Abstract
Although various types of imaging agents have been developed for photoacoustic (PA) imaging, relatively few imaging agents exhibit high selectivity/sensitivity to the tumor microenvironment for on-demand PA imaging and therapy. Herein, molybdenum-based polyoxometalate (POM) clusters with the highest oxidation state of Mo(VI) (denoted as Ox-POM) were designed as novel agents for redox-activated PA imaging-guided photothermal therapy (PTT). Capable of escaping from recognition and capture by the liver and spleen, these renal clearable clusters with ultra-small size (hydrodynamic size: 1.9 nm) can accumulate in the tumor, self-assemble into larger nanoclusters at low pH, and are reduced to NIR absorptive agents in the tumor microenvironment. Studies in 4T1 tumor-bearing mice indicated that these clusters could be employed for bio-responsive PA imaging-guided tumor ablation in vivo. Our finding is expected to establish a new physicochemical paradigm for the design of PA imaging agents based on clusters, bridging the conventional concepts of “molecule” and “nano” in the bio-imaging field.
Keywords: Redox-responsive probes, polyoxometalate cluster, photoacoustic imaging, photothermal therapy, theranostic agent
TOC image
On-demand tumor diagnosis and therapy (i.e., theranostics) trigged by intrinsic physiological microenvironment characteristics (e.g., redox state, pH, enzymes, ions, etc.) have attracted considerable attention in recent years.1–4 Such therapies can simultaneously reduce the damage of anti-cancer agents to normal tissues and improve their therapeutic efficacy. Among the various physiological parameters, redox status plays a major role in many pathological conditions including chronic inflammation, stroke, and cancer.5, 6 It has been demonstrated that glutathione (GSH) is the most abundant reducing agent in the tumor, and levels of GSH in cancer tissues are significantly higher than that in normal tissues.7
Development of novel imaging methods or probes to detect and monitor redox status in vivo is highly desirable. By employing redox-activated molecules or nanoparticle-based probes, various methods for monitoring the redox environment have been reported, including optical imaging with high sensitivity and magnetic resonance imaging (MRI) with high resolution.8–12 As one of the fastest growing imaging modalities over the past decade, photoacoustic (PA) imaging combines the advantages of high sensitivity of optical imaging and high resolution of ultrasonic imaging.13, 14 In addition, it can usually be combined with photothermal therapy (PTT) for cancer treatment because both use the same near-infrared (NIR) absorbing materials.15, 16 However, PA imaging agents with redox-activated diagnostic and therapeutic functions, which could be of high significance for on-demand tumor treatment, have not been developed to date.
Various types of nano-agents have been explored as exogenous agents for PA imaging/PTT, including carbon-based materials,17–19 gold nanomaterials,15, 20–23 sulfides,24–26 and polymeric nanoparticles.27–30 A common concern about nano-agents for PA imaging is the non-specific uptake of nano-agents by mononuclear phagocyte systems (e.g., liver/spleen) and low accumulation in tumors due to the poor enhanced permeability and retention (EPR) effect. To address this issue, self-assembled PA agents have been designed to remain as small molecules during circulation in the bloodstream and then self-assemble into larger nanostructures in the tumor, triggered by the tumor microenvironment.31 Various bioresponsive contrast agents have been developed for PA imaging of reactive oxygen species,28 enzyme activity,32,33 pH,34 and bacterial infection.30 For example, Dragulescu-Andrasi et al. reported a probe with enzyme-induced self-aggregation, which could provide PA signal to detect enzymatic activity in living subjects.32 With their self-adaptive electronic structure for enhanced NIR absorption, the Mo-based polyoxometalate (POM) clusters can form larger structures through hydrogen bond formation due to acid-induced protonation.35, 36 This pH-responsive assembly and photothermal conversion of POM clusters with a size range between “molecule” and “nano” endows them promise as photothermal agents for cancer treatment.37 The electron relaxation polarization caused by the charge transfer between Mo(VI) and Mo(V) is regarded as the origin of the typical NIR absorption of the POM clusters.38 The readily reducible characteristics of Mo(VI) into lower oxidation states (e.g., Mo(V)) highlight the potential of POM clusters as an agent for redox-activated bio-imaging.
In this work, we report an intelligent theranostic agent based on POM clusters with the highest oxidation state of Mo(VI) (denoted as Ox-POM) for pH-responsive assembly and redox-activated PA imaging-guided PTT (Scheme 1). These Ox-POMs show no NIR absorption in their original chemical form, but exhibit strong NIR absorption in the tumor redox microenvironment because Mo(VI) is reduced to Mo(V). The rapidly renal-clearable Ox-POM can self-assemble into much larger nanostructures under acidic conditions for enhanced intratumoral accumulation, as demonstrated in our previous work,37 and herein we further employ the structures as redox-activated agents for PA imaging-guided PTT of cancer. Importantly, these clusters can escape from recognition and capture by the liver and spleen and are mainly excreted through the kidneys, representing an intelligent redox-activated theranostic agent with significant clinical prospects.
Scheme 1.
