Abstract
This article reports on the changes of oxidation indices and minor components of low free fatty acid (FFA) and freshly extracted crude palm oils after storage at ambient (28 ± 1 C) and 60 C for 77 days. The changes in peroxide value (PV), FFA, extinction coefficient at 233 and 269 nm (K 233 and K 269), bleachability index (DOBI), carotene and vitamin E contents were monitored. PV, FFA, K 233 and K 269 of both oil samples increased as storage progressed while the values of carotene and vitamin E contents decreased. At the end of storage period at 60 °C, the carotene content of low FFA crude palm oil was 4.24 ppm. The storage conditions used led to the loss of entire vitamin E fractions of both oil samples as well as a reduction in DOBI values except for freshly extracted crude palm oil stored at ambient temperature.
Keywords: Low free fatty acid, Freshly extracted, Crude palm oil, Storage, Oxidation indices, Vitamin E
Introduction
Ninety percent of palm oil is used worldwide in the food industry such as margarine, deep fat frying, shortening, ice creams, and cocoa butter substitutes in chocolate (Corley and Tinker 2003; Hassan 1988; Idris and Samsuddin 1993). Palm oil contains a high proportion of palmitic acid as well as considerable quantities of oleic and linoleic acids, which gives it a higher unsaturated fatty acid content compared to coconut oil and palm kernel oil (Ayre and Hulbert 1996; Cottrell 1991; Gunstone et al. 1986; Satchithanandam et al. 1993). Leong (1994) suggested that good quality palm oil products used as raw materials contributes to better properties of end-products such as longer shelf life, consistent end-product quality and consumer acceptance.
Oils and fats are a mixture of triacylglycerols of different types of fatty acids, with palm oil products containing mainly oleic and palmitic acids. One of the major problems affecting palm oil, like other edible oils, is lipid oxidation, which is the cause of important deteriorative changes in their chemical, sensory and nutritional properties (Velasco and Dobarganes 2002). Oxidative deterioration of oil and fat not only results in the development of a pungent and offensive off-flavour, but also the destruction of vitamins (A, D, E, K, C), essential fatty acids, chlorophylls, carotenes, amino acids, proteins, and enzymes by the production of toxic or physiologically active compounds (Gardner 1979). In contrast to animal fats, which are predominantly saturated and hence do not react readily with other chemicals, unsaturated vegetable oils are more reactive (Matalgyto and Al-Khalifa 1998). Oxidation normally occurs slowly at the initial stage and accelerates when the oil is exposed to heat, light and trace metals. An increase in dissolved oxygen content (DOC) will also accelerate the oxidation process.
As double bonds in unsaturated fatty acids may induce faster oxidation, the more double bonds present, the less stable the oil. Therefore, palm oil is relatively more stable than polyunsaturated oils such as soy bean oil and corn oil due to its lower percentage of unsaturated fatty acids. In addition, crude palm oil contains carotenoids at concentrations of 500–700 ppm (Chong 1994) and vitamin E at concentrations of 600–1000 ppm (Tan and Oh 1981). These components are dietary essentials whose main function is to act as antioxidants, a substance that prevents oxidation.
Hydroxyperoxides are the primary products of the oxidation process, which further degrades into secondary oxidation products—a complex mixture of aldehydes, ketones and free fatty acids that cause undesirable flavours and reduces the value of the oil. Besides oxidative rancidity, hydrolytic reactions catalyzed by lipases from food or from microorganisms will lead to deteriorative changes in oil. The effects of hydrolytic reactions can be minimized by cold storage, good transportation, careful packaging and sterilization; while auto-oxidation can be inhibited by vacuum packaging, packing under an inert gas to exclude oxygen and refrigeration/freezing (Naz et al. 2004).
According to Velasco and Dobarganes (2002), there are several types of measurements of oxidative stability at elevated temperatures such as the Schaal oven test, Active Oxygen Method (AOM), oxygen uptake method (oxidograph) and Oil Stability Index (OSI). Chemical tests often used to monitor the progress of lipid oxidation include peroxide value (PV), anisidine value (AnV), iodine value (IV), thiobarbituric acid (TBA) value, and levels of conjugated dienes (CD) and hexanal (Hex) formed during oxidation (Frankel 1998).
This research was aimed at monitoring chemical changes that takes place in low free fatty acid crude palm oil (low-FFA-CPO) extracted from palm fruits as compared to freshly extracted crude palm oil during dark-storage at ambient (28 °C) and elevated temperature (60 °C) for 77 days.
