Abstract
Green coffee extract, GCE (Coffee canephora) was used at 1.0, 1.5 and 2.0% levels for making bioactive rich bread. The processed GCE from the green coffee beans had 21.42% gallic acid equivalents (GAE) total polyphenols (TPP), 37.28% chlorogenic acid (CGA) and 92.73% radical scavenging activity (RSA), at 100 ppm concentration of GCE and caffeine content (1.75%). Rheological, physico-sensory and antioxidant properties of GCE incorporated breads were analysed and compared with control bread. The results revealed not much significant change in the rheological characteristics of dough up to 1.5% level; an increase in bread volume; greenness of bread crumb and mostly unchanged textural characteristics of the bread with increased addition of GCE from 0 to 2.0%. Sensory evaluation showed that maximum level of incorporation of GCE without adverse effect on the overall quality of bread (especially taste) was at 1.5% level. The contents of TPP, RSA and CGA increased by 12, 6 and 42 times when compared to control bread and had the highest amount of 4–5 caffeoylquinic acid.
Keywords: Green coffee beans, Rheology, Chlorogenic acid, Bread
Introduction
Bakery products are important sources of energy and nutrients such as complex carbohydrates, proteins, minerals and vitamins. Normal bread contains most of the nutrients but lacks in antioxidant rich polyphenolic compounds due to the use of refined wheat flour. Conventional milling of wheat grains is based on separating the endosperm (which produces white flour when milled) from the bran layers and embryo. The aleuronic cells, along with the other bran layers and the embryo, are removed to form the bran fractions (Stevenson et al. 2012). Nutritionally, bran fractions produced by milling are rich in fiber, minerals, vitamin B6, thiamine, folate, vitamin E and some phytochemicals, in particular antioxidants such as phenolic compounds (Shewry 2009). Breads with elevated levels of antioxidants are in high demand because of their roles in the maintenance and enhancement of health and protection against many diseases (Sivam et al. 2012). Antioxidant rich spices, herbs and green part of plants are being explored to enrich nutritional characteristics of the bread (Raba et al. 2007; Lim et al. 2011; Goh et al. 2015).
The coffee plant from the genus Coffea belongs to the Rubiaceae family. Two plant species Coffea arabica L. (arabica coffee) and Coffea canephora (robusta coffee) account for more than 99% of commercial coffee production in the world. The main constituents of green coffee beans are carbohydrates (up to 50%), both soluble (galactomannan, arabinogalactan) and insoluble (cellulose); phenolic compounds such as chlorogenic acids (CGA), proteins, lipids, caffeine and minerals (Bicchi et al. 1995; Fischer et al. 2001; Naidu et al. 2008; Chu 2012; Wei et al. 2012). It has been recognized that benefits from consuming green coffee infusions are mainly related to the presence of phenolic compounds, especially CGA which has antioxidant activity (Bicchi et al. 1995; Suzuki et al. 2002; Naidu et al. 2008). CGA refer to the esters of hydroxycinnamic acids (caffeic acid, ferulic acid and p-coumaric acid) with quinic acid (Clifford et al. 2003). About 5–12 g of CGA is present in 100 g of green coffee (Upadhyay and Mohan Rao 2013; Farah et al. 2005, 2008). Recent studies demonstrated that the consumption of green coffee extracts produced antihypertensive effect in rats and humans (Suzuki et al. 2002; Kozuma et al. 2005), inhibitory effect on fat accumulation and body weight in mice and humans (Shimoda et al. 2006; Dellalibera et al. 2006), resulted in an improvement in human vasoreactivity (Ochiai et al. 2004) and modulation of glucose metabolism in humans (Blum et al. 2007). Such biological properties have been attributed to CGA present in green coffee. The concentration of CGA can be increased to 3–4 times by processing green coffee beans into an extract.
Dziki et al. (2015) reported that partial replacement of wheat flour with up to 3% ground green coffee bean (GCB) powder in bread making gave satisfactory overall consumer acceptability and concluded that breads enriched with GCB possessed higher antiradical activity than control samples. A study conducted by Farah et al. (2008) showed that the major CGA compounds present in green coffee are highly bioavailable in humans. Caffeoylquinic acid (CQA) and diCQA, which are major CGA compounds in coffee are differentially absorbed and/or metabolized throughout the gastrointestinal tract. According to van der Werf et al. (2014), the total radical scavenging capacity in coffee decline with roasting due to auto-degradation of phenolic compounds. Use of green coffee in bread was tried to assess the basic biological activities in human cells in culture. It was concluded that supplementation of bread with green coffee improved the chemo-protective and anti-genotoxic activities in human cells (Glei et al. 2006). Several authors have tried other sources such as green tea extract, garlic, basil and ginger to improve the antioxidant properties and nutritional characteristics of bread. According to Goh et al. (2015), addition of green tea extract (GTE) at 0.45, 1, and 2% concentration levels resulted in significant reduction in the glycaemic potential of baked and steamed breads. Bread fortified with 2% GTE showed significantly lower level of glucose release during the first 90 min of pancreatic digestion as well as lower content of rapidly digested starch (RDS) content. The potential of transforming bread into a low GI food using GTE fortification was proven to be promising. Raba et al. (2007) used different ratios of garlic powder and sweet basil for bread enrichment and reported that the polyphenols level increased from 0.18 to 0.22 mM gallic acid/100 g, 0.19–0.28 mM gallic acid equivalent/100 g for bread incorporated with garlic and basil respectively. Bread showed good rheological characteristics with doubled antioxidant content compared to the control bread and the highest sensorial acceptability with 3% of ginger powder (Balestra et al. 2011).
Thus, the objectives of the study were to evaluate the effect of bioactive rich green coffee extract on rheological, physico-sensory and antioxidant properties of the bread.
