Abstract
Non-enzymatic browning (NEB) in canned peach halves in syrup during storage was investigated. Absorbance at 420 nm (A 420), colorimetric parameters (CIELab, TCD and La/b), fructose, glucose and sucrose, total sugar, organic acids, ascorbic acid (AA), dehydroascorbic acid, and 2,3-diketogulonic acid were used to estimate the extent of NEB during 1 year of storage at 30 °C and the relationships between each of these parameters and A 420 were established. The investigation was carried out to explore the possibility of replacing the E330 commonly used as acidifier by turbid or clarified lemon juice (TLJ or CLJ) to obtain a product having good nutrition with better retention of quality. The a, La/b, glucose and fructose were positively correlated with A 420 and all proved to be good indicators of browning development. Overall results showed that replacement of acidifier E330 with CLJ for controlling pH in canned peach halves in syrup had some advantages.
Keywords: Ascorbic acid degradation, Absorbance, Color indicators, Sugars, Maillard reaction
Introduction
Non-enzymatic browning (NEB) is one of the most important chemical reactions responsible for quality and color changes during the heating or prolonged storage of fruit and vegetable products (Arena et al. 2001; Bharate and Bharate 2014). NEB is referred as a Maillard reaction when is initiated by the condensation of the carbonyl group of reducing sugars with free amino groups of amino acids and/or proteins. This chemical process produces a brown color in foods without the activity of enzymes. Melanins and other chemicals are responsible for the brown color. It is generally understood that the degradation products of L-ascorbic acid (AA) and/or sugars, e.g. furfural, 5-(hydroxymethyl) furfural (HMF), or other carbonyl compounds, participates in juice browning and polymerize with each other or react with amino acids to yield browning materials (Kennedy et al. 1990; Lee and Nagy 1988a; Martins et al. 2001; Sawamura et al. 1991).
AA degradation was associated with browning of orange juice a long time ago. Likewise, it was demonstrated that amino acids accelerate AA breakdown, and in the presence of amine it is dehydroascorbic acid (DHA) the reactive intermediate in the pathway to furfural and brown pigment production. AA is readily converted to DHA acid by mild oxidation, but the loss of vitamin activity only arises after hydrolysis of the lactone to form 2,3-diketogulonic acid (DKG) (Tannebaum et al. 1985). Under anaerobic conditions, AA reacts via its keto tautomer which is in equilibrium with its anion and undergoes delactonisation to form DKG. Decarboxylation of DKG results in the formation of xylosone and 3-deoxypentaone, which is formed by β-elimination at the C4 of DKG, followed by decarboxylation. It is at this point that the pathways begin to assume the features of NEB reactions.
NEB during manufacture and storage is of vital interest for food industry. Different indicators were assayed to determine the extent of browning (Garza et al. 1999). These included absorbance measurements at given wavelengths, being 420 nm, one of the most frequently used, as well as colorimetric parameters and qualitative and quantitative determinations of intermediate and final products, such as AA degradation compounds, sugars (glucose, fructose and sucrose) and HMF. The aim of this study was to establish whether the colorimetric parameters CIE (Committee International d’Eclairage) Lab or combinations of these three values, such as the total color difference (TCD) and La/b, as well as the variation in determined compounds which intervene in the NEB reactions, can be used to evaluate the extent of these reactions in canned peach halves produced at industrial-scale and stored at room temperature (30 °C) during 1 year. As complementing previous studies (Saura et al. 2003), the present investigation was carried out to explore the possibility of replacing the citric acid (E330) commonly used as acidifier by turbid or clarified lemon juice (TLJ or CLJ) in the canning of peach halves in syrup to obtain a product having good nutrition with better retention of quality during storage. Citric acid might participate in chemical reactions leading to the formation of brown pigments in the presence of AA, irrespective of pH value. Clegg (1966) showed that citric acid neither acted as a catalyst nor yielded reactive carbonyl compounds under aerobic incubation conditions, but he suggested that citric acid, or a derivative, was incorporated into some of the developed brown pigments in model systems which simulated lemon juice (LJ). On the contrary, several studies (González-Molina et al. 2009, 2012; Mena et al. 2013) emphasize the anti-browning activity of lemon juice in combination with different natural color sources rich in bioactive products.
Materials and methods
Fruit material
The variety of peach chosen for the current experiment, Sudanell, has a free stone, uniform and large size, symmetric shape, yellow pulp, and firm but melting texture. This selection has other implications as well; in general, freestone peaches are recognized as more aromatic than clingstone peaches. The fresh peaches used were grown in Northeastern Spain (Lleida, Catalonia) and had a total soluble solids (TSS) concentration between 9.6 and 10.4°Brix and a pH between 3.61 and 3.65.
Clarification of LJ
The clarified juice was obtained by the tangential- or cross-flow filtration method described by Saura et al. (1991). Experiments were performed on a Minitan cross-flow filtration unit (EMD Millipore, Bedford, Massachusetts MA 01739, USA), equipped with 4 Durapore cartridges of 0.45 µm of pore diameter. Each cartridge provided 0.24 m2 membrane area. The unit was operated as a recirculating batch system, so both retentate and permeate streams were recirculated back into the feed tank. Feed stream was pumped from a temperature controlled tank, 30 ± 2 °C into the filtration cartridges at a rate of 0.5 L/min. Ultrazym 100 G (Novo Nordisk, Bagsvaerd, Denmark) was added to the feed tank at a concentration of 500 mg/L. A valve in the return line controlled transmembrane pressure.