Materials and methods
Sample preparation
Fresh ripe oil palm bunches (Elaeis guineensis) were obtained from Labu Estate, Selangor, Malaysia, for the extraction of low-FFA-CPO. Extraction of low-FFA-CPO was carried out according to the method used by Tan et al. (2009). Oil palm bunches were cut into spikelets and subjected to drying at 66.8 °C for 12.8 h. After drying, palm fruits were separated from stalks and palm mesocarp was obtained by slicing manually. Palm mesocarp was further dried for 30 min at 66.8 °C. Low-FFA-CPO was then extracted by using a laboratory scale screw press. Warm water (~50 to 60 °C) was added to the extracted oil to remove dirt and fibre and the oil was skimmed from the upper layer of the oil–water mixture. One representative sample was extracted per day at three different days. The initial composition and quality indices of low-FFA-CPO were measured in triplicate for each sample.
Freshly extracted crude palm oil (CPO) (500 ml in screw-capped clear bottles) was obtained from Tanah Merah Palm Oil Mill, Port Dickson, Malaysia and the sampling method was the same as the sampling method for low-FFA-CPO. The average free fatty acid content of the oil was 3.47%. All chemicals used were either of high-performance liquid chromatography (HPLC) or analytical grade.
For the storage study, amber glass bottles (50 ml) were filled with 20 g of oil samples and tightly capped. Three samples were taken for each condition and all samples were stored in the dark at two different storage temperatures; ambient temperature (28 ± 1 °C) and accelerated temperature using an oven at 60 °C (Gan et al. 2005). Samples were taken at each scheduled time and kept in a freezer (~−18 °C) prior to analyses. Adequate glass bottles for each oil sample were used so that no sample was reused once withdrawn from storage for further analysis. All samples were stored for 77 days and analyses were carried out after 7, 21, 35, 49, 63 and 77 days.
Quality indices
Chemical analyses
Determination of peroxide value, free fatty acids (FFA), and ultra-violet absorption expressed as specific UV extinction in light were carried out according to standard analytical methods described in AOCS official methods Cd 8-53, Ca 5a-40 and Ch 5-91, respectively (AOCS 1996). DOBI (Deterioration of Bleachability Index) and carotene content were determined using MPOB test methods p2.9 and p2.6, respectively (MPOB Test Methods 2005).
FFA content, expressed as a percentage (m/m) of palmitic acid, was determined by dissolving 2 g of sample with neutralized iso-propanol into a conical flask and titrated with 0.1 M standard sodium hydroxide until the appearance of the first permanent pink colour.
K 233 and K 269 extinction coefficients were measured from absorption at 233 and 269 nm, respectively, with an UV/VIS spectrophotometer (Perkin-Elmer, Lambda 12). Using the same UV/VIS spectrophotometer, carotene content was measured by taking absorption readings at 446 nm. DOBI value was determined by weighing 0.1 g of completely melted and homogenized oil sample into a 25 ml volumetric flask, dissolved and made up to volume with iso-octane. The absorbance at 446 and 269 nm of samples against the solvent used was then measured by means of a spectrophotometer. Results for DOBI values were expressed as the numerical ratio of spectrophotometric absorbance at 446–269 nm (Swoboda 1981).
Peroxide value, expressed in milliequivalents of active oxygen per kilogram of oil (meq/kg), was determined as follows: two grams of oil samples was dissolved in acetic/iso-octane (3:2) and left to react with saturated potassium iodide solution; the free iodine was then titrated with a sodium thiosulphate solution.
Determination of tocopherols and tocotrienols
Two grams of oil samples were dissolved in 10 ml of hexane and 20 μl of the mixture was then injected into an HPLC system (Hewlett Packard, Series 1100, California, USA), equipped with a UV detector according to AOCS Method Ce 8-89 (AOCS 2004). A silica Gel column (Purospher STAR RP-18 encapped column; Merck, Darmstadt, Germany) with particle sizes of 3 µm was used to separate the compounds and the oven temperature was set at 40 °C. The flow rate of the mobile phase (0.5% 2-propanol/hexane) was set at 1 ml/min. Tocopherols and tocotrienols peaks were determined based on the retention time of standards.
Oxidative stability
The Rancimat method was used to evaluate the oxidative susceptibility of both crude oil samples. Samples that were stored in the freezer were heated in an oven at 60 °C prior to analyses. Approximately 2.5 g of melted sample was weighed and the stability was expressed as the induction time (hours) measured with the Rancimat 679 apparatus (Metrohm, Switzerland) according to the AOCS Official Method Cd 12b-92 (AOCS 1997).
Statistical analysis
Statistical treatment of the data was performed using one-way analysis of variance (Minitab 13 software package, State College, USA). Separation of means was performed by Tukey’s test at the 0.05 significance level.