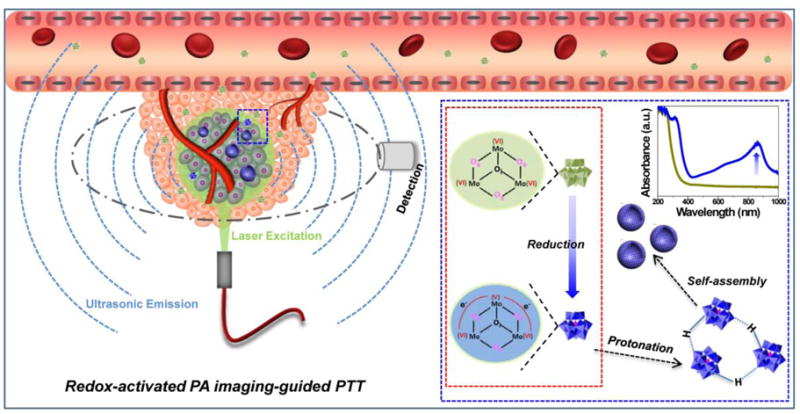
Schematic diagram of the Ox-POM in response to the tumor microenvironment for redox-activated PA imaging-guided PTT.
The colorless and highly hydrophilic Ox-POMs, obtained from an easy, fast, and large-scale synthesis process, were highly stable in various media (Figure S1–S3). Such easy fabrication and large-scale synthesis at a low cost will undoubtedly promote the future investigation of these laboratory-synthesized Ox-POMs toward potential clinical studies. The clusters were highly uniform with an average diameter of ~1 nm at pH 7.4 as observed in transmission electron microscopy (TEM) image (Figure 1a). Their energy-dispersive X-ray (EDX) spectrum demonstrated the existence of all the essential chemical elements (Mo, P, and O) of these clusters (Figure S4). Interestingly, these clusters were found to self-assemble into larger but monodisperse spherical assemblies with a diameter of ~25 nm in mildly acidic conditions (pH = 6.5) as TEM showed the collapsed nano-vesicles during the drying process (Figure 1b), suggesting a hollow sphere formation by non-covalent linkage among these cluster blocks.35, 36 These nano-vesicles were further aggregated into larger structures through additional acidification at pH 5.0 and remained hydrophilic (Figure 1c). This process of pH-responsive self-assembly was also monitored by dynamic light scattering (DLS) measurement (Figure 1d), with their hydrated size increasing from 1.9 nm (pH = 7.4) to 29.3 nm (pH = 6.5) and to ~ 0.43 μm at pH 5.0. Our previous work revealed that the hydrogen bond formation of POM macroanions through acid-induced protonation, with resulting decreased electrostatic repulsions and increased attractive forces, was likely responsible for such pH-driven self-assembly.37
Figure 1.
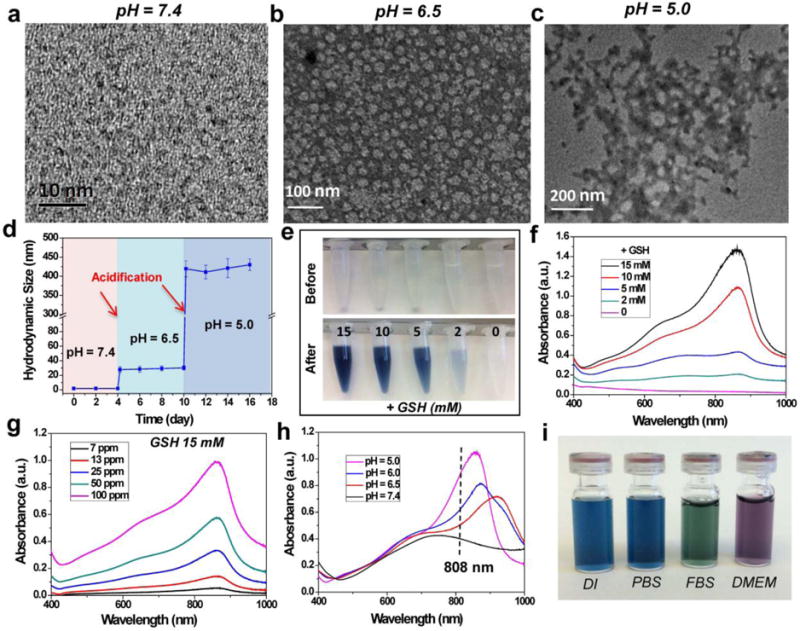
Synthesis and characterization of Ox-POM. a - c) TEM images of clusters at pH = 7.4, 6.5, and 5.0. d) DLS measurements of Ox-POM clusters with successive acidifications from pH = 7.4 to pH = 6.5 and to pH = 5.0. e) Photographs of Ox-POM clusters dispersed in deionized water before and after incubation with various concentrations of GSH and f) the corresponding UV-vis spectra. g) UV-vis absorptions of Ox-POM clusters with various Mo concentrations after incubation with GSH (15 mM). h) UV−vis-NIR absorptions of Ox-POM clusters (100 ppm Mo) at various pH values after incubation with GSH (15 mM). i) Photographs of GSH-reduced Ox-POM clusters dispersed in various media.
