Abstract
In present study, three varieties (G 80, Ageta 112 and HG 365) of guar bean (Cyamopsis tetragonoloba) were analysed for proximate analysis and were processed by different methods (dehusking, soaking, autoclaving, extrusion and germination) to reduce its antinutritional factors. Processed guar flours were studied for antinutritional factors (tannins, phytic acid and polyphenols) and protein fractions. The highest protein, ash and polyphenols contents were observed in Ageta 112. G 80 contained the lowest tannin and phytic acid content. High temperature treatments (i.e. autoclaving at 110 °C/10 min, 120 psi and extrusion—Clextral, Twin screw extruder) were found to be most effective in reducing the tannin and polyphenol content. More than 90% reduction in tannins was observed with high temperature treatments in HG 365. Phytic acid fraction increased slightly on soaking, however, extensive reduction was observed with other treatments. Globulins formed the major protein fraction in guar bean and various processing treatments significantly affected the protein fractions. Autoclaving was observed to be the best treatment to reduce antinutritional factors in guar bean and thereafter, its utilization in food.
Keywords: Guar, extrusion, autoclaving, antinutritional factors, protein fraction, globulins
Introduction
Cyamopsis tetragonoloba, commonly known as guar bean or cluster bean is majorly contributed to the world by Indian fields, accounting to 80% of global guar bean production. This drought resistant annual pod bearing crop belonging to Fabaceae family and subfamily Faboideae (Sharma et al. 2011), fetches foreign currency because of its galactomannan content. The guar gum finds application in array of fields viz food, pharmaceuticals, paper, cosmetics, mining, etc. Recently the application of guar gum in fracking process for extraction of oil has given an immense boost to guar crop production. The estimated production of guar bean in India for the year 2015–2016 is 27,51,423 metric tons with area under cultivation of 55,81,216 ha. Rajasthan leads the production among different states in India with estimated production of 22,23,474 metric tons for year 2015–2016, followed by Haryana, Punjab, Gujrat and other states (http://www.guargumcultivation.com). The guar bean seed comprises of the seed coat (14–17%), the endosperm (35–42%), and the germ (43–47%). The prime important constituent of guar bean i.e. galactomannan is present in the spherical-shaped endosperm (Sharma and Gummagolmath 2012).
Major portion of Guar is recovered as guar meal also known as Korma and Churi during the process of seeds into guar gum split, which is used as cattle feed (Sharma and Gummagolmath 2012). Guar is good source of iron and other elements like zinc, copper are also present in fair amount. Cis-linolenic acid is the most predominant fatty acids in guar bean, followed by cis-oleic acid and palmitic (Badr et al. 2014). The composition of guar bean has been accredited for its pharmacological properties like anticancerous, antidiabeic, anti ulcer, anti coagulant, anti microbial, anti asthamatic and anti inflammatory properties (Sharma et al. 2011).
Presence of beany flavour and toxicity of guar due to phenols restricts its consumption as food (Badr et al. 2014). Tannin in plants play a defensive role to environmental attack and have been reported to have potential antioxidant properties due to chain-breaking ability rather than to chelating activity with transition metals. Phytic acid is formed during maturation of the plant seed and represents 60–90% of total phosphate in dormant seeds. It serves as the principal storage form of phosphorus in plant tissues and has also shown anticarcinogenic properties, and blood glucose lowering effect by reducing the rate of starch digestion and slowing gastric emptying (Rusydi and Azrina 2012). However, its antnutritional effects are widely studied as they bind with essential nutrients and reduces their availability. Thus various studies have been executed to reduce antinutritional factors in different legumes. Processing of legumes to reduce the antinutritional factors affects the protein fraction i.e. albumin, globulins, prolamins and glutelins, which accounts to the functionality of proteins.
Thus, the objective of this study was to process guar bean by different methods in order to remove anti-nutritional compounds and assess the effects of treatments on protein fraction.
Materials and methods
Raw materials
Varieties of C. tetragonoloba i.e. G 80, Ageta 112 and HG 365 grown in fields of Punjab Agricultural University, Ludhiana, India, in crop year 2013 were chosen for study. The seeds were cleaned manually to remove damaged seeds and other filthy materials. The cleaned seeds were then stored in polyethylene bags for further use. All chemicals used were of analytical grade.