Results and discussion
Initial composition
Table 1 shows the values of the quality indices and the composition of CPO and low-FFA-CPO at the beginning of the experiment. Both CPO and low-FFA-CPO were of good quality, as indicated by their initial zero PV (expressed as meq O2 per kg) and low FFA (3.47 and 1.02%, respectively). Results indicate that the FFA content of low-FFA-CPO was significantly lower than CPO because of the inactivation of oil palm lipase through the drying process during the extraction of low-FFA-CPO (Tan et al. 2009). The inactivation of lipase restricted the degradation of triacylglycerols in the oil palm fruits and therefore, the formation of FFA in low-FFA-CPO was reduced.
Table 1.
Initial composition and quality indices of crude palm oil and low free fatty acid crude palm oil
| Quality indices | Crude palm oil | Low free fatty acid crude palm oil |
|---|---|---|
| Free acidity (%) | 3.47 ± 0.01a | 1.02 ± 0.00b |
| Peroxide value (meq O2/kg) | 0.00 ± 0.00a | 0.00 ± 0.00a |
| K 233 | 1.11 ± 0.01a | 1.27 ± 0.02b |
| K 269 | 0.26 ± 0.01a | 0.10 ± 0.01b |
| Deterioration of bleachability index (DOBI) | 3.07 ± 0.02a | 4.38 ± 0.13b |
| Carotene content* | 668 ± 1.11a | 1224.5 ± 2.80b |
| α-Tocopherol* | 235.5 ± 0.71a | 276.5 ± 2.12b |
| α-Tocotrienol* | 387.5 ± 0.71a | 310.50 ± 0.71b |
| γ-Tocotrienol* | 455.5 ± 0.71a | 387.50 ± 2.12b |
| δ-Tocotrienol* | 144.5 ± 0.71a | 254.50 ± 0.71b |
| Stability (h) | 27.8 ± 0.71a | 24.30 ± 0.42b |
Mean values with different letters in the same row are statistically different (p < 0.05)
* Expressed as ppm
There was a significant difference (p < 0.05) in the carotene content of CPO (668 ppm) and that of low-FFA-CPO (1224 ppm) samples, with the latter almost double in β-carotene content compared to CPO. This is most likely due to the fact that the extraction methods for both oils were different. The extraction process for the production of conventional CPO was carried out at high temperatures ranging from 80 to 140 °C (Bockisch 1998) whilst low-FFA-CPO were produced at lower temperatures with a maximum temperature of 67 °C (Tan et al. 2009). Therefore, the amount of carotene retained in low-FFA-CPO was higher than in CPO.
From Table 1, the concentrations of α-tocopherol and δ-tocotrienol in low-FFA-CPO (276.5; 254.5 ppm) were significantly higher (p < 0.05) than CPO (235.5; 144.5 ppm). On the other hand, the concentrations of α-tocotrienol and γ-tocotrienol in low-FFA-CPO (310.5; 387.5 ppm) were significantly (p < 0.05) lower as compared to CPO (387.5; 455.5 ppm). The total concentration of all vitamin E fractions in both low-FFA-CPO and CPO were 1229 and 1223 ppm, respectively, in which tocotrienols accounted for 77% in low-FFA-CPO and 81% in CPO. Low-FFA-CPO and CPO had high vitamin E concentrations because both oils were in crude forms. Vitamin E would be reduced once refined and it would partition preferentially into the olein fraction (Obahiagbon 2012).
The DOBI value is the numerical ratio of spectrophotometric absorbance at 446 nm to absorbance at 269 nm for a solution of crude oil in iso-octane (Swoboda 1981). DOBI numerical values are observed as <1 for bad, 1–2 for poor, 2–3 for average, and >3 for good crude oils (Swoboda 1981). Both CPO and low-FFA-CPO in this study can be categorized in the ‘good’ group as the DOBI values were more than 3 (Table 1). This indicates that deterioration due to oxidation was either negligible or did not take place in the production of both oils.
