Abstract
Background
The 2013 WHO guidelines incorporated simplified and more effective antiretroviral regimens for the purposes of preventing mother-to-child transmission of HIV. With ideal implementation of these recommendations, perinatal HIV transmission could be reduced to less than 2%. However, loss to follow-up (LTFU) has the potential to erode the success of programs and a number of studies report high rates of LTFU within the prevention of mother-to-child transmission (PMTCT) care cascade. We evaluated the timing and magnitude of LTFU in a large programmatic PMTCT cohort in Nigeria in order to focus future efforts to reduce loss in this high burden setting.
Methods
From 2004-2014, the APIN/Harvard PEPFAR program supported antenatal HIV screening for nearly one million pregnant women and provided PMTCT care to over 30,000 women. The care cascade for women enrolling in the PMTCT program includes antenatal, delivery, and infant follow-up services through 12-18 months of life. In this retrospective cohort analysis, we examined data collected between 2004-2014 from 31 clinical sites in Nigeria and assessed the numbers of mothers and infants enrolled and LTFU at various points along the care cascade.
Results
Among 31,504 women (median age 30, IQR: 27-34) entering PMTCT care during the antenatal period, 20,679 (66%) completed the entire cascade of services including antenatal, delivery, and at least one infant follow-up visit. The median gestational age at presentation for antenatal care services was 23 weeks (IQR: 17-29). The median infant age at last follow-up visit was 12 months (IQR: 5-18). The greatest loss in the PMTCT care cascade occurred prior to delivery care (21%), with a further 16% lost prior to first infant visit. Of the 38,223 women who entered at any point along the PMTCT cascade, an HIV DNA PCR was available for 20,202 (53%) of their infants. Among infants for whom DNA PCR results were available, the rate of HIV transmission for infants whose mothers received any antenatal and/or delivery care was 2.8% versus 20.0% if their mother received none.
Conclusions
In this large cohort analysis, the proportion of women LTFU in the PMTCT care cascade was lower than that reported in previous cohort analyses. Nevertheless, this proportion remains unacceptably high and inhibits the program from maximally achieving the goals of PMTCT care. We also provide the largest analysis to date on rates of perinatal HIV transmission, with low rates among women receiving NNRTI- or PI-based regimens, approaching that reported in clinical trials. However, among mothers who received any antenatal care, infant outcomes were unknown for 48%, and women presented later in pregnancy than that recommended by current guidelines. Implementation research to evaluate ways to improve integration of services, particularly transitions from antenatal to delivery and pediatric care, are critically needed to reduce LTFU within PMTCT programs and achieve the ultimate goal of eliminating pediatric HIV infection.
Introduction
With currently available antiretroviral (ARV) regimens for the prevention of mother-to-child transmission (PMTCT) of HIV, perinatal transmission may be reduced from 20-45% without intervention to less than 2% [1,2]. Based on considerable progress in the expansion of antiretroviral therapy (ART) and PMTCT services globally, as well as implementation of simplified and more effective PMTCT regimens outlined in the 2013 World Health Organization (WHO) guidelines [3], the prospect of eliminating pediatric HIV is closer than ever [4].
In 2011, the Joint United Nations Programme on HIV/AIDS (UNAIDS) announced the “Countdown to Zero” initiative, ambitiously aiming to eliminate pediatric HIV infection by 2015 [5]. However, despite improved access to ARVs, utilization of PMTCT services remains suboptimal. In 2012, just 62% of HIV-infected pregnant women received ARVs to reduce MTCT [6,7]; in 2011, only 35% of infants born to HIV-infected mothers underwent HIV testing in the first two months of life [7]. Although the number of children born with HIV infection has declined by 58% since 2002, an estimated 240,000 infants were born with HIV in 2013 [8,9], falling far short of what could be achieved with available biomedical interventions.
Among women accessing PMTCT care services, loss to follow-up (LTFU) within the care cascade inhibits optimal outcomes. Studies examining LTFU within PMTCT programs in resource-limited settings vary widely [10-20], but range from 11% [20] to 75% [12]. In a 2013 meta-analysis that examined 25 studies, the pooled proportion of patients LTFU between antenatal registration and delivery was 49.1%, while LTFU for infants within 3 months of delivery was 33.9% [15]. These analyses provide important insight regarding points of loss within the care cascade, however, many of the studies involve small cohorts, the largest reporting on just over 3000 infants [15,16].
Nigeria is home to over 170 million people, with a greater number of HIV-infected pregnant women in need of PMTCT services annually than any other country in the world [21]. At the same time, the rate of PMTCT uptake in Nigeria is among the lowest at 20% [22] and there are more children living with HIV than in any other country [23]. Thus, issues of PMTCT care uptake and retention within Nigeria are critical to the global PMTCT response. In this study, we provide the largest analysis to date of LTFU within the PMTCT care cascade, examining a decade of data on over 30,000 pregnant HIV-infected women in Nigeria.
Materials and Methods
Since 2004, the Harvard School of Public Health has partnered with AIDS Prevention Initiative in Nigeria (APIN) utilizing President's Emergency Plan for AIDS Relief (PEPFAR) funds to support HIV care and treatment services as well as PMTCT care throughout Nigeria. The APIN PEPFAR program has screened almost one million pregnant women for HIV and, based on estimates from 2012, provided nearly 20% of all reported PMTCT care in Nigeria [22]. Women found to be HIV-infected are enrolled in the PMTCT program, in which the care cascade includes antenatal, delivery, and infant follow-up services through 12-18 months of life. In this study, we determine the numbers of mothers and infants (mother-baby pairs) accessing care during the three phases of the cascade and assess rates of LTFU.
