Abstract
This research was focused on preservation strategies applied to develop fish burgers enriched with tomato flour and extra-virgin olive oil. The effects of three different gas mixtures (5:95 O2/CO2; 10:60:30 O2/CO2/N2 and 5:50:45 O2/CO2/N2) on burger quality were analyzed by monitoring microbial cell load of main spoilage microorganisms, pH and sensory properties. As expected, modified atmosphere packaging significantly affected mesophilic bacteria with a reduction of about 2 log cycles for samples under 5% O2 and 95% CO2. Afterward, the best gas mixture was used in combination with various natural antimicrobial compounds (thymol, grape fruit seed extract and biocitrus). The biocitrus showed the strike balance between microbial and sensory quality, thus suggesting to be adopted for dipping treatment of the entire fish fillet before the mincing process. Later all the strategies tested individually were combined and samples were monitored for microbiological and sensory quality. Results obtained showed that dipping treatment of fillet in biocitrus solution (20,000 ppm) under modified conditions extended the shelf life by 8 days compared to the control sample, without affecting the sensory acceptability.
Keywords: Fish burger, Modified atmosphere packaging, Natural antimicrobial compounds, Shelf life, Food preservation
Introduction
Fish is highly nutritious, tasty and easily digested comparable to meat and dairy products (FAO 2015). Unfortunately, fresh fish is more susceptible to rapid spoilage. The freshness loss is caused by enzymatic and chemical reactions, together with high microbial activity (Gram and Huss 1996). The growing demand for freshness requires the adoption of proper technologies of production and preservation, in as much as processing and storage conditions may greatly affect product shelf life (Arvanitoyannis et al. 2005). Many strategies have been developed to inhibit spoilage phenomena in food but a few of them have been applied with success to fish and in particular to processed fish products (Del Nobile et al. 2009).
To date, modified atmosphere packaging (MAP) in combination with refrigeration is reported to provide a substantial shelf life extension of fresh fish, thus appearing the most common technology for fish preservation (Sivertsvik 2007). However, MAP is not always enough to preserve processed food and requires combination with other preservation strategies. Among the reported hurdle approaches of food technology, the combination of MAP with natural active compounds seems a very promising preservation technique for fish products (Giatrakou et al. 2008; Kostaki et al. 2009; Speranza et al. 2013).
Among active compounds of natural origin, the essential oils (EOs), also called volatile or ethereal oils (FDA 2005), found great application in food (Bajpai et al. 2012). Specifically, in fish sector these substances have been widely used as ingredients of active dipping, or have been encapsulated in coating (Gómez-Estaca et al. 2010). It is important that these substances do not adversely affect food acceptability, due to undesirable sensory impact. Therefore, it is necessary to study proper options to found the best method to apply active compounds and the most suitable concentration to exert desired properties, without affecting sensory quality.
The objective of the present work was to extend the shelf life of fresh fish burgers. To the aim, the influence of MAP and active compounds was assessed by monitoring microbial and sensory quality of fish products stored at refrigerated temperature.
Materials and methods
Raw materials
Aquaculture sea bass (Dicentrarchus labrax) was caught in the Mediterranean sea on March for the first step (I), on April for the second step (II) and on May for the third step (III). Fishes were kindly provided by a local fish company (Lepore Mare, Fasano, Italy), slaughtered by immersion in ice-cold water (hypothermia) and packed in insulated polystyrene boxes with ice. Then, they were transported to the laboratory within 2 h from the moment of capture. Once at the laboratory, fish were decapitated, cleaned, filleted and skinned.
Fish burgers preparation
Mincing of skin-off fillets was performed by a domestic food processor (Multiquick 5, Braun, Germany). Tomatoes flour (10 g) (Fiordelisi, Foggia, Italy) was added in the form of powder dissolved in sterile water (100 ml) to minced fish containing salt, parsley and basil (0.5 g), oregano (0.1 g), cappers (3 g) and potato starch (5 g). Moreover, a whey protein foam (3.75 g) (Farmalabor, Canosa di Puglia, Italy), prepared according to a previous work of Conte et al. (2011) was soaked with extra-virgin olive oil (15 ml) and also added to the formulation. All the ingredients were homogenized in a bowl mixer (Multichef, Ariete, Firenze, Italy) with a spiral dough hook for 5 min. Fish burgers were prepared by hand (40 g, 30–40 mm diameter).