Throughout this clarification process, the total amount of volatile compounds decreases, for example from 612 mg/kg in the fresh squeezed juice down to 269 mg/kg at the end of the clarification steps. The contents of pectins and proteins underwent significant reduction while the contents of sugars and organic acids did not differ substantially from those found in the starting juice.
Manufacturing of canned peaches
Peaches were chemically peeled through immersion of the fruits in 20% NaOH at a temperature of 90 °C for about 1 min; no antioxidant agent was added. Then, peaches were automatically placed into 0.5 kg cans without any type of internal coating; the can netweight was 425–430 g, and the product dry weight 250–260 g. In a regular manufacturing process of canned peaches, the preservation liquid is added by automatic machinery at temperatures of about 100 °C. In this way, this syrup remains in its pool for several min at a high temperature and in contact with atmospheric oxygen. Under these conditions, the LJ will get easily oxidized. In order to avoid NEB reactions of LJ, the addition of LJs and E330 was carried out at room temperature and before the addition of the preservation liquid. At this point, the following products were added (product per can): (a) 10 mL of 5.8% E330, (b) 8.9 mL of TLJ, or (c) 9.1 mL of CLJ. Then, the preservation liquid (mixture of water and sucrose and glucose syrups) was added at room temperature. Its main properties were: initial (before addition to cans) and final (after approximately 60 day of equilibrium with fruits) TSS 34–35°Brix and 20–22°Brix, respectively; initial and final pH values were 2.7 and 3.7, respectively. Immediately after the addition of preservation liquid cans were heated to 95 °C for 2 min. Then, the full cans were introduced in an automatic closing machine, which removed any oxygen left in the headspace before closing the containers. Later, the cans were sterilized for 12 min in boiling water.
A total of 132 cans of 0.5 kg were elaborated and divided in 3 different treatments or series of 44 cans each: 1st series: addition of E330 (control); 2nd series: addition of TLJ; 3rd series: addition of CLJ. All quality parameters of samples were studied at different times of storage (30 °C): 0, 31, 67, 105, 143, 167, 181, 257, 302 and 364 day. The values shown in this manuscript are the arithmetic means of 6 cans per series.
Color measurement
Color was measured using a Minolta CR-10 colorimeter. The CIELab color space is presently one of the most popular color spaces for measuring object color and is widely used in virtually all fields. In this color space, L indicates lightness and ranges from 0 (black) to 100 (white); a indicates redness and ranges from −60 (green) to +60 (red); and b indicates yellowness and ranges from −60 (blue) to +60 (yellow). The instrumental color measurements were performed on 2 spots from the peeled surface of different peach halves after draining and rinsing of preservation liquid.
The color changes during storage of canned peach halves were also expressed as a single numerical value that defines the magnitude of the TCD, according to the following equation (Aguiló-Aguayo et al. 2009):
| 1 |
where a o, b o and L o are the CIELab values of the sample at storage time 0.
High-performance liquid chromatography (HPLC) analysis of sugars
The injection method is used directly after the flocculation of pectin and proteins contained in 25 mL preservation liquid with equal volume of methanol. Samples were centrifuged at 15000×g for 15 min and then the supernatant was filtered through a 0.45 μm PVDF syringe filter.
The detection is also straightforward; with a refractive index detector RID 6A in addition to a Shimadzu HPLC system equipped with two Shimadzu LC 6A pumps (Shimadzu Corp., Kyoto, Japan). A µBondapak Amino (NH2) column (10 µm, 3.9 × 300 mm) (Waters Associates, Milford, Massachusetts, USA) was used. The mobile phase was water:acetonitrile (15:85). The flow used was 2 mL/min. Sugars were characterized and quantified by chromatographic comparison with analytical standards. Results were expressed as g/L.
High-performance ion-exclusion chromatography of organic acids
The content of each can of peach halves in syrup is crushed until obtaining a uniform homogenate. For organic acid determination, 25 mL of peach in syrup homogenate was mixed with 200 µL of Ultrazym 100G (100 ppm) during 9 h at 0 °C. Samples were centrifuged at 15000×g for 15 min and then 3 mL of the supernatant was passed through a solid phase separation C18 Sep-Pak cartridge (Waters Associates) pre-activated with equal volumes of methanol, air and water (10:10:10 v/v).
High-performance ion-exclusion chromatography of organic acids contained in the extracts were analyzed using a model LC pump T-414 (Kontron Instruments, Zurich, Switzerland) coupled with a LC-858 photodiode array detector (Perkin Elmer, Waltham, Massachusetts, USA) and a Interaction ORH-801 (Interaction Chemicals Inc., Mountain View, California, USA) column (5 µm, 6.5 × 300 mm). A 20 µL sample was analyzed using a mobile phase of 0.1% H2SO4 at a flow rate of 0.6 mL/min. Organic acids were characterized and quantified by UV–vis spectra (210 nm) by comparison with analytical standards. Results were expressed as g/L.