Study patients
This retrospective cohort analysis examined PMTCT service utilization at 31 clinical sites in Nigeria from January 2004 through June 2014. Women entered the PMTCT program primarily through referral from the APIN adult ART clinic when they became pregnant, or through routine HIV screening in antenatal clinics. Guidelines for ART eligibility and PMTCT prophylaxis regimens have changed over time. A general overview of the evolution of programmatic PMTCT guidelines within the APIN PEPFAR program is provided in Table 1.
Table 1. Evolution of programmatic PMTCT interventions over time in the APIN PEPFAR program, 2004-2014 [3,24-28].
| Year | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WHO CD4 threshold for ART eligibility (adult) | <200 cells/mm3 | ≤350 | ≤500 | ||||||||
| Programmatic CD4 threshold for ART eligibility (adult) | <200 | ≤350 | |||||||||
| Programmatic regimen for ART-eligible pregnant women | AZT/3TC/NVP<br>or<br>d4T/3TC/NVP | AZT/3TC/(NVP or EFVb)<br>For HBV coinfected: TDF/(3TC or FTC)/(NVP or EFVb) | EFV/TDF/(3TC or FTC)d | ||||||||
| Programmatic prophylaxis for ART ineligible pregnant women | Presenting 28-33 weeks gestation: AZT bid + sdNVP at labora<br><br>Presenting ≥34 weeks: AZT/3TC bid + sdNVP at labora<br><br>sdNVP for women presenting in labor | From 14 weeks gestation on: AZT bid + sdNVP in laborc<br>Or<br>Triple ART prophylaxis: LPV/r+AZT+3TC EFV+AZT+(3TC or FTC)<br>EFV+TDF+(3TC or FTC)<br>ABC+3TC+AZT | EFV/TDF/(3TC or FTC)e | ||||||||
Abbreviations: ABC, abacavir; AZT, zidovudine; 3TC, lamivudine; EFV, efavirenz; FTC, emtricitabine; LPV/r, lopinavir-ritonavir; NVP, nevirapine; TDF, tenofovir; sdNVP, single-dose NVP.
plus AZT bid “tail” for one week post-delivery
At this time, EFV was not recommended during the 1st trimester
plus AZT/3TC bid in labor and AZT/3TC bid for 7-day “tail” post-delivery
Restriction on EFV use during 1st trimester eliminated.
Started at any time in pregnancy, labor, or after delivery and continued until one week after cessation of all breastfeeding
Data collection
Patient data were collected on standardized clinic forms by trained physicians or healthcare providers and entered into a customized electronic medical record database (FileMaker Pro, Santa Clara, CA) by trained data staff at each clinical site. All pregnant women enrolling in the PMTCT program were given a unique patient identifier that allowed for linkage of records throughout the care cascade. This identifier is separate from the patient ID given to individuals enrolled in the adult ART program. The adult ART ID is maintained throughout a patient's lifetime of ART care, whereas the PMTCT ID is relevant only during the patient's pregnancy and infant follow-up and a new PMTCT ID number is assigned with each subsequent pregnancy. The infant identifier is linked to the mother's to aid in follow-up and tracking. For mothers that were simultaneously enrolled in the adult ART program, PMTCT IDs were used to link to adult ART database systems to abstract additional data.
Definition of loss to follow-up and care compliance
For the purposes of this study, compliance with the care cascade was defined as having documentation of care at each of the three stages of PMTCT care. Specifically, a woman was considered compliant if she has at least one antenatal visit, a documented delivery note, and at least one infant follow-up visit. LTFU was defined as having no additional patient records after a particular stage of care. There are no standard definitions for retention-in-care or LTFU within the PMTCT care cascade [29], but increasingly definitions that incorporate adherence to scheduled visits are being used [30]. Although our definition of care compliance does not account for inconsistent attendance or adherence to ARVs, it requires that women access care across several different clinical settings (antenatal clinics, labor and delivery, and infant follow-up clinics). In developing our definition, we believe that this, in combination with infant outcomes data, provides a valuable assessment of the continuity and quality of care in our program.
Statistical analysis
Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC). Loss to follow-up outcomes within the PMTCT care cascade were detailed using descriptive information, primarily numbers and proportions of women reported as LTFU at each stage. Baseline characteristics of women who completed the entire care cascade versus those with incomplete compliance were also compared. Baseline characteristics are reported as medians [interquartile range, IQR] or as proportions, where appropriate. Student's t-test was used for normally distributed continuous variables and the Wilcoxon rank sum test for non-normally distributed continuous variables. For binary or categorical outcome variables, the Chi-square test was used. The Pearson Product-Moment Correlation was used to evaluate for a linear trend in compliance rate by enrollment year. Variables at the p<0.05 were considered statistically significant.
Ethical considerations
At the time of enrollment into the APIN PEPFAR PMTCT program, all patients completed a consent for care form and were given the option to allow their de-identified data to be used for future evaluations. Only patients who provided consent for use of their data in future research were included in this analysis. The institutional review board (IRB) of the Harvard School of Public Health and the APIN regulatory affairs committee approved this study.