Subsequently, the Ox-POM clusters were incubated with GSH at different concentrations to evaluate their redox-activated properties. As shown in Figure 1e–f, the pure solution of Ox-POM clusters was colorless and exhibited no NIR absorption. After reduction with GSH, the color changed to blue, which deepened monotonically with increasing GSH concentration. The corresponding absorbance showed a peak in the NIR area, which enhanced gradually as the GSH concentration increased (Figure 1f). The same phenomenon was also observed for various concentrations of Ox-POM after incubation with a fixed concentration of GSH (Figure 1g, Figure S5). These reduced clusters with blue color exhibited high dispersity in different solvents including deionized water, PBS, FBS and DMEM (Figure 1i). Cysteine (Cys), another reducing agent, was also used as a model to demonstrate the common redox-activated effect of these Ox-POM clusters. With the addition of an increasing amount of Cys to the Ox-POM solution, the NIR absorption of Ox-POM increased gradually as a result of the reduction of Mo(VI) (Figure S6). This enhanced NIR absorption of the Ox-POM is founded on the reduction of Mo(VI) to Mo(V) following GSH or Cys incubation. The increased reduction of Ox-POM will facilitate the delocalized electron density and occupiable cation site of Mo(V) through the reversible and multiple steps of electron exchange, which will simultaneously strengthen the electron relaxation polarization,37 leading to enhanced NIR absorption. These Ox-POMs couldn’t be reduced in the blood, and extremely low hemolysis rate was found after incubation with red blood cells (Figure S7). Also, pH-dependent absorption of Ox-POM after GSH incubation was observed, where the acid-induced self-assembly of POM resulted in an enhanced absorption peak (Figure 1h). The significant blue-shift towards 808 nm, one of the most widely clinically used NIR lasers, makes these clusters a promising photothermal agent for tumor ablation therapy.
Ox-POM clusters were incubated with varying concentrations of GSH (control, 2, 10, and 15 mM) to evaluate their redox-activated PA imaging performance. Representative PA images of the four samples with 870 nm excitation are shown in Figure 2a. No PA signal was detected for pure Ox-POM without GSH incubation. The samples with higher levels of reduction exhibited higher PA signal intensities, further confirmed by the PA spectra of the sample solutions (Figure 2b). Furthermore, the photothermal conversion performance of these samples was investigated under 808 nm laser irradiation at a power density of 1.5 W/cm2. As shown in Figure 2c–d, the co-culture solutions with higher GSH concentrations showed higher temperatures of up to 55 °C within 5 min of irradiation, while the temperature of Ox-POM without GSH showed little change, demonstrating that the clusters after redox-activation can efficiently convert NIR laser energy into thermal energy. More detailed experiments revealed that the temperature enhancement was proportional to the concentration of clusters and the power density of the laser (Figure S8).
Figure 2.
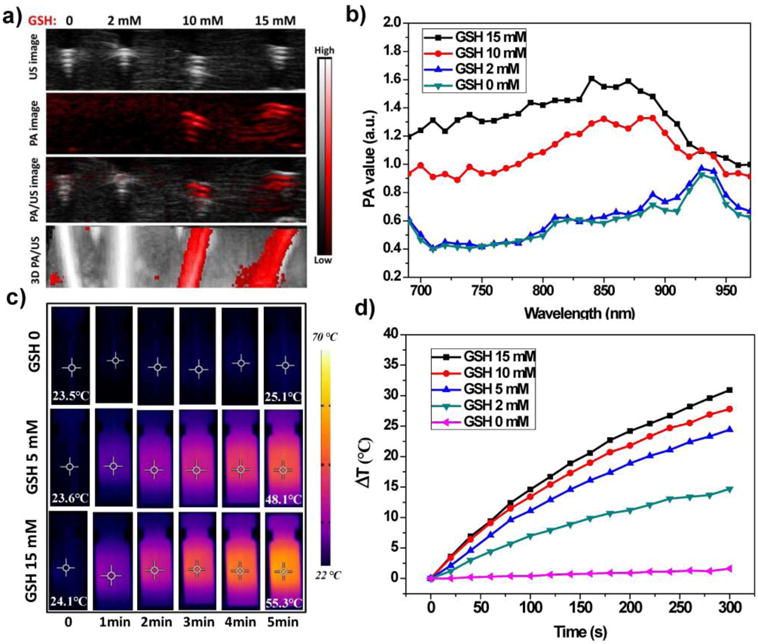
Redox-activated PA and photothermal properties of Ox-POM clusters. a) PA imaging and b) PA spectra of Ox-POM clusters after incubation with various concentrations of GSH (control, 2, 10, and 15 mM). c) Thermal images of various solutions and d) photothermal heating curves of Ox-POM clusters (100 ppm Mo, incubated with various concentrations of GSH) with 808 nm laser irradiation for 5 min at the power intensity of 1.5 w/cm2.
