Abstract
Bioactive compounds and antioxidant activity were evaluated from industrial Jalapeño pepper byproducts and simulated non processed byproducts from two Mexican states (Chihuahua and Sinaloa) to determine their value added potential as commercial food ingredients. Aqueous 80% ethanol produced about 13% of dry extract of polar compounds. Total phenolic content increased and capsaicin and dihydrocapsaicin decreased on scalding samples (80 °C, 2 min) without affecting ascorbic acid. The major phenolic compounds, rutin, epicatechin and catechin comprised 90% of the total compounds detected by HPLC of each Jalapeño pepper byproducts. ORAC analysis showed that the origin and scalding process affected the antioxidant activity which correlated strongly with capsaicin content. Although scalding decreased capsaicinoids (up to 42%), phenolic content by (up to 16%), and the antioxidant activity (variable). Jalapeño pepper byproduct is a good source of compounds with antioxidant activity, and still an attractive ingredient to develop useful innovative products with potential food/non-food applications simultaneously reducing food loss and waste.
Keywords: Byproduct, Valorization, Jalapeño pepper, Antioxidant activity, Bioactive compounds
Introduction
Jalapeño is one of the varieties of peppers most preferred by consumers in Mexico where 836,246 t are produced annually (SIAP 2015). Most of the harvest (60%) is pickled and canned, and about 20% is consumed raw (Galicia-Cabrera 2005). One of the most popular industrialized Jalapeño pepper product is the pickled non-fermented sliced and canned pepper and considerable amount (more than 500 t, only in the Northwest region of Mexico) of byproduct is generated during their preparation (Sandoval-Castro et al. 2014). This byproduct-consisting of seeds and placenta of Jalapeño pepper-is eliminated during the preliminary operation in pickling after scalding (Galicia-Cabrera 2005) and currently not exploited but discarded as waste. Such waste increases the contribution of vegetables as a commodity to the economic cost of food loss and waste (23% of total cost) with considerable carbon footprint (21% of total) (FAO 2013). Cost-effective and environmentally friendly reuse is one of the strategies advocated to reduce food loss and waste due to processing from local facilities (FAO 2014). Therefore, adding value to the Jalapeño pepper byproduct is a viable solution to reduce food loss and waste by simultaneously developing useful innovative products.
Anatomically, pepper consists primarily of the pericarp that includes the exocarp (skin), mesocarp and endocarp and seeds that are attached to the placenta (Bosland and Votava 2012). The pericarp contains up to 20% (dry weight) cellulose and other fibrous material, whereas the skins, a by-product of canning green New Mexican pods contain 77% soluble fiber and 80% total dietary fiber. The dry pericarp and seeds contain 16–17 and 18% protein, respectively. The placenta consists of vesicles known for the production of capsaicinoids and oleoresin (Bosland and Votava 2012). Extracts from red pepper pericarp exhibit strong ferrous-chelating and high free radical (OH– and DPPH) scavenging activity, but weak superoxide dismutase (SOD) activity due to the presence of high total phenolic and flavonoid contents (Bosland and Votava 2012). Total phenolic content of ethanol (80%) extracts and their antiradical potential from the pericarp were reported to be higher than from placenta (2175 vs 2082 mg chlorogenic acid equivalents per kg of fresh pepper). However, phenolic acid derivatives predominated in the placenta, particularly the ten times higher trans-p-feruloyl-β-D glucoside levels than in the pericarp (Materska 2014).
Pepper seeds have been investigated as an alternative source of bioactive compounds in an effort to reduce/reuse byproduct or wastes of the pepper processing industry (Silva et al. 2013). A similar study examined utilization of seed waste as an antioxidant due to increased red pepper production in Korea and global consumption (Sim and Sil 2008). Our waste reduction research has focused on byproducts utilization from food industries by identifying them as sources of bioactive compounds with potential industrial application. In this regard, we demonstrated the potential use of tomato peel, seeds and byproducts from the tomato processing industry in neighboring region (Valdez-Morales et al. 2014). The current investigation aims to valorize Jalapeño industrial byproduct by characterizing the effects of the scalding process on hydrophilic compounds, phenolic, ascorbic acid and capsaicinoids contents and in vitro antioxidant capacity of industrial Jalapeño pepper byproducts from two different local sources/origins. This characterization of Jalapeño pepper byproducts may lead to their enhanced utilization and/or development of novel products for potential food/non-food applications.