Results
Study population
Between January 2004 and June 2014, 31,504 HIV-infected pregnant women enrolled in the PMTCT program during the antenatal period (Figure 1). The median maternal age at presentation for antenatal services was 30.2 years (IQR, 26.8-33.7 years) and the median gestational age was 23 weeks (IQR, 17-29 weeks). The median baseline CD4+ cell count was 362 cells/mm3 (IQR, 234-518 cells/mm3) and plasma HIV-1 RNA level was 846 copies/mL (IQR, 200-19,552 copies/mL). The proportion of women already receiving ART at the time of enrollment is unknown. However, of the 31,504 women who received any antenatal care, baseline VL measurements were available for 21,966 (70%), and of those, 10,077 (46%) had undetectable VL values (i.e. VL <400 copies/mL). This, in addition to the relatively high baseline CD4 count, suggests that a significant portion of women were receiving ART at entry into the PMTCT program.
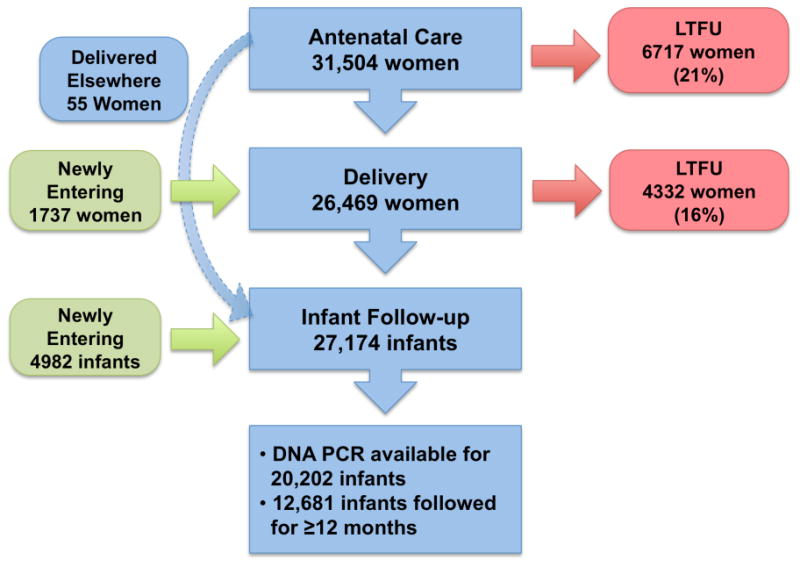
Figure 1. Rates of LTFU within PMTCT Care Cascade.
Patient characteristics by enrollment year
Table 2 shows the evolution of patient characteristics over time. After a period of intensive scale-up in 2004 and 2005, maternal age at presentation has remained relatively stable, while CD4 count at presentation increased and both gestational age and VL at presentation trended down. With implementation of the revised Nigerian PMTCT guidelines in 2010 and 2012 (Table 1), use of mono- or dual-NRTI prophylaxis has decreased over time while the proportion of women receiving NNRTI-based ART has increased. Further, the proportion of women receiving no ARVs has declined significantly over time (p-value for trend <0.0001). The proportion of infants seen in follow-up increased over time to 2011; however, infant follow-up data for women enrolled in 2013 and 2014 is incomplete due to insufficient time for 18 months of infant follow-up.
Table 2. Baseline characteristics, ARV status, and infant follow-up by year of enrollment.
| Variable | Median (IQR) or percentage by year of enrollment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013A | 2014A | Total | |
| Number of patients per enrollment year | 26 | 141 | 1048 | 2297 | 3686 | 4025 | 4295 | 4926 | 5255 | 4224 | 1581B | 31,504 |
| Maternal age at 1st visit (years) | 31 (30-33) | 29 (27-32) | 29 (26-32) | 30 (26-33) | 29 (26-33) | 30 (26-33) | 30 (27-34) | 30 (27-34) | 31 (28-34) | 31 (28-35) | 32 (28-35) | 30 (27-34) |
| Gestational age at 1st visit (weeks) | 18 (12-27) | 25 (17-31) | 26 (18-33) | 25 (17-31) | 24 (17-30) | 24 (16-30) | 23 (17-29) | 22 (16-28) | 22 (17-28) | 22 (16-28) | 22 (17-28) | 23 (17-29) |
| CD4 count at baseline (cells/mm3) | 140 (120-402) | 282 (179-386) | 310 (193-459) | 316 (207-479) | 335 (219-493) | 345 (228-503) | 349 (231-490) | 356 (234-512) | 387 (251-546) | 391 (254-549) | 415 (272-573) | 362 (234-518) |
| Viral load at baseline (copies/mL) | 9946 (200-31438) | 18538 (820-89635) | 5198 (315-39269) | 2792 (200-26317) | 2673 (200-23231) | 1932 (200-22319) | 918 (200-17802) | 417 (200-17131) | 200 (200-13225) | 200 (200-13719) | 200 (200-6953) | 846 (200-19552) |
| ARV Status, (%) | ||||||||||||
| NNRTI-based ART/prophylaxis | 50% | 71% | 50% | 47% | 44% | 49% | 64% | 84% | 87% | 90% | 92% | 70% |
| PI-based ART | - | 1% | 3% | 3% | 4% | 5% | 6% | 9% | 10% | 8% | 7% | 7% |
| Triple-NRTI ART | - | - | 0.6% | 0.7% | 0.4% | 0.3% | 0.2% | 0.4% | 0.3% | 0.05% | - | 0.3% |
| Mono/dual-NRTI prophylaxis | - | - | 14% | 22% | 23% | 24% | 20% | 2% | 0.3% | 0.2% | - | 11% |
| Single-dose NVP only | - | - | 0.7% | 0.5% | 0.4% | 0.6% | 0.2% | - | 0.02% | - | - | 0.2% |
| No ARVs received or none documented | 50% | 28% | 31% | 27% | 28% | 22% | 10% | 4% | 2% | 2% | 1% | 12% |
| No. (%) of infants with ≥1 follow-up visit | - | 5 (4%) | 430 (41%) | 1551 (68%) | 2360 (64%) | 2716 (67%) | 3005 (70%) | 3627 (74%) | 3843 (73%) | 2692 (64%) | 554 (35%) | 20783 (66%) |
| Median duration of infant follow-up (months) | - | 21 (12-21) | 17 (8-18) | 13 (6-18) | 13 (5-18) | 15 (6-18) | 17 (7-18) | 16 (9-18) | 12 (6-18) | 6 (3-10) | 2 (1-3) | 12 (5-18) |
| % of infants with ≥1 follow-up who have DNA PCR result | - | 20% | 28% | 58% | 69% | 81% | 87% | 89% | 87% | 63% | 21% | 76% |
Reported infant follow-up data is incomplete as there is insufficient time from maternal enrollment to delivery and 18 months follow-up for all infants (data was only analyzed through June 2014).
