Abstract
The objective of the present study was to investigate the effects of superchilling with modified atmosphere packaging on the physicochemical properties and shelf life of swimming crab. As the storage time increased, the rates at which the total aerobic plate count, total volatile basic nitrogen, pH, peroxide value and thiobarbituric acid-reactive substances value increase were significantly lower for the superchilling with modified atmosphere packaging (SCS + MAP) treatment compared to superchilling storage (SCS) and chilling storage (CS). With increasing storage time, the carbonyl content of the proteins increased from 1.21 nmol/mg of protein (0 day) to 2.03, 1.87, 1.66 nmol carbonyl/mg protein on the 6th day for CS, SCS and SCS + MAP, respectively. The disulfide bonds increased in a similar manner, and the total sulfhydryl content, salt extractable protein and Ca-ATPase stability decreased. Sodium dodecyl sulphate polyacrylamide gel elcetrophoresis (SDS-PAGE) and microstructure analysis also indicated that SCS + MAP could reduce the degree of protein degradation. These results suggested that superchilling with modified atmosphere packaging offers an effective approach to slowdown protein and lipid oxidation, and extends the shelf life of swimming crab. However, superchilling with high-CO2 packaging had a negative effect on the surface hydrophobicity and drip loss of swimming crab.
Keywords: Swimming crab, Superchilling, Modified atmosphere packaging, Carbon dioxide, Shelf life
Introduction
The swimming crab (Portunus trituberculatus) is found in the coastal waters of Japan, Korea, and China, and the global production of swimming crab in 2012 was 429,959 t (FAO 2014). Crab is highly perishable due to the biochemical, microbiological or physical changes that occur during post-mortem storage. Crab meat deteriorates rapidly, even at refrigerated temperatures, which results in a limited shelf life of the product (Ashie et al. 1996). Previously frozen then thawed muscle tissue has significantly reduced textural quality and water-binding capacity due to ice crystal formation (Cheung et al. 2009), so offering an alternative and promising technique for the preservation of crab is needed.
Superchilling is a method of preserving food by partial ice crystallization. Compared to traditional chilling, superchilling extends food shelf-life at least by 1.4–5 times (Duun and Rustad 2008). During the superchilling process, ice crystals are formed in the outer layer of the superchilled product and about 5–30% of water begins to form ice inside the products, and then a 1–3 mm layer of ice would be formed on the product surface. The formed ice will then serve as an ice reservoir to absorb heat from the product interior, and no external ice is required during transportation or storage (Wu et al. 2014). Until now, superchilling preservation technology has been successfully applied in tilapia and other aquatic product (Cyprian et al. 2013; Wu et al. 2014). However, the application of superchilling in crab storage is still unknown.
Aside from temperature controls, modified atmosphere packaging (MAP) has grown in recent years as an efficient method to delay microbial growth and enzymatic spoilage. During MAP and refrigerated storage, the shelf-life of aquatic products was increased for periods between 10 and 22 days (Masniyom 2011). Low oxygen can retard several types of deteriorative reactions, and most of the aerobic bacteria growth was inhibited in low oxygen conditions. Carbon dioxide has a bacteriostatic effect and is the most important component in the choice of a gas mixture. The possible mechanisms for CO2 inhibition include dissolved CO2 leading to a decrease in the intracellular pH and enzyme activities or altering the membrane properties and functions (Devlieghere et al. 1998). The inhibition of the growth of microorganisms in MAP foods is significantly regulated by the concentration of dissolved CO2 into the product, and it is necessary to introduce a minimum concentration of 20–30% of CO2 to the head space of packages to obtain this effect (Stiles 1991). Therefore, a higher CO2 concentration of MAP should be considered due to the high solubility of CO2 in both water and fats and within the product.
Crab quality is a complex concept, including a range of factors that depend on consumers’ quality perception, market preferences, storage conditions, and so on. Our previous study indicated that modified atmosphere packaging had good effect on the seafood quality. However, more than 60% CO2 in MAP enhanced the deterioration of crab quality (Sun et al. 2016). The objective of the present study was to assess the potential benefits of superchilling at −3 ± 1 °C with a modified atmosphere packaging (with 60% CO2, 10% O2 and 30% N2) system by evaluating the physicochemical attributes and shelf life of crab during storage.
Materials and methods
Preparation of crab samples
Female swimming crab (Portunus trituberculatus) with an average weight of 293.3 ± 40.1 g were purchased from a local aquatic market and were transported live to the laboratory. All crabs were painlessly killed using −20 °C sterile ice. The 270 crab samples were individually fixed in a special, semi-closed plastic box, and then randomly assigned into three groups, and each was subjected to processing in triplicate. The group 1 and 2 samples were individually air packaged in polyamide/polyethylene pouches (90 μm thickness, 25 cm × 17 cm; O2 transmission 10–20 mL/(m2d) at 25 °C, 1 atm pressure) and stored at 4 ± 1 °C (chilling storage, CS) or −3 ± 1 °C (superchilling storage, SCS), respectively. The group 3 samples were individually packaged with mixed gas 10%O2/60%CO2/30%N2 and stored at −3 ± 1 °C (superchilling combined with modified atmosphere packaging, SCS + MAP). Given the different storage period of the three treatment consideration, samples were taken every day for CS treatment, and 0, 3, 4, 6, 9, 12, 15 days for SCS and SCS + MAP, and 18 days only for SCS + MAP. At each sampling time, crabs were artificially remove gills and viscera and to get meat near the side of the shell (not including legs meat). The whole experiment was replicated three times.