Materials and methods
Materials
Robusta cherry green coffee beans (Coffee canephora) procured from the local market of Mysuru, Karnataka, (India) were ground and sieved (<720 μm), using a hammer mill (CMC-CM; Cadmach Machinery Private Limited, Ahmedabad, India). The ground coffee powder was packed in low-density polyethylene pouches and preserved for further analysis. Commercial wheat flour, compressed yeast, sunflower oil, salt and sugar procured from the local market, Mysuru were used in the studies.
Chemicals
Reference standards, such as caffeine, chlorogenic acid, 1,1-diphenyl-2-picrylhydrazyl (DPPH), butylated hydroxyanisole (BHA) were purchased from Sigma Chemical Co., St. Louis, MO. Gallic acid (Loba Chemie, Mumbai), Folin Ciocalteu’s, FC phenol reagent (Merck, Mumbai), anhydrous sodium carbonate, 99.5%, LR (Ranbaxy, New Delhi) and solvents such as hexane, methanol (Qualigens Fine Chemicals, Mumbai). All the chemicals used were of analytical grade.
Methods
Aqueous extraction methodology
The ground green coffee sample was defatted with hexane (1:6; w/v) at 60–80 °C for 8 h in a soxhlet extraction system. Defatted green coffee powder was subjected to decaffeination with ethyl acetate (1:6; w/v) at 80 °C for 16 h in soxhlet extraction system. The decaffeinated powder was extracted using a rotary evaporator (Heidolph, RE 120; Heidolph Insruments, Schwabach, Germany) at atmospheric pressure using water (1:5; w/v) at three different temperatures of 60, 70 and 80 °C for 1 h. The slurry was filtered to get a clear extract and concentrated at 80 °C for 30 min. The extracts were dried in vacuum tray dryer and used for qualitative and quantitative analysis.
Analytical methodologies
Total polyphenols (TPP)
TPP content of the GCE was determined by using FC reagent. The samples (0.1 ml) were mixed with FC reagent (0.5 ml) and saturated sodium carbonate solution (7.5 ml). The sample solution was made up to 10 ml with distilled water, incubated at room temperature for 60 min and the absorbance was measured at 765 nm. TPP content was expressed as gallic acid equivalents, GAE (Swain and Hillis 1959).
Radical scavenging activity (RSA)
The RSA of GCE was evaluated according to the procedure described by Blois (1958) with slight modifications (Abdille et al. 2005). Sample solution (1 ml) at various concentrations (50, 100 and 200 ppm) was mixed with 0.1 mM methanolic solution (4 ml) of DPPH and allowed to stand at 27 °C for 20 min in dark. The control was prepared as above, without GCE and methanol was used for baseline correction. The optical density (OD) of the samples was measured at 517 nm. The RSA was expressed as the inhibition percentage and was calculated using the formula:
Chlorogenic acids (CGA)
Chlorogenic acid was estimated by UV spectrophotometry before and after lead acetate treatment of the coffee extract, followed by measurement of the absorbance at 325 nm according to AOAC 2000. GCE (1 g) was taken in a 20 ml test tube, extracted with petroleum ether to remove fat; the residue was extracted with water and filtered. The filtrate was suitably diluted (A). Then 100 ml of the sample solution was treated with saturated potassium acetate solution (2 ml) and basic lead acetate solution (10 ml) and transferred to a 250 ml volumetric flask and filtered (B). Absorbance at 325 nm of the solutions A and B was measured using UV–visible spectrophotometer (Cintra 10, GBC, Australia) immediately using water as a blank. From standard curve, apparent concentration of chlorogenic acid in solution without lead treatment (Co); apparent concentration in filtrate after lead treatment (C1) was determined. From latter value, 0.00045 mg/ml was subtracted to correct for solubility of lead chlorogenate. The chlorogenic acid content of the extract was calculated using a standard graph.
Caffeine
The GCE solution was extracted with chloroform in the solvent ratio of 1:4 (v/v), the combined extracts were dried and the absorbance was measured at 275 nm in a spectrophotometer (Cintra 10, GBC, Dandenong, Australia). The quantity of caffeine was calculated from a standard graph, prepared using a reference standard (AOAC 2000).
Estimation of isomers of chlorogenic acid (CGA) in green coffee extract
The isomers of CGA were analysed according to the procedure mentioned by Balyaya and Clifford (1995) with slight modifications. GCE (25 mg) was dissolved in triple distilled water (10 ml). The solution was filtered through the membrane (0.45 µm) and injected (20 µL) into HPLC. The HPLC system consisting of Waters 515 binary HPLC pump, Waters 2998 photodiode array detector (PDA) controlled by Empower software and C18 column (Waters, particle size 5 µm, i.d. 4.6 mm, length 250 mm and pore size 100 Å) was used. The samples were eluted with a gradient elution of mobile phase A (0.5% trifluoroacetic acid) and B (45% acetonitrile in A). Mobile phase A was kept 100% at 0 min and mobile phase B increased slowly to 100% in 56 min at a flow rate of 1 mL min−1. GCE, fractions along with standards were injected and peak areas were recorded.
Wheat flour
Physicochemical characteristics
The characteristics of the wheat flour such as moisture, ash, fat, protein, dry gluten, falling number, Zeleny’s-sedimentation value and dietary fibre were determined using AACC methods (2000).
Rheological characteristics of wheat flour
Based on the preliminary study on the use of GCE and its effect on the bread quality along with the functionality, three levels of GCE i.e., 1, 1.5 and 2% were selected and their effect on rheological characteristics of wheat flour were studied using farinograph, amylograph and alveograph according to AACC methods (2000).
Preparation of bread
Effect of different levels of GCE on bread-making characteristics of wheat flour was studied using the following formulation: wheat flour: 100 g; GCE: 1/1.5/2.0 g; compressed yeast: 2.0 g, salt: 1.0 g; sugar: 3.0 g, sunflower oil : 1.0 g and water: farinograph water absorption. Breads in quadruplicate were prepared by mixing the ingredients in a Hobart mixer (Model N-50, Hobart, GmbH, Offenburg, Germany) with a flat blade for 4 min at 61 rpm. The dough was fermented for 90 min at 30 °C and 75% relative humidity (RH), remixed, rounded, again fermented for 25 min, moulded, proofed for 55 min at 30 °C, 85% RH, baked for 25 min at 220 °C, cooled and packed.
