Abstract
Thymbra capitata and Thymus species are commonly known as thyme in Spain and they are currently used as culinary herbs, as well as for ornamental, aromatizing and traditional medicinal purposes. Given the economic importance of thyme oils, many thyme species have been studied, and their essential oils (EOs) and other volatile-containing extracts have been chemically characterized. The aim of this study was to evaluate the antioxidant, antimicrobial and cytotoxic activities as well as the phytochemical composition of the EOs from fruits and flowers of T. capitata and Thymus species. High antioxidant capacity has been observed and related to the relative amounts of terpenoid and phenolic with good antioxidant properties. The antioxidant activities decreased as follows: TAC > TZ > TC > TM. The results of antimicrobial activity showed that all tested EOs were active against all tested microbial species, including Gram-positive and negative bacteria. The minimum inhibition concentration values obtained ranged from 0.1 to 0.01 μL/mL. Furthermore, all EOs assayed induced cell death in both human epitheloid cervix carcinoma and histiocytic leukemia cell lines. Overall, the results of this study indicates that the EOs from fruits and flowers of T. capitata and Thymus species posses interesting antioxidant properties and represent a potential source of medicine for the treatment of infectious diseases and cancer.
Keywords: Essential oils, Antioxidant, Antimicrobial, Cytotoxicity, Thymus, Thymbra
Introduction
Bioactive substances derived from foods and plants have recently attracted much attention with regard to human health, which is due to their low toxicity, limited costs, and broad availability. Moreover, extraction is one of the most widely used unit operations in the food industry. It is mainly used for obtaining certain desired bioactive components initially retained in a food matrix (Pinelo et al. 2005). In this context, essential oils (EOs) obtained from plants contain a large and complex variety of compounds, mostly hydrocarbons and oxygenated-types, with potential antioxidant, anticancer and antimicrobial properties that may be utilized in pharmaceutical, food, cosmetic, and other industries (Miguel 2010b). EOs are generally derived from one or more plant parts, such as flowers, leaves, stems, bark, wood, roots, seeds, fruits, rhizomes and gums or oleoresin exudations.
Thyme is the common name of many taxa belonging to the Thymbra and Thymus genera. Thymbra and Thymus species are frequent in the west Mediterranean region, which is considered to be the centre of origin of the genus Thymus. These species have extended further westwards from the Iberian Peninsula and northwest Africa to the Macaronesian region in the Atlantic Ocean (Figueiredo et al. 2010).
EOs can be defined as volatile products of complex nature, produced by certain vegetables to confer their pleasant characteristic aroma. Officially, EOs are known products that can be obtained by dragging current of steam or by expression of pericarps in certain fruits. Usually, they are known as essences, although this denomination is broader because it encompasses other substances obtained by diverse extraction methods (Bruneton 2001). Chemical compounds of EOs depend on several factors. Firstly, the botanic origin, because every species has different chemical compounds, and even in the same species it could be found several chemical races (chemotypes). Quantitative and qualitative characteristics of species vary according to vegetative cycle. Lastly, it is important to note that extraction can alter EO composition with respect to vegetal origin (Bruneton 2001).
EOs are used in a wide variety of consumer goods such as detergents, soaps, toilet products, cosmetics, pharmaceuticals, perfumes, confectionery food products, soft drinks, distilled alcoholic beverages (hard drinks) and insecticides. World production and consumption of EOs and perfumes are increasing very fast. Production technology is an essential element to improve the overall yield and quality of EO. The traditional technologies pertaining to EO processing are of great significance and are still being used in many parts of the globe. Water distillation, water and steam distillation, cohobation and maceration are the most traditional and commonly used methods. Thus, hydrodistillation is one of the less drastic processes for EOs obtaining. It could induce esters hydrolysis and reunification, isomerizations, razemisations, or oxidations, which are caused by water, acids or temperature of this process. Furthermore, the state of extraction of prime material could influence composition, highlighting the importance of storage and preparation of vegetal material.
Based on the properties of EO, different uses have been developed in several fields. Food industry has applied EOs in preservation of products due to their antiseptic properties (butter, fruits, meat, etc.) or as dressing (liquors, olives, meat, fish, and natural antioxidant for olive oil). Cosmetic industry also includes the use of these products in perfumes and creams. Knowledge acquired along the time on EO has been applied for seeking pharmacological utilities. Among them, it is important to underline its antiseptic, burnt-healing, spasmolytic, anti-inflammatory and antimutagenic activities. For that reason, the aim of this work has been to characterize the chemical composition, antioxidant activity, cytotoxic activity and antimicrobial activity of four species of Thymbra and Thymus spp.
Materials and methods
Biological material
Samplings of fruits and flowers of various species of Thymus and Thymbra (TC: Thymus caespititius; TAC: Thymbra capitata; TM: Thymus mastichina; TZ: Thymus zygis) were picked up from Badajoz´s province during 2012 season. The UTM coordinates of the central area were x = 702,192.29, y = 4,303,406.71. The climate of the area is Mediterranean; the average annual rainfall was 404 mm, mostly distributed outside of a 4-months summer drought period. The samplings were carried out in the morning, taking samples randomly, in different parts of the plants with three repetitions. After harvesting, all samples were immediately transported to the Technological Institute of Food and Agriculture of Extremadura (INTAEX) in ventilated storage trays to avoid compositional changes. The plant materials were dried in a dark room and, after that, they were stored in paper bags until study. Herbarium sheets of each studied populations were also stored. Dried fruits and flowers were ground in a knife mill. Then, powdered samples were sieved to select particles between 0.50 and 3.0 mm, and they were stored at room temperature in vacuum conditions until further use.