Microbiological growth, pH and TVB-N analysis
Ten grams of the samples was aseptically weighed and homogenized with 90 mL of sterile 0.9% physiological saline for 1 min. A ten-fold serial dilution of crab homogenates were used for microbial analyses. The total bacteria were enumerated using nutrient agar, incubated at 37 °C for 48 h. The results were expressed as the log10 CFU/g of the sample. Another 10-g sample of the crab muscle was homogenized thoroughly with 90 mL of distilled water and the homogenate was used for pH determination using a digital pH meter. The total volatile basic nitrogen (TVB-N) content was determined as described by Hong et al. (2012). The results were expressed as mg N per 100 g of crab muscle.
Peroxide value and TBARS analysis
The peroxide value (PV) and thiobarbituric acid-reactive substances (TBARS) were determined according to the method of Wongwichian et al. (2015). PV assay was performed with no modification, and TBARS assay was performed with a slight modification. Briefly, a ground sample (10 g) was homogenized with 30 mL of a solution containing 0.375% (w/v) thiobarbituric acid, 15% (w/v) trichloroacetic acid and 0.25 mol/L HCl. The mixture was heated in a boiling water bath for 10 min and cooled and centrifuged at 3600g at 25 °C for 20 min. The absorbance of the supernatant was measured at 532 nm. Each determination was performed in triplicate, and the TBARS value was expressed as equivalents of mg malonaldehyde/kg of sample.
Preparation of myofibrillar protein procedure
Myofibrillar protein was prepared according to the followed procedure. Twenty milligrams of swimming crab meat was homogenized for 2 min with 10 volumes of Tris–HCl buffer (50 mM KCl-20 mM Tris–HCl, pH 7.0). The homogenate was centrifuged at 10,000g at 4 °C for 15 min, and the precipitate was added to Tris–HCl buffer (0.6 M KCl, 20 mM Tris–HCl, pH 7.0) and then homogenized. Next, the homogenate was extracted at 4 °C for 60 min and then centrifuged at 10,000g for 20 min at 4 °C. The obtained supernatant was the myofibrillar protein solution used in this study. The protein concentration was determined using the Bradford (1976) method.
Determination of protein total sulfhydryl content
The total sulfhydryl (SH) content was analyzed using 5, 5′-dithiobis (2-nitrobenzoic acid) (DTNB) according to the method described by Benjakul et al. (1997) with some modifications. First, myofibrillar protein from the crab were diluted to 2 mg/mL in 0.1 M phosphate buffer (pH 7.4), and then, 1 mL of myofibrillar protein was added to 9 mL 0.2 M Tris–HCl buffer (pH 6.8, 8 M urea, 2% SDS, and 10 mM EDTA). A 4-mL aliquot of the mixture was added to 0.4 mL of a 0.1% DTNB solution and incubated at 40 °C for 25 min. The absorbance was measured at 412 nm with a spectrophotometer. The total SH content was estimated using a molar extinction coefficient of 13,600/M/cm and was expressed as nmol/mg protein.
Determination of protein disulphide bond content
The disulfide bond content was determined according to the method of Benjakul et al. (2003). To 0.5 mL of a sample solution, 3.0 mL of a freshly prepared 2-nitro-5-thi-osulphobenzoate (NTSB) assay solution was added. The mixture was incubated at 25 °C for 30 min. The absorbance at 412 nm was read. The disulfide bond content was calculated using an extinction coefficient of 13,900/M/cm.
Determination of protein carbonyls
The protein carbonyl content was evaluated according to the method of Soyer et al. (2010). The results are expressed as nmol carbonyls/mg protein using an absorption coefficient of 22,000/M/cm for the hydrazones.
Determination of salt extractable protein
Salt extractable protein (SEP) was measured as outlined by Xiong et al. (2009). The SEP content was expressed as the percentage of the protein concentration of each sampling to the initial protein concentration.
Ca-ATPase analysis
The Ca-ATPase activity was measured according to the method of Rawdkuen et al. (2010) with a slight modification. The swimming crab myofibrillar protein was diluted to 1–2 mg mL with 0.6 M KCl (pH 7.0). One milliliter of the protein solution was added to 0.6 mL of 0.5 M Tris–HCl (pH 7.0). Next, 10 mM CaCl2 was added to the mixture for the Ca-ATPase activity assay to a total volume of 9.5 mL. The assay solution was incubated at 25 °C for 10 min in the presence of 0.5 mL of 20 mM ATP, and the reaction was terminated by adding 5 mL of chilled 15% trichloroacetic acid. The reaction solution was then centrifuged at 4000g for 5 min and then the supernatant was measured for inorganic phosphate (Pi) content using a colorimetric quantitative method. The results were expressed as nmol phosphate/mg protein/min. A series of NaH2PO4 solutions (0.0–1.0 mM) was used to prepare the standard curve for phosphate calculation.
SDS-PAGE
The crab myofibrillar protein samples were subjected to Sodium dodecyl sulphate polyacrylamide gel elcetrophoresis (SDS-PAGE) according to the method of Laemmli (1970). The samples were dissolved in SDS-PAGE sample buffer with 10% β-mercaptoethanol (β-ME) to obtain an approximately 1 mg/mL protein concentration. The samples were loaded onto the polyacrylamide gel with a 12% running gel and 5% stacking gel and subjected to electrophoresis at a constant current of 15 mA per gel.
