRP/Bcrp (Jonker et al. 2002; Maliepaard et al. 2001). These transporters export a wide range of substrates and can reduce the amount of a chemical that reaches the systemic circulation. Additional intestinal transporters, including MRP3/Mrp3 (Mutch et al. 2004; Rost et al. 2002; Scheffer et al. 2002) and the organic solute transporter heterodimer, OST/Ostα/β (Dawson et al. 2005; Rao et al. 2008), are tasked with secreting endogenous and exogenous compounds from the enterocyte into the portal circulation, where they then travel to the liver.
It has been reported that gastrointestinal motility is reduced in women with advancing gestation, leading to an increase in gastric residence time and prolonged opportunity for the uptake of nutrients (Parry et al. 1970). This adaptation has also been observed in pregnant mice, pseudopregnant mice, and male rats treated with progesterone, suggesting that, similar to humans, this hormone plays a key regulatory role (Datta et al. 1974; Liu et al. 2002; Wald et al. 1981; Wald et al. 1982). Prior studies have demonstrated the ability of steroid hormones to specifically influence hepatic drug metabolizing enzyme and transporter expression (reviewed in Jeong 2010). In the third trimester of pregnancy, estradiol and progesterone reach peak concentrations of 0.1 and 1 μM (Jeong 2010), respectively. Recently, levels of progesterone metabolites were detected in healthy pregnant women at concentrations of 5–7 μM (non-pregnant value 0.08 μM) (Abu-Hayyeh et al. 2010; Abu-Hayyeh et al. 2013). While the effect of these hormones on metabolism and transport in the liver has been studied extensively in vivo and in vitro, little data exists regarding their impact in the intestine. Further, the ethical concerns and limited availability of tissues from pregnant women necessitate the use of a combination of in vivo and in vitro techniques to answer questions regarding metabolic changes in this special population.
The farnesoid X receptor (FXR/Fxr) and pregnane X receptor (PXR/Pxr) regulate bile acid homeostasis, in addition to genes that are responsible for the metabolism and excretion of xenobiotics. Both nuclear receptors are highly expressed in the liver and small intestine, and respond directly or indirectly to hormone exposure. In the ileum, Fxr induces the expression of the fibroblast growth factor 15 (Fgf15), small heterodimer partner (Shp) and intestinal bile acid binding protein (I-babp), important factors that regulate bile acid trafficking and synthesis (Inagaki et al. 2005; Oelkers and Dawson 1995). Fxr also controls bile acid efflux into the portal circulation through direct transactivation of the Ostα/β genes (Frankenberg et al. 2006; Landrier et al. 2006). Pxr has many target genes involved in endobiotic and xenobiotic disposition, including the human Cytochrome P450 (CYP) 3A4/mouse Cyp3a11. In addition to Cyp3a11, Pxr can regulate the expression of apical and basolateral efflux transporters in the small intestine, including Mdr1, Mrp2/3, and Bcrp (Jigorel et al. 2006; Martin et al. 2008). Alterations to both the intestinal Fxr and Pxr signaling pathways during pregnancy may have critical implications for bile acid and xenobiotic disposition.
The LS174T cell line has been gaining use as an in vitro human intestinal model that more stably expresses a number of nuclear receptors, drug metabolizing enzymes, and xenobiotic transporters compared to the Caco-2 cell line (Pfrunder et al. 2003). Modulation of MDR1 expression, localization, and function by the PXR prototypical inducer rifampicin and the inhibitor ketoconazole has been thoroughly explored in naïve LS174T cells (Kota et al. 2010). Moreover, the induction of FGF19, I-BABP, and SHP gene expression by FXR agonists has been established in LS174T cells transiently transfected with the human FXR gene (LS174T-FXR) (Vaquero et al. 2013). While LS174T cells required transfection with the FXR gene to probe its activity, FXR expression in Caco-2 cells is dependent on a high degree of differentiation and extended time in culture (De Gottardi et al. 2004; Vaquero et al. 2013). Important to investigating sex hormone-mediated regulation of disposition genes is the fact that naïve LS174T cells express the functional proteins of both the estrogen and progesterone receptors (Hendrickse et al. 1993).
The liver has been the primary tissue investigated to better understand how pregnancy influences drug metabolism and transport. Mechanistic studies have extensively described the interaction of steroid and placental hormones with hepatic enzymes and transporters. However, there is a need to understand the molecular adaptations in intestinal nuclear receptor pathways such as Fxr and Pxr during pregnancy. The purpose of the current study was to 1) determine the temporal expression of key ileal efflux transporters regulated by Fxr and Pxr during late pregnancy in mice and 2) identify potential sex hormones that mediate pregnancy-related changes in efflux transporters using an intestinal cell line.
Materials and methods
Chemicals
Unless otherwise specified, chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Animal treatment
Adult male and female C57BL/6 mice (strain 027) were purchased from Charles River Laboratories at 8–12 weeks of age (Wilmington, MA). All mice were housed in an Association for Assessment and Accreditation of Laboratory Animal Care accredited animal care facility in temperature-, light- and humidity-controlled rooms. Studies were approved by the Rutgers University Institutional Animal Care and Use Committee, and were in accordance with national guidelines. A subset of female mice were mated overnight with male mice and checked for the presence of a vaginal sperm plug the next morning (designated gestation day 0). The remaining female mice were used as virgin controls. Mice had access to standard chow and water ad libitum. Ileum of the small intestines were collected from virgin and pregnant time-matched mice on gestation days 14, 17 and 19, snap frozen in liquid nitrogen and stored at −80°C. Ileal fragments were utilised for all studies.
Cell culture and treatment
Human colon adenocarcinoma LS174T cells (ATCC, Manassas, VA) were cultured in 24-well plates with phenol red-free Minimum Essential Media (Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum at 37°C in an atmosphere containing 5% CO2. On day 4, cells were transiently transfected with the pCMV-ICIS human NR1H4/FXR plasmid (LS174T-FXR, Open Biosystems, Huntsville, AL) (Li et al., 2012) using Lipofectamine LTX according to the manufacturer’s recommendations (ThermoFisher Scientific, Rockford, IL). On day 5, cells were cultured in media supplemented with 10% charcoal stripped fetal bovine serum and treated with vehicle (0.1% DMSO), 0.1 μM 17β-estradiol, 1 μM progesterone, 7 μM epiallopregnanolone-sulfate (PM5S, Steraloids, Newport, RI), 0.3 μM placental lactogen (NHPP, Torrance, CA), 13.3 nM testosterone, 7 pM growth hormone, 0.8 μM cortisol or 50 nM dehydroepiandrosterone (Steraloids), concentrations that represent third trimester levels in humans (Abu-Hayyeh et al., 2010; Jeong, 2010). Total RNA was collected from cells on day 6.