Bacterial cultures were obtained from the Spanish type culture collection (CECT) of Valencia University. The following bacterial strains were used in the screening of the antimicrobial spectrum of the essential oil: Listeria innocua 910, Staphylococcus aureus subsp. aureus 976, Salmonella enterica subsp. enteric 409, Escherichia coli 45 and Bacillus cereus 495.
Human epitheloid cervix carcinoma (HeLa) cell line (ECACC No. 93021013; Dorset, UK) was grown in Dulbecco’s Modified Eagle medium (DMEM; Lonza, Basel, Switzerland) supplemented with 2 mM l-glutamine (Lonza), 1 mM sodium pyruvate (Lonza), 10% heat-inactivated fetal bovine serum (Lonza), 100 U/mL penicillin, and 10 μg/mL streptomycin. Human histiocytic leukemia (U937) cell line (ECACC No. 85011440) was grown in RPMI 1640 medium (Lonza) supplemented with 2 mM l-glutamine, 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin and 100 µg/mL streptomycin. Cells were cultured in a humidified atmosphere containing 5% CO2 at 37 °C.
Essential oils extraction
The EOs were produced separately from fruits and flowers by distillation according to the European Pharmacopoeia (Council of Europe. 1997). In all of them, water is heated to produce steam, which carries the most volatile chemicals of the aromatic material with it. The steam is then chilled (in a condenser) and the resulting distillate is collected. The EOs were floated on top of the hydrosol (the distilled water component) and were separated off. The oils were stored away from the light in amber-coloured glass bottles at 4 °C until analysis (within 1 month).
Chemicals
For antioxidant activity determination, the standard antioxidant used was 6-hidroxy-2,5,7,8-tetramethyl-chroman-2-carboxylic acid (Trolox) from Sigma–Aldrich (Steinheim, Germany) and 2,2′azobis-(3-ethylbenzothiazoline-6-sulfonic acid; ABTS) was from Fluka Chemicals (Madrid, Spain).
Chemical composition determination
The analysis of the volatile components of the EOs of T. capitata and Thymus species were performed with a Hewlett Packard 6890 gas chromatograph equipped with a flame ionization detector (FID) and a 30 m × 0.32 mm × 0.25 µm (film thickness) of 5% phenyl-methyl-silicone column. The injection volume was 3 µL of essential oil, in split mode (12:9) and the injector temperature was kept at 250 °C. The oven temperature was programmed at 3 °C/min from 70 to 240 °C and then held it for 2 min. Carrier gas flow is nitrogen at 0.4 mL/min with 2.5 psi of pressure. The identification of individual constituents was accomplished by comparing their retention indices with those of authentic standards.
Antioxidant activity assay
For the sample preparation, an aliquot of filtered EOs were weigh (2.5 g) and then, was dissolved in 6 mL of n-hexane. A diol-bonded phase cartridge (Supelco Co., Bellefonte, PA, US) was placed in a vacuum elution apparatus and conditioned by the consecutive addition of 6 mL of methanol and 6 mL of n-hexane. The oil solution was applied to the column, and the solvent was pulled through, leaving the sample on the solid phase. The sample container was washed with 6 mL of n-hexane (2 × 3 mL), which were run out of the cartridge. The sample container was washed again with 4 mL of n-hexane/ethyl acetate (85:15, v/v), which were run out of the cartridge and discarded. The column was eluted with 15 mL of methanol and the solvent was evaporated in a rotary evaporator at room temperature and low speed at reduced pressure until dryness. The phenolic residue was dissolved in 2 mL of methanol (Mateos et al. 2001).
The capacity of radical scavenging of the oil samples were assessed by the ABTS·+ method (Cano et al. 2000). Briefly, 1 mL of the radical cation ABTS (2.2′-azinobis (3-ethylbenzoithiazolone 6-sulphonate)) was placed in a spectrometric cuvette and 20 μL of the phenolic extract were added. The initial absorbance value at 730 nm was measured by using an UV–Vis spectrophotometer model HP8453 (Agilent, Madrid, Spain) and then compared with the absorbance obtained after 20 min of reaction. The results were expressed as a TAC (antioxidant equivalent Trolox) value as g Trolox/L essential oil on a calibration curve using different Trolox concentration.
Antimicrobial activity assay
The antimicrobial activity values were studied for the EOs following the procedure previously established by Delgado-Adámez et al. with some minor modifications (Delgado Adámez et al. 2012). The EOs were first dissolved in 10% DMSO and then diluted to the highest concentration (0.1% v/v) to be tested, and were serially diluted from 0.1 to 0.001% (v/v). The target microorganisms were cultured in Mueller–Hinton broth (MHB) at 37 °C for 24 h. To measure the CFU, 180 µL of MHB containing ~105 CFU/mL diluted bacteria (1/1000 ratio in McFarland standard turbidity) plus 20 µL of EO solutions were added per well in a 96-well plate. Then, the microplates were incubated at 37 °C for 24 h under aerophilic conditions. Absorbances at 450 nm were measured at 0 and 24 h in a plate reader. Turbidity readings were related to bacterial growth. The inhibitory effect was calculated using the following formula:
where:
ΔAbsReference is the increase in absorbance of control sample.
ΔAbsAssay is the increase in absorbance of test sample.