Drip loss analysis
Drip loss analysis was performed on five crab samples for the actual sampling day and expressed as the percentage of the weight difference of the crab sample before and after storage: Drip loss (%) = (WA − WB)/WA × 100, and where WA and WB denote the weight of the crab sample before and after t days of storage, respectively.
Freezing point and microstructure analysis
The freezing point of swimming crab was determined by differential scanning calorimetry (DSC200PC, Netzsch, Bavaria, Germany) according to the method of Liu et al. (2013). The microstructure of crab muscle was determined using a scanning electron microscope (JSM-840, JEOL Tokyo, Japan). The samples were sputter-coated with gold (Sputter coater SPI-Module, PA, USA), and the specimens were observed at an acceleration voltage of 15 kV.
Statistical analysis
The Origin 8.0 for Windows software (OriginLab Inc., Hampton, MA, USA) was used to explore the statistical significance of the results obtained, including multivariate contrasts and multiple comparisons by the Tukey’s test. A probability value of P < 0.05 was considered to be significant. All experiments were performed in triplicate (n = 3). The figures were designed by Origin Lab 9.1 (Origin Lab Co, Northampton, MA, USA).
Results and discussion
Microbiological growth, pH and TVB-N
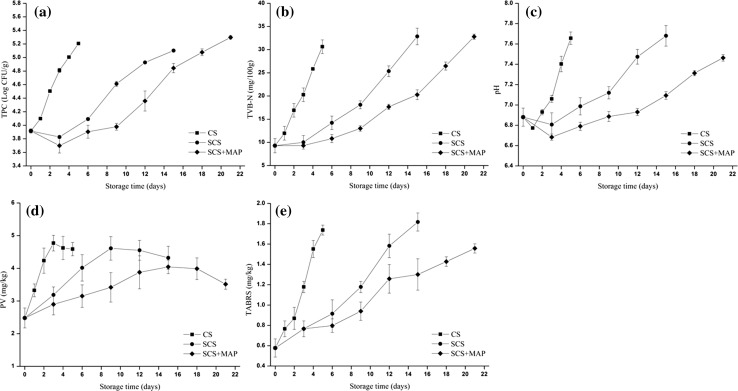
The initial total aerobic plate count (TPC) was 3.96 log CFU/g for fresh crab (Fig. 1a). TPC count in the CS treatment was significantly higher than SCS and SCS + MAP samples at the same storage time points beyond the first day. TPC count reached log105 cfu/g at day 4, day 12–15 and day 15–18 in SC, SCS and SCS + MAP samples, respectively, suggesting the SCS and SCS + MAP treatment significantly inhibit bacteria growth during storage especially at day 4 (P < 0.05). Comparison with the recommended limits (log106 cfu/g) for refrigerated and frozen crab meat (ICMSF 1986) showed that no samples reached the spoilage level in the present study. This result was not in agreement with a previous study, which revealed that bacteria in crab meat reached approximately log106.5 cfu/g at day 14 during chilled storage (Lorentzen et al. 2014). This difference might due to the different species, capture location and processing conditions resulting in different nutrients content of crab samples.
Fig. 1.
Effects of CS, SCS and SCS + MAP on the total aerobic plate count (a), total volatile basic nitrogen (b), pH (c), peroxide value (d) and thiobarbituric acid reactive substances value (e) of swimming crab. The error bars indicate the standard deviation obtained from three analyses. CS chilling storage, SCS superchilling storage, SCS + MAP superchilling storage with modified atmosphere packaging
The total volatile basic nitrogen (TVB-N) and pH are traditional indicators for the quality of crab product. In general, the rise of TVB-N and pH in all treatments was observed during storage (Fig. 1b, c). The increase of pH in crab was significantly inhibited by SCS and SCS + MAP treatment at day 4 (P < 0.05). The pH values did not exceed a maximum level of 8.20 (Anacleto et al. 2011) at day 15 and day 21 in SCS and SCS + MAP samples, respectively. The TVB-N increased rapidly for the CS treatment, and the value reached 30.64 mg N/100 g on the 5th day of storage, while the corresponding values of the SCS and SCS + MAP treated samples were only 14.21 and 10.82 mg N/100 g on the 6th day of storage, respectively. The TVB-N contents reached 30 mg N/100 g limits (Ocaño-Higuera et al. 2011) at day 12–15 and day 18–21 in SCS and SCS + MAP samples, respectively.
According to Masniyom (2011), higher CO2 concentration potentially inhibited the growth of mainly gram negative microorganisms and decreased deamination capacity of bacteria, resulting in lower volatile compounds production. This fact corroborates our microbiological results (Fig. 1a), since samples submitted to SCS + MAP treatment present in general a lower number of colonies in the stationary phase. In addition, there was a significant difference (P < 0.05) in the amount of total TVB-N produced in SCS + MAP treatment samples, which reasserts the efficacy of superchilling with low O2 and high CO2 in modified atmosphere packaging to reduce TVB-N production during storage and to extend shelf life.