Test I-Screening of modified atmosphere packaging
Fish burgers (40 g) obtained as previously described, were packaged in commercially available bags (Nylon/Polyethylene) with thickness of 95 μm, provided by Cartonpack (Bari, Italy) under three different modified conditions: 5%:95% O2:CO2 (M1 sample), 10%:60%:30% O2:CO2:N2 (M2 sample) and 5%:50%:45% O2:CO2N2 (M3 sample). A Reepack RV 200 sealer equipped with a gas mixer Mix 9001 ME (PBI dansensor, Milan) was adopted to realize MAP. The gas mixtures were chosen from literature data on fish (Sivertsvik et al. 2002; Kostaki et al. 2009; Speranza et al. 2013). Packages of fish burgers were also hermetically sealed by means of a thermal sealer (Gandus sealers, Milan, Italy) under aerobically conditions, as the controls. All the packages were stored at 4 °C.
Test II-Screening of antimicrobial compounds
Three natural antimicrobial compounds were used: red thyme essential oil (Farmalabor, Canosa di Puglia, Italy), Grape Fruit Seed Exctract (GFSE, Probena s.l, Zaragoza, Spain) and biocitrus (Biosecur F440, Sochim International, Milan, Italy), all food grade. In order to screen the antimicrobial effects, each compound was added to fish mixture to obtain final concentration of 5000, 10,000 and 15,000 ppm. Afterward, burgers were prepared by hand (40 g). As the controls, samples without any antimicrobial compounds were also prepared. In the same test, a dipping for 60 s in biocitrus solution of the entire fish fillet before the mincing process was also applied. The concentrations of biocitrus were 10,000, 15,000 and 20,000 ppm. After dipping the fillets were allowed to drain for 2 min at 10 °C on a pre-sterilized metal net, then minced and mixed with the other ingredients to make the burgers.
All the samples analyzed were packaged under MAP (M1) and stored for 15 days at 4 °C .
Test III-Final combined treatments
On the basis of the results recorded in the previous steps, the fish fillets were dipped in the biocitrus solution (20,000 ppm), prior to mincing. Then, 5000 ppm of biocitrus were also added to the fish mixture with all the ingredients and then burgers were realized (Dipp-Dough). As further samples, burgers with sole biocitrus added into the formulation (5000 ppm) (Dough) and burgers whose fillets were previously dipped in 20,000 ppm of biocitrus solution (Dipp) were also prepared. Burgers without any active substance were used as the controls. All the samples were packaged in air and MAP (M1) and stored for 30 days at 4 °C.
Headspace gas composition
O2 and CO2 concentrations were monitored by a PBI Dansensor analyzer (Checkmate 3, Ringsted, Denmark). To avoid modifications in the headspace gas composition due to gas sampling, each package was used only for a single measurement. Two bags were used for each measurement.
Microbiological analyses and pH determination
For microbiological analyses, about 20 g of sample was aseptically removed from each package, placed in a stomacher bag, diluted with 0.9% NaCl solution and homogenized with a Stomacher LAB Blender 400 (Pbi International, Milan, Italy). Decimal dilutions were carried out using the same diluent. The media and the conditions used were: Plate Count Agar (PCA) incubated at 30 °C for 24–48 h and 7 °C for 10 days for mesophilic and psychrotrophic bacteria, respectively; Pseudomonas Agar Base (PAB), added with cetrimide–fucidin–cephaloridine (C–F–C) selective supplement, incubated at 25 °C for 48 h for Pseudomonas spp.; pour plated Iron Agar (IA), incubated at 25 °C for 3 days, for hydrogen sulphide-producing bacteria (HSPB); spread plated chilled IA, supplemented with 5 g/l NaCl and incubated at 15 °C for 7 days, for psychrotolerant and heat labile aerobic bacteria (PHAB). The conditions used during the counts of HSPB and PHAB were those suggested by the NCFA, with regard to fish and fishery products (NCFA 2006). All media were supplied from Oxoid (Milan, Italy). The microbiological analyses were carried out twice on two different batches.
The measurement of pH, conducted in duplicate, was performed on the first homogenized dilution of the fish samples with a pH meter (Crison, Barcelona, Spain).
Sensory analyses
The attributes of fish burgers (odor, color, appearance, texture and overall quality) were evaluated by a panel of eight experienced judges. According to the method of Paulus et al. (1979), a 9-point scale was used to quantify each attribute, where a score of 9 corresponded to “very good quality,” scores of 7–8 to “good quality” and a score of 6 to “sufficient quality.” The value equal to 5 represented the acceptability threshold, while a score of 1–4 corresponded to “unacceptable quality.”
Shelf life calculation
In order to determine the shelf life of fish burgers in the different steps, the microbial acceptability limit (MAL) (i.e., the storage time at which the viable cell concentration reaches the threshold) and the sensory acceptability limit (SAL) (i.e., the storage time at which the overall quality reaches the threshold) were calculated and compared. To measure both MAL and SAL values, a re-parameterized Gompertz equation was fitted to both microbiological (mesophilic bacteria) and sensory (overall quality) data, according to previous studies also reported in the literature (Conte et al. 2009).