AA, DHA and DKG determination
From the peach in syrup homogenate, 25 mL were clarified by adding 200 µL of Ultrazym 100G (5000 ppm). The samples were stored in hermetic containers for 9 h at 0 °C. Later, 3 mL of solution was filtered through a C18 Sep-Pak cartridge (Waters Associates) conditioned with water and methanol. The first millilitre of the solution was discarded. Then, 40 µL of the solution was injected into HPLC. The mobile phase was water:EDTA 97.5:2.5 (with metaphosphoric acid 2.5 g/L), and 15 mL/L of thiodiglycol (FlukaChemie AG, Busch, Germany) was also added, adjusting the pH to 7. The flow rate used was 0.5 mL/min. A Spherisorb ODS2 column (5 µm, 4.6 × 25 mm) (Tracer Analitica, S.A., Barcelona, Spain) was also used. The injected sample volume was 40 µL. The post column reaction was carried out with 1.4-ditio-DL-Treitol (Fluka Chemie AG), 300 mg/L in phosphate buffer at pH 6.5 which was mixed after passing through the column at a flow rate of 0.5 mL/min. Later, it was transported through a tephlon tube of 1/16″ internal diameter and 0.010″ external diameter, interwoven in a special way to favour the mixture and submerged in water at 80 °C. The function of the post column reaction is to reduce the DHA to AA in order to detect the length of the measurement wave, 267 nm. This method was considered as effective for the simultaneous detection of AA and DHA (Ziegler et al. 1987). The patterns used were Merck reagents (Darmstadt, Germany). The DKG pattern was obtained by adding NaOH 0.5 N to a solution of DHA until pH 7, as described by Doner and Hicks (1981).
Determination of soluble brown pigments (BPs)
Browning development was determined by measuring the soluble BPs formed during storage. After dilution of 1 mL preservation liquid with distilled water, the absorbance was read at 420 nm wavelength by using a Unicam Helios Delta 9423 UVD 1000E spectrophotometer (Cambridge, UK).
Statistical analysis
All measurements were done in duplicate and results expressed as the mean value ± standard deviation (SD). Statgraphics® Plus for Windows 3.0 (Statistical Graphic Corp. and Graphic Software Systems Inc., Rockville, Maryland, USA) was used for Statistical Analysis, including Analysis of Variance (ANOVA), Fisher’s least significant difference (LSD) procedure to discriminate among the means, and Regression Analysis to describe the relationship between variables.
Results and discussion
Evolution of colorimetric parameters
The browning changes of canned peaches during storage were determined using the CIELab color system, an objective tool for color perception. Results are presented in Fig. 1a–c. No statistical differences (P ≥ 0.05) were found for the L and b parameters, regardless of acidifier used in canned peach halves in syrup. However, a values for the samples acidified with E330 were always higher (P = 0.0000) than for LJ-treated peaches. These results agreed with those previously reported by Trifiro and Landi (1965) in nectars acidified with LJ and could be related to the addition of AA and flavanone glycosides (eriocitrin and hesperidin) which have protective action against UV-induced oxidative reactions (Tikekar et al. 2011). AA is widely considered as an antioxidant. The juice industry sometimes adds AA, in concentrations of approximately 0.01%, to protect the color of juices. Results could also be related to the destruction of chlorophyll and the subsequent synthesis of carotenes (orange-colored plant pigments) (Robertson et al. 1990). Finally, canned peach halves become redder when heated as occur with the peach puree (Ávila and Silva 1999). However, these relationships have no technological meaning because the heat treatment, ascorbic acid content and chlorophyll degradation were similar in all three types of samples.
Fig. 1.
Evolution of colorimetric parameters CIEL (a), a (b) and b (c) in canned peach halves in syrup during 1 year of storage a 30 °C. Acidifier E330 (filled circle), turbid lemon juice (filled triangle) and clarified lemon juice (filled square)
On the other hand, 61.1% of trained panelists considered the peach halves acidulated with E330 darker than those acidulated with LJ. This sensory evaluation implied that the acids added with LJ caused a whitening process of the peach halves, being this effect more evident for the samples treated with CLJ (55.5%). An increase in the intensity of redness could be perceived as a darker color by the trained panel (Saura et al. 2004).
TCD and various combinations, such as Lab, L/ab, La/b and Lb/a of tristimulus CIELab values have been used to represent the change in visual color of food products (Adekunte et al. 2010; Shin and Bhowmik 1995). The long process of storage led also to differences in TCD (P = 0.0002) and La/b (P = 0.0000) values of canned peach halves. Higher TCD values were obtained in samples acidified with TLJ (6.07 ± 2.01) compared to those CLJ and E330-treated which exhibited TCD values from 0.89 to 3.04 and 1.15 to 5.90, respectively. TCD values between 1.5–3.0, 3.0–6.0 and 6.0–12.0 could be classified as noticeable, well visible and great color difference (Renard and Maingonnat 2012). In contrast the La/b values for the samples acidified with E330 were higher (5.42 ± 1.77) than for LJ–treated peach halves (0.27 ± 1.25). Consequently the La/b parameter proved to be good indicator of the total color change of canned peach halves stored at 30 °C during 1 year.