Maternal enrollment data is incomplete for 2014 as this cohort only included women enrolled through June 2014.
Loss to follow-up during the PMTCT care cascade
Among the 31,504 women entering PMTCT care during the antenatal period, 20,679 (66%) completed the entire cascade of services, including antenatal, delivery, and at least one infant follow-up visit (Figure 1). The greatest loss in the PMTCT care cascade occurred at delivery, with 21% of women LTFU after receiving some antenatal care. Among the 6717 women who attended at least one antenatal visit but were lost to any subsequent care, 43% had only one antenatal visit. Among 26,469 women who received antenatal and delivery or delivery care only, 4332 (16%) did not subsequently return for infant follow-up care. A small percentage (less than 1%; n=55) of women received antenatal care and did not deliver at one of the program sites, but then returned with their infants.
Late entry into the care cascade
A small proportion of women entered care for the first time during labor and delivery (1737 women; 4.5%), whereas nearly 20% of infants (4982 infants) followed in the exposed infant program were brought for care after their delivery with no documented maternal antenatal or delivery care.
Characteristics of women compliant with care cascade
The baseline characteristics of women who were compliant with the care cascade were compared to those with incomplete compliance (Table 3). Although all characteristics achieved statistical significance due to the large sample size, there were no clinically significant differences between the two groups in terms of maternal age at first antenatal visit, gestational age, CD4+ cell count, and viral load at baseline. An overall comparison of maternal ARVs by regimen type (eg. NNRTI- or PI-based ART, triple-NRTI ART, mono- or dual-NRTI prophylaxis, single-dose nevirapine, or no ARVs received or none documented) was significantly different between the two groups (p<0.0001). Women who were compliant with the care cascade were significantly more likely than women with incomplete compliance to receive any ARV therapy (i.e. NNRTI- or PI-based ART, triple-NRTI ART, or mono/dual-NRTI prophylaxis). A small proportion of women (0.2% in both groups) received only single-dose nevirapine. Among women in the incomplete compliance group nearly 20% did not receive any ARVs compared with 8% in the complete compliance group (p<0.0001).
Table 3. Baseline Characteristics for Women with CompleteA versus Incomplete Compliance with PMTCT Care Cascade.
| Variable | Completed PMTCT Care Cascade (N=20,679) | Incomplete Compliance with Care Cascade (N=10,825) | |||
|---|---|---|---|---|---|
| Median (IQR)B | N available for analysis | Median (IQR)B | N available for analysis | P-value | |
| Maternal age at 1st visit (years) | 30 (27-34) | 20,111 | 30 (26-34) | 10,244 | <0.0001 |
| Gestational Age at 1st visit (weeks) | 23 (17-29) | 19,005 | 22 (16-29) | 9776 | <0.001 |
| CD4 count at baseline (cells/mm3) | 363 (240-516) | 17,904 | 359 (223-522) | 8620 | 0.039 |
| Viral load at baseline (copies/mL) | 603 (200-16026) | 15,109 | 1626 (200-29638) | 6857 | <0.0001 |
| ARV Status, (%) | 20,679 | 10,825 | <0.0001D | ||
| NNRTI-based ART/prophylaxis | 72% | 65% | <0.0001 | ||
| PI-based ART | 7% | 6% | <0.0001 | ||
| Triple-NRTI ART | 0.4% | 0.2% | <0.02 | ||
| Mono/dual-NRTI prophylaxis | 12% | 10% | <0.0001 | ||
| Single-dose NVP only | 0.2% | 0.2% | 0.6 | ||
| No ARVs received or none documentedC | 8% | 19% | <0.0001 | ||
Complete compliance requires a minimum of one antenatal, delivery, and at least 1 infant follow-up visit.
All values reported in median (IQR) unless otherwise specified
No evidence of ARV receipt in database due to multiple factors: LTFU, non-eligible and <28 weeks (2006 guidelines), not documented.
Chi-square test was used for overall comparison of ARV regimens between those with complete and incomplete care compliance; Student's t-test was used for individual ARV comparisons.