Processing of seeds
The guar bean seeds were subjected to various treatments in order to reduce the antinutritional factors viz. dehusking, soaking, autoclaving, extrusion processing and germination.
Dehusking
The seeds were roasted for 2 min and the outer fibrous layer of seeds i.e. husk were removed using the barley pearler for 2 min.
Soaking
Seeds were soaked in water at room temperature (27–30 °C) for 18 h. Thereafter, the water was drained and the seeds were wiped with muslin cloth, followed by drying at 50 °C in hot air oven.
Autoclaving
The seeds were pressure cooked in an autoclave at a temperature of 110 °C for 10 min at 120 psi. The treated guar bean was then dried at 60 °C in hot air oven.
Extrusion processing
The seeds of guar bean were milled in Cemotech Mill (Model-3303 Perten, Finland) to a particle size enough to pass through 200 µ mesh sieve. Flour obtained were subjected to extrusion processing using Clextral BC 21 (Clextral, Firminy, France), co-rotating intermeshing twin screw extruder having 400 mm useful length and 2.5 mm screw diameter and was equipped with a single screw volumetric feeder. Barrel temperature was set at 25, 75, 100 and 170 °C for 1st, 2nd, 3rd and 4th zones respectively. Screw speed was maintained at 500 rpm. Moisture content of samples was adjusted to 17%. Feeder speed was set to feed at 20 kg/h. The die was fitted with one circular insert having 3 mm diameter. The resultant product was cooled, ground to prepare it in flour form followed by packing in polypropylene bags.
Germination
Guar bean seeds were sterilized by soaking in ethanol for 1 min followed by soaking in distilled water (1:10, w/v) at 30 ± 5 °C for 8 h. The soaked, drained seeds were kept between thick layers of muslin cloth and allowed to germinate in the dark for 3 days. The germinated seeds were then dried at 50 °C in hot air oven and stored in cool, dark place for further use.
Preparation of samples
The raw seeds were roasted in open stainless steel vessel at 120 °C for 2 min followed by milling in Cemotech Mill (Model-3303 Perten, Finland). The treated dried legume samples were milled in Cemotech Mill (Model-3303 Perten, Finland) to flour fineness and stored in labeled high density polyethylene bags in cool and dark place for subsequent use in analysis.
Chemical composition
Physico-chemical characteristics such as moisture, ash, protein (% N × 6.25) content were determined as per the approved methods (American Association of Cereal Chemists AACC 2000). Fat and fibre content were determined using Soxtech (model-2045 Foss) and Fibertech (model-2023 Foss). Total carbohydrate content was determined in percentage by the difference.
Protein fractionation
Fractionation of protein into albumin, globulins, glutelins and prolamines was done as per Landry and Moureaux (1970).
Antinutritional properties
Raw and treated guar flour were analyzed to determine the antinutritional components i.e. tannins, total polyphenols and phytic acid.
Tannins
Tannins were determined by method outlined by Saxena et al. (2013). 0.5 g of sample was transferred to a 250 mL conical flask and 75 mL water was added. The flask was heated gently and boiled for 30 min followed by centrifugation at 2000 rpm for 20 min. The supernatant was collected in 100 mL volumetric flask and volume was made up. 1 mL of the sample extract was transferred to 100 mL volumetric flask containing 75 mL water, followed by addition of 5 mL of Folin–Denis reagent, 10 mL of sodium carbonate solution and then diluted to 100 mL with water and shaken well. The absorbance was read at 700 nm after 30 min using spectrophotometer (Thermo Spectronic 200, India). Standard curve was plotted and tannins were estimated.
Total polyphenols
Total polyphenols in guar flour was determined on a dry matter basis using the Folin–Ciocalteu assay (Singleton et al. 1999). The method is based on the color reaction of Folin–Ciocalteu reagent with hydroxyl groups. Absorbance was measured at 765 nm using a spectrophotometer (Thermo Spectronic 200, India).