BP formation
Absorbance at 420 nm (A 420) has been extensively used by other authors to study NEB of different fruit juices: peach (Buedo et al. 2001; Garza et al. 1999; Ibarz et al. 1993), pear (Beveridge and Harrison 1984), apple (Garza et al. 1996) or orange (Johnson et al. 1995). Statistically significant differences at the 95% confidence level (P = 0.0008) were observed among the A 420 values for the three types of samples. The A 420 values for the samples acidified with E330 (0.08 ± 0.01) were higher than for the LJ–treated ones (0.06 ± 0.01). Kinetic changes of spectrophotometric measurements are shown in Fig. 2. The curve of NEB reaction for the samples acidified with E330 was a straight line, indicating apparently a zero-order kinetic. As expected, A 420 values increased by increasing storage time. The correlation between A 420 and period of storage was as follows: y = 0.0001x + 0.0608 with a regression coefficient R 2 equals 0.8769. These results agreed with those previously reported by Burdurlu and Karadeniz (2003) and Giner et al. (2013) in Golden Delicious juice concentrate and thermal pasteurized tomato juice, respectively. In contrast, browning development followed a first-order reaction in carrot juice concentrate and cold break tomato paste after 150-day storage at 25 °C and 37 °C as reported by Wang et al. (2006) and Liu et al. (2010).
Fig. 2.
Spectrophotometric color changes (absorbance at 420 nm) in canned peach halves in syrup during 1 year of storage a 30 °C. Acidifier E330 (filled circle), turbid lemon juice (filled triangle) and clarified lemon juice (filled square)
Kinetics of NEB could not be determined in stored TLJ and CLJ samples. In fact, regression coefficients (0.6598 and 0.0419) showed that no browning development occurred practically in these samples.
Changes in sugar contents
The evolution of glucose, fructose, sucrose and total sugar contents throughout storage at 30 °C is shown in Figs. 3a–d. It should be emphasized the rapid decline of sucrose content due to hydrolysis in acidic medium to yield equimolar amounts of glucose and fructose. Sucrose does not participate in NEB reactions because it lacks reducing ends, but their hydrolysis products can participate because they are reducers. Initial sucrose content in E330 samples was lower than in LJ-treated ones, showing that its hydrolysis was lower in latters, possibly due to more rapid dissolution and dispersion of crystalline E330. Similar results were reported by Lee and Nagy (1988b) for grapefruit juice. It can be concluded that as a result of hydrolysis of sucrose, levels of glucose and fructose increased until it practically disappeared.
Fig. 3.
Changes of glucose (a), fructose (b), sucrose (c) and total sugar (d) contents in canned peach halves in syrup during 1 year of storage a 30 °C. Acidifier E330 (filled circle), turbid lemon juice (filled triangle) and clarified lemon juice (filled square)
In general, the glucose content was higher than that of fructose and showed statistical differences (P = 0.0417) among the three types of samples. In fact, E330 and TLJ-treated samples were richer in glucose (9.44 ± 2.57 g/L) than CLJ-treated ones (5.02 ± 2.02 g/L). After 1 year of storage, glucose concentration was significantly increased in a 22.20, 322.37, and 227.50% for the samples acidified with E330, TLJ, and CLJ, respectively. As can be seen this increase was more intense in the LJ-treated samples. According to results of Lee and Nagy (1988b), the reducing sugar content for all samples increased with small fluctuations with increasing storage time.
Storage time had also a significant effect on the total sugar which was significantly decreased (between 15.25 and 23.61%) in accordance with that reported by Wang et al. (2006) and Liu et al. (2010) for carrot juice concentrate and cold break tomato paste. Both the inversion reaction of sucrose and Maillard reaction occur during storage. The glucose and fructose contents should be reduced by Maillard reaction, but they increased herein. This unconformity was caused by the two chemical reactions during storage, indicating that reaction rate of sucrose inversion was greater than that of Maillard reaction. Therefore, the decrease in sucrose was attributed to the sucrose inversion in glucose and fructose at high temperature and low pH, while the decrease in total sugar was resulted from Maillard reaction.