RNA isolation and quantitative PCR
Total RNAs were extracted from mouse ilea and LS174T-FXR cells using the RNeasy Mini Kit (Qiagen, Valencia, CA), and complementary DNA (cDNA) was generated using High Capacity cDNA Synthesis (Applied Biosystems, Foster City, CA). RNA purity and concentration were assessed using a NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific). Messenger RNA expression was quantified by real time-qPCR using the SYBR Green-based method (Applied Biosystems) for detection of amplified products. qPCR was performed in a 384-well plate format using the ViiA7 Real Time PCR machine (Life Technologies, Grand Island, NY). Ct values were converted to delta delta Ct values by adjusting to a reference gene (Ribosomal Protein L13A, RPL13A/Rpl13a) (Livak and Schmittgen 2001). Specific forward and reverse primer sequences are listed in Table 1.
Table 1.
qPCR primer sequences
| Primer | Forward (5′ to 3′) | Reverse (5′ to 3′) |
|---|---|---|
| MOUSE | ||
|
| ||
| Cyp3a11 | CCCAGTGGGGATAATGAGTAA | CTTGCCTTTCTTTGCCTTCT |
| Fgf15 | GCCATCAAGGACGTCAGCA | CTTCCTCCGAGTAGCGAATCAG |
| Fxrα | TCCGGACATTCAACCATCAC | TCACTGCACATCCCAGATCTC |
| I-babp | CCCCAACTATCACCAGACTTC | ACATCCCCGATGGTGGAGAT |
| Mdr1a | TGCCCCACCAATTTGACACCCT | ATCCAGTGCGGCCTGAACCA |
| Mrp2 | AGCAGGTGTTCGTTGTGTGT | AGCCAAGTGCATAGGTAGAGAAT |
| Mrp3 | CTGGGTCCCCTGCATCTAC | GCCGTCTTGAGCCTGGATAAC |
| Ostα | CACTGGCTCAGTTGCCATTT | GCATACGGCATAAAACGAGGT |
| Ostβ | GCAAACAGAAATCGAAAGAAGC | TCTGGCAGAAAGACAAGTGAT |
| Pxr | GCAGCATCTGGAACTACCAA | CTGGTCCTCAATAGGCAGGT |
| Rpl13a | CAAGAAAAAGCGGATGGTGG | TCCGTAACCTCAAGATCTGC |
| Shp | CGATCCTCTTCAACCCAGATG | AGGGCTCCAAGACTTCACACA |
|
| ||
| HUMAN | ||
|
| ||
| CYP3A4 | CACAAACCGGAGGCCTTTTGGTC | GTCTCTGCTTCCCGCCTCAGAT |
| FGF19 | TCGGAGGAAGACTGTGCTTTC | CCTCTCGGATCGGTACACATTG |
| FXRα | CGCCTGACTGAATTACGGACA | TCACTGCACGTCCCAGATTTC |
| I-BABP | CTCCAGCGATGTAATCGAAA | CCCCCATTGTCTGTATGTTG |
| MDR1 | TTGAAATGAAAATGTTGTCTGG | CAAAGAAACAACGGTTCGG |
| MRP2 | AGCCATGCAGTTTTCTGAGGCCT | TGGTGCCCTTGATGGTGTGC |
| MRP3 | CTTCCTGGTGACCCTGATCACCCT | TGCTGGATCCGTTTCAGAGACACA |
| OSTα | GGAGCACAGCTCTATGGATCA | TCAGGATGAGGAGAACCTGGA |
| PXR | ATCTCCTACTTCAGGGACTT | AGCTTCTTCAGCATGTAGTG |
| RPL13A | GGTGCAGGTCCTGGTGCTTGA | GGCCTCGGGAAGGGTTGGTG |
| SHP | ATCCTCTTCAACCCCGATGTG | GTCGGAATGGACTTGAGGGT |
Western blot analysis
Ilea were homogenised in sucrose-Tris buffer (pH=7.4–7.5) using the TissueLyser LT Adapter (Qiagen), per the manufacturer’s protocol. Protein concentrations were determined by the BCA assay (Pierce Biotechnology, Rockford, IL). Forty micrograms of homogenate were loaded onto a SDS-PAGE gel (8%, Life Technologies). Semi-quantification of expression was determined using primary antibodies raised against Mdr1 (1:2000, C219, Abcam, Cambridge, MA), Mrp2 (1:600, Mrp2III-5, Alexis), Bcrp (1:5000, BXP-53, Alexis), Ostα (1:1000) and Ostβ (1:1000) followed by incubation with a species-appropriate secondary antibody (Aleksunes et al. 2012; Rao et al. 2008). Primary antibodies for Ostα and Ostβ were supplied by Dr. Paul Dawson (Emory University, Atlanta, GA). The intensity of band luminescence was acquired using a FluorChem E System Imager (ProteinSimple, Santa Clara, CA). Na+/K+ ATPase (1:2000, sc-28800, Santa Cruz) was used as a loading control.
Statistical analysis
Data are presented as mean ± SE. Statistical analysis was performed using GraphPad Prism v6 (La Jolla, CA). Messenger RNA and protein expression were analyzed by student’s t-test (in vivo studies) or 1-way ANOVA followed by a Newman-Keul’s multiple comparison post-hoc test (in vitro studies), to compare overall mean differences between groups. Significance was set at p≤0.05.