Phytic acid
Phytic acid was determined according to method given by Sadasivam and Manickam (1992). One gram of guar flour was extracted with nitric acid by continuous shaking, filtered and made up to suitable volume with water. To 1.4 mL of the filtrate, 1 mL of ferric ammonium sulphate solution (21.6 mg in 100 mL water) was added, mixed and placed in a boiling water bath for 20 min. The contents were cooled and 5 mL of isoamyl alcohol was added and mixed. To this, 0.1 mL ammonia solution was added, shaken thoroughly and centrifuged at 3000 rpm for 10 min. The alcoholic layer was separated and the colour intensity was read at 465 nm against amyl alcohol blank after 15 min. Sodium phytate standards were run along with the sample. The standard curve of phytic acid was prepared for measurement the concentration of phytic acid in our samples [plotting the concentration of different Fe (NO) (mg) against the corresponding reading of Spectrophotometer (Thermo Spectronic 200, India) in Absorbance], the phytate phosphorus was calculated from the concentration of ferric iron assuming 4:6 (iron:phosphorus molar ratio). The results were expressed as mg phytic acid/100 g dry weight using following equation:
where A = optical density, C = concentration corresponding to optical density, S = weight of sample.
Statistical analysis
The statistical procedures were performed using SPSS version 16.0 (Statistical Package for Social Sciences, SPSS Inc, Chicago, USA). One-way analysis of variance (ANOVA) was carried out and significant differences (p < 0.05) were determined by Tukey’s.
Results and discussion
Quality and acceptance of product is significantly affected by the proximate composition. The result pertaining to composition of guar flour of different varieties is presented in Table 1. The values for moisture contents in guar beans ranged from (6.12 to 7.61%). Analysis revealed non significant variation in protein content of guar bean variety ranging from 24.55 to 26.78%. Ahmed et al. (2006) reported higher (52.6%) protein as compared to the varieties undertaken in present study, however, the results were similar to the findings of Pathak et al. (2011). Fat content varied from 2.7 to 3% and crude fibre ranged between 8.44 and 9.81%. The results were supported by studies of Pathak et al. (2011) and Ahmed et al. (2006).
Table 1.
Proximate composition of guar flour (on dry basis)
| Variety | Protein (%)c | Fat (%) | Fibre (%) | Ash (%) | Carbohydrates (%) |
|---|---|---|---|---|---|
| G 80 | 24.55a ± 0.94 | 3.06a ± 0.21 | 9.78a ± 0.44 | 3.59b ± 0.43 | 54.72a ± 1.32 |
| Ageta 112 | 26.78a ± 0.49 | 2.98a ± 0.27 | 8.44b ± 0.25 | 5.29a ± 0.39 | 53.33a ± 0.67 |
| HG 365 | 25.68a ± 0.93 | 2.70a ± 0.11 | 9.81a ± 0.31 | 3.76b ± 0.64 | 53.65a ± 1.10 |
Means followed by same letter within a column do not differ significantly (p ≤ 0.05)
Means (±SD) of triplicate analysis
Total nitrogen X 6.25
Ash content of Ageta 112 (5.29%) varied significantly from G 80 and HG 365. The carbohydrate content was higher (up to 54.4%) as compared to observations reported by Ahmed et al. (2006) (i.e. 23.7%). The variation in results could be subjected to varietal difference.
Effect of treatments on antinutritional factors of guar flour
The antinutritional factors play a defensive role during the growth of plant, however, its consumption in humans and animals can cause ill effects. Table 2 depicts the antinutritional factors (i.e. tannins, polyphenols and phytic acid) for raw and treated guar bean seeds. The higher content of total free phenolics and tannins is known to inhibit the activity of the digestive enzymes such as α-amylase, trypsin, chymotrypsin and lipase. Thus, interfere with the digestion and absorption of dietary proteins, carbohydrates, minerals and other nutrients, such as vitamin B12 (Vijayakumari et al. 2007; Doss et al. 2011) and cause damage to the mucosa of the digestive tract (Liener 1994). High temperature treatments i.e. autoclaving and extrusion processing were observed in extensive reduction levels to 141.5 and 134.5 mg/100 g, respectively. Alonso et al. (2000) found a reduction of 83.6% of tannins in extruded seeds of Pisum sativum. Various research studies have found similar results of reduction in tannins on heat treatments (Mittal et al. 2012; Vijayakumari et al. 2007; Doss et al. 2011). High temperatures change the molecular structures which possibly have a lower extractability due to a certain degree of polymerization (Van der Poel et al. 1991). In addition, they can be destroyed or the chemical reactivity can be changed after heat-processing (Barroga et al. 1985). Thermal treatments such as steaming, reconstitution or extrusion imply a qualitative change in the chemical structure of tannins (Alonso et al. 2000). Presence of tannins in testa allows its significant removal on dehulling, which accounted for a reduction % up to 72.37% in dehusked HG 365 guar variety. Soaking and germination treatment were effective in reducing tannin content which possibly was contributed due to the water soluble nature of tannins. Similar results were reported by Gurumoorthi and Uma (2011).