Statistical analysis
The values of MAL and SAL of the investigated samples were compared by one-way ANOVA, respectively. A Duncan’s multiple range test, with the option of homogeneous groups (P < 0.05), was used to determine significance among differences. Statistica 7.1 for Windows (StatSoft Inc., Tulsa, OK, USA) was used for this purpose.
Results and discussion
Step I-Modified atmosphere packaging selection
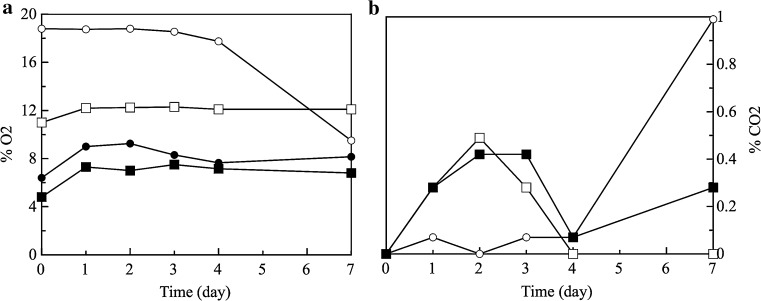
Figure 1a, b shows the evolution of gas concentration in the headspace for M1, M2, M3 and Ctrl samples. O2 levels in the packages with air slowly decreased during the first 4 days, thus remaining above 17% (Fig. 1a) and then decreasing again up to approximately 9%. Kostaki et al. (2009) reported similar results on sea bass fillets stored in air. From the Fig. 1 it can be seen that comparable trends among samples packaged under MAP were observed. In particular, CO2 accumulation and O2 depletion remained constant throughout the storage (7 days).
Fig. 1.
O2 (a) and CO2 (b) concentrations in the package headspace of fish burgers stored at 4 °C for 7 days: (open circle) packaging in air, (filled circle) 5% O2 + 95% CO2, (open square) 10% O2 + 60% CO2 + 30% N2 and (filled square) 5% O2 + 50% CO2 + 45% N2. Data shown are mean ± standard deviation
As regard microbial growth, Table 1 reports the cell load at the end of the sampling (7th day). Total mesophilic bacteria were initially equal to 4.4 log CFU/g for all the investigated samples, thus indicating a good fish quality. During storage a faster increase of mesophilic population was observed in the fish burgers packaged in air (7.9 log CFU/g). The overall quality rapidly decreased and exceed the sensory threshold after 3 days of storage (data not shown). It is well established that inhibition of bacterial growth is due to CO2 action, no substantial differences on mesophilic cell loads were found among samples packaged in air or under MAP (M2 and M3). This was probably due to low CO2 concentration set in the above-mentioned samples which were found statistically similar to the control sample also from a sensory point of view (data not shown). Among the screened samples, there is a trend on the inhibition of mesophilic growth and the higher concentrations of CO2 set for M1 (Fig. 1b) were the most effective. As can be inferred from Table 1, M1 sample never reached the microbial limit for the entire observation period. Our findings are in agreement with previous reports that also suggest that MAP could provide a substantial shelf life extension of fresh fish (Torrieri et al. 2006; Sivertsvik 2007; Del Nobile et al. 2009). The sensory characteristic were better preserved with M1 gas mixture and sample was refused about 4 days after the reference sample (data not shown).
Table 1.
Microbial counts (log cfu/g) of fish burgers during 10 days of storage (Step I)
| Samples | Mesophiles | HSPB | PHAB | Pseudomonas | Psychrotrophic | LAB | Enterobacteriaceae |
|---|---|---|---|---|---|---|---|
| Ctrl-Air | 7.93 ± 0.30a | 8.41 ± 0.10a | 8.81 ± 0.27a | 9.01 ± 0.15a | 8.39 ± 0.12a | 7.02 ± 0.21a | 5.66 ± 0.20a |
| M1 | 6.93 ± 0.07b | 5.41 ± 0.09c | 6.32 ± 0.05b | 5.15 ± 0.08b | 6.43 ± 0.14c | 5.28 ± 0.22b | 3.22 ± 0.15b |
| M2 | 7.16 ± 0.28a,b | 6.16 ± 0.37b | 6.57 ± 0.03b | 5.47 ± 0.04b | 6.50 ± 0.17c | 5.83 ± 0.24c | 4.11 ± 0.17c |
| M3 | 7.70 ± 0.71a,b | 5.12 ± 0.13c | 6.76 ± 0.66b | 4.85 ± 0.52b | 7.07 ± 0.35b | 5.92 ± 0.25c | 4.36 ± 0.24c |
Mean values ± standard deviation
Means in the same column followed by different superscript letter are significantly different (P < 0.05)
Concerning the specific spoilage organisms (SSOs) responsible of offensive, fishy, rotten H2S-off odors and flavors of spoiled marine fish (Gram and Huss 1996), such as Pseudomonas spp., hydrogen sulphide-producing bacteria (HSPB) and psychrotolerant and heat labile aerobic bacteria (PHAB), all the gas mixtures used in this study were able to affect the above-mentioned microbial growth (see Table 1). In fact, the main spoilage microorganisms were initially below the sensitivity limit of the plate counting method (102 log CFU/g) but only in the control sample proliferated to high concentrations, more than 6–8 log CFU/g. Differently, fish burgers packaged under MAP showed a reduction of 2 (PHAB), 3 (HSPB and psycrotrophic bacteria) or 4 (Pseudomonas spp.) log cycles, compared to the control sample.