Physical and sensory characteristics
Loaf volume of bread was determined by the rapeseed displacement method using standard volume-measuring apparatus (National Manufacturing Co., Lincoln, Netherland). The moisture content of bread samples was analysed according to AACC method (2000). The texture analyzer LR-5 K (Lloyd Instruments Ltd., Hampshire, England) was used for measuring hardness, chewiness, cohesiveness and springiness of bread crumb. The following conditions were set: load cell: 5 kg; square shaped bread crumb pieces measuring 4 cm on all sides with a sample thickness of 1 inch; 80 mm diameter circular probe; 100 mm/min cross head speed and 50% compression of the sample height. The crumb colour of bread was measured in terms of lightness (L) and colour (+a: red, −a: green, +b: yellow, −b: blue) using Hunter Lab colour measuring system (Labscan XE, Hunter Associates Laboratory Inc., Reston, Virginia, USA). A standard white board made from barium sulphate (100% reflectance) was used as a perfectly white object for setting the instrument with illuminant. Bread samples were placed in the sample holder and the reflectance was auto-recorded for the wavelength ranging from 360 to 800 nm. Sensory evaluation of bread was carried out by twelve men and twelve female panelists ranging in age from 30 to 55 years. The panelists were trained in four sessions, involving 2 h of training in each session. Each panelist evaluated four samples of bread in four replicates by assigning scores for crust color: 1 = dull brown, 10 = golden brown; crust shape: 1 = flat, uneven, 10 = normal; crumb color: 1 = dull white, 10 = greenish white; crumb grain: 1 = very coarse, 10 = very fine; texture:1 = very firm, 10 = very soft; mouthfeel: 1 = gummy/dry, 10 = no residue in mouth; taste: 1 = astringent taste, 10 = pleasant taste. The overall quality score was taken as the combined score of seven quality attributes.
Extracts preparation
After baking, bread samples were allowed to cool to room temperature (30 ± 2 °C, 65% RH). for 4 h. Subsequently, the samples were sliced (about 1.5 cm thick) and kept at −20 °C until analysis. After thawing, the slices were dried, ground in a mortar with a pestle and screened through a 0.5 mm sieve to obtain fine powder. The dried bread powders were defatted at two successive extractions using hexane. 2.5 g of defatted dried bread samples was taken and boiled in 75 ml of distilled water for 30 min. The boiled samples were made up to 100 ml with water. The solution was centrifuged at 6000 rpm for 20 min at 25 °C. The obtained supernatant was used for further analysis.
Estimation of isomers of chlorogenic acids in bread
The isomers of CGA in GCE incorporated breads were analysed using HPLC according to the method described by Balyaya and Clifford (1995) with slight modification. The analysis was carried out in similar conditions as mentioned in the HPLC analysis of CGA isomers of the GCE. The analysis of each sample was carried out in triplicate, and the average peak area was used to calculate the CGA content in breads.
Antioxidant properties
Control and breads with 1, 1.5 and 2% GCE were analyzed for moisture content (method 44-15), TPP (FC Reagent method), RSA (Blois 1958), CGA and caffeine (AOAC, 2000). All estimations were done in triplicates.
Statistical analysis
All the analyses were carried out in triplicate, and the results were provided as mean values with standard deviation. The obtained data was subjected to statistical analysis and the means, compared by Duncan’s New Multiple Range test (p ≤ 0.05), are presented.
Results and discussions
Yield of aqueous extracts and quantitative analysis of GCE
The extraction yields were in the range of 18.39–19.41% with water as a solvent of extraction. The yield decreased with the increase in temperature from 60 to 80 °C. Highest yield of 19.41% was obtained at 60 °C. The various polyphenols present in the coffee were caffeic acid, protocatecuic acid, gallic acid, quinic acid, pyrogallol etc. apart from CGA (Varnam and Shutherland 1994). TPP content in extracts of 60, 70 and 80 °C was present in the range of 20.04–21.42% GAE. There was a decrease in TPP with the increase in temperature from 60 to 80 °C. This may be due to degradation of phenolic compounds at higher temperatures. RSA in GCE was measured in terms of percentage inhibition of DPPH radical scavenging. Extracts at the different concentration ranging from 50 to 200 ppm were taken, and RSA was evaluated. RSA of different extracts at different temperature conditions using water as a solvent are presented in Table 1. The extract showed RSA of 90.25% even at 50 ppm concentration. The RSA at all concentrations of 50, 100 and 200 ppm was found to be higher in 60 °C extract. CGA content decreased with the increase in temperature from 60 to 80 °C. The highest amount of CGA (37.28%) was obtained at 60 °C. This could be due to degradation of CGA at higher temperature. The caffeine content increased with the increase in temperature from 60 to 80 °C. The content of caffeine in water extract at 60 °C was 1.75%. The lower content of caffeine is desirable. Involvement of decaffeination also has reduced caffeine content in the extract. These results showed that the extraction yield, CGA, RSA, TPP decreased and caffeine increased with the increase in temperature from 60 to 80 °C. Hence the sample extracted at 60 °C with the highest antioxidant activity was used for further studies.
Table 1.