Changes in quality indices during storage
FFA, K233, K269 and DOBI value
Table 2 shows the changes in FFA content of low-FFA-CPO and CPO over the course of the storage period. At the end of the storage period for oil samples stored at 60 °C, the FFA content of low-FFA-CPO and CPO were 1.15 and 3.85% respectively. Although the oil samples were stored at elevated temperatures, these values did not exceed the FFA limit as stated in Codex Alimentarius which is 5%. This might be due to the fact that FFA would only increase rapidly in bruised mesocarp (Corley and Tinker 2003). In the production of CPO used in this study, sterilization was carried after the palm fruit bunches were harvested. This was done to kill microorganisms and inactivate endogenous lipase that can cleave the bond between glycerol and fatty acids in triacylglycerols. After extraction, the oil was dried to 0.1% moisture content where autocatalytic hydrolysis of triacylglycerols becomes negligible (De Graaf 1976). Even though there was a significant difference (p < 0.05) between the samples, the FFA content of the oil samples that were stored at 60 °C in this study was less than 5% because no lipolytic microorganisms or lipases were added. Similarly, the FFA content of refined, bleached and deodorized palm olein stored at 60 °C was 0.22% after 52 days as reported by Gan et al. (2005).
Table 2.
Evolution of K 233, K 269, DOBI and carotene content of low-FFA-CPO and CPO
| System | Days | FFA (%) | K 233 | K 269 | DOBI | |
|---|---|---|---|---|---|---|
| Low-FFA-CPO | 28 ± 1 °C | 0 | 1.02 ± 0.00a | 1.27 ± 0.02a | 0.10 ± 0.01a | 4.38 ± 0.13a |
| 7 | 1.25 ± 0.16a | 1.29 ± 0.14a | 0.10 ± 0.01a | 4.44 ± 0.20a | ||
| 21 | 1.28 ± 0.16a | 1.95 ± 0.12b | 0.13 ± 0.01b | 4.21 ± 0.18a | ||
| 35 | 1.12 ± 0.56a | 1.78 ± 0.66ab | 0.13 ± 0.01b | 4.15 ± 0.31a | ||
| 49 | 1.59 ± 0.17a | 2.68 ± 0.02c | 0.13 ± 0.01b | 4.61 ± 0.41a | ||
| 63 | 1.65 ± 0.33a | 3.12 ± 0.22d | 0.18 ± 0.03c | 3.77 ± 0.21ab | ||
| 77 | 1.89 ± 0.70a | 3.61 ± 0.18e | 0.21 ± 0.04c | 3.54 ± 0.16b | ||
| 60 °C | 0 | 1.02 ± 0.00ab | 1.27 ± 0.02a | 0.10 ± 0.01a | 4.38 ± 0.13a | |
| 7 | 1.18 ± 0.12b | 2.52 ± 0.03b | 0.12 ± 0.07a | 4.37 ± 0.35a | ||
| 21 | 0.83 ± 0.16a | 5.55 ± 0.21c | 0.29 ± 0.05b | 2.73 ± 0.30b | ||
| 35 | 1.02 ± 0.14ab | 7.46 ± 0.07d | 0.52 ± 0.12c | 1.28 ± 0.27c | ||
| 49 | 0.79 ± 0.17a | 8.31 ± 0.09e | 0.75 ± 0.15cd | 0.21 ± 0.01d | ||
| 63 | 1.06 ± 0.16ab | 8.84 ± 0.14f | 0.98 ± 0.12d | 0.06 ± 0.01e | ||
| 77 | 1.15 ± 0.23b | 8.38 ± 0.15g | 2.05 ± 0.18e | 0.03 ± 0.02e | ||
| CPO | 28 ± 1 °C | 0 | 3.47 ± 0.01a | 1.11 ± 0.01a | 0.26 ± 0.01a | 3.07 ± 0.02a |
| 7 | 3.49 ± 0.13a | 1.17 ± 0.01b | 0.25 ± 0.01a | 3.05 ± 0.04a | ||
| 21 | 3.55 ± 0.14a | 1.24 ± 0.01c | 0.26 ± 0.01a | 3 ± 0.04a | ||
| 35 | 3.88 ± 0.13a | 1.27 ± 0.07c | 0.26 ± 0.03a | 3.02 ± 0.21a | ||
| 49 | 3.87 ± 0.23a | 1.35 ± 0.03d | 0.25 ± 0.01a | 3.07 ± 0.06a | ||
| 63 | 3.68 ± 0.14a | 1.42 ± 0.02e | 0.25 ± 0.01a | 3.02 ± 0.08a | ||
| 77 | 3.78 ± 0.28a | 1.53 ± 0.02f | 0.26 ± 0.01a | 2.98 ± 0.07a | ||
| 60 °C | 0 | 3.47 ± 0.01a | 1.11 ± 0.01a | 0.26 ± 0.01a | 3.07 ± 0.02a | |
| 7 | 3.42 ± 0.22a | 1.76 ± 0.03b | 0.28 ± 0.01a | 2.83 ± 0.04b | ||
| 21 | 3.26 ± 0.45a | 3.07 ± 0.02c | 0.31 ± 0.01b | 2.53 ± 0.04c | ||
| 35 | 3.91 ± 0.10a | 4.74 ± 0.04d | 0.39 ± 0.01c | 2.01 ± 0.01d | ||
| 49 | 3.97 ± 0.18a | 7.26 ± 0.07e | 0.67 ± 0.01d | 0.72 ± 0.06e | ||
| 63 | 3.71 ± 0.18a | 7.52 ± 0.02f | 0.9 ± 0.01e | 0.1 ± 0.01f | ||
| 77 | 3.85 ± 0.07a | 7.71 ± 0.28g | 1.13 ± 0.07f | 0.02 ± 0.01g |
Mean values with different letters in the same column for each system are statistically different (p < 0.05)