For the Enterobacteriaceae cell concentration, counts in MAP samples were lower compared to other microbial species, in agreement with results obtained for other type of fishes stored under MAP, such as hake steaks (Ordonez et al. 2000), and swordfish fillets (Giatrakou et al. 2008). On the contrary, LAB showed a high growth rate under MAP conditions (5 log CFU/g), thus suggesting that MAP did not inhibit their growth. On the basis of the results recorded in this step, the low levels of O2 (about 5%) and the high levels of CO2 (95%) represent the gas composition chosen for the next steps.
Step II-Antimicrobial compounds selection
Table 2 summarizes parameters obtained from fitting of microbiological and sensory data of all the investigated samples. As regard the microbiological quality, on the first day of storage, the mesophilic bacteria were approximately 4.7 log CFU/g and exceeded 7 log CFU/g on the 6th day of storage. On the other hand, all samples with antimicrobial compounds at 10,000 and 15,000 ppm never reached the microbial threshold, thus confirming the effects of the natural compounds against bacteria (Smith-Palmer et al. 1998; Viuda-Martos et al. 2008). Mejlholm and Dalgaard (2002) also reported the inhibition of aerobic microbial growth when oregano oil was combined with MAP to preserve cod fillets. Thyme oil and GFSE exerted the lowest preservative effect when were added to fish mix at 5000 ppm. Among the screened compounds, Biocitrus-5000 sample was the most effective for the inhibition of microbial growth until the 13th days of storage. In fact, literature data confirm the pronounced antimicrobial effect of citrus essential oils (Tassou et al. 1995; Caccioni et al. 1998; Dorman and Deans 2000; Lanciotti et al. 2004).
Table 2.
Microbial acceptability limit (MAL), sensory acceptability limit (SAL) and shelf life (mean ± SD) of fish burgers (Step II)
| Samples | MALMesophiles (days) | SAL (days) | Shelf life (days) |
|---|---|---|---|
| Ctrl-Map | 11.68 ± 0.62a | 6.37 ± 0.24a | 6.37 ± 0.24a |
| Biocitrus-5000 | 13.34 ± 0.55b | 8.90 ± 0.44c | 8.90 ± 0.44c |
| Biocitrus-10000 | >5 | 5.33 ± 0.11a,b | 5.33 ± 0.11a,b |
| Biocitrus-15000 | >5 | 4.57 ± 1.13b | 4.57 ± 1.13b |
| Ctrl-Map | 6.15 ± 0.34a | 4.40 ± 0.17a | 4.40 ± 0.17a |
| GFSE-5000 | 8.46 ± 0.87b | 6.27 ± 0.19b | 6.27 ± 0.19b |
| GFSE-10000 | >8 | 6.33 ± 0.40b | 6.33 ± 0.40b |
| GFSE-15000 | >8 | 5.89 ± 0.34b | 5.89 ± 0.34b |
| Ctrl-Map | 5.55 ± 0.54a | 8.06 ± 0.00a | 5.55 ± 0.54a |
| Thyme-5000 | 5.71 ± 1.44a | 8.24 ± 0.35a | 5.71 ± 1.44a |
| Thyme-10000 | >5 | 3.12 ± 2.53b | 3.12 ± 2.53b |
| Thyme-15000 | >5 | 1.77 ± 0.00b | 1.77 ± 0.00b |
| Ctrl-Map | 8.45 ± 0.37a | 5.74 ± 0.80a | 5.74 ± 0.80a |
| Dipp-Biocitrus-10000 | 10.74 ± 0.43b | 5.27 ± 0.47b | 5.27 ± 0.47b |
| Dipp-Biocitrus-15000 | 11.61 ± 0.52b,c | 5.45 ± 0.35b | 5.45 ± 0.35b |
| Dipp-Biocitrus-20000 | 12.03 ± 0.88d | 7.31 ± 0.32a | 7.31 ± 0.32a |
Mean values ± standard deviation
Means in the same column followed by different superscript letter are significantly different (P < 0.05)
As regard the specific spoilage organisms (Pseudomonads, HSPB and PHAB), a very low initial population were recorded. In particular, HSPB and Pseudomonads spp. reached final counts of about 2 and 4 log CFU/g, respectively. The antimicrobial substances tested, as well as the MAP conditions inhibited their growth by 2 log cycles, compared to the control sample (data not shown) and leading the population at low concentrations, far from 6 log CFU/g (HSPB) and 8–9 log CFU/g (Pseudomonas spp.) that are spoilage concentrations of chilled fish (Gram and Huss 1996; NCFA 2006). Whilst, Enterobacteriaceae showed a very low initial load, below the sensitivity limit by plate count method (<102 CFU/g), indicating good hygiene of marine environment where fish were caught in, as well as good fishing practices and handling (data not shown).