Chemical characteristics of GCE
| GCE | 60 °C | 70 °C | 80 °C |
|---|---|---|---|
| Extraction yield (%) | 19.41 ± 0.12a | 18.81 ± 0.09a | 18.39 ± 0.06a |
| TPP (%) | 21.42 ± 0.31a | 21.03 ± 0.22a | 20.04 ± 0.27a |
| CGA (%) | 37.28 ± 0.24a | 36.24 ± 0.33a | 36.18 ± 0.31a |
| Caffeine (%) | 1.75 ± 0.05a | 1.82 ± 0.03b | 1.87 ± 0.03b |
| RSA (%) at different ppm levels | |||
| 50 | 90.25 ± 0.13b | 90.12 ± 0.07a | 89.93 ± 0.08a |
| 100 | 92.73 ± 0.09b | 91.21 ± 0.17a | 91.06 ± 0.22a |
| 200 | 94.38 ± 0.11b | 93.26 ± 0.12b | 93.12 ± 0.14a |
Values are mean ± SD of triplicate analysis. Values not having similar superscripts in the same row are significantly (p < 0.05) different
GCE green coffee extract, TPP total polyphenols, CGA chlorogenic acid, RSA radical scavenging activity
Quality characteristics of wheat flour
The wheat flour used in this study had 11.38% moisture, 0.51% total ash, 1.28% fat, 11.40% protein, 10.38% dry gluten, 521 s falling number, 24 ml sedimentation value and 3.41% dietary fibre. These results indicated that the wheat flour selected was of medium strength.
Effect of 1, 1.5 and 2% GCE blends on rheological characteristics of wheat flour
Farinograph, amylograph and alveograph characteristics
The control and wheat flour with 1.0, 1.5 and 2.0% GCE had 59.7, 59.6, 59.3 and 58.1% farinograph water absorption, dough stability (5.6, 5.2, 5.0 and 4.1 min); amylograph pasting temperature (60.9, 60.8, 60.6 and 60.3 °C), peak viscosity (808, 814, 829, 842 BU); alveograph maximum overpressure. P (75, 70, 68 and 58 mm), average abscissa at rupture, L (38, 40, 42 and 53 mm) and balance of tenacity and extensibility, P/L (1.97, 1.75, 1.62 and 1.09). These data indicated that addition of GCE up to 1.5% level did not alter the rheological characteristics of dough however above 1.5% level, significant decrease in water absorption, dough stability during mixing, dough tenacity, dough balance of tenacity, extensibility, increased dough extensibility and swelling capacity of starch during heating was observed.
Bread making
The moisture content of control and breads with 1, 1.5 and 2.0% GCE varied from 34.5 to 34.8% indicating not much change in the moisture content with the addition of GCE. However, use of increasing amount of GCE from 0 to 2.0% increased volume from 525 to 565 ml. The increase in the volume of breads with GCE may be due to the improvement brought by GCE in the balance of tenacity and extensibility of dough, as shown by alveograph data (P/L) and increase in swelling capacity of starch during heating. The Hunter Lab colour L value representing lightness for control bread was 67.76; it significantly decreased to 59.97, 59.17 and 58.65 with the addition of 1.0, 1.5 and 2.0% GCE respectively. The −a value, representing greenness increased from −0.05 to −0.46. The yellowness of the crumb colour as indicated by b values is presented in Table 2. The b value for control bread was 12.1 and bread with 1–2% GCE varied from 11.45 to 10.60. The above results indicate that use of GCE decreased the lightness, yellowness and increased the greenness of bread crumb. The texture profile analysis parameters of breads presented in Table 2 show that addition of GCE did not alter significantly the hardness, cohesiveness, springiness and gumminess of the bread.
Table 2.
Effect of GCE on the physico-sensory characteristics of breads
| Bread | Control | GCE (%) | ||
|---|---|---|---|---|
| 1 | 1.5 | 2 | ||
| Moisture (%) | 34.5 ± 0.08a | 34.8 ± 0.05a | 34.6 ± 0.12a | 34.5 ± 0.09a |
| Volume (ml) | 525 ± 5.01a | 530 ± 5.52a | 540 ± 5.0ab | 565 ± 5.51b |
| Colour | ||||
| L | 67.76 ± 0.19c | 59.97 ± 0.11b | 59.17 ± 0.21b | 58.65 ± 0.17a |
| a | −0.05 ± 0.01a | −0.11 ± 0.01b | −0.37 ± 0.02c | −0.46 ± 0.02d |
| b | 12.1 ± 0.16c | 11.45 ± 0.14b | 11.02 ± 0.21b | 10.60 ± 0.13a |
| TPA parameters | ||||
| Hardness (N) | 4.81 ± 0.19a | 4.73 ± 0.22a | 4.6 ± 0.24a | 4.38 ± 0.26a |
| Cohesiveness | 0.62 ± 0.02a | 0.6 ± 0.01a | 0.6 ± 0.04a | 0.59 ± 0.07a |
| Springiness (mm) | 12.32 ± 0.36a | 12.23 ± 0.33a | 12.22 ± 0.31a | 12.18 ± 0.38a |
| Gumminess (N) | 2.45 ± 0.04a | 2.55 ± 0.03a | 2.81 ± 0.02a | 2.86 ± 0.01a |
| OQS (70) | 63.5 ± 0.18d | 62 ± 0.22c | 60 ± 0.31b | 57.5 ± 0.28a |
OQS overall quality score of all combined crust and crumb characteristics, GCE green coffee extract, TPA texture profile analysis. Values in the row with the same letter in superscript are not significantly different from each other at p ≤ 0.05
L lightness, higher values indicate lighter colour, a, greenness; b, yellowness; higher colour intensity is indicated by higher values
Bread making characteristics of 0, 1, 1.5 and 2.0% GCE showed that with the addition of GCE, the colour of the crust changed from golden brown to slightly dark brown; the normal shape of bread was not affected. The crumb colour which was creamish white changed to pale green with the addition of GCE. The control as well as breads with different levels of GCE had medium fine crumb grain and softer texture. The mouth feel of control and breads with 1, 1.5 and 2.0% GCE showed no residue formation during chewing. There was mild green coffee taste was observed in bread with 1.5% GCE; however the adverse effect of GCE on taste was noticed in bread with 2.0% GCE, which had slight astringent taste. The overall quality score (Table 2) representing the combined score of all crust and crumb parameters was 63.5 for control bread, bread with 1.0% GCE (62) 1.5% GCE (60) and 2.0% GCE (57.5). It can be concluded from these results that the maximum level of incorporation of GCE on the overall quality of bread with special reference to the taste and without adverse effect was at 1.5% level.