Results
Expression of Intestinal Signaling Pathways in Pregnant Mice
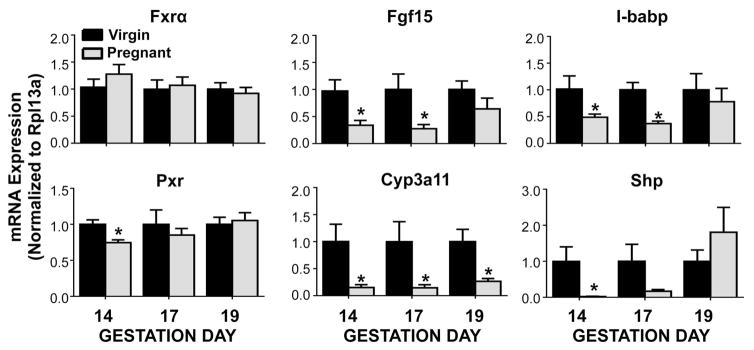
Similar to previous reports analyzing livers from pregnant mice (Milona et al., 2010a; Aleksunes et al., 2013), the mRNA expression of the nuclear receptor, Fxr, was unchanged in ileum on gestation days 14 through 19 (Figure 1). Though Fxr expression was unchanged, the expression of all three of its target genes, including Fgf15, I-babp, and Shp was suppressed on gestation days 14 and 17 by 50–95%, but approached virgin control levels by gestation day 19. Pxr mRNA was slightly reduced by 25% in the ileum of mice on gestation day 14. Likewise, mRNA expression of the prototypical Pxr target gene, Cyp3a11, was down-regulated by 75 to 85% at all three gestational time points.
Figure 1.
Gene expression of nuclear receptor signaling pathways in pregnant mice. mRNA expression was quantified in ileum of virgin and pregnant mice on gestation days 14, 17 and 19. Data were normalised to virgin controls (set to 1.0) at each time point and presented as mean relative expression ± SE (n=5–6). Black bars represent virgin mice, and grey bars represent pregnant mice. Asterisks (*) represent statistically significant difference (p ≤ 0.05) compared with time-matched virgin controls.
Expression of Intestinal Efflux Transporters in Pregnant Mice
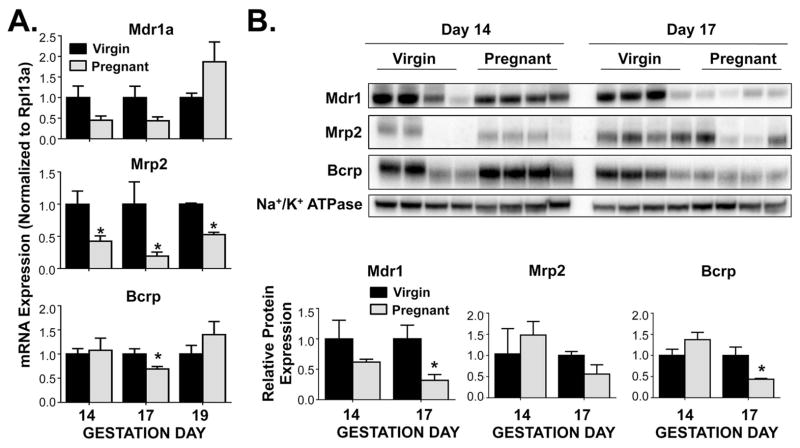
Efflux transporter mRNA and protein expression profiles varied across time points in pregnant mice. Interestingly, there was a down-regulation of the luminal efflux transporters Mdr1a, Mrp2, and Bcrp (Figure 2). There was a trend for decreased Mdr1a mRNA on gestation days 14 and 17. Likewise, Mdr1 protein expression was suppressed on gestation day 17 by 70% when compared to virgin controls. Mrp2 mRNA expression was significantly reduced by 60%, 70% and 45% on gestation days 14, 17 and 19, respectively. Both Bcrp mRNA and protein expression were decreased 30% and 55%, respectively, on gestation day 17 compared to time-matched virgin controls.
Figure 2.
Expression of ileal apical efflux transporters in pregnant mice. (A) mRNA and (B) protein expression were quantified in ileum of virgin and pregnant mice on gestation days 14, 17 and 19 (mRNA) or gestation days 14 and 17 (protein). Western blot staining was performed using whole ileal homogenates. Representative images are shown. Band density was semi-quantified. Data were normalised to virgin controls (set to 1.0) at each time point and presented as mean relative expression ± SE (n=5–6 for mRNA, n=4 for protein). Black bars represent virgin mice, and grey bars represent pregnant mice. Asterisks (*) represent statistically significant difference (p ≤ 0.05) compared with time-matched virgin controls.
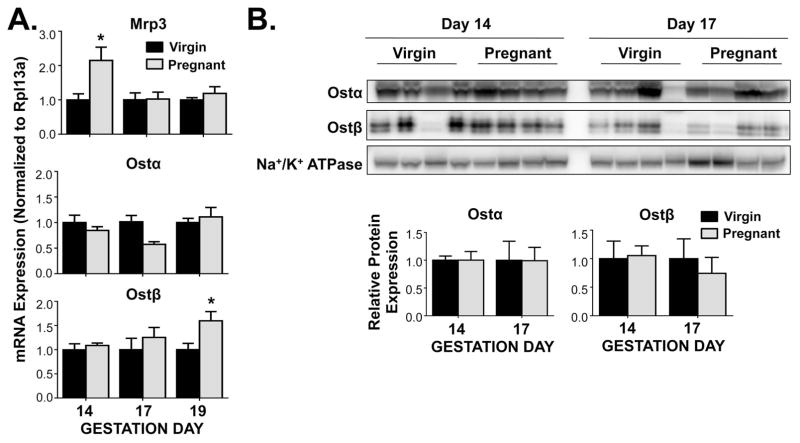
Ileal expression of the basolateral efflux transporters Mrp3, Ostα, and Ostβ was differentially regulated during gestation (Figure 3). Mrp3 mRNA levels were enhanced 2-fold on gestation day 14, returning to control levels on days 17 and 19. Ostα mRNA expression tended to be decreased on gestation day 17 by 40%, though mRNA of its heterodimerizing partner, Ostβ, steadily increased throughout gestation, with a notable 1.6-fold up-regulation on gestation day 19. However, no changes in the expression of Ostα and Ostβ proteins were observed.
Figure 3.