A further inhibition of bacterial growth was observed when the entire fillets were dipped in the active solution prior to form the burger samples. Thus, compared to the control samples, a microbiological shelf life extension of 2, 3 and 4 days was achieved when solutions of biocitrus at 10,000, 15,000 and 20,000 ppm were utilized, respectively. Fisher and Phillips (2008) also suggested the use of citrus EOs as an ideal alternative to chemical-based food antimicrobials in fish products.
As regard the sensory quality, the addition of antimicrobial compounds into the fish products or the treatment of the entire fillets affected the sensory quality (Table 2). As well documented, the use of active substances may impart a certain unpleasant flavor to foodstuff. Sivertsvik et al. (2002) reported the occurrence of different phenomena such as, discoloration, excessive exudate or softening of texture during storage of fishery product. In our study, thyme, GFSE and biocitrus at high concentrations impart a strong odor which was responsible of unpleasant acceptability and a dark surface. In contrast, the lower concentration used (5000 ppm) increased storage time respect to the control samples. As regard the dipping treatment, the sensory decay was better controlled only at high concentrations (20,000 ppm). On the basis of the results recorded in this trial, biocitrus was chosen as antimicrobial compound to be used also in the subsequent experimental test.
Step III-Combined treatments to prolong the shelf life
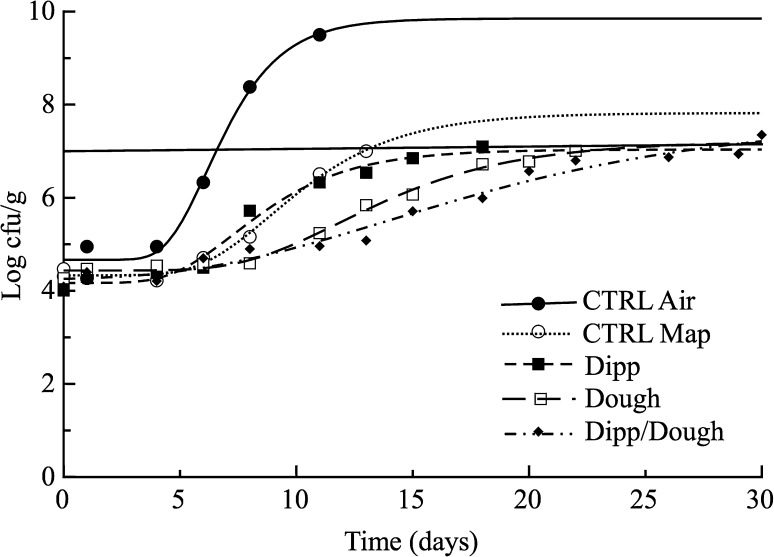
Figure 2 shows the viable cell concentration of mesophiles for fish burger samples, whereas the horizontal solid line is the microbial threshold. The quality was monitored until the product reached either its microbial or sensory acceptability threshold. The MALmesophiles values are listed in Table 3. As it can be inferred from data, the highest viable cell concentrations were recorded for both reference samples packaged in air and MAP, that went beyond 7 log CFU/g, considered as the upper acceptability limit for fresh marine species (International Commission on Microbiological Specifications for Foods 1986). Similar results were also found by Kostaki et al. (2009) on sea bass fillets packaged with a gas mixture composed by 60% CO2/30% N2/10% O2 that reached 7 log CFU/g on day 12 of storage, while samples with a less CO2 concentration (40%) reached the same value on day 9. The antimicrobial activity of CO2 can be related to the sensibility of certain aerobic spoilage microorganisms to high gas concentrations (Stammen et al. 1990). On the other hand, relevant differences were observed among the other active samples. In particular, all the treatments proposed were effective in inhibiting the mesophiles and resulted in a MAL extension of 4 (Dipp), 10 (Dough) and 14 (Dipp-Dough) days respect to the control sample packed under MAP (Ctrl-Map). Dipp-Dough treatment was the most effective strategy in terms of shelf life extension of fish burgers (Table 3), due to biocitrus components, especially citral and linalool, known to exert a valid antimicrobial activity (Caccioni et al. 1998).