Table 2.
Effect of treatments on antinutritional factors of guar flour
| Treatment | Tanins (mg/100 g) | Polyphenols (mg/100 g) | Phytic acid (mg/100 g) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| G 80 | Ageta | HG | G 80 | Ageta | HG | G 80 | Ageta | HG | |
| Raw | 940.2a ± 0.8 | 1294.9a ± 0.7 | 2479.2a ± 0.7 | 66.7a ± 0.8 | 84.8a ± 1.0 | 39.7a ± 0.9 | 587.6a ± 0.7 | 849.0a ± 0.8 | 898.5b ± 0.5 |
| Dehusked | 448.3d ± 0.9 | 572.6c ± 0.4 | 684.8c ± 0.5 | 37.4b ± 0.9 | 39.4c ± 1.1 | 22.6b ± 0.8 | 594.7a ± 0.8 | 855.9a ± 0.7 | 927.5a ± 0.8 |
| (−52.3) | (−55.7) | (−72.37) | (−43.9) | (−53.5) | (−43.07) | (+1.2) | (+0.8) | (+3.2) | |
| Soaked | 645.9b ± 0.6 | 797.4b ± 0.8 | 994.7b ± 0.6 | 33.1c ± 0.7 | 46.3b ± 0.8 | 24.2b ± 0.9 | 505.9b ± 0.7 | 796.0b ± 0.4 | 788.5c ± 0.8 |
| (−31.3) | (−38.4) | (−59.8) | (−50.5) | (−45.3) | (−39.0) | (−13.9) | (−6.2) | (−12.2) | |
| Autoclaved | 141.5e ± 0.8 | 134.9d ± 0.4 | 194.0d ± 0.7 | 10.6d ± 1.0 | 12.0e ± 0.9 | 16.6c ± 1.1 | 326.6d ± 0.8 | 592.0c ± 0.3 | 578.0d ± 0.7 |
| (−84.9) | (−89.5) | (−92.1) | (−84.0) | (−85.8) | (−58.2) | (−44.4) | (−30.3) | (−35.7) | |
| Extruded | 134.5e ± 0.3 | 130.8d ± 0.7 | 198.3d ± 0.6 | 7.3e ± 0.7 | 8.9f ± 1.1 | 5.3d ± 0.8 | 346.5c ± 0.5 | 591.2c ± 0.8 | 582.1d ± 0.6 |
| (−85.7) | (−89.8) | (−91.9) | (−88.9) | (−89.4) | (−86.5) | (−41.0) | (−30.4) | (−35.2) | |
| Germinated | 511.6c ± 0.8 | 592.8c ± 0.3 | 698.2c ± 0.2 | 32.2c ± 0.9 | 24.2d ± 0.8 | 22.3b ± 1.1 | 311.82e ± 0.4 | 477.63d ± 0.7 | 552e ± 0.9 |
| (−45.5) | (−54.2) | (−71.8) | (−51.7) | (−71.5) | (−43.8) | (−46.9) | (−43.7) | (−38.6) | |
Means (±SD) of triplicate analysis
Means followed by same letter within a column do not differ significantly (p ≤ 0.05)
Values in parentheses shows percent increase (+) or decrease (−) from raw sample
A reduction level up to 93.25% of tannins were reported in germinated chickpea by Mittal et al. 2012. High temperature treatments i.e. autoclaving and extrusion were observed to be most efficient treatments for reduction of tannins. The reduction effect of high temperature treatments was most pronounced in variety HG 365. Ahmed et al. (2006) reported 1750 mg/100 g of tannin concentration in raw guar bean seed and similar to our results concluded autoclaving to be most efficient treatment for removal of tannins.