Lipid oxidation
Changes in the peroxide value (PV) and thiobarbituric acid reactive substances (TBARS) values of crab samples during storage are shown in Fig. 1d, e, respectively. An increase in PV was observed in all samples during the early days of storage. The increase in PV indicates that the samples were in the propagation stage of lipid oxidation with a lower rate of decomposition of hydroperoxide (Shi et al. 2014). CS and SCS samples displayed higher PV compared to SCS + MAP samples throughout the entire storage time. The continuous increase in PV was observed up to day 15 for SCS + MAP, while the PV of CS and SCS decreased with increasing storage time. The decrease in PV at the late storage times was probably due to the decomposition of hydroperoxide into other oxygenated compounds, which are considered secondary oxidation products (Maqsood and Benjakul 2010).
The TBARS content in all the samples increased substantially during storage (P < 0.05). Ruiz-Capillas and Moral established that the minimum value of the TBA index detectable by panelists was 1.44 mg MDA/kg (Ruiz-Capillas and Moral 2001). The TBARS content reached 1.5 mg MDA/kg of muscle at day 4, day 12 and day 21 in CS, SCS and SCS + MAP samples, respectively, suggesting that SCS treatment was more effective in exhibiting the oxidation procedure than that of CS. Lipid oxidation could be initiated and accelerated by different mechanisms, including the production of singlet oxygen and enzymatic and non-enzymatic generation of free radicals and active oxygen (Kubow 1992). Statistically significantly lower (P < 0.05) values of TBARS were observed in the SCS + MAP treatment after 6 days of storage compared to SCS. The results indicated that the presence of oxygen has a definite influence on the level of lipid oxidation (Giménez et al. 2002) and the decrease of oxygen content results in lower TBARS values.
Total sulfhydryl content (SH), disulfide bound and carbonyl content
Protein oxidation is known to have a number of negative effects on the attributes of meat quality. The changes in protein oxidation and denaturation parameters are shown in Table 1. As storage progressed, protein oxidation was evident by decreases in the total sulfhydryl content and increases in the disulfide bond and carbonyl content. A significant (P < 0.05) decrease in the total –SH content was observed during the first 3 days of storage for all treatments. The decrease in total –SH content is due to the formation of disulfide bonds through oxidation of –SH groups or disulfide interchanges (Thawornchinsombut and Park 2005). Among different swimming crab samples, SCS + MAP had significantly a higher (P < 0.05) –SH content from day 6 to day 15. It has been provide that oxygen promotes fat and protein deteriorative reactions (Sandhya 2010). A lower loss of –SH in SCS + MAP may have been due to lower oxidation caused by the low oxygen of modified atmosphere packaging.
Table 1.
Effects of CS, SCS and SCS + MAP on the protein oxidation and denaturation parameters of swimming crab
| Parameter | Storage conditions | Storage time (days) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 3 | 5/6* | 9 | 12 | 15 | ||
| Total sulfhydryl content (nmol/mg pro) | CS | 75.0 ± 1.3A | 56.2 ± 3.8Bb | 54.8 ± 4.5Bab | – | – | – |
| SCS | 75.0 ± 1.3A | 62.4 ± 2.5Bab | 53.1 ± 1.8Cb | 49.1 ± 1.9Cb | 47.5 ± 3.5Cb | 47.6 ± 2.6Cb | |
| SCS + MAP | 75.0 ± 1.3A | 66.1 ± 3.9Ba | 60.2 ± 1.2BCa | 57.6 ± 2.9CDa | 56.7 ± 1.1Da | 55.6 ± 0.8Da | |
| Disulfide bond (nmol/mg pro) | CS | 41.9 ± 4.1A | 57.1 ± 4.7Bb | 64.0 ± 4.4Cb | – | – | – |
| SCS | 41.9 ± 4.1A | 44.3 ± 4.7Aa | 54.1 ± 2.9Ba | 65.7 ± 3.1Cb | 73.3 ± 0.9Db | 77.1 ± 4.3Db | |
| SCS + MAP | 41.9 ± 4.1A | 43.5 ± 1.7Aa | 52.8 ± 4.7Ba | 60.2 ± 2.3BCa | 62.6 ± 1.5Ca | 67.2 ± 2.1Da | |
| Carbonyl groups (nmol/mg pro) | CS | 1.21 ± 0.19A | 1.88 ± 0.14Bb | 2.03 ± 0..15Bb | – | – | – |
| SCS | 1.21 ± 0.19A | 1.47 ± 0.05Aa | 1.87 ± 0.06Bb | 2.44 ± 0.20Cb | 2.64 ± 0.09Cb | 2.68 ± 0.15Cb | |
| SCS + MAP | 1.21 ± 0.19A | 1.42 ± 0.18ABa | 1.66 ± 0.07Ba | 2.06 ± 0.14Ca | 2.19 ± 0.20Ca | 2.15 ± 0.08Ca | |
| Salt extractable protein (%) | CS | 100.0 ± 0.0A | 82.6 ± 9.9Bb | 78.5 ± 9.9Cb | – | – | – |
| SCS | 100.0 ± 0.0A | 95.6 ± 9.9Ba | 86.0 ± 9.9Ca | 76.7 ± 9.9Da | 72.9 ± 9.9Da | 70.7 ± 9.9Da | |
| SCS + MAP | 100.0 ± 0.0A | 94.5 ± 9.9Ba | 84.3 ± 9.9Ca | 72.6 ± 9.9Db | 67.0 ± 9.9Eb | 64.2 ± 9.9Eb | |
| Ca-ATPase (nmol Pi/mg pro.min) | CS | 146.0 ± 3.5A | 99.0 ± 1.0Bb | 91.3 ± 0.5Cb | – | – | – |
| SCS | 146.0 ± 3.5A | 129.7 ± 1.5Bab | 106.3 ± 3.5Cab | 86.7 ± 3.8Db | 75.0 ± 3.0Eb | 71.3 ± 0.5Eb | |
| SCS + MAP | 146.0 ± 3.5A | 135.0 ± 4.6Ba | 116.3 ± 6.5Ca | 101.7 ± 0.5Da | 94.0 ± 4.6Ea | 89.0 ± 3.6Ea | |
*5 days is for CS, and 6 days for SCS and SCS + MAP treatment. Mean values with different letters within columns for each parameter (a, b, c) or within rows (A, B, C) differ significantly at P < 0.05. CS chilling storage, SCS superchilling storage; SCS + MAP superchilling storage with modified atmosphere packaging
Changes of the carbonyl content showed a similar trend with the disulfide bonds. The initial carbonyl content was 1.21 nmol carbonyl/mg protein, which increased to 2.03, 1.87, 1.66 nmol carbonyl/mg protein (P < 0.05) on the 6th day for CS, SCS and SCS + MAP, respectively. Moreover, SCS + MAP had a lower (P < 0.05) –C=O content from day 6 to day 15. These results indicated that the formation of carbonyls without modified atmosphere packaging was more sensitive during chilling and superchilling storage.