Expression of ileal basolateral efflux transporters in pregnant mice. (A) mRNA and (B) protein expression were quantified in ileum of virgin and pregnant mice on gestation days 14, 17 and 19 (mRNA) or gestation days 14 and 17 (protein). Western blot staining was performed using whole ileal homogenates. Representative images are shown. Band density was semi-quantified. Data were normalised to virgin controls (set to 1.0) at each time point and presented as mean relative expression ± SE (n=5–6 for mRNA, n=4 for protein). Black bars represent virgin mice, and grey bars represent pregnant mice. Asterisks (*) represent statistically significant difference (p ≤ 0.05) compared with time-matched virgin controls.
Expression of Intestinal Signaling Pathways in Sex Hormone-Treated LS174T-FXR Cells
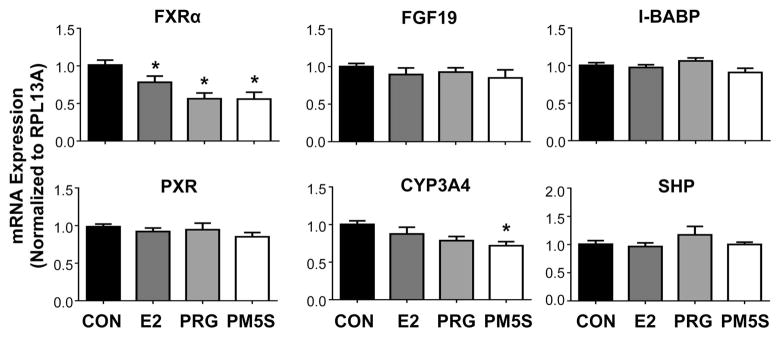
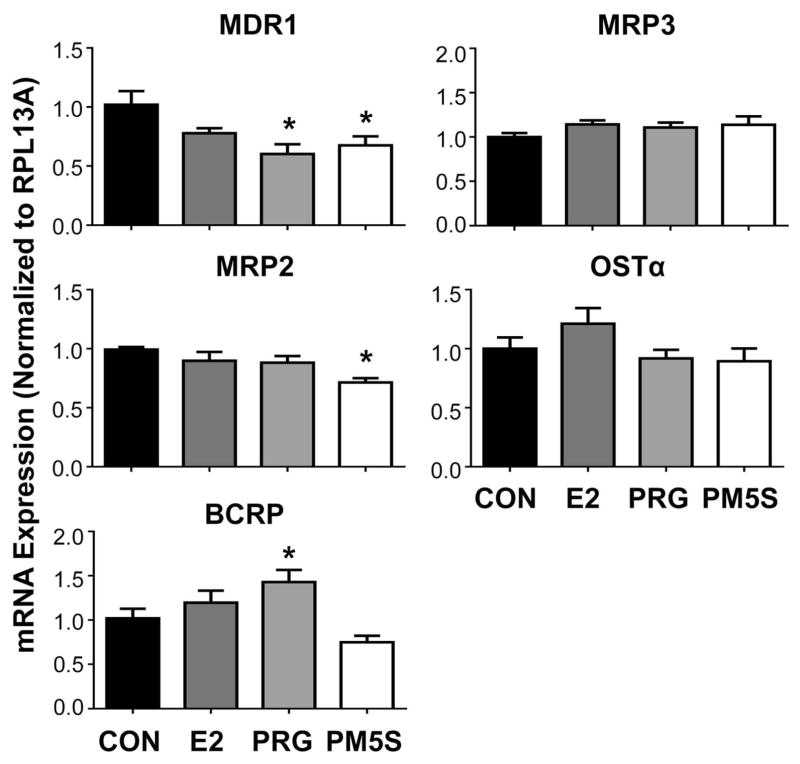
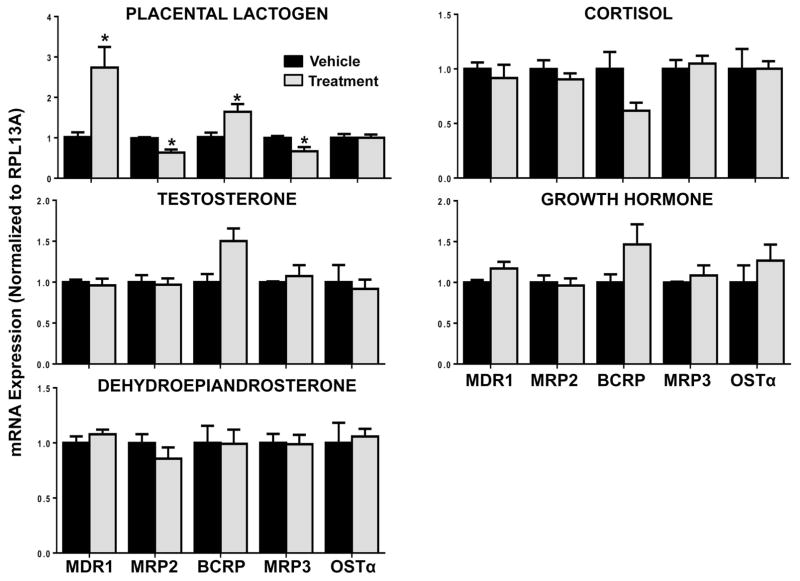
Unlike in pregnant mice, FXRα mRNA was reduced by treatment with the individual hormones estradiol (20%), progesterone (45%) and PM5S (45%) in LS174T-FXR cells (Figure 4). However, no reduction in downstream target gene expression (FGF19, I-BABP, SHP) was noted. Messenger RNA levels of CYP3A4, the prototypical target gene of human PXR and ortholog of Cyp3a11, were reduced by 30% with PM5S treatment, but not progesterone. Likewise, MDR1 and MRP2 transporter mRNAs were down-regulated by 30–40% in the presence of either progesterone or its metabolite PM5S (Figure 5). Alternatively, BCRP was up-regulated 40% by progesterone. In LS174T-FXR cells, placental lactogen induced MDR1 (2.7-fold) and BCRP (1.6-fold) and down-regulated MRP2 and MRP3 (35%; Figure 6). The androgens testosterone and dehydroepiandrosterone, as well as cortisol and pituitary growth hormone did not alter efflux transporter expression in LS174T-FXR cells (Figure 6).
Figure 4.