Phenolic compounds were reported to decreases the digestibility of proteins, carbohydrates and the availability of vitamins and minerals (Liener 1994). Also it has been reported to damage the mucosa of digestive tract and reduced nutritional absorption such as vitamin B12. Polyphenol content of guar bean seeds varied from 39.7 to 84.8 mg/100 g. The outcome noted were higher than the results reported by Ahmed et al. (2006) (i.e. 25 mg/100 g). Presence of polyphenols in seed coat allowed its removal during dehulling and thus lowering its level up to 75.8%. Water soluble nature of polyphenols contributed to leaching of phenols during soaking (Vijayakumari et al. 2007). Significant reduction in polyphenol content was observed on soaking of guar bean seeds. Ahmed et al. (2006) reported an increase in polyphenols after autoclaving the seeds, which is in contradiction to our observation. Results from the present study found autoclaving and extrusion treatments to be best treatments for removal of polyphenols. However, Germinated seeds exhibited significantly lower levels of phenols than the raw guar bean seed which was due to enzymatic degradation during germination (Rao and Deosthale 1982). Alonso et al. 2000 reported extrusion processing to be the best treatment for removal of antinutrients. Other researchers (Doss et al. 2011; Vijayakumari et al. 2007; Shimelis et al. 2007) have concluded autoclaving as best treatment for reduction of antinutrients.
Phytate content present in guar bean varieties ranged from 587.6 to 898.5 mg/100 g, which was higher than the results reported by Ahmed et al. (2006). Removal of outer husk layer during dehulling of guar bean seeds showed a non significant reduction of phytic acid, which was possibly due to concentration of phytic acid in cotyledon of seed. Autoclaving and extrusion processing exhibited significant reduction in phytic acid. Guar variety G 80 revealed 44.4 and 41.0% reductions on autoclaving and extrusion processing, respectively. Reduction in phytates may be due in part to hydrolysis of the inositol hexaphosphate to penta- and tetraphosphates. High extrusion temperatures can affect the molecular structure of condensed tannins and polyphenols. This chemical modification may alter tannins’ solubility or chemical reactivity (Marzo et al. 2002). However, germination was found to be most effective in reducing the phytic acid levels in guar bean seeds. Phytic acid levels were lowered by 46.9% in germinated G 80 Our findings are in agreement with results of Mubarak (2005) and Vidal-Valverde et al. (1998). Honke et al. (1998) observed a gradual reduction in inositol hexaphosphate during germination of peas, lupins and faba beans. Phytic acid serves as an important reserve of phosphorus which is generated by the action of phytase during seed germination, resulting in an increase in available phosphorus and, hence, improving the mineral’s bioavailability.