Antioxidant properties of bread
Total polyphenols
The major bioactives in the green coffee bean are polyphenols including CGA, which is well known, besides other polyphenols for its strong antioxidant activity. The TPP content of GCE used in the study was 21.42% GAE (Table 3). The TPP content increased significantly with increasing GCE levels in bread from 1 to 2%. Control bread had 0.02% GAE of TPP, whereas bread prepared with 1% GCE had 0.16% GAE, 1.5% GCE (0.25% GAE) and 2% GCE (0.34% GAE) of TPP. This shows that the amount of non-degraded total polyphenols were 75, 78 and 79% respectively. Highest degradation was observed at 2% GCE addition level. Since 1.5% GCE incorporated bread was sensorially acceptable with highest overall quality score, the increase in TPP was 12 times higher than control bread. Alvarez-Jubete et al. (2010) reported that wheat bread contained 29.1 mg GAE/100 g total phenols, and it showed antioxidant activity with DPPH radical scavenging and ferric ion reducing methods. Cheynier (2005) observed that phenolics are quite heat unstable and reactive compounds and the baking process results in reduction of phenolic compounds.
Table 3.
Total polyphenols, chlorogenic acid, radical scavenging activity and caffeine of bread supplemented with green coffee extract
| Parameters | Bread | |||
|---|---|---|---|---|
| Control | GCE (%) | |||
| 1 | 1.5 | 2 | ||
| TPP (%) | 0.02 ± 0.00a | 0.16 ± 0.01b | 0.25 ± 0.01c | 0.34 ± 0.02d |
| RSA (%) | ||||
| 50 ppm | 8.21 ± 0.01a | 61.31 ± 0.02b | 62.74 ± 0.02c | 64.74 ± 0.03d |
| 100 ppm | 10.14 ± 0.01a | 63.72 ± 0.03b | 65.24 ± 0.04c | 66.41 ± 0.05d |
| 200 ppm | 12.74 ± 0.02a | 65.29 ± 0.04b | 66.78 ± 0.02c | 68.46 ± 0.01d |
| CGA (%) | – | 0.28 ± 0.05a | 0.42 ± 0.06b | 0.54 ± 0.05c |
| Caffeine (%) | – | 0.015 ± 0.01a | 0.022 ± 0.00b | 0.029 ± 0.01c |
Values are means of three replicates ± standard deviation. Values in the row with the same letter in superscript are not significantly different from each other at p ≤ 0.05. TPP total polyphenols, RSA radical scavenging activity, CGA chlorogenic acid, GCE green coffee extract
Radical scavenging activity
The RSA of GCE was found to be quite high, as discussed previously (Table 3). Control bread without GCE had 8.21% RSA at 50 ppm, 10.14% (100 ppm) and 12.74% (200 ppm). An addition of 1% GCE in bread increased RSA to 61.31% at 50 ppm, 63.72% at 100 ppm and 65.29% at 200 ppm. Addition of 1.5% GCE in bread increased RSA to 62.74% (50 ppm), 65.24% (100 ppm) and 66.78% (200 ppm); 2% GCE in bread had 64.74% (50 ppm), 66.41(100 ppm) and 68.46 (200 ppm). It can be concluded from the above that addition of GCE resulted in a significant increase in RSA there by enhancing antioxidant potential of bread containing GCE.
Bread prepared without GCE had very low RSA (10.14% at 100 ppm) whereas bread prepared with 2% GCE had 66.41% (100 ppm) RSA, indicating a significant increase in RSA in bread. Reduction of RSA in bread with GCE was observed during baking. The extent of reduction in RSA was 30–40%. However despite the loss of RSA after baking, the bread with 1.5% GCE showed significantly higher RSA (six times) when compared with the control bread without GCE.
Chlorogenic acid
CGA content of GCE was 37.28% whereas the CGA was not detected in the control bread (Table 3). The addition of increasing amount of GCE from 1 to 2% increased the CGA content from 0.28 to 0.54%. CGA is a major bioactive compound of green coffee bean which has numerous health benefits like weight reduction by inhibition of fat absorption and activation of fat metabolism in the liver (Shimoda et al. 2006). Hence incorporation of GCE in bread could impart the health beneficial effect due to the presence of CGA.
Caffeine
Caffeine content in GCE was 1.75%. Breads with 1, 1.5 and 2% GCE had 0.015, 0.022 and 0.029% caffeine respectively (Table 3). The retention of caffeine content in bread with GCE at the levels of 1, 1.5 and 2% was 86, 84 and 83% respectively. It is interesting to know that the amount of degraded caffeine was lower when the concentration of GCE in bread was lesser.
These results showed that with the addition of 1.5% GCE, TPP increased by 12 times, RSA at 100 ppm (6 times) and CGA (42 times).This was due to the presence of 21.42% GAE TPP, 92.73% RSA (100 ppm) and 37.28% CGA in GCE. The bread supplemented with 1.5% GCE was found to be having health benefits of CGA on the basis of these parameters.