Magnitude of loss to follow-up by year of enrollment
We were also interested in examining if compliance improved over calendar year. After a period of low compliance due to programmatic scale-up in 2004-2005, compliance with the care cascade significantly improved across enrollment years with compliance of 72.9% in 2012 (p-value for trend <0.0001, Figure 2). Data from 2013 and 2014 were excluded from this trend analysis due to insufficient time for complete follow-up of all mother-infant pairs.
Figure 2. Compliance with PMTCT care cascade by year of enrollment*.
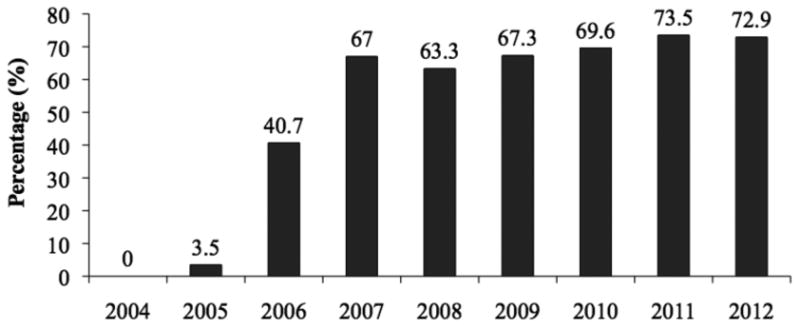
* PEPFAR services began in 2004, hence low initial enrollment (see Table 2) and linkage between care during the initial scale-up period.
Infant follow-up and outcomes
The median age at first and last infant follow-up visit was 0.7 months (3 weeks) (IQR, 0.4-1.7 months) and 11.5 months (IQR, 4.9-18.0 months), respectively. The median number of visits per infant in the exposed infant program was 5 (IQR, 3-9 visits). Among the 27,174 infants followed in the exposed infant program, 12,681 (47%) were followed until at least 12 months of age. The median infant age at first HIV DNA PCR test was 1.6 months (IQR, 1.4-3.1 months).
Among the 38,223 mothers who accessed care at any point along the cascade, infant DNA PCR results were available for 20,202 (52.9%). Among the 27,174 infants with at least one infant follow-up visit, PCR results were available for 74%. Within the group of infants born to mothers who received any antenatal or delivery care, the proportion found to be HIV-infected was 2.8% (474 of 17,248 available DNA PCR results). By comparison, among the group of exposed infants whose mothers had not received PMTCT care during the antenatal or delivery periods, HIV DNA PCR results were available for 2954 (59%) and 20.0% of those infants (591 of 2954) were found to be HIV-positive.
Infant outcome by maternal ARV regimen
Among those children for whom DNA PCR results were available, the HIV transmission rate was 2.2% and 1.9% with maternal receipt of NNRTI- and PI-based regimens, respectively. Among those women who received mono- or dual-NRTI prophylaxis regimens, the rate of transmission was 4.7% (95 of 2031 infants). Additionally, a small number of women received triple-NRTI therapy and transmission was 6.1% (4 of 66 infants). Among the 24.3% of women who either did not receive any ARVs during pregnancy or delivery or for whom the maternal ARV regimen was not documented, the rate of transmission was 15.8% (661 of 4185 women who either received some antenatal and/or delivery care but for whom receipt of ARVs was not documented, or women who entered the care cascade for the first time after delivery for exposed-infant care).
Discussion
This is the largest cohort analysis, to date, evaluating retention in care within the PMTCT care cascade. Among more than 31,000 women, the rate of compliance with the care cascade was 66%. Although this proportion of women retained in PMTCT care is higher than retention statistics from many published cohorts [10-20], LTFU remains a significant barrier to achieving optimal program outcomes. Our findings also emphasize the ongoing need to identify HIV-infected women earlier in pregnancy, which remains a particular challenge in Nigeria, where PMTCT service uptake remains critically low and one in five births occurs at home with “no one present” [31]. Additionally, we found that 41% of women with only one antenatal visit failed to bring their infants in for follow-up, as opposed to 29% with more than one visit. Finally, our data show that programs can improve over time, as retention reached 72.9% in 2012. Multiple factors contributed to this improvement over time including the program's emphasis on clinical training and quality improvement activities, streamlining PMTCT protocols, and expanding infrastructure, among others. Additionally, whether retention continues to improve with implementation of the simplified 2013 WHO guidelines is unknown and additional program evaluations will be useful in understanding the impact.
Although the interpretation of infant outcomes from this study is limited due to LTFU and missing data, with over 20,000 DNA PCR results available, this is one of the largest reports of infant transmission rates, to date. HIV transmission among infants of mothers who were known to have received NNRTI- or PI-based ART was 2.2% or less, similar to rates among clinical trials, suggesting that large-scale clinical programs have the potential to achieve excellent results. Among exposed infants whose mothers did not receive antenatal or delivery care, the rate of transmission was 20%. These findings are consistent with an early program evaluation [32], and emphasize the critical need to improve uptake and retention within the program.