Cytotoxicity assay
The cytotoxic effects of the different EOs on two human tumor cell lines were assayed by the MTT assay (Mosmann 1983). The cells were seeded at a density of 5 × 104 cells/well. The EOs were serially diluted in 1% dimethyl sulfoxide (DMSO) from 0.1 to 0.001% (v/v), and 5 μL liquid of each concentration was applied to the wells of a 24-well plate containing sub-confluent cell cultures. DMSO was used as a vehicle and the final DMSO concentration did not exceeded 0.1% (v/v). After 24 h of incubation, MTT solution (5 mg/mL) was then added to each well, and the formazan precipitate was dissolved in 200 μL DMSO after 1 h of incubation at 37 °C. The content of the wells was homogenized on a microplate shaker for 5 min. The optical densities (OD) were measured on a microplate reader (Infinite M200; Tecan Austria GmbH, Groedig, Austria) at a test wavelength of 490 nm and a reference wavelength of 650 nm to cancel out the effect of cell debris. All tests and analyses were run in duplicate and mean values were recorded. The cell survival curves were calculated as percentage of control values (untreated samples).
Statistical analysis
For statistical studies, SPSS 17.0 software was used (SPSS Inc. Chicago, IL, USA). Data are expressed as mean ± standard error of the mean (S.E.M.) and were analyzed using a one-way analysis of variance (ANOVA) followed by Tukey’s multiple range test. To evaluate the effects of phenological stage on chemical composition and anticancer, antioxidant and antimicrobial activities of the essential oils, paired student t-test was used. P < 0.05 was considered to indicate a statistically significant difference.
Results and discussion
Evaluation of the chemical composition of the EOs
Determination of compounds was higher than 70% in all the samples, reaching determination rates higher than 98% for T. capitata, 96% for T. mastichina and 91% for T. zygis (Table 1).
Table 1.
Chemical composition of essential oils from Thymbra capitata and Thymus species at different phenological stages
| Thymbra capitata | Thymus caespititius | Thymus mastichina | Thymus zygis | |||||
|---|---|---|---|---|---|---|---|---|
| Flower | Fruit | Flower | Fruit | Flower | Fruit | Flower | Fruit | |
| α-pinene | 0.82 ± 0.152 | 0.99 ± 0.11b | 2.25 ± 0.181 | 2.32 ± 0.25a | 0.27 ± 0.083 | 0.27 ± 0.07c | 0.45 ± 0.133 | 0.43 ± 0.06c |
| Camphene | 0.87 ± 0.113 | 1.00 ± 0.07bc | 0.94 ± 0.113 | 0.78 ± 0.10c | 5.15 ± 0.281A | 3.39 ± 0.19aB | 1.38 ± 0.102 | 1.18 ± 0.14c |
| β-myrcene | 1.61 ± 0.373 | 1.87 ± 0.12b | 3.17 ± 0.242A | 2.25 ± 0.09bB | 9.81 ± 0.701A | 5.69 ± 0.33aB | 2.87 ± 0.082A | 1.36 ± 0.13cB |
| Limonene + 1-8cineole | nd3 | ndc | 1.33 ± 0.142 | 0.88 ± 0.25b | 71.82 ± 1.221 | 78.37 ± 1.26a | 1.50 ± 0.242A | 0.90 ± 0.10bB |
| α-terpinene | 1.53 ± 0.101A | 1.09 ± 0.10aB | nd2 | ndb | nd2 | ndb | nd2 | ndb |
| p-cymene | 7.29 ± 0.313B | 9.10 ± 0.05bA | 10.66 ± 0.522 | 10.02 ± 1.82b | nd4 | ndc | 22.56 ± 1.351B | 29.68 ± 1.10aA |
| γ-terpinene | 4.83 ± 0.253A | 4.14 ± 0.31abB | 6.15 ± 0.522A | 3.54 ± 0.21bB | 0.53 ± 0.044B | 0.66 ± 0.04cA | 11.31 ± 0.641A | 4.53 ± 0.35aB |
| Linalool | 3.38 ± 0.471 | 3.25 ± 0.17a | 0.74 ± 0.062 | 0.91 ± 0.22b | 3.37 ± 0.211A | 1.30 ± 0.31bB | 1.50 ± 0.402 | 1.37 ± 0.14b |
| Terpinen-4-ol | 0.39 ± 0.203 | 0.21 ± 0.08d | 1.34 ± 0.122 | 1.39 ± 0.06b | 2.34 ± 0.261 | 2.15 ± 0.18a | 0.91 ± 0.1223 | 0.88 ± 0.12c |
| α-terpineol | 0.88 ± 0.253 | 0.82 ± 0.09c | 42.44 ± 1.551B | 53.20 ± 0.84aA | 5.32 ± 0.562 | 5.05 ± 0.16b | tr4 | trd |
| Carvacrol | 75.51 ± 1.581 | 74.27 ± 2.00a | 0.32 ± 0.103 | 0.63 ± 0.22b | nd4 | ndc | 4.29 ± 0.202A | 1.08 ± 0.14bB |
| β-caryophyllene oxide | 1.29 ± 0.151 | 1.36 ± 0.10a | nd2 | ndb | nd2 | ndb | nd2 | ndb |
| Thymol | nd3 | ndc | 0.82 ± 0.122 | 0.92 ± 0.09b | nd3 | ndc | 45.09 ± 3.921B | 52.70 ± 2.32aB |
| % total | 98.41 | 98.09 | 70.16 | 76.84 | 98.59 | 96.89 | 91.87 | 94.11 |
Values were expressed as mean ± SEM. nd Not detected. tr. trace (<0.01). Within each column, values followed by the same capital letter in a column did not share significant differences at 5% (Student’s t-test). Within each row, values followed by the same lowercase letter or number did not share significant differences at 5% (Tukey’s test)
The main compounds vary depending mainly on species, being the most abundant molecules: carvacrol in the case of T. capitata (with values around 75%); α-terpineol for T. caespititus (with values of 42.44 ± 1.55% and 53.20 ± 0.84% for Eos from flower and fruit respectively); limonene + 1-8 cineole for T. mastichina (reaching 78.37 ± 1.26%); and thymol and p-cymene for T. zygis (Table 1).