Effect of treatment on protein fraction guar flour
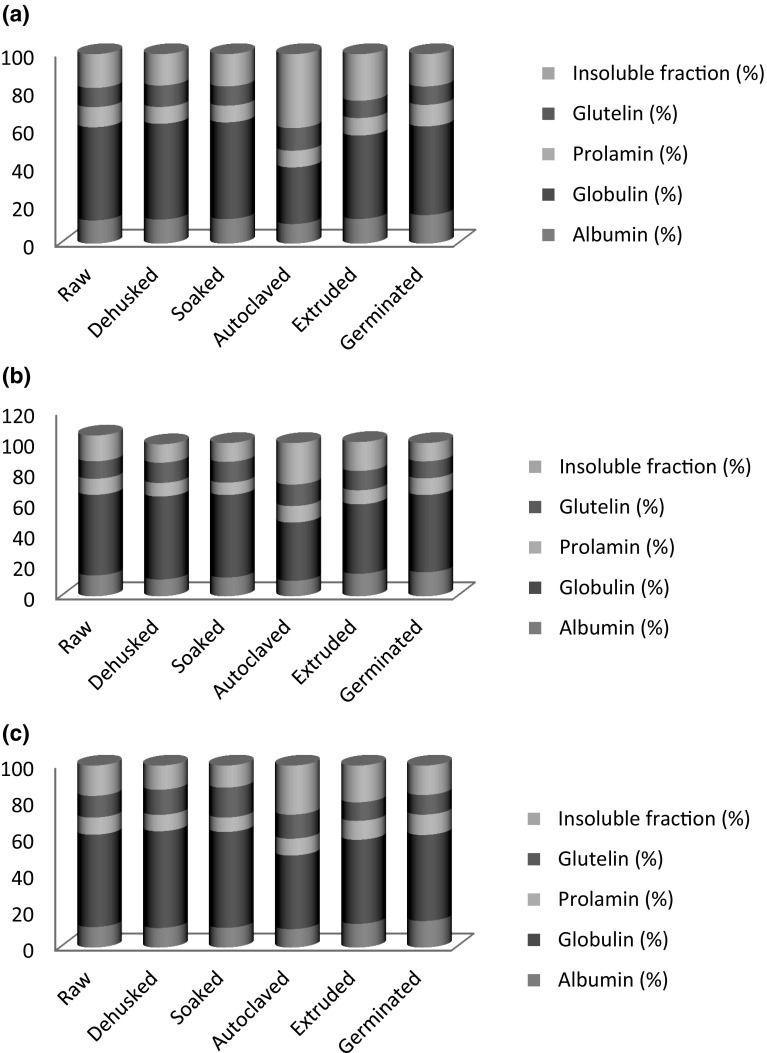
The proteins are classically divided on basis of solubility in different solvents: water-soluble (albumins), salt soluble (globulins), dilute alcohol soluble (prolamins) and dilute acetic acid soluble (glutelins) as per the classification of T B Osborne (1859–1929). These protein fractions play important roles in emulsification, salt tolerance, foaming properties, and functional characteristics that are of immense interest to the food processing industry (Gulewicz et al. 2008). Espino-Sevilla et al. (2013) from their study stated that glutelins and globulins were principally responsible for foam capacity and foam stability, and glutelin fraction in particular was the only fraction that exhibited emulsifying stability at pH 5, 6, and 7. Figure 1a–c represents the protein fractions of guar bean varieties G 80, Ageta 112 and HG 365 as affected by different treatments i.e. dehusking, soaking, autoclaving, extrusion and germination. Fractionation of protein revealed that globulins formed the dominant fraction followed by albumin. Germination and autoclaving demonstrated a significant effect on albumin fraction. Germination exhibited significant increase in albumin and prolamins protein fraction and decrease in globulins. De-Ruiz and Bressani (1990) reported globulin breakdown products during germination converted to nitrogen part calculated for albumin determination. The variation in the protein fractions observed was the consequence of change in molecular mass of the different proteins. The changes in protein conformation and complexation of proteins due to heat modified its solubility (Andualem and Gessesse 2013). Similar trend was reported by De-Ruiz and Bressani (1990), Afify et al. (2012), and Gulewicz et al. (2008). Abusin et al. (2009) studied faba beans and acclaimed significant reduction in albumins, globulins, prolamins on cooking but rise in glutelin fraction. Shivakumar and Murthy (2012) concluded germination of Tamarindus indica L. significantly reduced protein fractions, except for globulins.
Fig. 1.
Effect of different processing methods on protein fractions of guar flour a G 80, b Ageta 112 and c HG 365
Conclusion
Composition analysis revealed maximum protein and ash content in Ageta 112. HG 365 was observed with maximum tannins and phytic acid levels, whereas, polyphenols were highest in Ageta 112. Autoclaving and extrusion processing were most effective in reducing tannins and polyphenols, however, phytic acid was efficiently reduced by germination. Presence of galactomannans in guar bean flour makes the extrusion processing difficult, thus autoclaving was recommended as effective method for reducing the antinutritional factors. Globulins were the dominant fraction that increased on soaking and high temperature treatment reduced its level. Germination of guar beans increased the protein fraction. The suggested autoclaving method increased the glutelin fraction of protein that contribute to emulsifying properties and also shows better heat stability, thus better prospects in food processing.