Gene expression of nuclear receptor signaling pathways in intestinal cells treated with steroid hormones and metabolites. mRNA expression was quantified in LS174T-FXR cells treated with vehicle (CON, set to 1.0), estradiol (E2, 0.1 μM), progesterone (PRG, 1 μM) or epiallopregnanolone-sulfate (PM5S, 7 μM) for 24 hours. Data were normalised to vehicle and presented as mean relative expression ± SE (n=3 independent experiments). Asterisks (*) represent statistically significant difference (p ≤ 0.05) compared with vehicle-treated cells.
Figure 5.
Gene expression of efflux transporters in intestinal cells treated with steroid hormones and metabolites. mRNA expression was quantified in LS174T-FXR cells and treated with vehicle (CON, set to 1.0), estradiol (E2, 0.1 μM), progesterone (PRG, 1 μM) or epiallopregnanolone-sulfate (PM5S, 7 μM) for 24 hours. Data were normalised to vehicle and presented as mean relative expression ± SE (n=3 independent experiments). Asterisks (*) represent statistically significant difference (p ≤ 0.05) compared with vehicle-treated cells.
Figure 6.
Gene expression of efflux transporters in intestinal cells treated with steroid and placental hormones. mRNA expression was quantified in LS174T-FXR cells treated with vehicle (set to 1.0), placental lactogen (6 μg/mL), testosterone (13.3 nM), dehydroepiandrosterone (50 nM), cortisol (0.8 μM) or growth hormone (7 pM) for 24 hours. Data were normalised to vehicle and presented as mean relative expression ± SE (n=3 independent experiments). Asterisks (*) represent statistically significant difference (p ≤ 0.05) compared with vehicle-treated cells.
Discussion
This study provides an important assessment of Fxr- and Pxr-mediated transcriptional regulation in the ileum of pregnant mice and points to the potential for different sex hormones to mediate transcriptional changes. Suppression of the prototypical target genes, Fgf15 and Cyp3a11, in the ileum of pregnant mice suggests that the functions of Fxr and Pxr are reduced in late gestation, particularly on gestation days 14 and 17. An overall reduction in expression of the Fxr target genes, including I-babp and Shp, as well as apical efflux transporters including Mdr1, Mrp2, and Bcrp was observed. Interestingly, an induction of Mrp3 occurred on gestation day 14. Protein expression of transporters Mdr1, Mrp2, and Bcrp mirrored pregnancy-related changes at mRNA levels.
It has been demonstrated that different regions of the intestines have unique expression profiles of nuclear receptors (Modica et al. 2010). The colon more highly expresses PXR/Pxr, while the ileum comparatively expresses higher levels of FXR/Fxr (Lax et al. 2012; Modica et al. 2012). In addition, recent RNA-Sequencing quantification demonstrates that the ileum has high cumulative mRNA expression of uptake and efflux transporters (Fu et al. 2016). Therefore, the ileum was chosen as a section of the small intestine that coexpresses moderate to high levels of both nuclear receptors and their target transporters. Currently, there are no models available to study the human ileum in vitro, though primary epithelial cells isolated from the duodenum and jejunum are becoming commercially available. Unfortunately, the validity of primary enterocytes to study drug metabolizing enzymes and transporters has not been thoroughly tested. A previous report using the colon LS174T cell line demonstrated that genes regulated by FXR (FGF19, I-BABP and SHP) were not inducible by treatment with the FXR agonists chenodeoxycholic acid or GW4064 (Vaquero et al. 2013). However, transfection with the human FXR plasmid (LS174T-FXR cells) resulted in the up-regulation of FXR target genes with agonist treatment. In preliminary studies, transient transfection of LS174T cells with the FXR gene increased FXR mRNA levels from baseline (LS174T Ct values: 28–30 and LS174T-FXR Ct values: 11–13). Transient transfection did not alter baseline mRNA levels of any FXR or PXR target genes (data not shown). Therefore, in the current study, LS174T-FXR cells were utilised to 1) recapitulate nuclear receptor enrichment typically observed in the ileum (Modica et al. 2010) and 2) study the effects of candidate hormones on both FXR and PXR pathways in the same in vitro system. Interestingly, we observed a down-regulation of FXR target genes in the ilea of pregnant mice. However, no changes in these pathways were observed following various hormone treatments. It is possible that priming of FXR by adding bile acids to the culture media could reveal an interaction of bile acids and hormones in regulating downstream genes in LS174T cells. Additional studies are needed to determine alternative mechanisms to repress FXR signaling such as reduced recruitment of co-activators and enhanced availability of co-repressors.
Several reports suggest that the size of the intestines and absorptive area of the small intestine are increased in pregnant rodents (Cripps and Williams 1975; Prieto et al. 1994; Sabet Sarvestani et al. 2015). Therefore, it is possible that reduced expression of mRNA or protein may still yield the same total number of transporters in the intestinal tract. Nevertheless, down-regulation of metabolism and transport genes analyzed in mice was largely observed on gestation day 14–17, with resolution to virgin control levels by gestation day 19. Similar down-regulation has been noted for efflux transporters in the livers of mice during pregnancy (Aleksunes et al. 2012). Interestingly, the mRNA of uptake transporters expressed in the ileum of the small intestine were differentially regulated throughout the gestational time points assessed (Supplemental Table 1).
While this is the first study to perform a profiling of endobiotic and xenobiotic regulating factors and transporters throughout late gestation in the ileum of mice, several studies have been conducted in mice that highlight changes in their expression in the intestine during pregnancy. Previously published data suggest that mouse Fgf15 levels in the terminal ileum were unchanged in pregnancy (Milona et al. 2010). However, Fgf15 mRNA levels were only quantified on gestation day 18, and the current study demonstrates that levels are returning to those observed in virgin controls by this time point (Figure 1). Additional time course studies conducted in mice revealed that Cyp3a mRNAs, including Cyp3a11, in both the proximal and distal intestine were unchanged on gestation days 10, 15 and 19 (Zhang et al. 2008). The methods indicate that small intestines were only cut into two segments in this study (proximal and distal); suggesting that quantification of enzymes in the distal portion could be the combination of expression in both the jejunum and ileum, which may differ. In a prior study, Bcrp mRNA was induced in the small intestine of mice on gestation day 10, after which time expression returned to control levels (Wang et al. 2006). However, no change in protein expression was observed between pregnant and virgin mice (Wang et al. 2006). It should be noted that the current study utilised C57BL/6 mice, whereas the aforementioned analyses were performed in FVB mice, which could contribute to the observed differences. Discrepancies between studies further support the importance of systematically evaluating time-dependent changes in xenobiotic and bile acid pathways in specific regions of the small intestines in pregnant dams.