In general, chemical composition of EOs depends on the species, however, some intergenus differences may be suggested based on the fact that β-caryophyllene oxide, α–pinene, limonene + 1-8 cineole and α-terpineol percentage determined in T. capitata were statistically different to those in the rest of samples (all belonging to Thymus Genus). Whereas the differences due to phenological stage also vary depending on species, γ-terpinene determination varies in all the species between flower and fruit, being the influence of phenological stage specially marked in T. zygis.
Carvacrol can be denominated as a functionalized terpene, whose function is phenolic. Due to observed properties in EOs and its structure, carvacrol has been tested in several experiments, confirming its antimicrobial (Klein et al. 2013), antifungal (Abbaszadeh et al. 2014), and antioxidant activities (Kumar and Rawat 2013). In some studies carried out in plants from Iberian Peninsula α-terpinene has been determined as the main compound for T. caespititus (Miguel et al. 2004). Moreover, 1,8-cineole, also known as eucalyptol, is characterized as an oxygenated monoterpene with ether function. A long list of properties has been recognized for this compound. Among them, antimicrobial (de Sousa et al. 2015) and antifungal capacities (Vilela et al. 2009) have been identified. It has been also tested as insecticide (Kumar et al. 2013), and possesses anti-inflammatory capacity (Ehrnhöfer-Ressler et al. 2013). Despite the fact that elevated values of thymol are uncommon in literature, Gonçalves and co-workers found high values of thymol also in T. zygis, mainly the chemotypes thymol, linalool/1,8-cineole and carvacrol (Gonçalves et al. 2010). Thymol has antimicrobial, antifungal, antioxidant, and anti-inflammatory capacities, and also can activate or inactivate proteins. Regarding the stage of the vegetative cycle, Miguel and colleagues reported that there are not main differences in the chemical composition between May and December (Miguel et al. 1999).
Evaluation of the antioxidant activity of the EOs
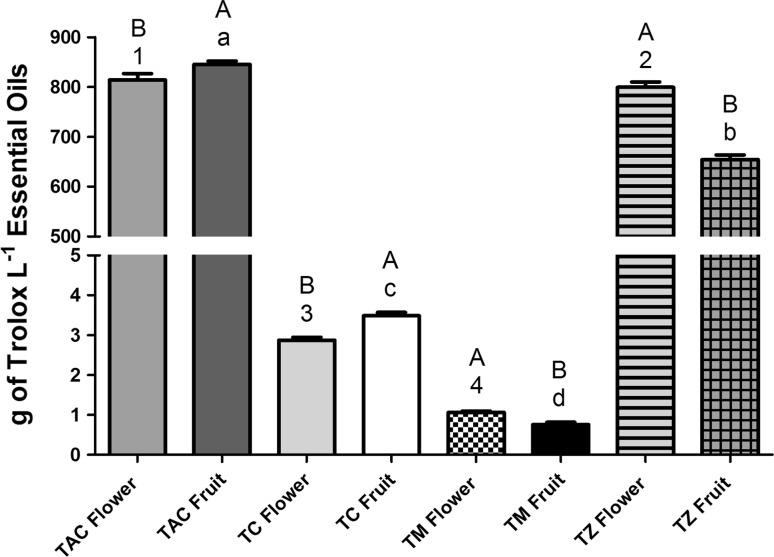
Antioxidant activity of the EOs from different parts of T. capitata and T. species is summarized in Fig. 1. The EOs exhibited varying degrees of scavenging ability, regardless of the phenological stage of the plant. The free radical scavenging activity of EO ranged from 0.75 ± 0.06 to 845.53 ± 6.77 g Trolox/L EO (Fig. 1). It was found that two out of four species (T. zygis and T. capitata) contained more than 650 g Trolox/L EO, while the other two species (T. caespititius and T. mastichina) were below 4 g Trolox/L EO. Among the eight EOs examined in this study, T. capitata fruit had the highest reducing power, while T. mastichina fruit had the lowest. Overall, the specific chemical composition of the EOs is presumably responsible for the variations in their antioxidant properties. Since there are a large number of different types of antioxidant compounds that might contribute to the total antioxidant capacity, it is not clear which components are responsible for the observed antioxidant capacity. The structure–activity relationships of compounds present in these species require further investigation. Thus, the terpenoid and phenolic compounds present in the EOs are closely associated with their antioxidant function, mainly due to their redox properties. Such properties may be exerted by various possible mechanisms: free radical-scavenging activity, hydrogen donors, transition metal chelating activity, and/or singlet oxygen quenching capacity (Miguel 2010b). However, the underlying action mechanism is still not clearly understood. On one hand, Amensour and colleagues have suggested that the antioxidant activity of EOs is mainly due to their redox properties, which play an important role in adsorbing and neutralizing free radicals, quenching singlet and triplet oxygen, or decomposing peroxides (Amensour et al. 2009). On the other hand, some works have also shown that the main components have less activity than the EO and suggested that the constituents of EO may act synergistically to scavenge free radicals (Amorati et al. 2013).
Fig. 1.