The blue-shift toward 808 nm (Figure 1h) of Ox-POM after redox activation motivated us to investigate the photothermal performance of these clusters at different pH values (pH = 4.0, 6.5, 7.4, and pure PBS of pH = 7.4). Such blue-shift from ~1030 nm toward 808 nm was observed because the gap between the highest occupied molecular orbital and the lowest unoccupied molecular orbital was broadened by the acidity via the protonation of the edge-sharing oxygen atoms.37 A more efficient photothermal conversion under 808 nm laser irradiation was obtained at lower pH values (Figure S9), highlighting the potential of these agents as promising agents for PTT cancer treatment since the pH in the tumor microenvironment is usually lower than that in normal tissues.39 In addition, the recycling temperature variations of the cluster dispersion were explored to evaluate the photothermal stability of these clusters under 808 nm laser irradiation for 5 min, followed by natural cooling to room temperature for five laser on/off cycles. No deterioration of the clusters’ photothermal performance was found during the recycling (Figure S10), which indicates the durable photothermal properties of these clusters.
The in vitro toxicity of Ox-POM was tested by a standard MTT assay after incubation of the clusters at varying concentrations (0, 50, 100, 250, and 500 μg/mL) with human embryonic kidney 293 (HEK293) and murine breast cancer 4T1 cells for 24 h and 48 h. The Ox-POM showed negligible toxicity in both cell lines, even at a high concentration of 500 μg/mL (Figure S11). Subsequently, the Ox-POM clusters reduced by different concentrations of GSH (5, 10, and 15 mM) were added into cell culture plates containing 4T1 cells, followed by irradiation with an 808 nm laser for 5 min. As shown in Figure 3a, with stronger reducing conditions or higher concentrations of Ox-POM clusters, more cells were killed upon NIR laser irradiation at the power density of 1.5 W/cm2. Furthermore, 4T1 cells were then treated with 808 nm laser of various power densities (0.7, 1.0, and 2.0 W/cm2) and a constant level of Ox-POM. It was found that the relative viabilities of the 4T1 cells decreased remarkably at elevated power density, as well as at higher cluster concentration levels (Figure 3b). In contrast, negligible cell killing was found for the groups without any treatment or treatment with NIR laser only. The cancer cells were also stained with trypan blue to differentiate dead cells from live cells (Figure 3c), corroborating the MTT results. These findings clearly demonstrate that the Ox-POM clusters reduced by GSH can be used as an effective photothermal agent for cancer cell ablation.
Figure 3.
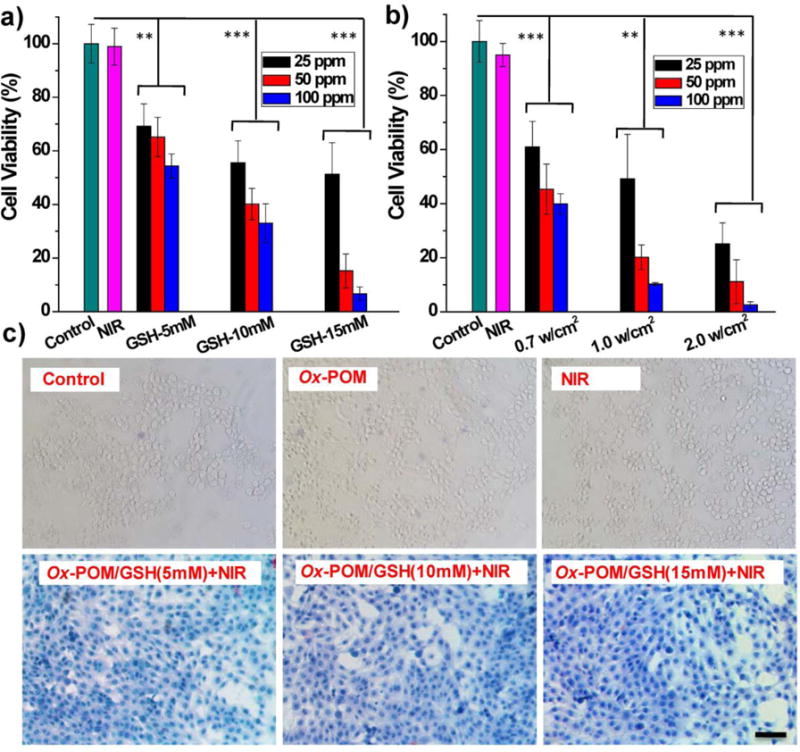
In vitro photothermal therapy. a) Relative viabilities of 4T1 cells after photothermal therapy at varied concentrations of Ox-POM in different reducing conditions (GSH: 15, 10, and 5 mM) under 808 nm NIR laser irradiation (1.5 W/cm2) for 5 min (n = 4, mean ± s.d., *P<0.05, ***P<0.001). b) Relative viabilities of 4T1 cells after photothermal therapy at varied concentrations of Ox-POM reduced with 15 mM GSH under 808 nm NIR laser irradiation of various power densities (2.0, 1.0, and 0.7 W/cm2) for 5 min (n = 4, mean ± s.d., *P<0.05, ***P<0.001). c) Optical microscopy images of trypan blue-stained cells after incubation with clusters and exposure to the 808 nm laser (1.5 W/cm2, 5 min) at different treatment conditions. Scale bar: 100 μm.