Fig. 2.
Evolution of mesophilic bacteria plotted as a function of storage time (Trial III). Data represent mean of two replicates ± standard deviation
Table 3.
Microbial acceptability limit (MAL), sensory acceptability limit (SAL) and shelf life (mean ± SD) of fish burgers (Step III)
| Samples | MALMesophiles (days) | SAL (days) | Shelf life (days) |
|---|---|---|---|
| Ctrl-Air | 6.55 ± 0.26a | 4.68 ± 0.13a | 4.68 ± 0.13a |
| Ctrl-Map | 12.88 ± 0.57b | 7.18 ± 1.00b | 7.18 ± 1.00b |
| Dipp-20000 | 16.29 ± 1.39c | 12.74 ± 2.02c | 12.74 ± 2.02c |
| Dough-5000 | 22.37 ± 1.81d | 8.11 ± 1.40b | 8.11 ± 1.40b |
| Dipp-Dough | 26.51 ± 1.32e | 7.76 ± 1.31b | 7.76 ± 1.31b |
Mean values ± standard deviation
Means in the same column followed by different superscript letter are significantly different (P < 0.05)
For the other investigated microbial groups, Pseudomonas spp. and PHAB counts were generally higher (6 log CFU/g) in samples kept under air and MAP conditions, compared with those obtained through the addition of biocitrus. In particular, a very low proliferation (2–3 log CFU/g, respectively) were recorded during the entire storage period for Dipp-20000, Dough-5000 and Dipp-Dough samples (data not shown). This effect may be due to the inhibitory action of phenolic compounds on Gram-negative bacteria, being the main constituents of citrus essential oils. As reported by Gram and Huss (1996) the microbiota of temperate water fish is dominated by Psychrotrophic Gram-negative, rod-shaped bacteria belonging, among others, to the genera Pseudomonas, Moraxella, Vibrionaceae and Shewanella. From the result, LAB counts were below the detection limit for the entire observation period. However, H2S-producing bacteria, as black colonies in Iron Agar and Enterobacteriaceae were inhibited to a greater extent by all the biocitrus treatments, registering 2 log CFU/g after 15 days of storage (data not shown).
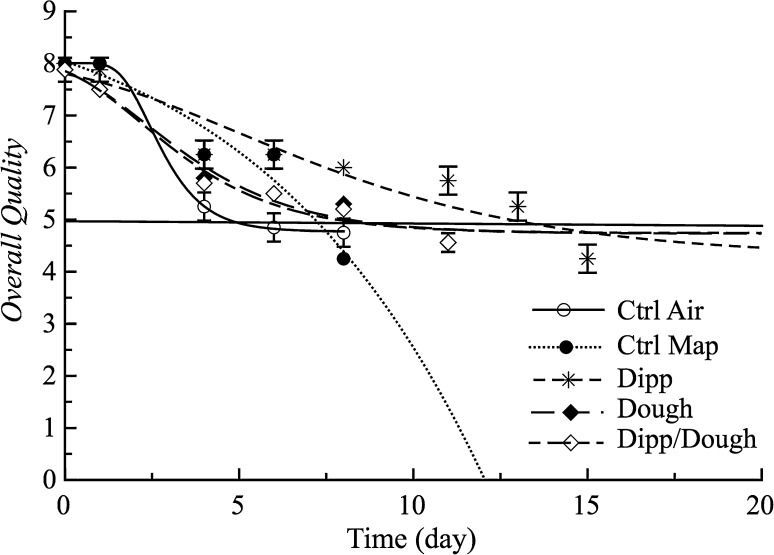
Figure 3 shows the fish burger overall quality for all the investigated samples. Curves shown in the figures were obtained by fitting the re-parameterized Gompertz equation to the experimental data, whereas the solid horizontal line is the overall quality threshold. The SAL values are also listed in Table 3. Sensory quality of fish burgers was very good at the beginning (score = 8) and as one could expect, it decreased during the storage. In particular, the overall quality of Ctrl-Air sample rapidly decreased, followed by the Ctrl-Map sample. The acceptability of these samples became lower than 5 after 4 (Ctrl-Air) and 7 (Ctrl-Map) days of storage. The above mentioned samples showed the worsening sensory attributes before exceeded the microbial threshold, thus confirming short shelf life of about 3 days (Fisher and Philipps 2008). As can be inferred from Table 3, no significant differences between Dough and Dipp-Dough samples were observed (P > 0.05). Despite a considerable antimicrobial effect, the afore-mentioned samples had an unpleasant color and odor that provoked their unacceptability around 8 days of storage whereas, Dipp samples remained acceptable for a longer period (12.74 days).