Total antioxidant capacity determined by ABTS·+ method of the essential oils from different Thymbra capitata and Thymus species parts according to organs and phenological stages. TC Thymus caespititius, TAC Thymbra capitata, TM Thymus mastichina, TZ Thymus zygis. Values expressed are mean ± SEM of six experiments. Values with the same lowercase letter or number are not significantly different (ANOVA, P < 0.05); values with the same capital letter are not significantly different (Student’s t-test, P < 0.05)
The major components identified in these eight EOs (Table 1) have reported antioxidant properties. Carvacrol and thymol were the main compounds found in EOs from T. capitata and T. zygis, respectively. Different studies evaluated the antioxidant activity of the volatile components present in the EOs assayed in the present study (Dorman et al. 2000). Thymol (1702.04 mg Trolox/100 mL) and carvacrol (1451.74 mg Trolox/100 mL) showed the highest antioxidant activity. The inhibition of oxidation by the EOs from plants of species was highly dependent on the content of carvacrol + thymol (Quiroga et al. 2015). In contrast to these two compounds, linalool (357.67 mg Trolox/100 mL), terpinen-4-ol (190.72 mg Trolox/100 mL) and sabinene (369.44 mg Trolox/100 mL), the main compounds in EOs from T. mastichina and T. caespititius, are compounds that exhibit lower antioxidant capacity compared to carvacrol and thymol (Dorman et al. 2000; Ruberto and Baratta 2000). Limonene, the major component of T. mastichina EOs, is a monocyclic terpene consumed by humans, e.g., in citrus fruit, carrots, coffee, orange, and nutmeg. Despite the fact that it is an isomer of terpinolene, α- and γ-terpinene (position of a double-bond), limonene curiously showed a moderate-to-low antioxidant activity in Ruberto’s study (Ruberto and Baratta 2000; Fig. 1). By comparison with the structure of germacrene-d, it appears that conjugation of the extracyclic methylene could be required for efficient radical scavenging; however, terpinolene and γ-terpinene are effective antioxidants despite non-conjugated double-bonds. Antioxidant properties have indeed been shown for limonene (Limem-Ben Amor et al. 2008). EOs of T. caespititius with α-terpineol as the major compound also showed radical-scavenging activity (Miguel 2010a).
The present results are in accordance with those of other authors who demonstrated that the antioxidant effect of aromatic plants is due to the presence of hydroxyl groups in their phenolic compounds, and that the number and pattern of hydroxyl groups are associated with the highest antioxidant activity (Heim et al. 2002).
Evaluation of the antimicrobial activity of the EOs
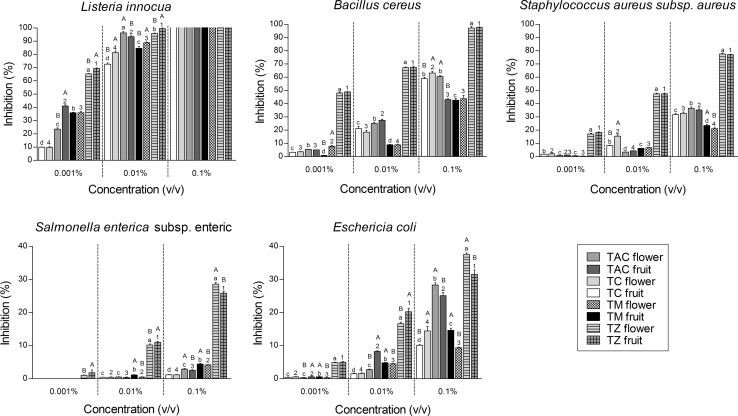
The antibacterial activity of EOs was assayed in vitro by a micro-dilution method against both Gram-positive and Gram-negative bacteria in order to determine the MIC, which is the lowest concentration inhibiting the growth of at least 90% of microbial strain (Fig. 2). All EOs analyzed showed antimicrobial activity against all bacteria. T. zygis EO showed the strongest antibacterial activity, while T. mastichina exhibited the lowest antibacterial potential. The oils have low activity on the growth of S. enterica subsp. enterica, which was only inhibited at high concentration, whereas the strongest inhibitory effect was achieved against L. innocua. The results were in concordance with the ones already published that showed that T. capitata and Thymus species has antibacterial activity against both Gram-negative and Gram-positive bacteria (Hosseini Behbahani et al. 2013).
Fig. 2.
Minimal inhibitory concentration (MIC) of Thymbra capitata and Thymus species essential oils at flowering and fruit maturation stage. TC Thymus caespititius, TAC Thymbra capitata, TM Thymus mastichina, TZ Thymus zygis. Values expressed are mean ± SEM of six experiments. Values with the same lowercase letter or number are not significantly different (ANOVA, P < 0.05); values with the same capital letter are not significantly different (Student’s t-test, P < 0.05)
Figure 2 presents the variation of the inhibition capacity with the concentration of EO in the medium. It can be seen that the inhibition percentage increases with EO concentration. For L. innocua, S. aureus subsp. aureus, B. cereus and E. coli the inhibitory effect of thyme oils were concentration-dependent. Statistically significant differences (P < 0.05) existed between all three doses used. In the case of S. enterica, differences were not significantly different (P > 0.05) between 0.01 and 0.001 μL/mL, but were substantially distinct (P < 0.05) between them and 0.1 μL/mL doses.
Antimicrobial activity of thyme oils can be justified by their main components, which are summarized in Table 1. Jaberian and co-workers reported that alkaloid and phenolic compounds in plants could be responsible for antibacterial and antioxidant activities (Jaberian et al. 2013). Since the EOs are complex and impure mixtures, their biological activities vary in accordance with the compounds that they include; the amount of small compounds should not be neglected. Interestingly, biological activity could be more powerful as a result of the synergistic effect that is formed by the compounds coming together. However, sometimes the antagonistic effect leading to lower activity can also be observed. The high percentage of thymol in T. zygis and carvacrol in T. capitata could be responsible for the antimicrobial activity of these plants. Several authors have claimed that the major component of thyme EO is thymol (Viuda-Martos et al. 2011), and the antimicrobial activity of this compound has been confirmed on bacteria such as E. coli, Pseudomonas spp., Shigella flexneri and Bacillus cereus (Marchese et al. 2016). Thymol has been shown to cause disruption of the cellular membrane, inhibition of ATPase activity, and release of intracellular ATP and other constituents (Marchese et al. 2016). Similarly, carvacrol is indicated as a high antibacterial and antifungal compound.