References
- AACC . Approved methods of American Association of Cereal Chemists. 10. Paul: The Association St; 2000. [Google Scholar]
- Abusin SAE, Hassan AB, Babiker EE. Nutritional evaluation of cooked faba bean (Vicia faba L.) and white bean (Phaseolus vulgaris L.) cultivars. Aust J Basic Appl Sci. 2009;3(3):2484–2490. [Google Scholar]
- Afify AEMMR, El-Beltagi HS, Abd El-Salam SM, Omran AA. Protein solubility, digestibility and fractionation after germination of sorghum varieties. PLoS ONE. 2012;7(2):31154. doi: 10.1371/journal.pone.0031154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed MB, Hamed RA, Ali ME, Hassan AB, Babiker EE. Proximate composition, antinutritional factors and protein fractions of guar gum seeds as influenced by processing treatments. Pak J Nutr. 2006;5:481–484. doi: 10.3923/pjn.2006.481.484. [DOI] [Google Scholar]
- Alonso R, Orue E, Zabalza M, Grant G, Marzo F. Effect of extrusion cooking on structure and functional properties of pea and kidney bean proteins. J Sci Food Agric. 2000;80:397–403. doi: 10.1002/1097-0010(200002)80:3<397::AID-JSFA542>3.0.CO;2-3. [DOI] [Google Scholar]
- Andualem B, Gessesse A. Effect of cooking on protein digestibility, fractions content and functional characteristics of defatted Millettia ferruginea seed flour. World Appl Sci J. 2013;27(9):1111–1118. [Google Scholar]
- Badr SEA, Abdelfattah MS, El-Sayed SH, Abd El-Aziz ASE, Sakr DM. Evaluation of anticancer, antimycoplasmal activities and chemical composition of guar (Cyamopsis tetragonoloba) seeds extract. Res J Pharm Biol Chem Sci. 2014;5(3):413–423. [Google Scholar]
- Barroga CF, Laurena AC, Mendoza MT. Polyphenols in mung bean (Vigna radiata L., Wilczek): determination and removal. J Agric Food Chem. 1985;33:1006–1009. doi: 10.1021/jf00065a056. [DOI] [Google Scholar]
- De Ruiz ASC, Bressani R. Effect of Germination on the chemical composition and nutritive value of amaranth grain. Cereal Chem. 1990;67(6):519–522. [Google Scholar]
- Doss A, Pugalenthi M, Vadivel VG, Subhashini G, Anitha Subash R. Effects of processing technique on the nutritional composition and antinutrients content of under-utilized food legume Canavalia ensiformis L. DC. Int Food Res J. 2011;18(3):965–970. [Google Scholar]
- Espino-Sevilla MT, Jaramillo-Flores ME, Hernandez-Gutierrez R, Mateos-Diaz JC, Andrews HE, Barba AP, Lopez JO, Rodrıguez SV, Cervantes ECL. Functional properties of Ditaxis heterantha proteins. Food Sci Nutr. 2013;1(3):254–265. doi: 10.1002/fsn3.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulewicz P, Cristina Martınez-Villaluenga, Frias J, Ciesiołka D, Gulewicz K, Vidal-Valverde C. Effect of germination on the protein fraction composition of different lupin seeds. Food Chem. 2008;107:830–844. doi: 10.1016/j.foodchem.2007.08.087. [DOI] [Google Scholar]
- Gurumoorthi P, Uma S. Heat-stable and heat-labile antinutritional profile in Mucuna pruriens var utilis: effected by germination. Int Food Res J. 2011;18(4):1421–1426. [Google Scholar]
- Honke J, Kozlowska H, Vidal-Valverde C, Frias J, Gorecki R. Changes in quantities of inositol phosphates during maturation and germination of legume seeds. Z Lebensm Unters Forsch A. 1998;206:279–283. doi: 10.1007/s002170050257. [DOI] [Google Scholar]
- Landry J, Moureaux T. Heterogeneity of corn seed glutelin: selective extraction and amino acid composition of the 3 isolated fractions. J Bull Soc Chem Biol. 1970;52:1021–1037. [PubMed] [Google Scholar]