Materials and methods
Biological material
Raw Jalapeño pepper (C. annuum) and a Jalapeño byproduct after the scalding process were obtained from La Costeña—a Vegetable Processing Industry located in Sinaloa Mexico. The peppers were produced in August and January 2014, and harvested from two production states Chihuahua and Sinaloa respectively, in Mexico. The byproduct was obtained after the scalding (80 °C, 2 min) and slicing processes. In order to compare the effect of scalding process on metabolites and their antioxidant activity, the raw Jalapeño pepper was sliced; the seeds together with the placenta were removed and combined to obtain a simulated raw byproduct, in the same proportions than that generated in the industry (Sandoval-Castro et al. 2014). Simulated raw Chihuahua (RCh) and Sinaloa (RSin), and scalded Chihuahua (SCh) and Sinaloa (SSin) byproducts were lyophilized and stored in the dark at −20 °C. Samples were pulverized in a TissueLyser II (Quiagen, F = 30 1/s, during 3 min), stored in the dark at −20 °C until use. Lipids were removed from the dry milled pepper samples with hexane using the Soxhlet extraction.
Chemical compounds, standards and ACS solvents were purchased from Sigma-Aldrich (St. Louis, MO, USA) and HPLC-grade acetonitrile and methanol, were purchased from Karal (Leon, Guanajuato, Mexico).
Ethanol extraction
Dry defatted samples (500 mg) were extracted with 80% (v/v) aqueous ethanol (5 mL) 30 min at room temperature on a sonicator (Fischer Scientific FS20, Massachusetts, USA). Aqueous ethanol (15 mL) was added to the mixture, agitated (400 rpm, 2 h, room temperature, Corning Magnetic Stirrer, MP9l, New York, USA) and centrifuged (1610 g, 10 min, Sorvall ST 16R, Thermo Scientific, Massachusetts, USA). The residue was re-extracted with aqueous ethanol (20 mL) with repeated agitation and centrifugation. The ethanol extracts were pooled, ethanol removed (vacuum rotary evaporation, 40 °C, Yamato RE-300, Santa Clara, CA, USA), extraction yield calculated and expressed as g of dry extract per kg of dry defatted material. The powdered extracts were reconstituted with 80% aqueous ethanol (20 mg per mL) for total phenolic and antioxidant activity determination and with absolute methanol for phenolic HPLC analysis. Extracts were stored in the dark at −20 °C until analysis.
Total phenolics
The modified Folin–Ciocalteau assay (Nurmi et al. 1996) adapted for microplates was used to determine total phenolic content of extracts. Absorbance was monitored at 760 nm (Thermo Scientific Multiskan GO spectrophotometer, Waltham, MA) using gallic acid (0–200 µg per mL) and (+)-catechin (50–300 µg per mL) as standards. The results were expressed as g gallic acid equivalents (GAE) and (+)-catechin (CAE) per kg of dry defatted byproduct (ddb).
Ascorbic acid and capsaicinoids analyses
Samples (500 mg, ddb) were extracted with acidified (0.125% formic acid, v/v) methanol according to the procedure of Qin et al. (2014). Briefly, samples were sonicated (15 min, Fisher Scientific FS20), centrifuged (3550 g, 15 min, room temperature) to recover the supernatant. Ascorbic acid was determined by incubating 500 μL extract in the dark for 1 h with 5 μL 0.05% 2-mercaptoethanol. The mixture was recovered and filtered (0.2 μm PTFE filter) prior to chromatographic separation on a Supelco Discovery HS C18 column (150 mm × 2.1 mm i.d., 3 μm; Sigma Aldrich, Co. USA.) at 25 °C (Alós et al. 2013).