Changes in K 233, K 269 and DOBI values of the two crude palm oils over 77 days of storage is shown in Table 2. The K 233 index increased in low-FFA-CPO and CPO samples at both ambient and 60 °C temperatures over the storage period. This test provides an indication of the purity and deterioration of oil. The auto-oxidation products of oil displays characteristic spectra in the ultraviolet region whereby linoleic hydroperoxide and conjugated dienes which result from decomposition show an absorption band at 233 nm. Meanwhile, secondary products of autoxidation and ethylenic diketones show an absorption band at 269 nm (MPOB test method 2005). The values of K 233 index of low-FFA-CPO and CPO after storage for 77 days at 28 ± 1 °C were 3.61 and 1.53, respectively. The significantly (p < 0.05) bigger change in K 233 index at 60 °C showed that oxidation of oil occurred at a greater rate at this temperature.
With respect to the K 269 index, CPO samples showed no significant difference (p > 0.05) throughout the storage period at 28 ± 1 °C and the K 269 index of low-FFA-CPO was less than 0.26 on day 77 at the temperature. As for storage at 60 °C, the values of K 269 index of low-FFA-CPO and CPO showed significant increments (p < 0.05). Lipids may undergo auto-oxidation, photo-oxidation, thermal oxidation and enzymatic oxidation under different conditions (Shahidi 2000). At 60 °C, thermal oxidation occurred and consequently more primary oxidation products were further degraded into secondary oxidation products.
Bleachability of an oil sample depends on the carotene content. However, bleachability is more affected by the oxidation state of the oil, level of antioxidants and presence of contaminants (Corley and Tinker 2003). Both low-FFA-CPO and CPO samples that were stored at 60 °C were in the ‘bad’ category after 49 days of storage as the DOBI value were less than 1 as shown in Table 2. There was a significant (p < 0.05) difference in the DOBI values for low-FFA-CPO stored at ambient temperature from the beginning to the end of the storage period. At the end of the storage time, the DOBI values for low-FFA-CPO and CPO stored at ambient temperature were 3.54 and 2.98, respectively. These results showed that oxidative deterioration in the oil samples was minimal at ambient temperature because the oil samples were still under the ‘good’ and ‘average’ categories, respectively.
Minor components—carotenoids and vitamin E
In addition to increasing the nutritive value of palm oil, carotenoids play a significant role in the stability even though the total percentage in the oil is less than one (Chong 1994). Since the production of low-FFA-CPO involved a processing temperature of less than 70 °C (which is half the commonly used processing temperature for the production of conventional CPO), the amount of natural antioxidants such as carotene and vitamin E that is retained in low-FFA-CPO is higher than conventional CPO (Tan et al. 2009). Storage at ambient temperatures had no effect on the carotene content of CPO (p > 0.05) and the deterioration of carotenoids in low-FFA-CPO was also minimal (15%) from the beginning of the storage, where the carotene content was 1039.3 ppm on day 77 (Fig. 1a).
Fig. 1.
Changes in carotene content (a) and vitamin E fractions (b)–(e) of low-FFA-CPO and CPO during storage. Temperature: Low-FFA-CPO-28, 28 ± 1 °C; Low-FFA-CPO-60, 60 °C; CPO-28, 28 ± 1 °C; CPO-60, 60 °C
Pigments are also involved in autoxidation and photo-oxidation mechanisms (Gutierrez et al. 1992). Carotenoids in crude palm oil, which are mainly in the forms of α- and β-carotenes, are easily destroyed thermally. As expected, for oils stored at 60 °C, the carotene content declined rapidly. The results as shown in Fig. 1a indicated that oils stored at 60 °C experienced a greater rate of oxidation than oils stored at ambient temperatures. At 60 °C, carotenoids in low-FFA-CPO degraded more rapidly than in CPO. Although the initial carotene content of low-FFA-CPO was higher than CPO, the carotene contents of both of the oil samples dropped to <10 ppm at day 77.