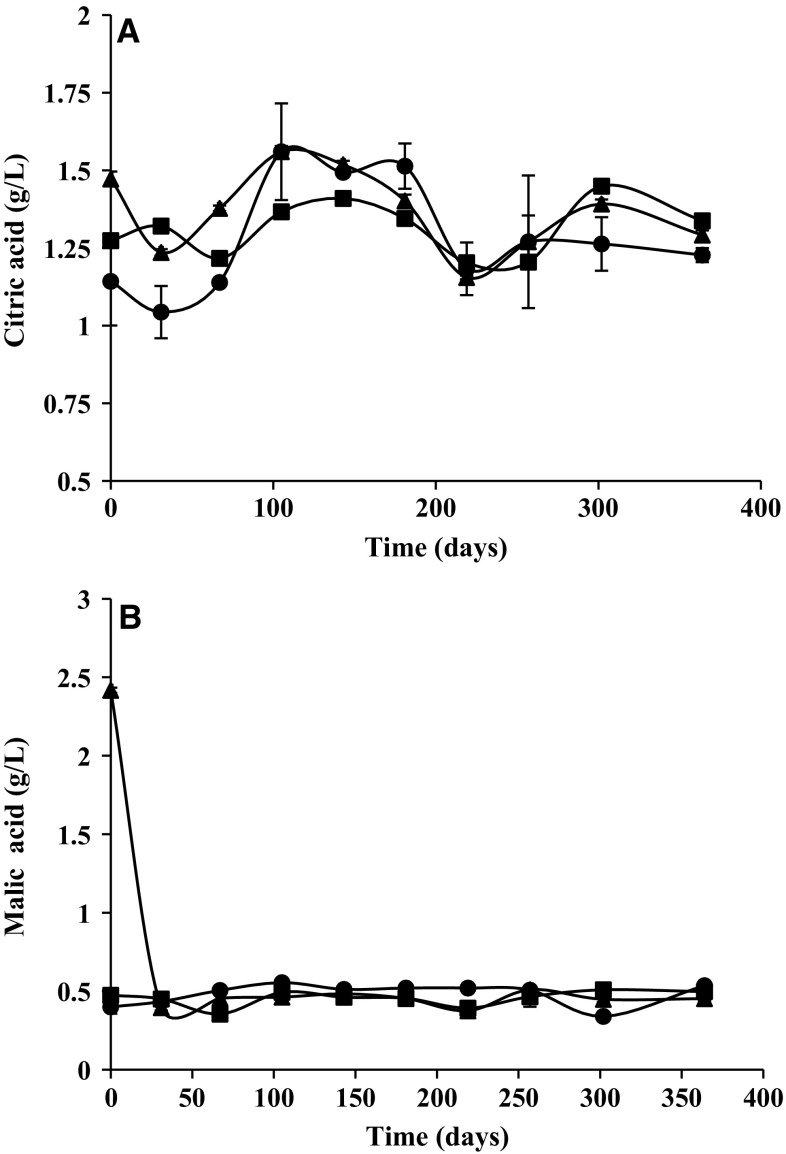
Organic acids
Figures 4a, b show the initial concentration of citric acid and malic acid in canned peach halves in syrup and its evolution during 1 year of storage at 30 °C. No statistical differences (P ≥ 0.05) were found for the citric and malic acid contents, regardless of acidifier used. The citric acid content ranged from 1.044 to 1.560 g/L, while the malic acid did from 0.340 to 0.555 g/L. Results obtained are similar to those found by Meredith et al. (1989). According to these authors by each 0.5 kg can of processed peaches, the citric and malic acid contents should be between 1013–1325 g and 450–497 g, respectively; and considering that preservation liquid provides about 435 g of citric acid, total content should be between 1.448 and 1.760 g/L.
Fig. 4.
Evolution of citric (a) and malic (b) acids in canned peach halves in syrup during 1 year of storage a 30 °C. Acidifier E330 (filled circle), turbid lemon juice (filled triangle) and clarified lemon juice (filled square)
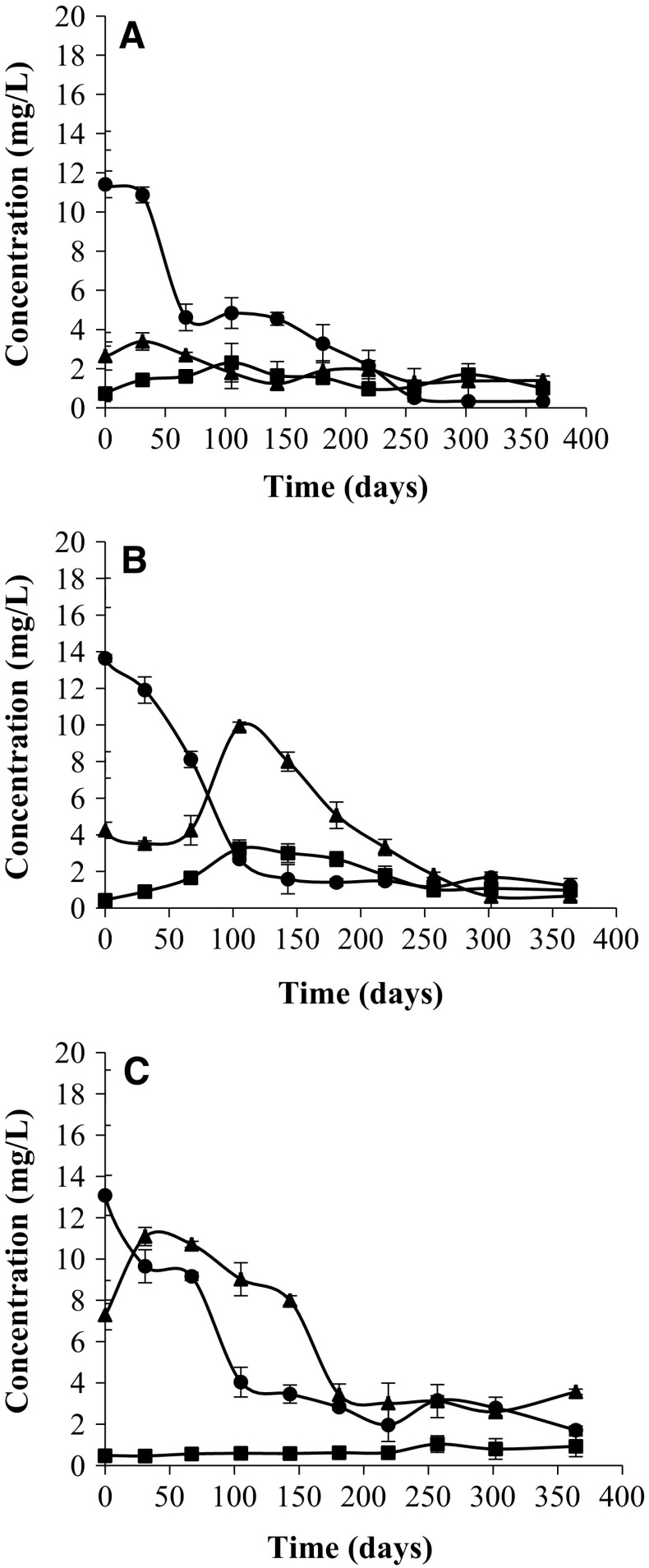
AA, DHA and DKG contents
Kinetic changes of AA, DHA and DKG contents in canned peach halves in syrup are shown in Fig. 5a–c. Degradation of AA was similar in all cases, independently of acidifying compound used. This pattern is consistent with that described in LJ. It is a degradative process with three consecutive stages and two products, as occurs in aerobic oxidative degradation of AA, giving DHA by a different route and later DKG by breaking the lactone ring.