Transactivation of xenobiotic disposition genes by progesterone and estradiol has been shown to be dose-dependent (Attardi et al. 2007; Ekena et al. 1998). Therefore, concentrations of steroid and placental hormones similar to those observed in the third trimester of pregnancy were used in cell culture treatments. The interaction of pregnenolone, as well as its metabolite, progesterone, with the ligand binding domain of PXR was identified as early as the 1990s (Kliewer et al. 1998). Progesterone activated PXR with an EC50 of 10 μM in CV-1 kidney cells transfected with the PXR expression plasmid (Kliewer et al. 1998). Progesterone metabolites have been shown to alter Pxr function in pregnancy. Interestingly, pregnant mice lacking Pxr did not exhibit the adaptations in vascular function typically observed in wild-type damns (Hagedorn et al. 2007). Further, authors showed that treatment of virgin Pxr-null mice with a subcutaneous implant of the progesterone metabolite 5β-dihydroprogesterone for 7 days did not mimic the pregnancy-related adaptations in vascular tone that were observed in virgin wild-type mice, pointing to Pxr-mediated signaling of progesterone metabolites (Hagedorn et al. 2007).
In the current study, repression of PXR/Pxr target genes (Cyp3a11/CYP3A4, Mdr1a/MDR1, Mrp2/MRP2) was detected in both pregnant mice and intestinal cells treated with progesterone and its metabolite. The noted expression pattern for ileal efflux transporters across gestation time points mirrors the reported peak in progesterone levels from gestation day 14–17 and decline to parturition in mice (McCormack and Greenwald 1974). It is hypothesised that progesterone is interfering with PXR/Pxr signaling, resulting in reduced expression of important downstream xenobiotic enzymes and transporters. While the relationship between PXR agonists and coactivator/corepressor recruitment is poorly understood, it is possible pregnancy-relevant concentrations of progesterone could lead to enhanced corepressor recruitment to PXR and warrants further analysis (Navaratnarajah et al. 2012). Additional studies are needed to determine whether there is a unique concentration-dependent relationship between progesterone and PXR; such that physiologically relevant concentrations of progesterone (<5 μM) and its metabolite PM5S can inhibit PXR, while high concentrations can activate signaling as previously reported (Blumberg et al. 1998; Kliewer et al. 1998).
Conclusions
The objective of this study was to assess the expression profiles of intestinal bile acid and xenobiotic disposition genes regulated by Fxr and Pxr during late pregnancy, and identify candidate sex hormones that may be responsible for altered nuclear receptor function. Candidate hormones were selected based on evidence in the literature of signaling through nuclear receptor pathways, and influence on drug metabolizing enzyme and transporter expression. Our findings suggest that there is interference with both Fxr and Pxr signaling during pregnancy, as evidenced by suppression of genes and proteins in both pathways. In vitro data support these in vivo observations indicating that interference with PXR/Pxr transcriptional regulation, likely by high progestin concentrations, may play a greater role in molecular adaptations of the intestine during pregnancy. Future studies should 1) assess the impact of mixtures of steroid and placental hormones on hepatic and intestinal drug metabolizing enzymes and transporters, as it is possible they can modify expression through shared nuclear receptor signaling pathways and 2) investigate whether reduced luminal efflux transporter expression during pregnancy influences intestinal drug absorption and the pharmacokinetic profiles of drugs.
Supplementary Material
Acknowledgments
The authors appreciate antibodies provided by Dr. Paul Dawson (Emory University, Atlanta, GA), and tissue collections performed by Myrna Trumbauer, Kristin Bircsak, Le Zhan and Jianliang Shen (Dept. of Pharmacology and Toxicology, Rutgers University).
Funding
This work was supported by the National Institute of Child Health and Human Development [NICHD, Grant F31HD082965], National Institute of Environmental Health Sciences [NIEHS, Grants R01ES020522, T32ES007148, P30ES002022], National Institute of General Medical Sciences [NIGMS, Grant R01GM104037] and an American Foundation for Pharmaceutical Education (AFPE) Predoctoral Fellowship in Pharmaceutical Science. NIEHS, NICHD, NIGMS, and AFPE did not have any role in the conduct of the study, interpretation of data or decision to publish. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or AFPE.
Footnotes
Declarations of interest
The authors report no conflicts of interest.