Salt extractable protein content
With the prolonged storage time, the SEP contents of CS, SCS, SCS + MAP decreased significantly (P < 0.05) (Table 1). Kong et al. (2016) reported that the change in SEP content was applied as a major parameter for the denaturation of proteins, which is related to the formation of hydrogen or hydrophobic bonds, as well as disulfide bonds and ionic interactions. On the 6th day of storage, the CS samples showed a lower salt extractable protein content than that of the SCS and SCS + MAP samples, suggesting that the superchilling temperature provided some protection against protein denaturation and aggregation.
Ca-ATPase activity
The decrease in Ca-ATPase activity has been widely used as an indicator of protein denaturation during frozen storage. The Ca-ATPase activity of the CS samples declined rapidly from an initial value of 146.0 nmol (pi)/mg pro.min at 6 days, decreasing by 37.5%. During storage for 15 days, the Ca-ATPase activity values of the SCS and SCS + MAP samples were 71.3 and 89.0 nmol (pi)/mg pro.min, 51.2 and 39.0% decreases, respectively, compared to their initial values. The combination of superchilling with modified atmosphere packaging was found to significantly reduce the Ca-ATPase activity decreases from day 6 to day 15 (P < 0.05). Thus, it was revealed that the modified atmosphere packaging could lessen the decrease in the Ca-ATPase activity and have a protective effect on the protein denaturation of swimming crab.
The loss in Ca-ATPase activity was caused by tertiary structural changes induced by ice crystals and the increased ionic strength of the system, as well as protein rearrangement via protein–protein interactions (Benjakul et al. 2003). Our results for the Ca-ATPase activity reduction and sulfhydryl oxidation were similar, suggesting that the sulfhydryl groups played an essential role in the ATPase activity and that the oxidation of the sulfhydryl groups caused the decrease in Ca-ATPase activity. Benjakul and Bauer (2000) also reported that the decreased Ca-ATPase activity may be associated with the oxidation of sulfhydryl groups on the myosin globular head. In addition, low oxygen of modified atmosphere packaging could reduce the formation of disulfide bonds, which could lessen protein aggregation and prevent protein denaturation.
SDS–PAGE
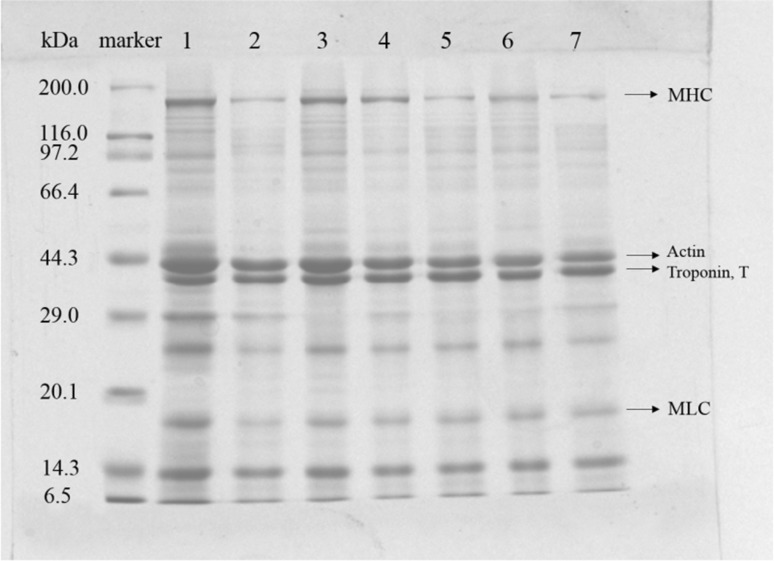
The patterns observed by SDS–PAGE for myofibrillar protein from the swimming crab during storage are shown in Fig. 2. The characteristic polypeptide bands of the myosin heavy chain (MHC), actin (A), troponin T, and myosin light chains (MLCs) were present in the gels. The band intensities of these proteins, especially MHC, decreased more rapidly in CS samples than in SCS and SCS + MAP samples at day 4. These results suggested that chilling storage caused more severe damage on myosin than superchilling storage. SCS + MAP could effectively maintain myosin integrity before day 4. After day 4, the SCS + MAP results at day 18 were similar to that of the CS results at day 4, indicating protein decreased at later storage period even at SCS + MAP conditions. Iwasaki et al. (2006) reported that the arrangement of filaments within the myofibril was the main contributor to water retention in the muscle cells. From the SDS–PAGE patterns, the MHC of CS samples decrease rapidly, which might explain changes in drip loss of CS samples even if they had no ice crystals damage.