While this program analysis sheds valuable light on issues of retention within a large PMTCT care program, several important limitations should be noted. First, the definition of compliance used in this study is not intended to represent ideal care. In fact, women in this analysis presented later than that recommended by the WHO, which recommends starting ARVs as early as possible during pregnancy, with a median gestational age at first visit of 23 weeks and the median number of antenatal visits was only two. Nevertheless, HIV transmission was low in this cohort. Another important limitation is the issue of missing data in this large database analysis. In particular, for approximately 12% of women who received any antenatal care, an ARV regimen was not documented. However, since rates of transmission among this group were only slightly higher than the rest of the cohort at 5.1%, this suggests that receipt of ARVs was somewhat under-documented. Finally, although care is taken within the program to assign and maintain unique patient identifiers within the PMTCT program, some overestimation of loss is possible if women did not mention prior engagement in care upon return for subsequent visits, in which case linkage between stages of care was lost.
This study emphasizes that implementation research is urgently needed to identify ways to improve retention within PMTCT care programs. In this study, baseline characteristics did not effectively identify women at risk of loss, so interventions toward improving retention should be applied uniformly. A variety of strategies to improve retention have been studied including, but not limited to, decentralization of services [33,34], integration of services [35-38], use of treatment partners [39], male involvement [18,40,41], accompaniment between clinics [42], active patient tracking [43], nutritional supplements [44,45], reimbursement of transportation costs [46], use of mobile technology for reminders [35], and reduced pill burden [47,48]. Additionally, streamlining treatment protocols has the potential to reduce loss by increasing the number of women initiated on treatment early, and the full impact of the 2013 WHO guidelines remains to be seen. The 2014 Nigerian integrated guidelines recommend several strategies to improve retention including decentralization and integration of services, task shifting, logistics management, among others [49]. Ultimately, a multifaceted approach aimed at addressing multiple barriers to follow-up across the PMTCT care cascade, with a particular focus on the transitions between stages of care are needed. The challenge is to determine which combination of strategies achieves the best outcomes while reaching the most women in a cost-effective manner.
Conclusions
To date, this is the largest cohort analysis presenting data on loss to follow-up within the PMTCT care cascade. Although retention was better than that seen in many smaller cohorts, loss of women from PMTCT care remains unacceptably high. Among infants whose mothers were compliant with the care cascade, outcomes were excellent, nearing transmission rates achieved in randomized controlled trials. These promising results should encourage PMTCT programs to further examine reasons for loss and implement interventions to reduce LTFU as a means of achieving the ultimate goal of eliminating perinatal HIV transmission.
Acknowledgments
This research was supported in part by the U.S. Department of Health and Human Services, Health Resources and Services Administration (U51HA02522) and the Centers for Disease Control and Prevention by cooperative agreement with APIN (U2GGH000924, 5U2GPS001058, and PS 001058). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the funding institution. The authors would like to acknowledge the hard work and dedication of the clinical, data, laboratory, and administrative staff at all of the APIN PEPFAR HIV care and treatment sites.
Footnotes
Conflict of Interest: None of the authors have any conflicts to declare.
References
- 1.Shapiro RL, Hughes MD, Ogwu A, et al. Antiretroviral Regimens in Pregnancy and Breast-Feeding in Botswana. N Engl J Med. 2010;362(24):2282–94. doi: 10.1056/NEJMoa0907736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chasela CS, Hudgens MG, Jamieson DJ, Kayira D, Hosseinipour MC, Kourtis AP, Martinson F, Tegha G, Knight RJ, et al. for the BAN Study Group. Maternal or Infant Antiretroviral Drugs to Reduce HIV-1 Transmission. N Engl J Med. 2010;362(24):2271–81. doi: 10.1056/NEJMoa0911486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach. WHO Press, World Health Organization; Geneva, Switzerland: Jun, 2013. p. 2013. [PubMed] [Google Scholar]
- 4.Mofenson LM. Protecting the Next Generation - Eliminating Perinatal HIV-1 Infection. N Engl J Med. 2010;362:2316–2318. doi: 10.1056/NEJMe1004406. [DOI] [PubMed] [Google Scholar]
- 5.Joint United Nations Programme on HIV/AIDS (UNAIDS) Countdown to zero: global plan for the elimination of new HIV infections among children by 2015 and keeping their mothers alive, 2011-2015. Geneva: UNAIDS; 2011. [Google Scholar]
- 6.UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2012. WHO Library Cataloguing-in-Publication Data; UNAIDS / JC2417E. 2012 [Google Scholar]
- 7.UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2013. WHO Library Cataloguing-in-Publication Data; UNAIDS / JC2502/1/E. 2013 Nov; [Google Scholar]
- 8.UNAIDS. The Gap Report. 2014 Jul; http://www.unaids.org/en/resources/campaigns/2014/2014gapreport/gapreport/
- 9.UNAIDS. The Gap Report: Epi Slides. 2014 Jul; http://www.unaids.org/en/media/unaids/contentassets/documents/document/2014/2014gapreportslides/01_Epi_slides_2014July.pdf.