Estimation of CGA isomers in GCE and bread supplemented with GCE
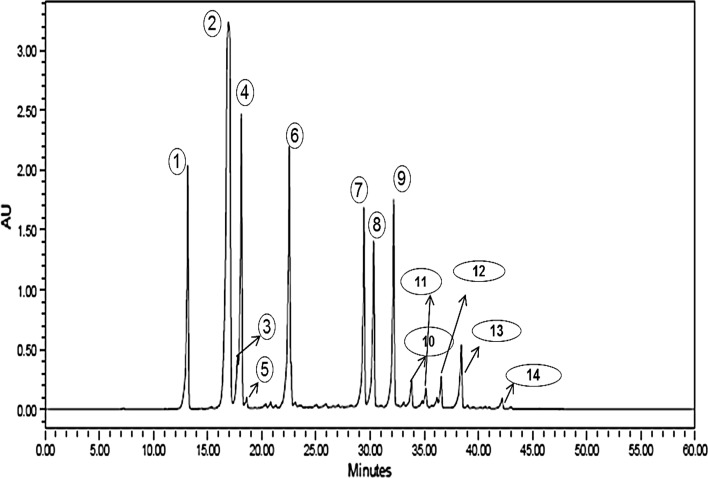
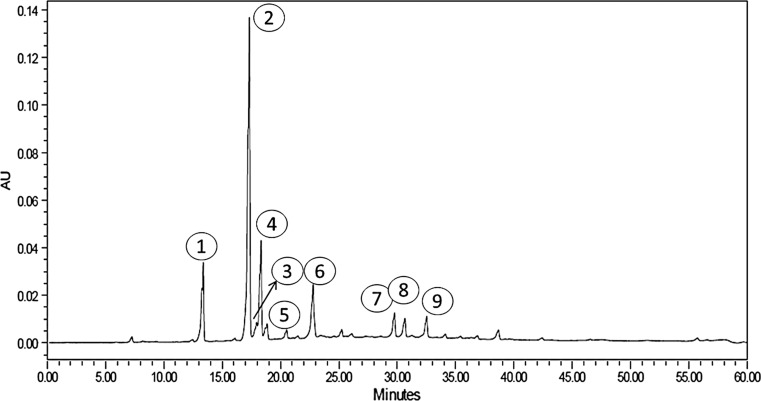
Among the different components of CGA in GCE, the highest amount of 4–5 CQA (12.74 g/100 g) was detected (Table 4; Fig. 1). The GCE used in doughnuts also showed the highest amount of 5-CQA isomer (Budryn et al. 2013). Besides 4–5 CQA, high contents of 5-FQA (4.41/100 g) were found. The contents of 5-p-CQA (3.62/100 g), 3-CQA (3.10/100 g), 4, 5-diCQA (3.01/100 g), 3, 4-diCQA (2.83/100 g) and 3,5-diCQA (2.56/100 g) were found in medium amounts; caffeoyl tryptophan (1.16/100 g), caffeic acid (0.97/100 g) were found in lower amounts; 3C,5FQA + 3F,5CQA (0.63/100 g), 4-FQA (0.60/100 g) and 3C,4FQA + 3F,4CQA (0.52/100 g) were found in lowest amounts. Two unidentifiable isomers, such as unknown1 (0.45/100 g) and unknown2 (0.20/100 g) were also found. In bread, with the addition of 1, 1.5 and 2% GCE, the highest amount of nondegraded CGA isomer (4–5 CQA) was found followed by 5-FQA; 5-p-CQA; 3,4-diCQA; 3-CQA; 3,5-diCQA; 4,5-diCQA; caffeic acid and 4-FQA. Other isomers such as 3C,4FQA + 3F,4CQA; 3C,5FQA + 3F,5CQA; Unknown1; Caffeoyl tryptophan and Unknown2 were not detected (Table 4; Fig. 2). With the increase in concentration of GCE from 1 to 2%, the retention of nondegraded CGA and degradation increased indicating that the degradation is lesser when the concentration of GCE was lower. Budryn et al. (2013) also found that the amount of non-degraded polyphenols was higher when the concentrations of extracts in doughnuts were smaller. Highest degradation was observed in bread with 2% GCE where the retention of CGA isomers was 80%. In bread with the 1.5% GCE, about 83% of CGA isomers added were retained. 3C,4FQA + 3F,4CQA, 3C,5FQA + 3F,5CQA, Unknown1, Caffeoyl tryptophan and Unknown2 were totally degraded. 3-CQA and caffeic acid were significantly changed. An addition of 1% of GCE caused a smaller degradation of CGA’s in bread; about 85% of total CGA isomers was left. Green tea cake produced by substituting 0–30% resulted in cake with good antioxidant activity, reducing power, scavenging ability on 1,1-diphenyl-2-picrylhydrazyl radicals and chelating ability on ferrous ions (Lu et al. 2010). Daily intake of 50 g or two slices of 4% turmeric bread can deliver approximately 4.6 mg of curcumin and 40.12 mg GAE of total phenolic compounds which can render additional health benefits to human body (Lim et al. 2011). Our studies indicate that daily intake of 50 g of bread with 1.5% GCE provides 210 mg of CGA, 125 mg of GAE total polyphenols with 65.24% radical scavenging activity.
Table 4.