Fig. 3.
Overall quality decay of burger samples (Trial III). The curves are the best fit to the experimental data
Conclusion
Results of this study suggested that when fish burgers were packaged in ordinary atmosphere a very short shelf life occurred, while the use of MAP condition (95% CO2/5% O2) extended product shelf life by 3 days. Moreover, the combined use of active MAP and biocitrus in the formulation allowed contributing to a substantial preservation of microbial quality, even though the sensory quality was compromised. A treatment of whole fish fillets in a solution of biocitrus (Dipp-20000 sample) prior to mincing, represents the best preservation strategy to control microbial quality decay for more than 12 days without affecting the sensory properties. To reach these results, the initial microbial load of fish, the respect of strict hygienic process conditions and the low storage temperature are important factors that concur to the final product quality.
Acknowledgements
This work was financially supported by the “Programma Operativo Nazionale Ricerca e Competitività 2007–2013 (D.D. Prot. n. 01/Ric. del 18.1.2010)”—PON01_01962: Tecnologie per la valorizzazione e l’estensione di shelf life di trasformati ittici ad elevato contenuto salutistico.
References
- Arvanitoyannis IS, Tsitsika EV, Panagiotaki P. Implementation of quality control methods (physicochemical, microbiological and sensory) in conjunction with multivariate analysis towards fish authenticity. Int J Food Sci Technol. 2005;40:237–263. doi: 10.1111/j.1365-2621.2004.00917.x. [DOI] [Google Scholar]
- Bajpai VK, Kwang-Hyun B, Chul KS. Control of Salmonella in foods by using essential oils. Food Res Int. 2012;45:722–734. doi: 10.1016/j.foodres.2011.04.052. [DOI] [Google Scholar]
- Caccioni DRL, Guizzardi M, Biondi DM, Agatino R, Ruberto G. Relationship between volatile components of citrus fruit essential oils and antimicrobial action on Penicillium digitatum and Penicillium italicum. Int J Food Microbiol. 1998;43(1–2):73–79. doi: 10.1016/S0168-1605(98)00099-3. [DOI] [PubMed] [Google Scholar]
- Conte A, Scrocco C, Brescia I, Del Nobile MA. Packaging strategies to prolong the shelf life of minimally processed lampascioni (Muscari comosum) J Food Eng. 2009;90:199–206. doi: 10.1016/j.jfoodeng.2008.06.023. [DOI] [Google Scholar]
- Conte A, Mastromatteo M, Cozzolino F, Lecce L, Del Nobile MA. Recipe optimization to produce functional food based on meat and fish. J Nutr Food Sci. 2011;S4:001. [Google Scholar]
- Dorman HJD, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol. 2000;88(2):308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- Fisher K, Phillips C. Potential antimicrobial uses of essential oils in food: is citrus the answer? Trends Food Sci Tech. 2008;19:156–164. doi: 10.1016/j.tifs.2007.11.006. [DOI] [Google Scholar]
- Del Nobile MA, Corbo MR, Speranza B, Sinigaglia M, Conte A, Caroprese M. Combined effect of MAP and active compounds on fresh blue fish burger. Int J Food Microbiol. 2009;135:281–287. doi: 10.1016/j.ijfoodmicro.2009.07.024. [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO) (2015) Fisheries and Aquaculture Department. http://www.fao.org/fishery/en. Accessed 27 July 2015
- Food and Drug Administration (2005) GRAS notifications. http://www.fda.gov. Accessed 28 June 2015
- Giatrakou V, Kykkidou S, Papavergou A, Kontominas MG, Savvaidis IN. Potential of oregano essential oil and MAP to extend the shelf life of fresh swordfish: a comparative study with ice storage. J Food Sci. 2008;73:167–173. doi: 10.1111/j.1750-3841.2008.00729.x. [DOI] [PubMed] [Google Scholar]
- Gómez-Estaca J, López de Lacey A, López-Caballero ME, Gómez-Guillén MC, Montero P. Biodegradable gelatine-chitosan films incorporated with essential oils as antimicrobial agents for fish preservation. Food Microbiol. 2010;27:889–896. doi: 10.1016/j.fm.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Gram L, Huss HH. Microbiological spoilage of fish and fish products. Int J Food Microbiol. 1996;33:121–137. doi: 10.1016/0168-1605(96)01134-8. [DOI] [PubMed] [Google Scholar]