Results presented in Fig. 2 showed that, among the five bacterial strains tested, B. cereus, L. innocua and S. aureus (Gram-positive) seems to be the most sensitive to the EO in terms of inhibition and MIC. The type of bacteria also has an influence on the effectiveness of the EOs. Gram-negative bacteria, in this case S. enterica and E. coli, were generally less susceptible than Gram-positive. The difference in susceptibility of the bacteria to an EO is thought to arise as a result of the differences in their cell membrane structure (Gilles et al. 2010). The cell envelopes of Gram-negative bacteria are more complex than the cell wall of Gram-positive bacteria. This phenomenon lies in the presence of external membrane structure which is particularly impermeable to EO molecules and the action of efflux mechanisms which results in protecting the bacteria against the action of EOs (Nazzaro et al. 2013).
Evaluation of the cytotoxic activity of the EOs
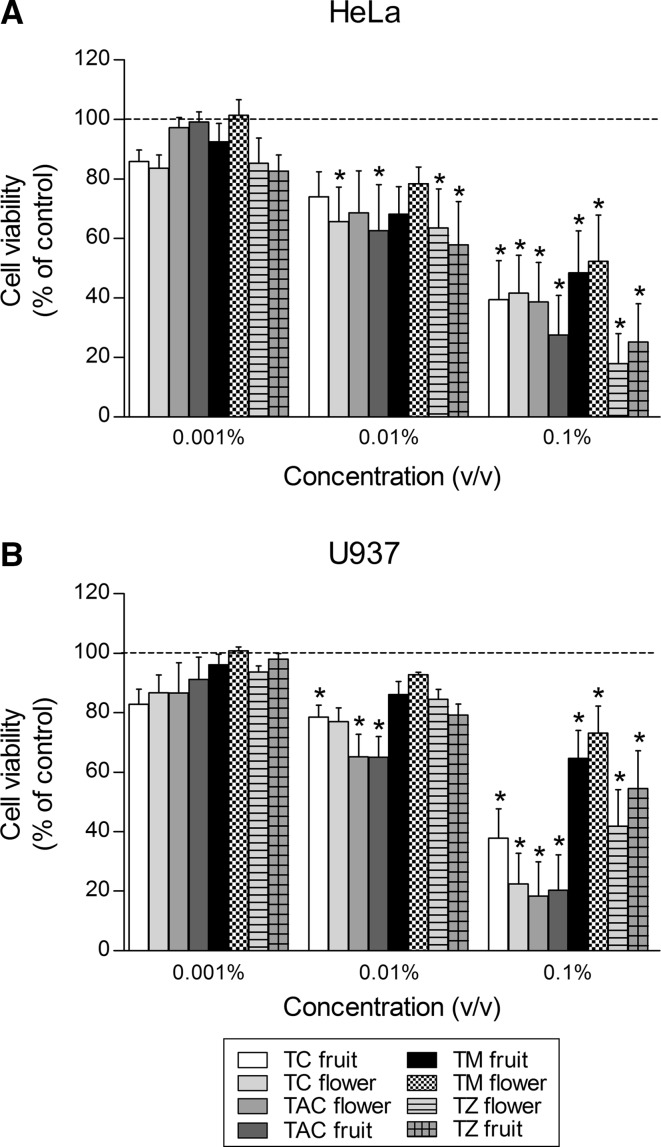
To investigate the cytotoxic activities, two human tumor cell lines, HeLa (adherent cells) and U937 (free-floating cells), were exposed to increasing concentrations of the various EOs. Cell viability was determined by the MTT assay. As shown in Fig. 3, the EOs revealed different cytotoxic activities towards the two human cancer cell lines investigated. In general, a dose-dependent decrease in the survival of both tumor cell lines was observed after treatment with all the EOs tested. At the highest concentration (0.1%, v/v), all EOs exhibited a substantial, statistically significant (P < 0.05) cytotoxic effect. Especially, T. zygis flower and fruit EOs caused the strongest cytotoxicity to HeLa (adherent) cells, thus reducing the cell viability by 82 and 75%, respectively. Similarly, T. capitata flower and fruit EOs as well as T. caespititius flower EO produced the most powerful cytotoxic effect to U937 (free-floating) cells, thereby decreasing the cell viability by 82, 80, and 78%, respectively. The intermediate concentration (0.01%, v/v) was partially efficient in diminishing cell viability. In fact, only four out of eight EOs, namely T. caespititius flower, T. capitata fruit, and T. zygis flower and fruit, showed statistically evident (P < 0.05) signs of cytotoxicity against HeLa cells, whereas only three out of eight EOs, i.e., T. caespititius fruit, and T. capitata flower and fruit, provoked a statistically significant (P < 0.05) diminution in U937 cell viability. At the lowest concentration tested (0.001%, v/v), EOs displayed poor cytotoxicity. Importantly, our results fit into previous investigations. Thus, EOs from different plants have been reported to present anticancer potential against mouth, breast, lung, prostate, liver, colon, and brain cancers, and even in leukemia. Not only EOs but also their constituents such as carvacrol, d-limonene, thymol and others have also been reported to possess cytotoxic effect on both cancer cell lines and in vivo studies (Deb et al. 2011; Jayakumar et al. 2012). Also, terpene analogues like terpinen-4-ol have been described to possess anticancer properties and induce apoptosis (Döll-Boscardin et al. 2012).