- 10.Hassan AS, Sakwa EM, Nabwera HM, et al. Dynamics and Constraints of Early Infant Diagnosis of HIV Infection in Rural Kenya. AIDS Behav. 2012;16(1):5–12. doi: 10.1007/s10461-010-9877-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurewa EN, Kandawasvika GQ, Mhlanga F, et al. Realities and Challenges of a Fiver Year Follow Up of Mother and Child Pairs on a PMTCT Program in Zimbabwe. Open AIDS J. 2011;5:51–58. doi: 10.2174/1874613601105010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook RE, Ciampa PJ, Sidat M, et al. Predictors of Successful Early Infant Diagnosis of HIV in a Rural District Hospital in Zambézia, Mozambique. J Acquir Immune Defic Syndr. 2011;56(4):e104–e109. doi: 10.1097/QAI.0b013e318207a535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manzi M, Zachariah R, Teck R, et al. High acceptability of voluntary counselling and HIV-testing but unacceptable loss to follow up in a prevention of mother-to-child HIV transmission programme in rural Malawi: scaling-up requires a different way of acting. Trop Med Int Health. 2005;10(12):1242–50. doi: 10.1111/j.1365-3156.2005.01526.x. [DOI] [PubMed] [Google Scholar]
- 14.Stringer EM, Ekouevi DK, Coetzee D, et al. Coverage of Nevirapine-Based Services to Prevent Mother-to-Child HIV Transmission in 4 African Countries. JAMA. 2010;304(3):293–302. doi: 10.1001/jama.2010.990. [DOI] [PubMed] [Google Scholar]
- 15.Sibanda EL, Weller IVD, Hakim JG, et al. The magnitude of loss to follow-up of HIV-exposed infants along the prevention of mother-to-child HIV transmission continuum of care: a systematic review and meta-analysis. AIDS. 2013;27(17):2787–97. doi: 10.1097/QAD.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moses A, Zimba C, Kamanga E, et al. Prevention of mother-to-child transmission: program changes and the effect on uptake of the HIVNET012 regimen in Malawi. AIDS. 2008;22(1):83–87. doi: 10.1097/QAD.0b013e3282f163b5. [DOI] [PubMed] [Google Scholar]
- 17.Chetty T, Knight S, Giddy J, et al. A retrospective study of Human Immunodeficiency Virus transmission, mortality and loss to follow-up among infants in the first 18 months of life in a prevention of mother-to-child transmission programme in an urban hospital in KwaZulu-Natal, South Africa. BMC Pediatr. 2012;12:146. doi: 10.1186/1471-2431-12-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wettstein C, Mugglin C, Egger M, et al. IeDEA Southern Africa Collaboration. Missed opportunities to prevent mother-to-child-transmission: systematic review and meta-analysis. AIDS. 2012 Nov 28;26(18):2361–73. doi: 10.1097/QAD.0b013e328359ab0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalembo FW, Zgambo M. Loss to Followup: AMajor Challenge to Successful Implementation of Prevention of Mother-to-Child Transmission of HIV-1 Programs in Sub-Saharan Africa. ISRN AIDS 2012. doi: 10.5402/2012/589817. Article ID 589817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panditrao M, Darak S, Kulkarni V, et al. Sociodemographic factors associated with loss to follow-up of HIV-infected women attending a private sector PMTCT program in Maharashtra, India. AIDS Care. 2011;23:593–600. doi: 10.1080/09540121.2010.516348. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. PMTCT Strategic Vision 2010-2015: Preventing mother-to-child transmission of HIV to reach the UNGASS and Millennium Development Goals. 2010 Available at http://www.who.int/hiv/pub/mtct/strategic_vision.pdf.
- 22.World Health Organization. Global Update on HIV Treatment 2013: Results, Impact and Opportunities. WHO report in partnership with UNICEF and UNAIDS. 2013 Jun; Available at http://www.who.int/hiv/pub/progressreports/update2013/en/
- 23.UNAIDS. Report on the Global AIDS Epidemic 2013. Available at http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf.
- 24.World Health Organization. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: recommendations for a public health approach; 2010 version. WHO Press, World Health Organization; Geneva, Switzerland: 2010. [PubMed] [Google Scholar]
- 25.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach; 2006 revision. WHO Press, World Health Organization; Geneva, Switzerland: 2006. [Google Scholar]
- 26.World Health Organization. Scaling up antiretroviral therapy in resource-limited settings: treatment guidelines for a public health approach; 2003 revision. World Health Organization; Geneva, Switzerland: 2004. [Google Scholar]
- 27.Federal Ministry of Health, Nigeria. National Guidelines on Prevention of Mother-to-Child Transmission of HIV (PMTCT) 2010 Nov; [Google Scholar]
- 28.Federal Ministry of Health, Nigeria. National Guidelines for HIV and AIDS Treatment and Care in Adolescents and Adults. Abuja, Nigeria: May, 2007. [Google Scholar]
- 29.Rollins NC, Becquet R, Orne-Gliemann J, et al. Defining and Analyzing Retention-in-Care Among Pregnant and Breastfeeding HIV-Infected Women: Unpacking the Data to Interpret and Improve PMTCT Outcomes. J Acquir Immune Defic Syndr. 2014;67:S150–S156. doi: 10.1097/QAI.0000000000000355. [DOI] [PubMed] [Google Scholar]
- 30.Oyeledun B, Oronsaye F, Oyelade T, et al. Increasing Retention in Care of HIV-Positive Women in PMTCT Services Through Continuous Quality Improvement–Breakthrough (CQI-BTS) Series in Primary and Secondary Health Care Facilities in Nigeria: A Cluster Randomized Controlled Trial. The Lafiyan Jikin Mata Study J Acquir Immune Defic Syndr. 2014;67:S125–S131. doi: 10.1097/QAI.0000000000000320. [DOI] [PubMed] [Google Scholar]