Fig. 5.
Degradation of ascorbic acid (filled circle) and dehydroascorbic (filled triangle) and diketogulonic (filled square) acid production in canned peach halves acidified with E330 (a), turbid lemon juice (b) or clarified lemon juice (c) during 1 year of storage at 30 °C
The initial AA content of LJ-treated samples, measured immediately after sterilization, is greater than in the samples acidified with E-330. This is so because TLJ and CLJ contributed with 5.29 and 5.33 mg AA by can respectively, accounting for about 11.2 and 11.5 mg/L. This setting is especially evident when the DHA content is compared. For LJ-series the DHA content is much higher than in the E330-series, and this is so since the day of manufacture, probably due to the preheating process an important part of AA which provides LJ is oxidized to DHA. Its content in CLJ samples is higher than TLJ ones, although levels are very close. However, the DKG content was slightly lower in LJ-samples.
The AA content showed a downward trend with time, descending from the beginning to almost negligible levels after 1 year of storage at 30 °C. Meanwhile, the DHA content reached a maximum after 31–105 day of storage and then decreased to low levels between 0.4 and 3.7 mg/L. During the first 105 day of storage there was a severe loss of AA caused probably by the presence of oxygen. This behavior could be due to air incorporated into the product during processing (Nagy 1980). Although preheating and steam jets got substantially reduce the oxygen content in the cans, something must remain dissolved in the syrup and trapped in the headspace. In this moment AA contents were 42.42, 30.89 and 19.65% from the initial for E-330, TLJ and CLJ samples. These reductions in AA content were corresponded to maximums of DKG. Once consumed the residual oxygen, the degradation of AA was much slower. Finally, the AA content was reduced to 2.98% from the initial in the E-330 samples; this reduction was lower in LJ samples although with AA losses of 86.93 and 91.06% for TLJ and CLJ respectively.
The results agree with those determined by Bui-Nguyen (1985) for peaches in syrup. Although the measured values were lower than those reported, between 35.7 and 41.4 mg/L, the authors did not distinguish between AA and DHA and took samples reinforced with AA.
To compare the different indicators of NEB variation in peach halves in syrup throughout 1 year of storage at 30 °C, the relationships between A 420 parameter and each of them were adequately fitted to a linear model (y = a + bx). A significant positive correlation was found between A 420 and a (correlation coefficient r = 0.5795; P = 0.0008 < 0.01), La/b (r = 0.5865; P = 0.0007), glucose (r = 0.5865; P = 0.0010) and fructose (r = −0.5711; P = 0.0015) parameters at 99% confidence level. These r values indicate a moderately strong relationship between the variables. Total sugar was also positively correlated with A 420 (r = 0.3981; P = 0.0359 < 0.05) at 95% confidence level. Relatively weak relationships between A 420 and AA (r = −0.3823; P = 0.0371) or DHA (r = −0.4015; P = 0.0279) contents were found.
Conclusion
A 420, colorimetric parameters (CIELab, TCD and La/b), fructose, glucose and sucrose, total sugar, organic acids, AA, DHA, and DKG were used to estimate the extent of NEB in canned peach halves in syrup throughout 1 year of storage at 30 °C and the relationships between each of these parameters and A 420 were established. The investigation was carried out to explore the possibility of replacing E330 commonly used as acidifier by TLJ or CLJ to obtain a product having good nutrition with better retention of quality during storage. The a, La/b and A 420 values increased significantly and were higher for the samples acidified with E330 than for LJ-treated ones. BP formation (A 420) in E330 samples increased according to a zero-order reaction kinetic, but no specific color development occurred in LJ-treated samples. Glucose and fructose increased significantly with increasing storage time, while sucrose and total sugar decreased significantly, being both processes more intense in LJ-treated samples. The AA content showed a downward trend with time; DHA content reached a maximum after 31–105 days of storage and then decreased, and DKG content always remained at low levels with maximums at 105-day storage. The AA reduction was lower in LJ samples.
The a, La/b, glucose, fructose and total sugar were positively correlated with A 420, while AA and DHA were negatively correlated. From these parameters, a, La/b, glucose and fructose proved to be good indicators of browning development in canned peach halves stored at 30 °C during 1 year. Overall results showed that replacement of acidifier E330 by CLJ for controlling pH in canned peach halves in syrup had important advantages, including reduced formation of brown pigments, greater retention of AA, the development of a more natural product without loss of sensory quality, and the opening of new uses for LJ in food industry.