Vitamin E fractions
Figure 1b–e displays the changes in vitamin E fractions of low-FFA-CPO and CPO during storage at two storage temperatures. There was no significant difference (p > 0.05) in α-tocopherol content in CPO samples that were stored at ambient temperature throughout the storage period. However, the α-tocopherol content in low-FFA-CPO samples that were stored at ambient temperatures reduced significantly from 276.5 ppm (Day 0) to 127.5 ppm (Day 77). Yi et al. (2011) reported that the concentrations of α-tocopherol and α-tocotrienol in palm olein/fish oil mixtures decreased during storage at 30 °C for 21 days. The overall pattern of the losses of α-tocopherol and α-tocotrienol may be due to oxidation mechanisms.
Both α-tocopherol and α-tocotrienol in both oils exhibited similar profiles of degradation over 77 days of storage at ambient temperatures and 60 °C. Rapid decline in the concentration of both vitamin E fractions for CPO samples is observed from Day 35 to 49 at 60 °C, as shown in Fig. 1b, c. The amounts of α-tocopherol and α-tocotrienol in low-FFA-CPO decreased at a greater rate when stored at 60 °C and these two fractions were not detected from Day 49 onwards. As expected, the decline of γ- and δ-tocotrienol concentrations occurred in both CPO and low-FFA-CPO samples at system 60 °C. However, these two antioxidants were destroyed earlier in low-FFA-CPO than CPO samples. It can be concluded that all vitamin E fractions in low-FFA-CPO and CPO were sensitive to heat as all of the fractions in both oils were completely destroyed upon storage at 60 °C for 77 days.
PV and oxidative stability
Figure 2 depicts the changes in PV and oxidative stability of low-FFA-CPO and CPO. In all samples, PVs were lower than the upper limit (15 meq/kg) established by Codex Alimentarius at the beginning of the assay. The PV of low-FFA-CPO stored at ambient temperatures increased significantly (p < 0.05) throughout the storage period. As for the CPO stored at ambient temperature, the PV remained the same as fresh CPO from Day 0 to 21 but results indicate a progressive increase in PV after 21 days of storage. The increment in the PVs of both oils could be attributed to the formation of hydroperoxides. Hydroperoxides have no flavour or odour of their own, but they are unstable and break down rapidly to other products such as aldehydes that have a strong, disagreeable flavour and odour (Shahidi 2005). Generally, when vegetable oils are exposed to oxidizing environments, they undergo oxidative degradation. Lipid oxidation occurs fairly slowly at room temperature as compared to accelerated temperature. Therefore, an accelerated method (Frankel 1998) was employed in this study to estimate the induction period of the oxidation reaction in a relatively short period of time.
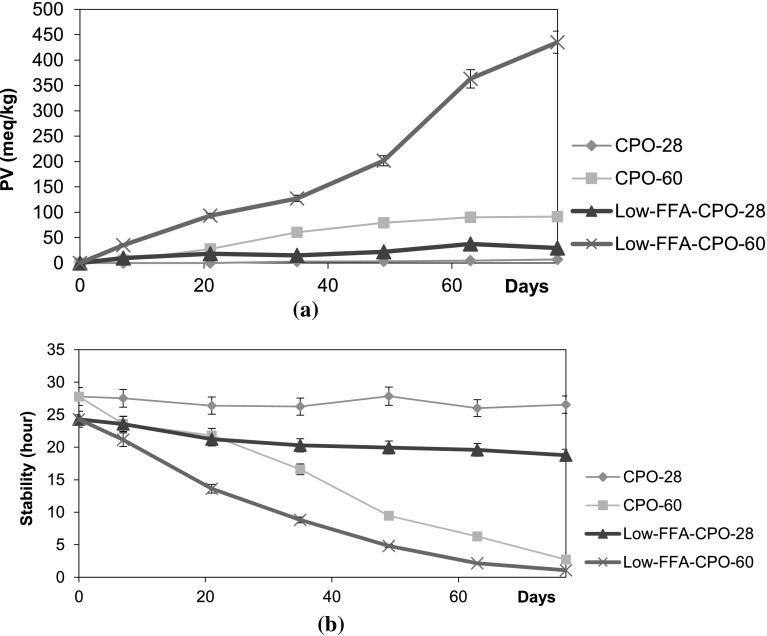
Fig. 2.