With encouraging in vitro findings, the circulation, biodistribution, and clearance of Ox-POM clusters were investigated via positron emission tomography (PET) imaging, a powerful, quantitative, and non-invasive imaging technique. The oxygen-rich Ox-POM clusters can be conveniently labeled with the highly oxophilic radionuclide 89Zr (in the chemical form of 89Zr4+),40, 41 and the labeling yield herein was measured to be as high as 90.4% (Figure S12). After intravenous injection of ~ 5 MBq of 89Zr-Ox-POM clusters into BALB/c mice, PET imaging was performed at various time points. Interestingly, the ultra-small sized 89Zr-Ox-POM showed low accumulation in the liver and spleen and was mainly excreted by the kidneys as strong PET signal was found in both the kidneys and bladder (Figure 4a). Our results consistent with previous reports that ultra-small sized particles (hydrodynamic size <5.5 nm) can escape from recognition and capture by the liver and are cleared via the kidneys, which filters metabolites from the bloodstream through the approximately 10 nm pores of the basal lamina.42 Quantitative data obtained from region-of-interest (ROI) analysis of these PET images indicated the excellent in vivo circulation behavior of these clusters with a long half-life of 2.79 ± 0.47 h (Figure 4b), which is highly desirable for both active and passive targeting of tumor tissue. The time point of the highest kidney uptake was 6 h postinjection (p.i.), at 23.7 ± 3.1 % ID/g, while the signals in the liver and spleen decreased gradually over the first 24 hours (Figure 4c). To further confirm the accuracy of PET quantification analysis, ex vivo biodistribution studies were carried out at 24 p.i. As shown in Figure 4d, the kidney uptake at 24 h p.i. of 89Zr-Ox-POM was 17.5 ± 3.2 % ID/g, while the accumulation in the liver and spleen was relatively low, with values of 8.7 ± 0.7 and 3.1 ± 1.1 % ID/g, respectively (n = 3). These results show that the clusters have excellent in vivo pharmacokinetic properties and can overcome the limitations of high uptake by mononuclear phagocyte systems (e.g., liver/spleen) and long in vivo retention time of many commonly-used nanoparticles.
Figure 4.
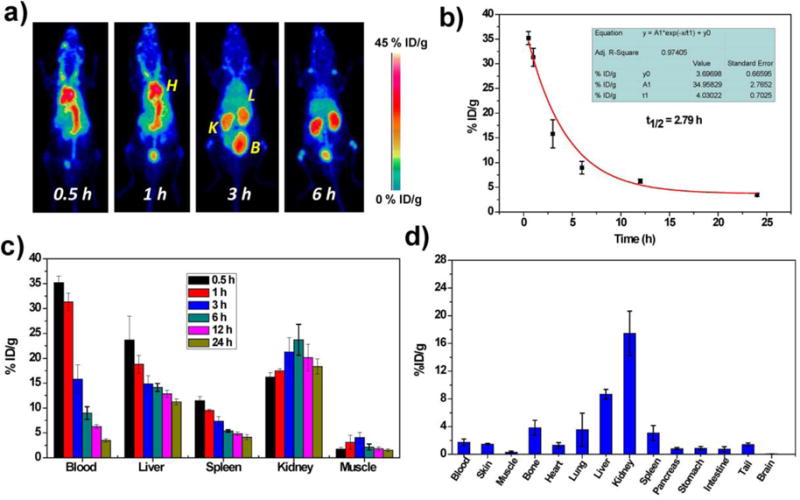
In vivo studies of Ox-POM clusters. a) Representative maximum intensity projection PET images of mice taken at various time point post intravenous injection of 89Zr-Ox-POM. H: heart; K: kidney; L: liver; B: bladder. b) Time-activity curves of 89Zr-Ox-POM in the blood (n = 3, mean ± s.d.). c) Quantification of 89Zr-Ox-POM uptake in the blood, liver, spleen, kidney, and muscle at various time points post-injection (n = 3, mean ± s.d.). d) Biodistribution of 89Zr-Ox-POM clusters at 24 h after intravenous injection into mice as determined by 89Zr radioactivity measurement in various tissues and organs (n = 3, mean ± s.d.).
The efficient redox-activated self-assembly of Ox-POM, strong photothermal conversion, and excellent in vivo pharmacokinetics encouraged us to employ Ox-POM for in vivo PA imaging-guided photothermal therapy of cancer. In vivo redox-activated PA imaging was conducted on 4T1-tumor-bearing mice prior to, and at 1 h, 3 h, 6 h, and 24 h after intravenous injection of Ox-POM clusters. As shown in Figure 5a, the PA signal was detected in the tumors as early as 1 h p.i., demonstrating the strong redox-activated effect of Ox-POM by the tumor redox status, which is regarded as one of the several physiologic properties differing tumor and normal tissues.43, 44 The signal intensity increased gradually from 1 h to 6 h p.i. (Figure S13) and showed excellent tumor tissue penetration. As both the extracellular tumor milieu (pH = 6.5 – 6.8) and endocytic organelles (pH = 5.0 – 6.0) in the tumor endothelial cells show a pH drop,1,39,45 the POM clusters can self-assemble into nano-aggregates in the tumor tissue to enhance tumor retention over time, which has been confirmed by TEM imaging of tumor tissues,37 as well as strong PA signal at an extended time of 24 h p.i. (Figure 5a). In line with the aforementioned in vitro results, these in vivo results confirm the pH-responsive self-assembly and redox-activated effect of Ox-POM clusters in the acidic and reducing tumor microenvironment.