Isomers of CGAa in GCE and bread supplemented with GCE
| Peak no | Compound name | RT | GCE (g/100 g) | Bread | ||
|---|---|---|---|---|---|---|
| GCE (mg/100 g) | ||||||
| 1% | 1.50% | 2% | ||||
| 1 | 3-CQA | 13.17 | 3.10 | 21.83 | 32.32 | 38.96 |
| 2 | 4–5 CQA | 16.93 | 12.74 | 121.26 | 176.43 | 229.55 |
| 3 | Caffeic acid | 18.11 | 0.97 | 5.55 | 7.93 | 9.95 |
| 4 | 5-p CoQA | 22.54 | 3.62 | 28.38 | 41.31 | 54.17 |
| 5 | 4-FQA | 22.78 | 0.60 | 4.58 | 6.78 | 8.69 |
| 6 | 5-FQA | 25.24 | 4.41 | 38.97 | 56.29 | 74.72 |
| 7 | 3,4-diCQA | 29.44 | 2.83 | 24.27 | 34.19 | 43.86 |
| 8 | 3,5-diCQA | 30.34 | 2.56 | 21.87 | 32.03 | 41.76 |
| 9 | 4,5-diCQA | 32.17 | 3.01 | 22.37 | 31.78 | 40.59 |
| 10 | 3C,4FQA + 3F,4CQA | 33.83 | 0.52 | nd | nd | nd |
| 11 | 3C,5FQA + 3F,5CQA | 35.15 | 0.63 | nd | nd | nd |
| 12 | Unknown1 | 36.57 | 0.45 | nd | nd | nd |
| 13 | Caffeoyl tryptophan | 38.42 | 1.16 | nd | nd | nd |
| 14 | Unknown2 | 42.18 | 0.20 | nd | nd | nd |
aValues are means of three replicates ± standard deviation. RT retention time, CGA chlorogenic acid, GCE green coffee extract, nd not detected. 3-CQA 3-O-caffeoylquinic acid, 4-5 CQA 4-O-caffeoylquinic acid and 5-O-caffeoylquinic acid; 5-pCoQA 5-O-p-coumaroylquinic acid, 4-FQA 4-O-feruloylquinic acid, 5-FQA 5-O-feruloylquinic acid, 3,4-diCQA 3,4-di-O-caffeoylquinic acid, 3,5-diCQA 3,5-di-O-caffeoylquinic acid, 4,5-diCQA 4,5-di-O-caffeoylquinic acid; 3C,4FQA + 3F,4CQA 3-O-caffeoyl-4-O-feruloyl quinic acid + 3-O-feruloyl-4-O-caffeoylquinic acid, 3C,5FQA + 3F,5CQA 3-O-caffeoyl-5-O-feruloylquinic acid + 3-O-feruloyl-5-O-caffeoylquinic acid
Fig. 1.
HPLC Chromatogram of isomers of chlorogenic acid (CGA) in green coffee extract (GCE). Numbers are explained in Table 4
Fig. 2.
HPLC chromatogram of isomers of chlorogenic acid (CGA) in bread supplemented with 1.5% green coffee extract (GCE). Numbers are explained in Table 4
Conclusion
Green coffee extract, rich in bioactive compounds with well-known health-beneficial properties was used for developing bioactive rich bread. The addition of GCE did not alter the rheological characteristics of wheat flour dough up to 1.5% level. Incorporation of 1.5% GCE significantly increased the TPP, CGA, RSA contents and therefore, antioxidant activity of the bread. Bread with 1.5% GCE was acceptable with mild coffee taste and had the highest amount of 4–5 CQA of CGA isomers. Hence, bread formulated with GCE showing effective antioxidant properties can be used to avail the health benefit.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- AACC . Approved methods of the AACC. 10. St. Paul: American Association for Cereal Chemists; 2000. [Google Scholar]
- Abdille MH, Singh RP, Jayaprakasha GK, Jena BS. Antioxidant activity of the extracts from Dillenia indica fruits. J Food Chem. 2005;90(4):891–896. doi: 10.1016/j.foodchem.2004.09.002. [DOI] [Google Scholar]
- Alvarez-Jubete L, Wijngaard H, Arendt EK, Gallagher E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buck wheat and wheat as affected by sprouting and baking. J Food Chem. 2010;119(2):770–778. doi: 10.1016/j.foodchem.2009.07.032. [DOI] [Google Scholar]
- AOAC . Official methods of analysis of AOAC International. Gaithersburg: Association of Official Analytical Chemists; 2000. [Google Scholar]
- Balestra F, Cocci E, Pinnavaia GG, Romani S. Evaluation of antioxidant, rheological and sensorial properties of wheat flour dough and bread containing ginger powder. J Food Sci Technol. 2011;44(3):700–705. [Google Scholar]
- Balyaya KJ, Clifford MN. Individual chlorogenic acids and caffeine contents in commercial grades of wet and dry processed Indian green robusta coffee. J Food Sci Technol. 1995;32:104–108. [Google Scholar]
- Bicchi CP, Binello AE, Pellegrino GM, Vanni AC. Characterization of green and roasted coffees through the chlorogenic acid fraction by HPLC-UV and principal component analysis. J Agric Food Chem. 1995;43(6):1549–1555. doi: 10.1021/jf00054a025. [DOI] [Google Scholar]
- Blois MS. Antioxidants determination by the use of a stable free radical. Nature. 1958;4617:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Blum J, Lemaire B, Lafay S. Effect of a green decaffeinated coffee extract on glycaemia—a pilot prospective clinical study. Nutra Food Res. 2007;6(13):7. [Google Scholar]
- Budryn G, Zyzelewicz D, Nebesny E, Oracz J, Krysiak W. Influence of addition of green tea and green coffee extracts on the properties of fine yeast pastry fried products. Food Res Int. 2013;50(1):149–160. doi: 10.1016/j.foodres.2012.10.006. [DOI] [Google Scholar]
- Cheynier V. Polyphenols in foods are more complex than often thought. Am J Clin Nutr. 2005;81(1):223S–229S. doi: 10.1093/ajcn/81.1.223S. [DOI] [PubMed] [Google Scholar]
- Chu YF. Coffee: emerging health effects and disease prevention. New York: Wiley; 2012. [Google Scholar]
- Clifford MN, Johnston KL, Knigh S, Kuhnert N. Hierarchical scheme for LC–MS n identification of chlorogenic acids. J Agric Food Chem. 2003;51(10):2900–2911. doi: 10.1021/jf026187q. [DOI] [PubMed] [Google Scholar]
- Dellalibera O, Lemaire B, Lafay S. Svetol®, green coffee extract, induces weight loss and increases the lean to fat mass ratio in volunteers with overweight problem. Phytotherapie. 2006;4(4):194–197. doi: 10.1007/s10298-006-0181-7. [DOI] [Google Scholar]