- International Commission on Microbiological Specifications for Foods (1986) Sampling plans for fish and shellfish. In: Microorganisms in foods 2, Sampling for microbiological analysis: principles and scientific applications, 2nd edn. University of Toronto Press, Toronto, pp 181–196
- Kostaki M, Giatrakou V, Savvaidis IN, Kontominas MG. Combined effect of MAP and thyme essential oil on the microbiological, chemical and sensory attributes of organically aquacultured sea bass (Dicentrarchus labrax) fillets. Food Microbiol. 2009;26:475–482. doi: 10.1016/j.fm.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Lanciotti R, Gianotti A, Patrignani F, Belletti N, Guerzoni ME, Gardini F. Use of natural aroma compounds to improve shelf-life and safety of minimally processed fruits. Trends Food Sci Technol. 2004;15:201–208. doi: 10.1016/j.tifs.2003.10.004. [DOI] [Google Scholar]
- Mejlholm O, Dalgaard P. Antimicrobial effect of essential oils on the seafood spoilage microorganism Photobacterium phosphoreum in liquid media and fish products. Lett Appl Microbiol. 2002;34:27–31. doi: 10.1046/j.1472-765x.2002.01033.x. [DOI] [PubMed] [Google Scholar]
- Nordic Committee on Food Analysis (2006) Aerobic Count and Specific Spoilage Organisms in Fish and Fish Products, NMKL Method No. 184, Espoo, Finland
- Ordonez JA, Lopez-Galvez DE, Fernandez M, Hierro H, Hoz L. Microbial and physicochemical modifications of hake (Merluccius merluccius) steaks stored under carbon dioxide enriched atmospheres. J Sci Food Agr. 2000;80:1831–1840. doi: 10.1002/1097-0010(200010)80:13<1831::AID-JSFA707>3.0.CO;2-Z. [DOI] [Google Scholar]
- Paulus K, Zacharias R, Robinson L, Geidel H. Kritische betrachtungen zur bewertenden pru fung mit skale als einem wesentlichen verfahren der sensorischen analyse. Lebensmittel-Wissenschaft und-Technologie. 1979;12:52–61. [Google Scholar]
- Sivertsvik M. The optimized modified atmosphere for packaging of pre-rigor filleted farmed cod (Gadus morhua) is 63 ml/100 ml oxygen and 37 ml/100 ml carbon dioxide. Lebensm Wiss Technol. 2007;40:430–438. doi: 10.1016/j.lwt.2005.12.010. [DOI] [Google Scholar]
- Sivertsvik M, Jeksrud WK, Rosnes JT. A review of modified atmosphere packaging of fish and fishery products-significance of microbial growth activities and safety. Int J Food Sci Tech. 2002;37:107–127. doi: 10.1046/j.1365-2621.2002.00548.x. [DOI] [Google Scholar]
- Smith-Palmer A, Stewart J, Fyfe L. Antimicrobial properties of plant essential oils and essences against five important food-borne pathogens. Lett Appl Microbiol. 1998;26:118–122. doi: 10.1046/j.1472-765X.1998.00303.x. [DOI] [PubMed] [Google Scholar]
- Speranza B, Bevilacqua A, Conte A, Del Nobile MA, Sinigaglia M, Corbo MR. Use of desirability approach to predict the inhibition of Pseudomonas fluorescens, Shewanella putrefaciens and Photobacterium phosphoreum in fish fillets through natural antimicrobials and modified atmosphere packaging. Food Bioprocess Technol. 2013;6:2319–2330. doi: 10.1007/s11947-012-0889-3. [DOI] [Google Scholar]
- Stammen K, Gerdes D, Caporaso F. Modified atmosphere packaging of seafood. Food Sci Nutr. 1990;29:301–331. doi: 10.1080/10408399009527530. [DOI] [PubMed] [Google Scholar]
- Tassou CC, Drosinos EH, Nychas GJE. Inhibition of resident microbial flora and pathogen inocula on cold fresh fish fillets in olive oil, oregano, and lemon juice under modified atmosphere and in air. J Food Protect. 1995;59:31–34. doi: 10.4315/0362-028X-59.1.31. [DOI] [PubMed] [Google Scholar]
- Torrieri E, Cavella S, Villani F, Masi P. Influence of modified atmosphere packaging on the chilled shelf life of gutted farmed bass (Dicentrarchus labrax) J Food Eng. 2006;77:1078–1086. doi: 10.1016/j.jfoodeng.2005.08.038. [DOI] [Google Scholar]
- Viuda-Martos M, Ruiz-Navajas Y, Fernández-López J, Pérez-Álvarez J. Antifungal activity of lemon (Citrus lemon L.), mandarin (Citrus reticulata L.), grapefruit (Citrus paradisi L.) and orange (Citrus sinensis L.) essential oils. Food Control. 2008;19:1130–1138. doi: 10.1016/j.foodcont.2007.12.003. [DOI] [Google Scholar]