PV (a) and oxidative stability (b) of low-FFA-CPO and CPO during storage. Temperature: Low-FFA-CPO-28, 28 ± 1 °C; Low-FFA-CPO-60, 60 °C; CPO-28, 28 ± 1 °C; CPO-60, 60 °C
Iodometric titration assay, which is based on the oxidation of the iodide ion (I−) by hydroperoxides (ROOH), is the basis of current standard methods for determination of PV (Antolovich et al. 2002). From Fig. 2a, both oils experienced a greater degree of deterioration when the oils were stored at 60 °C. The rate of oxidative deterioration of both low-FFA-CPO and CPO samples stored at this temperature rose rapidly from Day 7 to Day 63 and thereafter, decreased in rate which is similar to other peroxides in most storage studies (Gan et al. 2005; Rady and Msdkour 1995). Results show that the rate of increment in PV for low-FFA-CPO was higher than CPO. The PVs of low-FFA-CPO and CPO stored at 60 °C exceeded the upper limit established by Codex Alimentarius on Day 7 and Day 21, respectively. In the other words, the oils had been oxidized which indicates rancidity and the sensory characteristics of the oils were compromised due to the formation of unpleasant smells.
To determine the oxidation rate in each oil sample, the samples should be analyzed periodically since a single reaction criterion is not enough to account for oxidative changes at various stages under different conditions (Naz et al. 2004). Hence, besides PV, the oxidative stability of the oil samples was also analyzed using the Rancimat method. It can be observed from Fig. 2b that at ambient temperatures, the stability of low-FFA-CPO and CPO samples exhibited only slight changes over time. The values ranged from 18.8 to 24.3 h and 26.6 to 27.8 h for low-FFA-CPO and CPO, respectively. Meanwhile, the stability of both oils that were stored at 60 °C declined at a higher rate. The reduction of oxidative stability of both oil samples stored at 60 °C was more than 90% throughout the study. At the end of the storage period at 60 °C, the stability of low-FFA-CPO and CPO dropped to 1.1 and 2.7 h, respectively. This may be due to the occurrence of lipid oxidation which is an autocatalytic reaction where once started, the reaction is self-propagating and self-accelerating (Schaich 2005).
Conclusion
The oxidative stability of low-FFA-CPO under different conditions was determined where CPO was used as a control. The level of primary oxidation products was indicated by PV and K 233 while the level of secondary oxidation products was indicated by K 269. FFA and DOBI are also useful quality indices that act as oxidative indicators. There was no significant difference (p > 0.05) for the FFA of the oil samples stored at ambient temperature (28 ± 1 °C). Elevated temperature (60 °C) promoted the deterioration of oil samples. Minor components, namely carotene and vitamin E, in the mentioned oil samples decreased rapidly with system at 60 °C.
Acknowledgements
The authors would like to thank the Malaysian Palm Oil Board (MPOB) for providing financial and technical assistance for this study.
References
- Antolovich M, Prenzler PD, Patsalides E, Mcdonald S, Robards K. Methods for testing antioxidant activity. Analyst. 2002;127:183–198. doi: 10.1039/b009171p. [DOI] [PubMed] [Google Scholar]
- AOCS . Official methods and recommended practices of the American Oil Chemists’ Society. Champaign: American Oil Chemists’ Society; 1996. [Google Scholar]
- AOCS . Official methods and recommended practices of the American Oil Chemists’ Society. Champaign: American Oil Chemists’ Society; 1997. [Google Scholar]
- AOCS . Official methods and recommended practices of the American Oil Chemists’ Society. Champaign: American Oil Chemists’ Society; 2004. [Google Scholar]
- Ayre KJ, Hulbert AJ. Dietary fatty acid profile influences the composition of skeletal muscle phospholipids in rats. J Nutr. 1996;126:653–662. doi: 10.1093/jn/126.3.653. [DOI] [PubMed] [Google Scholar]
- Bockisch M (1998) Fats and oils handbook. AOCS Press, Champaign, USA
- Chong CL (1994) Chemical and physical properties of palm oil and palm kernel oil. In: Ariffin A, Ahmad MJ, Ghazali R, Mahidin MR (eds) Selected readings on palm oil and its uses. Palm Oil Research Institute of Malaysia, Malaysia, pp 60–67