Fig. 2.
Effects of CS, SCS and SCS + MAP on the SDS-PAGE of swimming crab protein. S standards, lane 1 raw sample; lane 2 storage for 4 days in CS; lane 3 storage for 4 days in SCS; lane 4 storage for 4 days in SCS +MAP; lane 5 storage for 12 days in SCS (E); lane 6 storage for 12 days in SCS + MAP, lane 7 storage for 18 days in SCS + MAP. MHC, myosin heavy chain (200 kDa); A actin (43 kDa); T troponin (37 kDa); MLCs myosin light chains (18 kDa). CS chilling storage; SCS superchilling storage; SCS + MAP superchilling storage with modified atmosphere packaging
Drip loss
Drip loss is unappealing to consumers as it may affect juiciness, flavor, appearance and texture. In this study, the drip loss of all of the samples increased significantly during storage time (P < 0.05) (Table 2). The losses ranged from 3.3% for the SCS samples to 5.0% for the CS samples on 6th day of storage. However, there was no significant difference among the treatments at day 6 (P > 0.05). However, after 6 days of storage, SCS and SCS + MAP had a significant effect on the drip loss. These results are similar to those reported by Liu et al. (2013) in grass carp fillets stored at −3 °C, and the drop loss was significantly higher than that of fillets stored at 0 °C due to cell damage, lower protein solubility and protein denaturation.
Table 2.
Effects of CS, SCS and SCS + MAP on drip loss of swimming crab
| Parameter | Storage conditions | Storage time (days) | ||||
|---|---|---|---|---|---|---|
| 3 | 5/6* | 9 | 12 | 15 | ||
| Drip loss (%) | CS | 2.7 ± 0.2Aab | 5.0 ± 0.6B | – | – | – |
| SCS | 2.3 ± 0.4Aa | 3.3 ± 1.5A | 5.1 ± 0.6BCa | 5.8 ± 0.6Ca | 7.4 ± 1.0D | |
| SCS + MAP | 3.0 ± 0.4Ab | 4.7 ± 0.7B | 6.8 ± 0.9Cb | 8.1 ± 0.5Db | 8.5 ± 0.9E | |
* 5 days is for CS, and 6 days for SCS and SCS + MAP treatment. Mean values with different letters within columns for each parameter (a, b, c) or within rows (A, B, C) differ significantly at P < 0.05. CS chilling storage, SCS superchilling storage, SCS + MAP superchilling storage with modified atmosphere packaging
Furthermore, superchilling with modified atmosphere packaging has been shown to reduce the drip loss of some seafood products. Cyprian et al. (2013) demonstrated that 50% CO2 packaging with superchilling can significantly reduce drip loss of fresh Nile tilapia fillets. However, in this study, SCS + MAP treatment increased drip loss compared to SCS. Our results are in good agreement with those of Masniyom et al. (2002), the higher the content of CO2 in MAP the higher the drip loss. This may be due to a greater loss of the water-holding capacity of muscle protein at lower pH values produced from the dissolution of CO2 in the muscle (Sivertsvik et al. 2002).
Freezing point and microstructure
According to swimming crab samples in the heat capacity versus temperature plot (Data not shown), the initial freezing point of swimming carb is −1.28 ± 0.36 °C. Considering temperature fluctuations resulting in thawing and re-freezing of the samples, −3 ± 1 °C was chosen as the superchilling temperature of this study.
Figure 3 shows the morphology variations in the muscle tissues of swimming crab samples stored using CS, SCS and SCS + MAP conditions. In the images, clear muscle fibers and nodules were observed in raw samples (Fig. 3a). After 4 days of storage, the nodules disappeared in the CS samples, and the muscle fibers pasted together with smooth edges (Fig. 3b). After 12 or 18 days of storage, similar phenomena were observed in the SCS and SCS + MAP samples (Fig. 3e–g). These observations indicated muscle corruption, leading to the development of muscle adhesions and degraded nodules. As a result, morphological changes were present in the muscle fibers. At day 4, SCS and SCS + MAP samples retained the appearance of clear muscle fiber and nodules. At day 12, SCS samples contained more compact fiber arrangements than that of SCS + MAP, suggesting that superchilling with modified atmosphere packaging could maintain the integrity of swimming crab muscle to some degree.
Fig. 3.
Microstructure of swimming crab meat. raw (a), storage for 4 days in CS (b), storage for 4 days in SCS (c), storage for 4 days in SCS + MAP (d), storage for 12 days in SCS (e), storage for 12 days in SCS + MAP (f), storage for 18 days in SCS + MAP (g). CS chilling storage, SCS superchilling storage; SCS + MAP superchilling storage with modified atmosphere packaging
Conclusion
In conclusion, the results of the present study indicate that superchilling with modified atmosphere packaging (10% O2/60% CO2/30% N2) helps prevent microbiological growth, reduces lipid and protein oxidation, and maintains tight morphology. Moreover, the shelf life of swimming crab was prolonged to 15–18 days by superchilling combined with modified atmosphere packaging. However, superchilling preservation technology is still in its infancy, and extensive research into optimization of the technique, temperature control and maintenance of the nutrition quality of aquatic products needs to be conducted.