Figure 5.
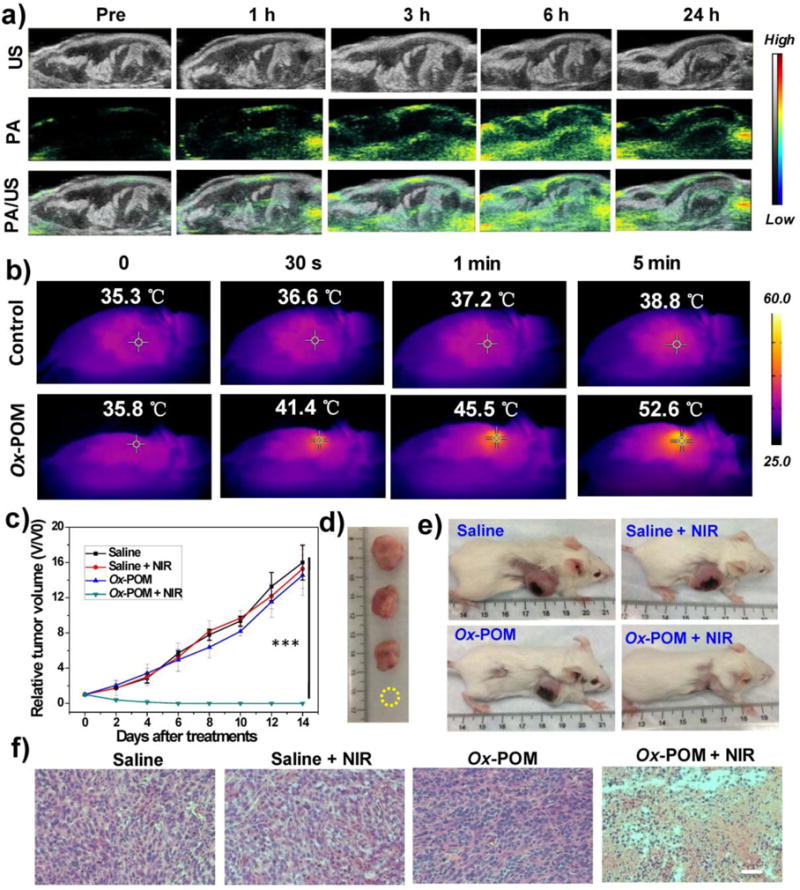
In vivo PA imaging and photothermal therapy in 4T1-tumor-bearing mice. (a) In vivo PA images of 4T1 tumor-bearing mice before and after intravenous injection of Ox-POM clusters. The entire view was tumor tissues. b) Photothermal images of 4T1 tumor-bearing mice before and after intravenous injection of Ox-POM clusters under continuous 808 nm laser irradiation for different durations. c) Tumor growth profiles of 4T1 tumors after each treatment (n = 4, mean ± s.d., ***P<0.001). d) Representative photographs of dissected tumors and e) mice at 14 day post treatments. f) H&E staining of tumor sections after various treatments. Scale bar: 100 μm.
Taking advantage of such redox-activated effects, in vivo photothermal imaging and therapy were performed under the exposure of an 808 nm laser for 5 min at 6 h p.i. of Ox-POM, as this time point demonstrated peak uptake of the nanoclusters in the tumor. The tumor temperature and thermal images were visualized with a thermal camera. As shown in Figure 5b, the temperature of the tumor rapidly increased to above 40 °C in 30 s and reached 52 °C in 5 min under laser irradiation, which is sufficient to thermally ablate the tumor, while the control group showed only limited temperature increase (Figure S14). For the treated group, 4T1 tumor growth was completely eliminated without subsequent recurrence for a prolonged period of up to 2 months (Figure 5c–e, Figures S15–16), whereas the control groups demonstrated rapid tumor growth. Hematoxylin and eosin (H&E) staining of tumors from different groups further confirmed the above results, where the treatment group shows the most tumor damage (Figure 5f). No abnormal behavior or significant weight loss was observed in any group (Figure S17), indicating minimal side effects of the clusters. The H&E stained images of different organs (heart, liver, spleen, lung, and kidney) showed no noticeable organ damage or inflammatory lesions over 30 days (Figure S18), suggesting the low in vivo toxicity of these clusters to normal tissue.
CONCLUSION
In summary, we present here the redox-activated Ox-POM clusters for tumor microenvironment-responsive PA imaging-guided tumor ablation, which exhibits high-performance PA imaging with outstanding photothermal conversion efficiency. The ultra-small Ox-POM clusters can escape from recognition and capture by the liver and spleen and are mainly excreted through the kidneys, which is highly desirable for reducing the potential toxic effects caused by the long-term accumulation of nanoparticles in various organs. Importantly, with long in vivo circulation half-life, these bioresponsive clusters can be passively accumulated in the tumor due to the EPR effect, self-assemble into larger nano sizes under the tumor’s acidic environment, and be reduced to NIR absorptive agents for efficient PA imaging-guided PTT of cancer, as was demonstrated by various in vitro and in vivo experiments. As a proof-of-concept, this finding is expected to establish a new class of redox-activated probes based on clusters, bridging the conventional concepts of “molecule” and “nano” in the bio-imaging field.