- Dziki D, Gawlik-Dziki U, Pecio L, Rozylo R, Swieca M, Krzykowski A, Rudy S. Ground green coffee beans as a functional food supplement—preliminary study. J Food Sci Technol. 2015;63(1):691–699. [Google Scholar]
- Farah A, de Paulis T, Trugo LC, Martin PR. Effect of roasting on the formation of chlorogenic acid lactones in coffee. J Agric Food Chem. 2005;53(5):1505–1513. doi: 10.1021/jf048701t. [DOI] [PubMed] [Google Scholar]
- Farah A, Monteiro M, Donangelo CM, Lafay S. Chlorogenic acids from green coffee extract are highly bioavailable in humans. J Nutr. 2008;138(12):2309–2315. doi: 10.3945/jn.108.095554. [DOI] [PubMed] [Google Scholar]
- Fischer M, Reimann S, Trovato V, Redgwell RJ. Polysaccharides of green arabica and robusta coffee beans. J Carbohydr Res. 2001;330(1):93–101. doi: 10.1016/S0008-6215(00)00272-X. [DOI] [PubMed] [Google Scholar]
- Glei M, Kirmse A, Habermann N, Persin C, Pool-Zobel BL. Bread enriched with green coffee extract has chemoprotective and antigenotoxic activities in human cells. J Nutr Cancer. 2006;56(2):182–192. doi: 10.1207/s15327914nc5602_9. [DOI] [PubMed] [Google Scholar]
- Goh R, Gao J, Ananingsih VK, Ranawana V, Henry CJ, Zhou W. Green tea catechins reduced the glycaemic potential of bread: an in vitro digestibility study. J Food Chem. 2015;180:203–210. doi: 10.1016/j.foodchem.2015.02.054. [DOI] [PubMed] [Google Scholar]
- Kozuma K, Tsuchiya S, Kohori J, Hase T, Tokimitsu I. Antihypertensive effect of green coffee bean extract on mildly hypertensive subjects. J Hypertens Res. 2005;28(9):711–718. doi: 10.1291/hypres.28.711. [DOI] [PubMed] [Google Scholar]
- Lim HS, Park SH, Ghafoor K, Hwang SY, Park J. Quality and antioxidant properties of bread containing turmeric (Curcuma longa L.) cultivated in South Korea. J Food Chem. 2011;124(4):1577–1582. doi: 10.1016/j.foodchem.2010.08.016. [DOI] [Google Scholar]
- Lu TM, Lee CC, Mau JL, Lin SD. Quality and antioxidant property of green tea sponge cake. J Food Chem. 2010;119(3):1090–1095. doi: 10.1016/j.foodchem.2009.08.015. [DOI] [Google Scholar]
- Naidu MM, Sulochanamma G, Sampathu SR, Srinivas P. Studies on extraction and antioxidant potential of green coffee. J Food Chem. 2008;107(1):377–384. doi: 10.1016/j.foodchem.2007.08.056. [DOI] [Google Scholar]
- Ochiai R, Jokura H, Suzuki A, Tokimitsu I, Ohishi M, Komai N, Rakugi H, Ogihara T. Green coffee bean extract improves human vasoreactivity. J Hypertens Res. 2004;27(10):731–737. doi: 10.1291/hypres.27.731. [DOI] [PubMed] [Google Scholar]
- Raba DN, Moigradean D, Poiana MA, Popa M, Jianu I. Antioxidant capacity and polyphenols content for garlic and basil flavored bread. J Agroalim Proc Technol. 2007;13(1):163–168. [Google Scholar]
- Shewry PR. The health grain programme opens new opportunities for improving wheat for nutrition and health. J Nutr Bull. 2009;34(2):225–231. doi: 10.1111/j.1467-3010.2009.01747.x. [DOI] [Google Scholar]
- Shimoda H, Seki E, Aitani M. Inhibitory effect of green coffee bean extract on fat accumulation and body weight gain in mice. J BMC Compl Altern Med. 2006;6(1):9. doi: 10.1186/1472-6882-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivam AS, Sun-Waterhouse D, Perera CO, Waterhouse GIN. Exploring the interactions between black currant polyphenols, pectin and wheat biopolymers in model breads; a FTIR and HPLC investigation. J Food Chem. 2012;131(3):802–810. doi: 10.1016/j.foodchem.2011.09.047. [DOI] [Google Scholar]
- Stevenson L, Phillips F, Osullivan K, Walton J. Wheat bran: its composition and benefits to health, a European perspective. Int J Food Sci Nutr. 2012;63(8):1001–1013. doi: 10.3109/09637486.2012.687366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Kagawa D, Ochiai R, Tokimitsu I, Saito I. Green coffee bean extract and its metabolites have a hypotensive effect in spontaneously hypertensive rats. J Hypertens Res. 2002;25(1):99–107. doi: 10.1291/hypres.25.99. [DOI] [PubMed] [Google Scholar]
- Swain T, Hillis WE. The phenolic constituents of Prunus domestica. I. The quantitative analysis of phenolic constituents. J Sci Food Agr. 1959;10(1):63–68. doi: 10.1002/jsfa.2740100110. [DOI] [Google Scholar]
- Upadhyay R, Mohan Rao LJ. An outlook on chlorogenic acids—occurrence, chemistry, technology, and biological activities. J Crit Rev Food Sci Nutr. 2013;53(9):968–984. doi: 10.1080/10408398.2011.576319. [DOI] [PubMed] [Google Scholar]
- van der Werf HM, Garnett T, Corson MS, Hayashi K, Huisingh D, Cederberg C. Towards eco-efficient agriculture and food systems: theory, praxis and future challenges. J Clean Prod. 2014;73:1–9. doi: 10.1016/j.jclepro.2014.04.017. [DOI] [Google Scholar]
- Varnam AH, Shutherland JP. Beverages: technology, chemistry and microbiology. Berlin: Springer Science and Business Media; 1994. pp. 191–255. [Google Scholar]
- Wei F, Furihata K, Koda M, Hu F, Kato R, Miyakawa T, Tanokura M. 13C NMR based metabolomics for the classification of green coffee beans according to variety and origin. J Agric Food Chem. 2012;60(40):10118–10125. doi: 10.1021/jf3033057. [DOI] [PubMed] [Google Scholar]