Fig. 3.
Dose-dependent cytotoxicity of different essential oils extracted from fruits and flowers of various species of Thymus and Thymbra. After 24 h of exposure to the essential oils, their citotoxicity towards HeLa (a) and U937 (b) cell lines was determined by the MTT assay. Values are presented as mean ± SEM of six independent experiments and expressed as percentage of control values (untreated samples). TC Thymus caespititius, TAC Thymbra capitata, TM Thymus mastichina, TZ Thymus zygis. * P < 0.05 compared to control values
Conclusion
The results demonstrated that the phenological stage of the plant influenced the chemical composition of the EOs of the T. capitata and Thymus species plants. The EOs from T. capitata and Thymus spp. exhibited strong antioxidant and antimicrobial activities. Moreover, results clearly showed that these EOs possed cytotoxic activity in vitro, since they exerted killing actions against tested cell lines, namely, HeLa (adherent cells) and U937 (free-floating cells). However, further studies are needed to elucidate their cytotoxic mechanism. Overall, these findings suggested that the EOs tested could be a potential source of pharmaceuticals.
Acknowledgements
J. Espino and M. Garrido hold a research post-doctoral fellowship from Government of Extremadura (jointly financed by the European Regional Development Fund (ERDF); Refs. PO14011 and PO14013, respectively).
Compliance with ethical standards
Conflict of interest
There are no conflicts of interest to disclose.
References
- Abbaszadeh S, Sharifzadeh A, Shokri H, et al. Antifungal efficacy of thymol, carvacrol, eugenol and menthol as alternative agents to control the growth of food-relevant fungi. J Mycol Médicale/J Med Mycol. 2014;24:e51–e56. doi: 10.1016/j.mycmed.2014.01.063. [DOI] [PubMed] [Google Scholar]
- Amensour M, Sendra E, Abrini J, et al. Total phenolic content and antioxidant activity of myrtle (Myrtus communis) extracts. Nat Prod Commun. 2009;4:819–824. [PubMed] [Google Scholar]
- Amorati R, Foti MC, Valgimigli L. Antioxidant activity of essential oils. J Agric Food Chem. 2013;61:10835–10847. doi: 10.1021/jf403496k. [DOI] [PubMed] [Google Scholar]
- Bruneton J (2001) Aceites esenciales. Factores de variabilidad de los aceites esenciales. In: Bruneton J (ed) Farmacognosia. Acribia S.A., Zaragoza, pp. 488–491
- Cano A, Acosta M, Arnao MB. A method to measure antioxidant activity in organic media: application to lipophilic vitamins. Redox Rep. 2000;5:365–370. doi: 10.1179/135100000101535933. [DOI] [PubMed] [Google Scholar]
- Council of Europe . European pharmacopoeia. 3. Strasbourg: Council of Europe; 1997. [Google Scholar]
- de Sousa JP, de Oliveira KÁR, de Figueiredo RCBQ, de Souza EL. Influence of carvacrol and 1,8-cineole on cell viability, membrane integrity, and morphology of aeromonas hydrophila cultivated in a vegetable-based broth. J Food Prot. 2015;78:424–429. doi: 10.4315/0362-028X.JFP-14-242. [DOI] [PubMed] [Google Scholar]
- Deb DD, Parimala G, Saravana Devi S, Chakraborty T. Effect of thymol on peripheral blood mononuclear cell PBMC and acute promyelotic cancer cell line HL-60. Chem Biol Interact. 2011;193:97–106. doi: 10.1016/j.cbi.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Delgado Adámez J, Gamero Samino E, Valdés Sánchez E, González-Gómez D. In vitro estimation of the antibacterial activity and antioxidant capacity of aqueous extracts from grape-seeds (Vitis vinifera L.) Food Control. 2012;24:136–141. doi: 10.1016/j.foodcont.2011.09.016. [DOI] [Google Scholar]
- Döll-Boscardin PM, Sartoratto A, Sales Maia BH, et al. In vitro cytotoxic potential of essential oils of Eucalyptus benthamii and its related terpenes on tumor cell lines. Evid Based Complement Alternat Med. 2012;2012:1–8. doi: 10.1155/2012/342652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman HJD, Surai P, Deans SG. In vitro antioxidant activity of a number of plant essential oils and phytoconstituents. J Essent Oil Res. 2000;12:241–248. [Google Scholar]
- Ehrnhöfer-Ressler MM, Fricke K, Pignitter M, et al. Identification of 1,8-cineole, borneol, camphor, and thujone as anti-inflammatory compounds in a Salvia officinalis L. infusion using human gingival fibroblasts. J Agric Food Chem. 2013;61:3451–3459. doi: 10.1021/jf305472t. [DOI] [PubMed] [Google Scholar]
- Figueiredo AC, Barroso JG, Pedro LG. Volatiles from Thymbra and Thymus species of the western mediterranean basin, portugal and macaronesia. Nat Prod Commun. 2010;5:1465–1476. [PubMed] [Google Scholar]
- Gilles M, Zhao J, An M, Agboola S. Chemical composition and antimicrobial properties of essential oils of three Australian Eucalyptus species. Food Chem. 2010;119:731–737. doi: 10.1016/j.foodchem.2009.07.021. [DOI] [Google Scholar]