Acknowledgements
This research was supported by Ningbo Agricultural Science and Technology Key Projects (2014C10009).
References
- Anacleto P, Teixeira B, Marques P, Pedro S, Nunes ML, Marques A. Shelf-life of cooked edible crab (Cancer pagurus) stored under refrigerated conditions. LWT Food Sci Technol. 2011;44:1376–1382. doi: 10.1016/j.lwt.2011.01.010. [DOI] [Google Scholar]
- Ashie INA, Smith JP, Simpson BK, Haard NF. Spoilage and shelf-life extension of fresh fish and shellfish. Crit Rev Food Sci. 1996;36:87–121. doi: 10.1080/10408399609527720. [DOI] [PubMed] [Google Scholar]
- Benjakul S, Bauer F. Physicochemical and enzymatic changes of cod muscle proteins subjected to different freeze thaw cycles. J Sci Food Agric. 2000;80:1143–1150. doi: 10.1002/1097-0010(200006)80:8<1143::AID-JSFA610>3.0.CO;2-C. [DOI] [Google Scholar]
- Benjakul S, Seymour TA, Morrissey MT, Haejung AN. Physicochemical changes in pacific whiting muscle proteins during iced storage. J Food Sci. 1997;62:729–733. doi: 10.1111/j.1365-2621.1997.tb15445.x. [DOI] [Google Scholar]
- Benjakul S, Visessanguan W, Thongkaew C, Tanaka M. Comparative study on physicochemical changes of muscle proteins from some tropical fish during frozen storage. Food Res Int. 2003;36:787–795. doi: 10.1016/S0963-9969(03)00073-5. [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quanlities of proteins utlizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cheung IWY, Liceaga AM, Li-Chan ECY. Pacific hake (Merluccius productus) hydrolysates as cryoprotective agents in frozen Pacific cod fillet mince. J Food Sci. 2009;74:C588–C594. doi: 10.1111/j.1750-3841.2009.01307.x. [DOI] [PubMed] [Google Scholar]
- Cyprian O, Lauzon HL, Jóhannsson R, Sveinsdóttir K, Arason S, Martinsdóttir E. Shelf life of air and modified atmosphere-packaged fresh tilapia (Oreochromis niloticus) fillets stored under chilled and superchilled conditions. Food Sci Nutr. 2013;1:130–140. doi: 10.1002/fsn3.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlieghere F, Debevere J, Van Impe J. Concentration of carbon dioxide in the water-phase as a parameter to model the effect of a modified atmosphere on microorganisms. Int J Food Microbiol. 1998;43:105–113. doi: 10.1016/S0168-1605(98)00101-9. [DOI] [PubMed] [Google Scholar]
- Duun AS, Rustad T. Quality of superchilled vacuum packed Atlantic salmon (Salmo salar) fillets stored at −1.4 and −3.6 °C. Food Chem. 2008;106:122–131. doi: 10.1016/j.foodchem.2007.05.051. [DOI] [Google Scholar]
- Food and Agriculture Organization of the United Nations (2014) FAO species identification and data programme. http://www.fao.org/fishery/statistics/en. Accessed 25 Aug 2014
- Giménez B, Roncalés P, Beltrán JA. Modified atmosphere packaging of filleted rainbow trout. J Sci Food Agric. 2002;82:1154–1159. doi: 10.1002/jsfa.1136. [DOI] [Google Scholar]
- Hong H, Luo YK, Zhou ZY, Shen HX. Effects of low concentration of salt and sucrose on the quality of bighead carp (Aristichthys nobilis) fillets stored at 4 °C. Food Chem. 2012;133:102–107. doi: 10.1016/j.foodchem.2012.01.002. [DOI] [Google Scholar]
- ICMSF (International Commission on Microbiological Specifications for Foods) Microorganisms in foods 2: sampling for microbiological analysis: principles and specific applications. 2. Canada: University of Toronto Press; 1986. pp. 1–293. [Google Scholar]
- Iwasaki T, Noshiroya K, Saitoh N, Okano K, Yamamoto K. Studies of the effect of hydrostatic pressure pretreatment on thermal gelation of chicken myofibrils and pork meat patty. Food Chem. 2006;95:474–483. doi: 10.1016/j.foodchem.2005.01.024. [DOI] [Google Scholar]
- Kong CL, Wang HY, Lia DP, Zhang YM, Pan JF, Zhua BW. Quality changes and predictive models of radial basis function neural networks for brined common carp (Cyprinus carpio) fillets during frozen storage. Food Chem. 2016;201:327–333. doi: 10.1016/j.foodchem.2016.01.088. [DOI] [PubMed] [Google Scholar]
- Kubow S. Routes of formation and toxic consequences of lipid oxidation in foods. Free Radical Bio Med. 1992;12:63–81. doi: 10.1016/0891-5849(92)90059-P. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu D, Liang L, Xia WS, Regenstein JM, Zhou P. Biochemical and physical changes of grass carp (Ctenopharyngodon idella) fillets stored at −3 and 0°C. Food Chem. 2013;140:105–114. doi: 10.1016/j.foodchem.2013.02.034. [DOI] [PubMed] [Google Scholar]