- Corley RHV, Tinker PB. The oil palm. Oxford: Blackwell Science Ltd.; 2003. [Google Scholar]
- Cottrell RC. Nutritional aspects of palm oil. Am J Clin Nutr. 1991;53:989–1009. doi: 10.1093/ajcn/53.4.989Sb. [DOI] [PubMed] [Google Scholar]
- De Graaf J. Composition, quality and end uses of palm oil. In: Corley RHV, Hardon JJ, Wood BJ, editors. Oil palm research. Amsterdam: Elsevier; 1976. pp. 493–503. [Google Scholar]
- Frankel EN. Lipid oxidation. Dundee: The Oily Press Ltd.; 1998. [Google Scholar]
- Gan HL, Tan CP, Che Man YB, Noraini I, Nazimah SAH. Monitoring the storage stability of RBD palm olein using the electronic nose. Food Chem. 2005;89:271–282. doi: 10.1016/j.foodchem.2004.02.034. [DOI] [Google Scholar]
- Gardner HW. Lipid hydroperoxide reactivity with proteins and amino acids: a review. J Agric Food Chem. 1979;27:220–229. doi: 10.1021/jf60222a034. [DOI] [Google Scholar]
- Gunstone FD, Harwood JL, Padley FB. The lipid handbook. London: Chapman and Hall; 1986. [Google Scholar]
- Gutierrez F, Garrido J, Gallardo L, Gandul B, Minguez MI. Action of chlorophylls on the stability of virgin olive oil. JAOCS. 1992;69:866–871. [Google Scholar]
- Hassan AH. Palm oil and health. Planter. 1988;64:505–519. [Google Scholar]
- Idris NA, Samsuddin S. Developments in food uses of palm oil: a brief review. Palmas. 1993;15:126–129. [Google Scholar]
- Leong WL (1994) Handling, storage and transportation of palm oil products. In: Ariffin A et al. (eds) Selected readings on palm oil and its uses. Palm Oil Research Institute of Malaysia, Malaysia, pp 91–105
- Matalgyto FS, Al-Khalifa AS. Effect of microwave oven heating on stability of some oil and fats. Arab Gulf J Sci Res. 1998;16:21–40. [Google Scholar]
- MPOB Test Methods (2005) Malaysian Palm Oil Board, Malaysia
- Naz S, Sheikh H, Siddiqi R, Sayeed SA. Oxidative stability of olive, corn and soybean oil under different conditions. Food Chem. 2004;88:253–259. doi: 10.1016/j.foodchem.2004.01.042. [DOI] [Google Scholar]
- Obahiagbon FI. A review: aspects of the African oil palm and the implications of its bioactives in human health. Am J Biochem Mol Biol. 2012;2:106–119. doi: 10.3923/ajbmb.2012.106.119. [DOI] [Google Scholar]
- Rady AH, Msdkour MA. Changes in physical and chemical properties of palm olein during heating. Grasas Aceites. 1995;46:270–275. doi: 10.3989/gya.1995.v46.i4-5.936. [DOI] [Google Scholar]
- Satchithanandam S, Reiks M, Clavert RJ, Cassidy MM, Kritchevsky D. Coconut oil and sesame oil affect lymphatic absorption of cholesterol and fatty acids in rats. J Nutr. 1993;123:1852–1858. doi: 10.1093/jn/123.11.1852. [DOI] [PubMed] [Google Scholar]
- Schaich KM. Lipid oxidation: theoretical aspects. In: Shahidi F, editor. Bailey’s industrial oil and fat products. Hoboken: Wiley; 2005. pp. 269–355. [Google Scholar]
- Shahidi F. Antioxidants in food and food antioxidants. Nahrung. 2000;44:158–163. doi: 10.1002/1521-3803(20000501)44:3<158::AID-FOOD158>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Shahidi F. Quality assurance of fats and oils. In: Shahidi F, editor. Bailey’s industrial oil and fat products. Hoboken, NJ: Wiley-Blackwell; 2005. pp. 565–575. [Google Scholar]
- Swoboda PAT (1981) UV–Vis spectrophotometric assays for palm oil quality. Paper presented at international conference on ‘Palm oil product technology in the eighties’, Kuala Lumpur
- Tan BK, Oh FC (1981) PORIM technology. No. 3. Palm Oil Research Institute of Malaysia, Malaysia
- Tan CH, Ghazali HM, Kuntom A, Tan CP, Ariffin AA. Extraction and physicochemical properties of low free fatty acid crude palm oil. Food Chem. 2009;113:645–650. doi: 10.1016/j.foodchem.2008.07.052. [DOI] [Google Scholar]
- Velasco J, Dobarganes C. Oxidative stability of virgin olive oil. Eur J Lipid Sci Technol. 2002;104:661–676. doi: 10.1002/1438-9312(200210)104:9/10<661::AID-EJLT661>3.0.CO;2-D. [DOI] [Google Scholar]
- Yi JR, Andersen ML, Skibsted LH. Interactions between tocopherols, tocotrienols and carotenoids during autoxidation of mixed palm olein and fish oil. Food Chem. 2011;127:1792–1797. doi: 10.1016/j.foodchem.2011.02.061. [DOI] [Google Scholar]