References
- Abu-Hayyeh S, Martinez-Becerra P, Sheikh Abdul Kadir SH, Selden C, Romero MR, Rees M, Marschall HU, Marin JJ, Williamson C. Inhibition of Na+-taurocholate Co-transporting polypeptide-mediated bile acid transport by cholestatic sulfated progesterone metabolites. J Biol Chem. 2010;285:16504–12. doi: 10.1074/jbc.M109.072140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Hayyeh S, Papacleovoulou G, Lovgren-Sandblom A, Tahir M, Oduwole O, Jamaludin NA, Ravat S, Nikolova V, Chambers J, Selden C, et al. Intrahepatic cholestasis of pregnancy levels of sulfated progesterone metabolites inhibit farnesoid X receptor resulting in a cholestatic phenotype. Hepatology. 2013;57:716–26. doi: 10.1002/hep.26055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksunes LM, Yeager RL, Wen X, Cui JY, Klaassen CD. Repression of hepatobiliary transporters and differential regulation of classic and alternative bile acid pathways in mice during pregnancy. Toxicol Sci. 2012;130:257–68. doi: 10.1093/toxsci/kfs248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attardi BJ, Zeleznik A, Simhan H, Chiao JP, Mattison DR, Caritis SN Obstetric-Fetal Pharmacology Research Unit N. Comparison of progesterone and glucocorticoid receptor binding and stimulation of gene expression by progesterone, 17-alpha hydroxyprogesterone caproate, and related progestins. Am J Obstet Gynecol. 2007;197:599.e1–7. doi: 10.1016/j.ajog.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg B, Sabbagh W, Jr, Juguilon H, Bolado J, Jr, van Meter CM, Ong ES, Evans RM. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998;12:3195–205. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cripps AW, Williams VJ. The effect of pregnancy and lactation on food intake, gastrointestinal anatomy and the absorptive capacity of the small intestine in the albino rat. Br J Nutr. 1975;33:17–32. doi: 10.1079/bjn19750005. [DOI] [PubMed] [Google Scholar]
- Datta S, Hey VM, Pleuvry BJ. Effects of pregnancy and associated hormones in mouse intestine, in vivo and in vitro. Pflugers Arch. 1974;346:87–95. doi: 10.1007/BF00587009. [DOI] [PubMed] [Google Scholar]
- Dawson PA, Hubbert M, Haywood J, Craddock AL, Zerangue N, Christian WV, Ballatori N. The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. J Biol Chem. 2005;280:6960–8. doi: 10.1074/jbc.M412752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gottardi A, Touri F, Maurer CA, Perez A, Maurhofer O, Ventre G, Bentzen CL, Niesor EJ, Dufour JF. The bile acid nuclear receptor FXR and the bile acid binding protein IBABP are differently expressed in colon cancer. Dig Dis Sci. 2004;49:982–9. doi: 10.1023/b:ddas.0000034558.78747.98. [DOI] [PubMed] [Google Scholar]
- Ekena K, Katzenellenbogen JA, Katzenellenbogen BS. Determinants of ligand specificity of estrogen receptor-alpha: estrogen versus androgen discrimination. J Biol Chem. 1998;273:693–9. doi: 10.1074/jbc.273.2.693. [DOI] [PubMed] [Google Scholar]
- Frankenberg T, Rao A, Chen F, Haywood J, Shneider BL, Dawson PA. Regulation of the mouse organic solute transporter alpha-beta, Ostalpha-Ostbeta, by bile acids. Am J Physiol Gastrointest Liver Physiol. 2006;290:G912–22. doi: 10.1152/ajpgi.00479.2005. [DOI] [PubMed] [Google Scholar]
- Fu ZD, Selwyn FP, Cui JY, Klaassen CD. RNA Sequencing Quantification of Xenobiotic-Processing Genes in Various Sections of the Intestine in Comparison to the Liver of Male Mice. Drug Metab Dispos. 2016;44:842–56. doi: 10.1124/dmd.115.068270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn KA, Cooke CL, Falck JR, Mitchell BF, Davidge ST. Regulation of vascular tone during pregnancy: a novel role for the pregnane X receptor. Hypertension. 2007;49:328–33. doi: 10.1161/01.HYP.0000253478.51950.27. [DOI] [PubMed] [Google Scholar]
- Hendrickse CW, Jones CE, Donovan IA, Neoptolemos JP, Baker PR. Oestrogen and progesterone receptors in colorectal cancer and human colonic cancer cell lines. Br J Surg. 1993;80:636–40. doi: 10.1002/bjs.1800800531. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–25. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Jeong H. Altered drug metabolism during pregnancy: hormonal regulation of drug-metabolizing enzymes. Expert Opin Drug Metab Toxicol. 2010;6:689–99. doi: 10.1517/17425251003677755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jigorel E, Le Vee M, Boursier-Neyret C, Parmentier Y, Fardel O. Differential regulation of sinusoidal and canalicular hepatic drug transporter expression by xenobiotics activating drug-sensing receptors in primary human hepatocytes. Drug Metab Dispos. 2006;34:1756–63. doi: 10.1124/dmd.106.010033. [DOI] [PubMed] [Google Scholar]
- Jonker JW, Buitelaar M, Wagenaar E, Van Der Valk MA, Scheffer GL, Scheper RJ, Plosch T, Kuipers F, Elferink RP, Rosing H, et al. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc Natl Acad Sci U S A. 2002;99:15649–54. doi: 10.1073/pnas.202607599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterstrom RH, et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- Kota BP, Tran VH, Allen J, Bebawy M, Roufogalis BD. Characterization of PXR mediated P-glycoprotein regulation in intestinal LS174T cells. Pharmacol Res. 2010;62:426–31. doi: 10.1016/j.phrs.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Landrier JF, Eloranta JJ, Vavricka SR, Kullak-Ublick GA. The nuclear receptor for bile acids, FXR, transactivates human organic solute transporter-alpha and -beta genes. Am J Physiol Gastrointest Liver Physiol. 2006;290:G476–85. doi: 10.1152/ajpgi.00430.2005. [DOI] [PubMed] [Google Scholar]
- Lax S, Schauer G, Prein K, Kapitan M, Silbert D, Berghold A, Berger A, Trauner M. Expression of the nuclear bile acid receptor/farnesoid X receptor is reduced in human colon carcinoma compared to nonneoplastic mucosa independent from site and may be associated with adverse prognosis. Int J Cancer. 2012;130:2232–9. doi: 10.1002/ijc.26293. [DOI] [PubMed] [Google Scholar]
- Liu CY, Chen LB, Liu PY, Xie DP, Wang PS. Effects of progesterone on gastric emptying and intestinal transit in male rats. World J Gastroenterol. 2002;8:338–41. doi: 10.3748/wjg.v8.i2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg AC, Schinkel AH, van De Vijver MJ, Scheper RJ, Schellens JH. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61:3458–64. [PubMed] [Google Scholar]