Acknowledgements
We gratefully acknowledge the excellent technical assistance and economical support from Mitra Sol Technologies S.L.
References
- Adekunte AO, Tiwari BK, Cullen PJ, Scannell AGM, O’Donnell CP. Effect of sonication on colour, ascorbic acid and yeast inactivation in tomato juice. Food Chem. 2010;122:500–507. doi: 10.1016/j.foodchem.2010.01.026. [DOI] [Google Scholar]
- Aguiló-Aguayo I, Soliva-Fortuny R, Martín-Belloso O. Avoiding non-enzymatic browning by high-intensity pulsed electric fields in strawberry, tomato and watermelon juices. J Food Eng. 2009;92:37–43. doi: 10.1016/j.jfoodeng.2008.10.017. [DOI] [Google Scholar]
- Arena E, Fallico B, Maccarone E. Thermal damage in blood orange juice: kinetics of 5-hydroxymethyl-2-furancarboxaldehyde formation. Int J Food Sci Technol. 2001;36:145–151. doi: 10.1046/j.1365-2621.2001.00436.x. [DOI] [Google Scholar]
- Ávila IMLB, Silva CLM. Modeling kinetics of thermal degradation of colour in peach puree. J Food Process Eng. 1999;39:161–166. doi: 10.1016/S0260-8774(98)00157-5. [DOI] [Google Scholar]
- Beveridge T, Harrison JE. Nonenzymatic browning in pear juice concentrate at elevated temperatures. J Food Sci. 1984;49:1335–1340. doi: 10.1111/j.1365-2621.1984.tb14984.x. [DOI] [Google Scholar]
- Bharate SS, Bharate SB. Non-enzymatic browning in citrus juice: chemical markers, their detection and ways to improve product quality. J Food Sci Technol. 2014;51(10):2271–2288. doi: 10.1007/s13197-012-0718-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buedo AP, Elustondo MP, Urbicain MJ. Non-enzymatic browning of peach juice concentrate during storage. Innov Food Sci Emerg Technol. 2001;1:255–260. doi: 10.1016/S1466-8564(00)00031-X. [DOI] [Google Scholar]
- Bui-Nguyen MH. Ascorbic acid and relates compounds. In: De Leenheer AP, Lambert WE, De Ruyter MGM, editors. Modern chromatographic analysis of the vitamins. New York: Marcel Dekker Inc; 1985. pp. 267–301. [Google Scholar]
- Burdurlu HS, Karadeniz F. Effect of storage on nonenzymatic browning of apple juice concentrates. Food Chem. 2003;80:91–97. doi: 10.1016/S0308-8146(02)00245-5. [DOI] [Google Scholar]
- Clegg KM. Citric acid and the browning of solutions containing ascorbic acid. J Sci Food Agric. 1966;17:546–549. doi: 10.1002/jsfa.2740171205. [DOI] [Google Scholar]
- Doner LW, Hicks KB. High-performance liquid chromatographic separation of ascorbic acid, erythorbic acid, dehydroascorbic acid, dehydroerythorbic acid, diketogulonic acid, and diketogluconic acid. Anal Biochem. 1981;115(1):225–230. doi: 10.1016/0003-2697(81)90550-9. [DOI] [PubMed] [Google Scholar]
- Garza S, Giner J, Martín O, Costa E, Ibarz A. Colour, sugars and HMF evolution during thermal treatment of apple juice. Food Sci Technol Int. 1996;2:101–110. doi: 10.1177/108201329600200207. [DOI] [Google Scholar]
- Garza S, Ibarz A, Pagán J, Giner J. Non-enzymatic browning in peach puree during heating. Food Res Int. 1999;32:335–343. doi: 10.1016/S0963-9969(99)00094-0. [DOI] [Google Scholar]
- Giner MJ, Hizarci Õ, Martí N, Saura D, Valero M. Novel approaches to reduce brown pigment formation and color changes in thermal pasteurized tomato juice. Eur Food Res Technol. 2013;236:507–515. doi: 10.1007/s00217-012-1900-y. [DOI] [Google Scholar]
- González-Molina E, Moreno DA, García-Viguera C. A new drink rich in healthy bioactives combining lemon and pomegranate juices. Food Chem. 2009;115(4):1364–1372. doi: 10.1016/j.foodchem.2009.01.056. [DOI] [Google Scholar]
- González-Molina E, Gironés-Vilaplana A, Mena P, Moreno DA, García-Viguera C. New beverages of lemon juice with elderberry and grape concentrates as a source of bioactive compounds. J Food Sci. 2012;77(6):C727–C733. doi: 10.1111/j.1750-3841.2012.02715.x. [DOI] [PubMed] [Google Scholar]
- Ibarz A, Miguelsanz R, Pagán J. The effect of high temperatures on nonenzymatic browning and formation of HMF in clarified peach juices. Fruit Process. 1993;3(7):262–265. [Google Scholar]
- Johnson JR, Braddock RJ, Chen CS. Kinetics of ascorbic acid loss and nonenzymatic browning in orange juice serum: experimental rate constants. J Food Sci. 1995;60:502–505. doi: 10.1111/j.1365-2621.1995.tb09812.x. [DOI] [Google Scholar]