Supplementary Material
Acknowledgments
This work was supported, in part, by the University of Wisconsin - Madison, the National Institutes of Health (NIBIB/NCI 1R01CA169365, 1R01EB021336, P30CA014520, S10-OD018505, T32GM008505), and the American Cancer Society (125246-RSG-13-099-01-CCE).
Footnotes
Details of experiments and additional supplementary figures. This information is available free of charge via the Internet at http://pubs.acs.org.
Conflict of Interest
The authors declare no competing financial interest.
References
- 1.Mi P, Kokuryo D, Cabral H, Wu H, Terada Y, Saga T, Aoki I, Nishiyama N, Kataoka K. Nat Nanotechnol. 2016;11:724–730. doi: 10.1038/nnano.2016.72. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Ye D, Wu M, Chen H, Zhang L, Shi J, Wang L. Adv Mater. 2014;26:7019–7026. doi: 10.1002/adma.201402572. [DOI] [PubMed] [Google Scholar]
- 3.Yavuz MS, Cheng Y, Chen J, Cobley CM, Zhang Q, Rycenga M, Xie J, Kim C, Song KH, Schwartz AG, Wang LV, Xia Y. Nat Mater. 2009;8:935–939. doi: 10.1038/nmat2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu Y, Aimetti AA, Langer R, Gu Z. Nat Rev Mater. 2016;2:16075. [Google Scholar]
- 5.Sarsour EH, Kumar MG, Chaudhuri L, Kalen AL, Goswami PC. Antioxid Redox Signal. 2009;11:2985–3011. doi: 10.1089/ars.2009.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubartelli A, Lotze MT. Trends Immunol. 2007;28:429–436. doi: 10.1016/j.it.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Lee MH, Yang Z, Lim CW, Lee YH, Dongbang S, Kang C, Kim JS. Chem Rev. 2013;113:5071–5109. doi: 10.1021/cr300358b. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Z, Fan H, Zhou G, Bai H, Liang H, Wang R, Zhang X, Tan W. J Am Chem Soc. 2014;136:11220–11223. doi: 10.1021/ja5029364. [DOI] [PubMed] [Google Scholar]
- 9.Lim SY, Hong KH, Kim DI, Kwon H, Kim HJ. J Am Chem Soc. 2014;136:7018–7025. doi: 10.1021/ja500962u. [DOI] [PubMed] [Google Scholar]
- 10.Tang Y, Song H, Su Y, Lv Y. Anal Chem. 2013;85:11876–11884. doi: 10.1021/ac403517u. [DOI] [PubMed] [Google Scholar]
- 11.Loving GS, Mukherjee S, Caravan P. J Am Chem Soc. 2013;135:4620–4623. doi: 10.1021/ja312610j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng R, Xie X, Vendrell M, Chang YT, Liu X. J Am Chem Soc. 2011;133:20168–20171. doi: 10.1021/ja2100774. [DOI] [PubMed] [Google Scholar]
- 13.Nie L, Chen X. Chem Soc Rev. 2014;43:7132–7170. doi: 10.1039/c4cs00086b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang LV, Hu S. Science. 2012;335:1458–1462. doi: 10.1126/science.1216210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JW, Galanzha EI, Shashkov EV, Moon HM, Zharov VP. Nat Nanotechnol. 2009;4(10):688–694. doi: 10.1038/nnano.2009.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun C, Wen L, Zeng J, Wang Y, Sun Q, Deng L, Zhao C, Li Z. Biomaterials. 2016;91:81–89. doi: 10.1016/j.biomaterials.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 17.De la Zerda A, Zavaleta C, Keren S, Vaithilingam S, Bodapati S, Liu Z, Levi J, Smith BR, Ma TJ, Oralkan O, Cheng Z, Chen X, Dai H, Khuri-Yakub BT, Gambhir SS. Nat Nanotechnol. 2008;3:557–562. doi: 10.1038/nnano.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie L, Wang G, Zhou H, Zhang F, Guo Z, Liu C, Zhang X, Zhu L. Biomaterials. 2016;103:219–228. doi: 10.1016/j.biomaterials.2016.06.058. [DOI] [PubMed] [Google Scholar]