- Gonçalves MJ, Cruz MT, Cavaleiro C, et al. Chemical, antifungal and cytotoxic evaluation of the essential oil of Thymus zygis subsp. sylvestris. Ind Crops Prod. 2010;32:70–75. doi: 10.1016/j.indcrop.2010.03.005. [DOI] [Google Scholar]
- Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002;13:572–584. doi: 10.1016/S0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- Hosseini Behbahani M, Ghasemi Y, Khoshnoud MJ, et al. Volatile oil composition and antimicrobial activity of two Thymus species. Pharmacogn J. 2013;5:77–79. doi: 10.1016/j.phcgj.2012.09.002. [DOI] [Google Scholar]
- Jaberian H, Piri K, Nazari J. Phytochemical composition and in vitro antimicrobial and antioxidant activities of some medicinal plants. Food Chem. 2013;136:237–244. doi: 10.1016/j.foodchem.2012.07.084. [DOI] [PubMed] [Google Scholar]
- Jayakumar S, Madankumar A, Asokkumar S, et al. Potential preventive effect of carvacrol against diethylnitrosamine-induced hepatocellular carcinoma in rats. Mol Cell Biochem. 2012;360:51–60. doi: 10.1007/s11010-011-1043-7. [DOI] [PubMed] [Google Scholar]
- Klein G, Rüben C, Upmann M. Antimicrobial activity of essential oil components against potential food spoilage microorganisms. Curr Microbiol. 2013;67:200–208. doi: 10.1007/s00284-013-0354-1. [DOI] [PubMed] [Google Scholar]
- Kumar D, Rawat DS. Synthesis and antioxidant activity of thymol and carvacrol based Schiff bases. Bioorg Med Chem Lett. 2013;23:641–645. doi: 10.1016/j.bmcl.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Kumar P, Mishra S, Malik A, Satya S. Housefly (Musca domestica L.) control potential of Cymbopogon citratus Stapf. (Poales: Poaceae) essential oil and monoterpenes (citral and 1,8-cineole) Parasitol Res. 2013;112:69–76. doi: 10.1007/s00436-012-3105-5. [DOI] [PubMed] [Google Scholar]
- Limem-Ben Amor I, Neffati A, Ben Sgaier M, et al. Antimicrobial activity of essential oils isolated from Phlomis crinita Cav. ssp. mauritanica Munby. J Am Oil Chem Soc. 2008;85:845–849. doi: 10.1007/s11746-008-1272-4. [DOI] [Google Scholar]
- Marchese A, Orhan IE, Daglia M, et al. Antibacterial and antifungal activities of thymol: a brief review of the literature. Food Chem. 2016;210:402–414. doi: 10.1016/j.foodchem.2016.04.111. [DOI] [PubMed] [Google Scholar]
- Mateos S, Valls J, Nadal M, Arola L. Estudio de madurez fenólica sobre diferentes variedades tintas y su relación con el color de los vinos. Tecnología del Vino. 2001;2:45–50. [Google Scholar]
- Miguel MG. Antioxidant activity of medicinal and aromatic plants. A review. Flavour Fragr J. 2010;25:291–312. doi: 10.1002/ffj.1961. [DOI] [Google Scholar]
- Miguel MG. Antioxidant and anti-inflammatory activities of essential oils: a short review. Molecules. 2010;15:9252–9287. doi: 10.3390/molecules15129252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel G, Guerrero C, Rodrigues H, et al. Study of the substrate and fertilization effects on the production of essentials oils by Thymus mastichina (L.) L. ssp. mastichina cultivated in pots. In: Anaç D, Martin-Prével P, et al., editors. Improved crop quality by nutrient management. Dordrecht: Springer; 1999. pp. 201–204. [Google Scholar]
- Miguel G, Simões M, Figueiredo AC, et al. Composition and antioxidant activities of the essential oils of Thymus caespititius, Thymus camphoratus and Thymus mastichina. Food Chem. 2004;86:183–188. doi: 10.1016/j.foodchem.2003.08.031. [DOI] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nazzaro F, Fratianni F, De Martino L, et al. Effect of essential oils on pathogenic bacteria. Pharmaceuticals. 2013;6:1451–1474. doi: 10.3390/ph6121451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinelo M, Rubilar M, Jerez M, et al. Effect of solvent, temperature, and solvent-to-solid ratio on the total phenolic content and antiradical activity of extracts from different components of grape pomace. J Agric Food Chem. 2005;53:2111–2117. doi: 10.1021/jf0488110. [DOI] [PubMed] [Google Scholar]
- Quiroga PR, Asensio CM, Nepote V. Antioxidant effects of the monoterpenes carvacrol, thymol and sabinene hydrate on chemical and sensory stability of roasted sunflower seeds. J Sci Food Agric. 2015;95:471–479. doi: 10.1002/jsfa.6744. [DOI] [PubMed] [Google Scholar]
- Ruberto G, Baratta MT. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000;69:167–174. doi: 10.1016/S0308-8146(99)00247-2. [DOI] [Google Scholar]
- Vilela GR, de Almeida GS, D’Arce MABR, et al. Activity of essential oil and its major compound, 1,8-cineole, from Eucalyptus globulus Labill., against the storage fungi Aspergillus flavus Link and Aspergillus parasiticus Speare. J Stored Prod Res. 2009;45:108–111. doi: 10.1016/j.jspr.2008.10.006. [DOI] [Google Scholar]
- Viuda-Martos M, Mohamady MA, Fernández-López J, et al. In vitro antioxidant and antibacterial activities of essentials oils obtained from Egyptian aromatic plants. Food Control. 2011;22:1715–1722. doi: 10.1016/j.foodcont.2011.04.003. [DOI] [Google Scholar]