- 31.Fapohunda BM, Orobaton NG. When Women Deliver with No One Present in Nigeria: Who, What, Where and So What? PLoS ONE. 2013;8(7):e69569. doi: 10.1371/journal.pone.0069569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adewole IF, Sagay AS, Meloni S, et al. Mother's Prophylaxis Regimen Strongly Predicts Risk of Early Mother-to-Child Transmission in Large ART Program in Nigeria. Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. 10-12 February, 2009; Poster Abstract T-153. [Google Scholar]
- 33.Massaquoi M, Zachariah R, Manzi M, et al. Patient retention and attrition on antiretroviral treatment at district level in rural Malawi. Trans R Soc Trop Med Hyg. 2009;103(6):594–600. doi: 10.1016/j.trstmh.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 34.De Baets AJ, Sifovo S, Pazvakavambwa IE. Early Identification and Care of HIV-Exposed and HIV-Infected Children in Rural Africa: The Role of Primary Health Care Centers. J Acquir Immune Defic Syndr. 2008;48(2):230–32. doi: 10.1097/QAI.0b013e31816e398b. [DOI] [PubMed] [Google Scholar]
- 35.Tudor Car L, van-Velthoven MH, Brusamento S, et al. Integrating prevention of mother-to-child HIV transmission (PMTCT) programmes with other health services for preventing HIV infection and improving HIV outcomes in developing countries. Cochrane Database of Systematic Reviews. 2011;(6) doi: 10.1002/14651858.CD008741.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Megazzini KM, Sinkala M, Vermund SH, et al. A cluster-randomized trial of enhanced labor ward-based PMTCT services to increase nevirapine coverage in Lusaka, Zambia. AIDS. 2010;24(3):447–55. doi: 10.1097/QAD.0b013e328334b285. [DOI] [PubMed] [Google Scholar]
- 37.Tsague L, Tsiouris FO, Carter RJ, et al. Comparing two service delivery models for the prevention of mother-to-child transmission (PMTCT) of HIV during transition from single-dose nevirapine to multi-drug antiretroviral regimens. BMC Public Health. 2010;10:753. doi: 10.1186/1471-2458-10-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thaineua V, Sirinirund P, Tanbanjong A, et al. From research to practice: use of short course zidovudine to prevent mother-to-child HIV transmission in the context of routine health care in Northern Thailand. Southeast Asian J Trop Med Public Health. 1998;29(3):429–42. [PubMed] [Google Scholar]
- 39.Futterman D, Shea J, Besser M, et al. Mamekhaya: A pilot study combining a cognitive-behavioral intervention and mentor mothers with PMTCT services in South Africa. AIDS Care. 2010;22(9):1093–1100. doi: 10.1080/09540121003600352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torpey K, Kabaso M, Kasonde P, et al. Increasing the uptake of prevention of mother-to-child transmission of HIV services in a resource-limited setting. BMC Health Serv Res. 2010;10:29. doi: 10.1186/1472-6963-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farquhar C, Kiarie JN, Richardson BA, et al. Antenatal couple counseling increases uptake of interventions to prevent HIV-1 transmission. J Acquir Immune Defic Syndr. 2004;37:1620–1626. doi: 10.1097/00126334-200412150-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ciampa PJ, Burlison JR, Blevins M, et al. Improving Retention in the Early Infant Diagnosis of HIV Program in Rural Mozambique by Better Service Integration. J Acquir Immune Defic Syndr. 2011;58:115–119. doi: 10.1097/QAI.0b013e31822149bf. [DOI] [PubMed] [Google Scholar]
- 43.Thomson KA, Cheti EO, Reid T. Implementation and outcomes of an active defaulter tracing system for HIV, prevention of mother to child transmission of HIV (PMTCT), and TB patients in Kibera, Nairobi, Kenya. Trans Royal Soc Trop Med and Hygiene. 2011;105(6):320–326. doi: 10.1016/j.trstmh.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 44.Braitstein P, Katshcke A, Shen C, et al. Retention of HIV-infected and HIV-exposed children in a comprehensive HIV clinical care programme in Western Kenya. Trop Med & Intnl Health. 2010;15(7):833–41. doi: 10.1111/j.1365-3156.2010.02539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kundu CK, Samanta M, Sarkar M, et al. Food Supplementation as an Incentive to Improve Pre-antiretroviral Therapy Clinic Adherence in HIV-Positive Children—Experience from Eastern India. J Trop Ped. 2012;58(1):31–37. doi: 10.1093/tropej/fmr026. [DOI] [PubMed] [Google Scholar]
- 46.Barker CE, Bird CE, Pradhan A, Shakya G. Support to the Safe Motherhood Programme in Nepal: an integrated approach. Reprod Health Matters. 2007;15:81–90. doi: 10.1016/S0968-8080(07)30331-5. [DOI] [PubMed] [Google Scholar]
- 47.Nachega JB, Parienti JJ, Uthman OA, et al. Lower Pill Burden and Once-daily Dosing Antiretroviral Treatment Regimens for HIV Infection: A Meta-Analysis of Randomized Controlled Trials. Clin Infect Dis. 2014;58(9):1297–1307. doi: 10.1093/cid/ciu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramjan R, Calmy A, Vitoria M, et al. Systematic review and meta-analysis: Patient and programme impact of fixed-dose combination antiretroviral therapy. Trop Med Int Health. 2014;19(5):501–513. doi: 10.1111/tmi.12297. [DOI] [PubMed] [Google Scholar]
- 49.National AIDS/STIs Control Programme Federal Ministry of Health. Integrated National Guidelines for HIV Prevention Treatment and Care. Federal Ministry of Health; Abuja, Nigeria: 2014. [Google Scholar]