- Martin P, Riley R, Back DJ, Owen A. Comparison of the induction profile for drug disposition proteins by typical nuclear receptor activators in human hepatic and intestinal cells. Br J Pharmacol. 2008;153:805–19. doi: 10.1038/sj.bjp.0707601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack JT, Greenwald GS. Progesterone and oestradiol-17beta concentrations in the peripheral plasma during pregnancy in the mouse. J Endocrinol. 1974;62:101–7. doi: 10.1677/joe.0.0620101. [DOI] [PubMed] [Google Scholar]
- Milona A, Owen BM, Cobbold JF, Willemsen EC, Cox IJ, Boudjelal M, Cairns W, Schoonjans K, Taylor-Robinson SD, Klomp LW, et al. Raised hepatic bile acid concentrations during pregnancy in mice are associated with reduced farnesoid X receptor function. Hepatology. 2010;52:1341–9. doi: 10.1002/hep.23849. [DOI] [PubMed] [Google Scholar]
- Modica S, Gofflot F, Murzilli S, D’Orazio A, Salvatore L, Pellegrini F, Nicolucci A, Tognoni G, Copetti M, Valanzano R, et al. The intestinal nuclear receptor signature with epithelial localization patterns and expression modulation in tumors. Gastroenterology. 2010;138:636–48. 48 e1–12. doi: 10.1053/j.gastro.2009.09.060. [DOI] [PubMed] [Google Scholar]
- Modica S, Petruzzelli M, Bellafante E, Murzilli S, Salvatore L, Celli N, Di Tullio G, Palasciano G, Moustafa T, Halilbasic E, et al. Selective activation of nuclear bile acid receptor FXR in the intestine protects mice against cholestasis. Gastroenterology. 2012;142:355–65. e1–4. doi: 10.1053/j.gastro.2011.10.028. [DOI] [PubMed] [Google Scholar]
- Mottino AD, Hoffman T, Jennes L, Vore M. Expression and localization of multidrug resistant protein mrp2 in rat small intestine. J Pharmacol Exp Ther. 2000;293:717–23. [PubMed] [Google Scholar]
- Mutch DM, Anderle P, Fiaux M, Mansourian R, Vidal K, Wahli W, Williamson G, Roberts MA. Regional variations in ABC transporter expression along the mouse intestinal tract. Physiol Genomics. 2004;17:11–20. doi: 10.1152/physiolgenomics.00150.2003. [DOI] [PubMed] [Google Scholar]
- Navaratnarajah P, Steele BL, Redinbo MR, Thompson NL. Rifampicin-independent interactions between the pregnane X receptor ligand binding domain and peptide fragments of coactivator and corepressor proteins. Biochemistry. 2012;51:19–31. doi: 10.1021/bi2011674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelkers P, Dawson PA. Cloning and chromosomal localization of the human ileal lipid-binding protein. Biochim Biophys Acta. 1995;1257:199–202. doi: 10.1016/0005-2760(95)00098-w. [DOI] [PubMed] [Google Scholar]
- Parry E, Shields R, Turnbull AC. Transit time in the small intestine in pregnancy. J Obstet Gynaecol Br Commonw. 1970;77:900–1. doi: 10.1111/j.1471-0528.1970.tb03423.x. [DOI] [PubMed] [Google Scholar]
- Pfrunder A, Gutmann H, Beglinger C, Drewe J. Gene expression of CYP3A4, ABC-transporters (MDR1 and MRP1-MRP5) and hPXR in three different human colon carcinoma cell lines. J Pharm Pharmacol. 2003;55:59–66. doi: 10.1111/j.2042-7158.2003.tb02434.x. [DOI] [PubMed] [Google Scholar]
- Prieto RM, Ferrer M, Fe JM, Rayo JM, Tur JA. Morphological adaptive changes of small intestinal tract regions due to pregnancy and lactation in rats. Ann Nutr Metab. 1994;38:295–300. doi: 10.1159/000177824. [DOI] [PubMed] [Google Scholar]
- Rao A, Haywood J, Craddock AL, Belinsky MG, Kruh GD, Dawson PA. The organic solute transporter alpha-beta, Ostalpha-Ostbeta, is essential for intestinal bile acid transport and homeostasis. Proc Natl Acad Sci U S A. 2008;105:3891–6. doi: 10.1073/pnas.0712328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rost D, Mahner S, Sugiyama Y, Stremmel W. Expression and localization of the multidrug resistance-associated protein 3 in rat small and large intestine. Am J Physiol Gastrointest Liver Physiol. 2002;282:G720–6. doi: 10.1152/ajpgi.00318.2001. [DOI] [PubMed] [Google Scholar]
- Sabet Sarvestani F, Rahmanifar F, Tamadon A. Histomorphometric changes of small intestine in pregnant rat. Vet Res Forum. 2015;6:69–73. [PMC free article] [PubMed] [Google Scholar]
- Scheffer GL, Kool M, de Haas M, de Vree JM, Pijnenborg AC, Bosman DK, Elferink RP, van der Valk P, Borst P, Scheper RJ. Tissue distribution and induction of human multidrug resistant protein 3. Lab Invest. 2002;82:193–201. doi: 10.1038/labinvest.3780411. [DOI] [PubMed] [Google Scholar]
- Ujhazy P, Ortiz D, Misra S, Li S, Moseley J, Jones H, Arias IM. Familial intrahepatic cholestasis 1: studies of localization and function. Hepatology. 2001;34:768–75. doi: 10.1053/jhep.2001.27663. [DOI] [PubMed] [Google Scholar]
- Vaquero J, Monte MJ, Dominguez M, Muntane J, Marin JJ. Differential activation of the human farnesoid X receptor depends on the pattern of expressed isoforms and the bile acid pool composition. Biochem Pharmacol. 2013;86:926–39. doi: 10.1016/j.bcp.2013.07.022. [DOI] [PubMed] [Google Scholar]
- Wald A, Van Thiel DH, Hoechstetter L, Gavaler JS, Egler KM, Verm R, Scott L, Lester R. Gastrointestinal transit: the effect of the menstrual cycle. Gastroenterology. 1981;80:1497–500. [PubMed] [Google Scholar]
- Wald A, Van Thiel DH, Hoechstetter L, Gavaler JS, Egler KM, Verm R, Scott L, Lester R. Effect of pregnancy on gastrointestinal transit. Dig Dis Sci. 1982;27:1015–8. doi: 10.1007/BF01391748. [DOI] [PubMed] [Google Scholar]
- Wang H, Wu X, Hudkins K, Mikheev A, Zhang H, Gupta A, Unadkat JD, Mao Q. Expression of the breast cancer resistance protein (Bcrp1/Abcg2) in tissues from pregnant mice: effects of pregnancy and correlations with nuclear receptors. Am J Physiol Endocrinol Metab. 2006;291:E1295–304. doi: 10.1152/ajpendo.00193.2006. [DOI] [PubMed] [Google Scholar]
- Zhang H, Wu X, Wang H, Mikheev AM, Mao Q, Unadkat JD. Effect of pregnancy on cytochrome P450 3a and P-glycoprotein expression and activity in the mouse: mechanisms, tissue specificity, and time course. Mol Pharmacol. 2008;74:714–23. doi: 10.1124/mol.107.043851. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.