- Liener IE. Implications of antinutritional components in soybean foods. Crit Rev Food Sci Nutr. 1994;34:31–67. doi: 10.1080/10408399409527649. [DOI] [PubMed] [Google Scholar]
- Marzo F, Alonso R, Urdaneta E, Arricibita FJ, Ibanez F. Nutritional quality of extruded kidney bean (Phaseolus vulgaris L. var. Pinto) and its effects on growth and skeletal muscle nitrogen fractions in rats. J Anim Sci. 2002;80:875–879. doi: 10.2527/2002.804875x. [DOI] [PubMed] [Google Scholar]
- Mittal R, Nagi HPS, Sharma P, Sharma S. Effect of processing on chemical composition and antinutritional factors in chickpea flour. J Food Sci Eng. 2012;2:180–186. [Google Scholar]
- Mubarak AE. Nutritional composition and antinutritional factors of mung bean seeds (Phaseolus aureus) as affected by some home traditional processes. Food Chem. 2005;89:489–495. doi: 10.1016/j.foodchem.2004.01.007. [DOI] [Google Scholar]
- Pathak R, Singh M, Henry A. Genetic diversity and interrelationship among clusterbean (Cyamopsis tetragonoloba) genotypes for qualitative traits. Indian J Agric Sci. 2011;81(5):402–406. [Google Scholar]
- Rao PU, Deosthale YG. Tannin content of pulses: varietal deferences and effects of germination and cooking. J Sci Food Agric. 1982;33:1013–1016. doi: 10.1002/jsfa.2740331012. [DOI] [Google Scholar]
- Rusydi MR, Azrina A. Effect of germination on total phenolic, tannin and phytic acid contents in soy bean and peanut. Int Food Res J. 2012;19(2):673–677. [Google Scholar]
- Sadasivam S, Manickam A. Biochemical methods for agricultural sciences. New Delhi: Wiley Eastern Ltd.; 1992. pp. 199–201. [Google Scholar]
- Saxena V, Mishra G, Saxena A, Vishwakarma KK. A comparative study on quantitative estimation of tannins in terminalia chebula, terminalia belerica, terminalia arjuna and saraca indica using spectrophotometer. Asian J Pharm Clin Res. 2013;6:148–149. [Google Scholar]
- Sharma P, Gummagolmath KC. Reforming guar industry in India: issues and strategies. Agric Econ Res Rev. 2012;25:37–48. doi: 10.1007/s40003-011-0003-5. [DOI] [Google Scholar]
- Sharma P, Dubey G, Kaushik S. Chemical and Medico-biological profile of Cyamopsis tetragonoloba (L) Taub: an overview. J Appl Pharm Sci. 2011;1(2):32–37. [Google Scholar]
- Shimelis E, Meaza M, Rakshit S. Physico-chemical properties, pasting behavior and functional characteristics of flours and starches from improved bean (Phaseolus vulgaris L.) varieties grown in East Africa. Agric Eng Int CIGR E-J. 2007;8:5–15. [Google Scholar]
- Shivakumar SP, Murthy KRS. Protein solubility and haemagglutinating activity of tamarind seed extracts. Adv Res Pharm Biol. 2012;2(3):305–309. [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteau reagent. Method Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Van der Poel AFB, Gravendeel S, Boel H. Effect of different processing methods on tannin content and in vitro protein digestibility of faba bean (Vicia faba L.) Anim Feed Sci Technol. 1991;33:49–58. doi: 10.1016/0377-8401(91)90045-T. [DOI] [Google Scholar]
- Vidal-Valverde C, Sotomayor JFC, Fernandez CDPM, Urbano G. Nutrients and antinutritional factors in faba beans as affected by processing. Z Lebensm Unters Forsch A. 1998;207:140–145. doi: 10.1007/s002170050308. [DOI] [Google Scholar]
- Vijayakumari K, Pugalenthi M, Vadive V. Effect of soaking and hydrothermal processing methods on the levels of antinutrients and in vitro protein digestibility of Bauhinia purpurea L. seeds. Food Chem. 2007;103:968–975. doi: 10.1016/j.foodchem.2006.07.071. [DOI] [Google Scholar]
- http://www.guargumcultivation.com