- 19.De la Zerda A, Liu Z, Bodapati S, Teed R, Vaithilingam S, Khuri-Yakub BT, Chen X, Dai H, Gambhir SS. Nano Lett. 2010;10:2168–2172. doi: 10.1021/nl100890d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen YS, Frey W, Kim S, Kruizinga P, Homan K, Emelianov S. Nano Lett. 2011;11:348–354. doi: 10.1021/nl1042006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, He J, Yang K, Yi C, Nie L, Khashab NM, Chen X, Nie Z. Angew Chem Int Ed. 2015;54:15809–15812. doi: 10.1002/anie.201508616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du Y, Jiang Q, Beziere N, Song L, Zhang Q, Peng D, Chi C, Yang X, Guo H, Diot G, Ntziachristos V, Ding B, Tian J. Adv Mater. 2016;28:10000–10007. doi: 10.1002/adma.201601710. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Su H, Cai J, Cheng D, Ma Y, Zhang J, Zhou C, Liu S, Shi H, Zhang Y, Zhang C. ACS Nano. 2016;10:10404–10417. doi: 10.1021/acsnano.6b06267. [DOI] [PubMed] [Google Scholar]
- 24.Zha Z, Zhang S, Deng Z, Li Y, Li C, Dai Z. Chem Commun. 2013;49:3455–3457. doi: 10.1039/c3cc40608c. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Liu C, Hu D, Wang F, Wu H, Gong X, Liu X, Song L, Sheng Z, Zheng H. Adv Funct Mater. 2016;26:8715–8725. [Google Scholar]
- 26.Lv G, Guo W, Zhang W, Zhang T, Li S, Chen S, Eltahan AS, Wang D, Wang Y, Zhang J, Wang PC, Chang J, Liang XJ. ACS Nano. 2016;10:9637–9645. doi: 10.1021/acsnano.6b05419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lovell JF, Jin CS, Huynh E, Jin H, Kim C, Rubinstein JL, Chan WC, Cao W, Wang LV, Zheng G. Nat Mater. 2011;10:324–332. doi: 10.1038/nmat2986. [DOI] [PubMed] [Google Scholar]
- 28.Pu K, Shuhendler AJ, Jokerst JV, Mei J, Gambhir SS, Bao Z, Rao J. Nat Nanotechnol. 2014;9:233–239. doi: 10.1038/nnano.2013.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miao Q, Lyu Y, Ding D, Pu K. Adv Mater. 2016;28:3662–3668. doi: 10.1002/adma.201505681. [DOI] [PubMed] [Google Scholar]
- 30.Li LL, Ma HL, Qi GB, Zhang D, Yu F, Hu Z, Wang H. Adv Mater. 2016;28:254–262. doi: 10.1002/adma.201503437. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Yang PP, Zhao XX, Wang H. Nanoscale. 2016;8:2488–2509. doi: 10.1039/c5nr07437a. [DOI] [PubMed] [Google Scholar]
- 32.Dragulescu-Andrasi A, Kothapalli SR, Tikhomirov GA, Rao J, Gambhir SS. J Am Chem Soc. 2013;135:11015–11022. doi: 10.1021/ja4010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang D, Qi GB, Zhao YX, Qiao SL, Yang C, Wang H. Adv Mater. 2015;27:6125–6130. doi: 10.1002/adma.201502598. [DOI] [PubMed] [Google Scholar]
- 34.Duan Z, Gao Y-J, Qiao Z-Y, Fan G, Liu Y, Zhang D, Wang H. J Mater Chem B. 2014;2:6271–6282. doi: 10.1039/c4tb00319e. [DOI] [PubMed] [Google Scholar]
- 35.Liu T, Diemann E, Li H, Dress AW, Muller A. Nature. 2003;426:59–62. doi: 10.1038/nature02036. [DOI] [PubMed] [Google Scholar]
- 36.Liu T, Langston ML, Li D, Pigga JM, Pichon C, Todea AM, Muller A. Science. 2011;331:1590–1592. doi: 10.1126/science.1201121. [DOI] [PubMed] [Google Scholar]
- 37.Zhang C, Bu W, Ni D, Zuo C, Cheng C, Li Q, Zhang L, Wang Z, Shi J. J Am Chem Soc. 2016;138:8156–8164. doi: 10.1021/jacs.6b03375. [DOI] [PubMed] [Google Scholar]
- 38.Buckley RI, Clark RJ. Coord Chem Rev. 1985;65:167–218. [Google Scholar]
- 39.Wang Y, Zhou K, Huang G, Hensley C, Huang X, Ma X, Zhao T, Sumer BD, DeBerardinis RJ, Gao J. Nat Mater. 2014;13:204–212. doi: 10.1038/nmat3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaffer TM, Wall MA, Harmsen S, Longo VA, Drain CM, Kircher MF, Grimm J. Nano Letters. 2015;15:864–868. doi: 10.1021/nl503522y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen F, Goel S, Valdovinos HF, Luo H, Hernandez R, Barnhart TE, Cai W. ACS Nano. 2015;9:7950–7959. doi: 10.1021/acsnano.5b00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV. Nat Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsumoto K, Hyodo F, Matsumoto A, Koretsky AP, Sowers AL, Mitchell JB, Krishna MC. Clin Cancer Res. 2006;12:2455–2462. doi: 10.1158/1078-0432.CCR-05-2747. [DOI] [PubMed] [Google Scholar]
- 44.Hyodo F, Matsumoto K, Matsumoto A, Mitchell JB, Krishna MC. Cancer Res. 2006;66:9921–9928. doi: 10.1158/0008-5472.CAN-06-0879. [DOI] [PubMed] [Google Scholar]
- 45.Tannock IF, Rotin D. Cancer Res. 1989;49:4373–4384. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