- Lorentzen G, Skuland AV, Sone I, Johansen JO, Rotabakk BT. Determination of the shelf life of cluster of the red king crab (Paralithodes camtschaticus) during chilled storage. Food Control. 2014;42:207–213. doi: 10.1016/j.foodcont.2014.02.019. [DOI] [Google Scholar]
- Maqsood S, Benjakul S. Synergistic effect of tannic acid and modified atmospheric packaging on the prevention of lipid oxidation and quality losses of refrigerated striped catfish slices. Food Chem. 2010;121:29–38. doi: 10.1016/j.foodchem.2009.11.086. [DOI] [Google Scholar]
- Masniyom P. Deterioration and shelf-life extension of fish and fishery products by modified atmosphere packaging. Songklanakarin J Sci Technol. 2011;33:181–192. [Google Scholar]
- Masniyom P, Benjakul S, Visessanguan W. Shelf-life extension of refrigerated seabass slices under modified atmosphere packaging. J Sci Food Agric. 2002;82:873–880. doi: 10.1002/jsfa.1108. [DOI] [Google Scholar]
- Ocaño-Higuera VM, Maeda-Martínez AN, Marquez-Ríos E, Ccnizales-Rodríguez DF, Castillo-Yáñez F, Ruíz-bustos E. Freshness assessment of ray fish stored in ice by biochemical, chemical and physical methods. Food Chem. 2011;125:49–54. doi: 10.1016/j.foodchem.2010.08.034. [DOI] [Google Scholar]
- Rawdkuen S, Jongjareonrak A, Phatcharat S, Benjakul S. Assessment of protein changes in farmed giant catfish (Pangasianodon gigas) muscles during refrigerated storage. Int J Food Sci Technol. 2010;45:985–994. doi: 10.1111/j.1365-2621.2010.02217.x. [DOI] [PubMed] [Google Scholar]
- Ruiz-Capillas C, Moral A. Residual effect of CO2 on hake (Merluccis merluccius L.) stored in modified and controlled atmospheres. Eur Food Res Technol. 2001;212:413–420. doi: 10.1007/s002170000270. [DOI] [Google Scholar]
- Sandhya Modified atmosphere packaging of fresh produce: Current status and future needs. LWT Food Sci Technol. 2010;43:381–392. doi: 10.1016/j.lwt.2009.05.018. [DOI] [Google Scholar]
- Shi C, Cui JY, Yin XF, Luo YK, Zhou ZY. Grape seed and clove bud extracts as natural antioxidants in silver carp (Hypophthalmichthys molitrix) fillets during chilled storage: effect on lipid and protein oxidation. Food Control. 2014;40:134–139. doi: 10.1016/j.foodcont.2013.12.001. [DOI] [Google Scholar]
- Sivertsvik M, Jeksrud WK, Rosnes JT. A review of modified atmosphere packaging of fish and fishery products-significance of microbial growth, activities and safety. Int J Food Sci Techol. 2002;37:107–127. doi: 10.1046/j.1365-2621.2002.00548.x. [DOI] [Google Scholar]
- Soyer A, Özalp B, Dalmıs Ü, Bilgin V. Effects of freezing temperature and duration of frozen storage on lipid and protein oxidation in chicken meat. Food Chem. 2010;120:1025–1030. doi: 10.1016/j.foodchem.2009.11.042. [DOI] [Google Scholar]
- Stiles ME (1991) Modified atmosphere packaging of meat, poultry and their products. In: Ooraikul B, Stiles ME (eds) Modified atmosphere packaging of food. London, pp 118–147
- Sun B, Zhao Y, Yu J, Ling J, Shang H, Liu Z. The combined efficacy of superchilling and high CO2 modified atmosphere packaging on shelf life and quality of swimming crab (Portunus trituberculatus) J Aquat Food Prod Technol. 2016 [Google Scholar]
- Thawornchinsombut S, Park JW. Role of ionic strength in biochemical properties of soluble fish proteins isolated from cryoprotected Pacific whiting mince. J Food Biochem. 2005;29:132–151. doi: 10.1111/j.1745-4514.2005.00005.x. [DOI] [Google Scholar]
- Wongwichian C, Klomklao S, Panpipat W, Benjakul S, Chijan M. Interrelationship between myoglobin and lipid oxidations in oxeye scad (Selar boops) muscle during iced storage. Food Chem. 2015;174:279–285. doi: 10.1016/j.foodchem.2014.11.071. [DOI] [PubMed] [Google Scholar]
- Wu CH, Yuan CH, Ye XQ, Hu YQ, Chen SG, Liu DH. A critical review on superchilling preservation technology in aquatic product. J Integr Agric. 2014;13:2788–2806. doi: 10.1016/S2095-3119(14)60841-8. [DOI] [Google Scholar]
- Xiong GQ, Cheng W, Ye LX, Du X, Zhou M, Lin R, Geng SR, Chen ML, Corke H, Cai YZ. Effects of konjac glucomannan on physicochemical properties of myofibrillar protein and surimi gels from grass carp (Ctenopharyngodon idella) Food Chem. 2009;116:413–418. doi: 10.1016/j.foodchem.2009.02.056. [DOI] [Google Scholar]