- Kennedy JF, Rivera ZS, Lloyd LL, Warner FP, Jumel K. Studies on non-enzymic browning in orange juice using a model system based on freshly squeezed orange juice. J Sci Food Agric. 1990;52:85–95. doi: 10.1002/jsfa.2740520110. [DOI] [Google Scholar]
- Lee HS, Nagy S. Quality changes and nonenzymic browning intermediates in grapefruit juice during storage. J Food Sci. 1988;53:168–180. doi: 10.1111/j.1365-2621.1988.tb10201.x. [DOI] [Google Scholar]
- Lee HS, Nagy S. Relationship of sugar degradation to detrimental changes in citrus juice quality. Food Technol. 1988;42:91–94. [Google Scholar]
- Liu F, Zhou L, Cao X, Wang H, Liao X. Effects of storage temperature on quality changes of cold break tomato paste during storage. Trans Chin Soc Agric Eng. 2010;26(8):343–349. [Google Scholar]
- Martins SIFS, Jongen WMF, van Boekel MAJS. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci Technol. 2001;11:364–373. doi: 10.1016/S0924-2244(01)00022-X. [DOI] [Google Scholar]
- Mena P, Martí N, Saura D, Valero M, García-Viguera C. Combinatory effect of thermal treatment and blending on the quality of pomegranate juices. Food Bioprocess Technol. 2013;6:3186–3199. doi: 10.1007/s11947-012-0961-z. [DOI] [Google Scholar]
- Meredith FI, Robertson JA, Horvat RJ. Changes in physical and chemical parameters associated with quality and postharvest ripening of harvested peaches. J Agric Food Chem. 1989;37:1210–1214. doi: 10.1021/jf00089a002. [DOI] [Google Scholar]
- Nagy S. Vitamin C contents of citrus fruit and their products: a review. J Agric Food Chem. 1980;28:8–18. doi: 10.1021/jf60227a026. [DOI] [PubMed] [Google Scholar]
- Renard CMGC, Maingonnat J-F. Thermal processing of fruits and fruit juices. In: Sun D-W, editor. Thermal food processing: New technologies and quality issues. 2. Boca Raton: CRC Press; 2012. pp. 413–440. [Google Scholar]
- Robertson JA, Meredith FI, Horvat RJ, Senten SD. Effects of cool storage and maturity on the physical and chemical characteristics and volatile constituents of peaches (cv. Crashaven) J Agric Food Chem. 1990;38:620–624. doi: 10.1021/jf00093a008. [DOI] [Google Scholar]
- Saura D, Ros JM, Canovas JA, Gomez E, Laencina J. Tangential flow filtration with enzymatic treatment in the clarification of lemon juice. Mededelingen Van de Faculteit Landbouwwetenschappen, Rijksuniversiteit Gent. 1991;56:1705–1707. [Google Scholar]
- Saura D, Laencina J, Pérez-López AJ, Lizama V, Carbonell-Barrachina AA. Aroma of canned peach halves acidified with clarified lemon juice. J Food Sci. 2003;68(3):1080–1085. doi: 10.1111/j.1365-2621.2003.tb08292.x. [DOI] [Google Scholar]
- Saura D, Martí N, Laencina J, Lizama V, Carbonell-Barrachina AA. Sensory evaluation of canned peach halves acidified with clarified lemon juice. J Food Sci. 2004;69(2):74–78. [Google Scholar]
- Sawamura M, Takemoto K, Li ZF. 14C studies on browning of dehydroascorbic acid in an aqueous solution. J Am Chem Soc. 1991;39:1735–1737. [Google Scholar]
- Shin S, Bhowmik SR. Thermal kinetics of colour changes in pea puree. J Food Eng. 1995;24:77–86. doi: 10.1016/0260-8774(94)P1609-2. [DOI] [Google Scholar]
- Tannebaum SR, Archer MC, Young VR. Vitamins and minerals. In: Fennema OR, editor. Food chemistry. New York: Marcel Dekker; 1985. pp. 488–493. [Google Scholar]
- Tikekar RV, Anantheswaran RC, Elias RJ, LaBorde LF. Ultraviolet-induced oxidation of ascorbic acid in a model juice system: identification of degradation products. J Agric Food Chem. 2011;59:8244–8248. doi: 10.1021/jf201000x. [DOI] [PubMed] [Google Scholar]
- Trifiro E, Landi S. Sull’acidificazione dei nettari di fruta con suceo di limone. Ind Conserve. 1965;40:90–93. [Google Scholar]
- Wang HY, Hu XS, Chen F, Wu JH, Zhang ZH, Liao XJ, Wang ZF. Kinetic analysis of non-enzymatic browning in carrot juice concentrate during storage. Eur Food Res Technol. 2006;223:282–289. doi: 10.1007/s00217-005-0202-z. [DOI] [Google Scholar]
- Ziegler SJ, Meier B, Sticher O. Rapid and sensitive determination of dehydroascorbic acid in addition to ascorbic acid by reverse-phase high-perfomance liquid chromatography using a post-column reduction system. J Chromatogr A. 1987;391:419–426. doi: 10.1016/S0021-9673(01)94343-2. [DOI] [PubMed] [Google Scholar]