HPLC analysis was performed using a Dionex UltiMate 3000 liquid chromatograph with isocratic (water acidified with acetic acid [pH 2.4]) elution at 0.3 mL min−1 flow rate and 245 nm detection. Ascorbic acid (0.05–0.5 mg per mL) was used as reference standard and results expressed as g per kg ddb.
Capsaicinoids were separated under an isocratic elution using the same HPLC system as above with two solvent systems: (a) acetonitrile: methanol (70:30, v/v; 0.14 mL min−1) and (b) water (0.06 mL min−1) at 0.2 mL min−1 and 25 °C. Capsaicin (20 ng per mL) and dihydrocapsaicin (40 ng per mL) in methanol solutions were used as standards and results expressed in g per kg ddb.
HPLC analysis of phenolic acids
Phenolic acids (5 mg per mL) in the reconstituted methanol extracts were analyzed on an HPLC (Dionex UltiMate 3000) system equipped with a titanium quaternary pump (LPG-3400AB), an autosampler (WSSIN-3000TBPL), a photodiode array detector (DAD-3000, Thermo Fisher Scientific, New York, NY) and Chromeleon 7.0 software (Dionex, Thermo Fisher Scientific, New York, NY). The separation was carried out with an Acclaim® 120 A C18 column (250 mm × 4.6 mm i.d., 5 μm; Thermo Fisher Scientific, New York, NY, USA). Chromatographic separation was performed according to established procedures (Espinosa-Alonso et al. 2006; Valdez-Morales et al. 2014) with 10 μL extract using two solvent systems: (a) water acidified with acetic acid (pH 2.8) and (b) acetonitrile at room temperature. A linear gradient solvent B 5–95% for 40 min was used for eluting the phenolic compounds followed by return to initial condition. The chromatograms were recorded at 260, 280, 300, 342, 350 and 375 nm. Phenolics were quantified using caffeic, chlorogenic, ferulic, gallic, 3,4-hydroxybenzoic, 2,5-hydroxybenzoic, o-coumaric, p-coumaric, sinapic, syringic, trans-cinnamic and vanillic acids; and flavonoids: (+) catechin hydrate, 6,7-dimethyl hydroxycoumarin, (−)-epicatechin, 7-hydroxycoumarin, naringenin, quercetin, quercetin 3-β-D-glucoside, and rutin hydrate as standards. Samples were filtered through a 0.2 μm PTFE filter prior to injection and results expressed in g per kg ddb.
Antioxidant activity
Trolox equivalent antioxidant capacity (TEAC) assay
TEAC assay based on ABTS·+ was performed according to the method of Pellegrinini et al. (2003). ABTS·+ work solution was diluted with 80% ethanol to obtain an absorbance value of 0.70 ± 0.02 at 734 nm. Aqueous 80% ethanol extract, blank and controls/standard (20 μL) was mixed with ABTS·+ work solution (200 μL), gently vortexed during 20 s, and absorbance monitored at 734 nm. Trolox (0–500 μM) was used as standard, and the results expressed as mmol TE per kg ddb.
DPPH method
Antioxidant activity was determined by the DPPH method adapted for use with microplates as previously described (Cardador-Martínez et al. 2006). Briefly, a solution of DPPH (200 μL, 0.24 µM) prepared in 80% methanol was mixed with sample extract (20 μL). Absorbance at 515 nm was measured with a spectrophotometer (Thermo Scientific Multiskan GO) from 0 to 60 min. Trolox (50–500 μM) was used as standards, and the results expressed as mmol TE per kg ddb.
IC50 values were calculated for ABTS and DPPH assays using the semi-logarithmic (y = * ln (x) ± b) and linear (y = mx + b) equations, respectively, versus % ARA at different concentrations (0.1, 0.5, 1.5, 7.5, 10, 12.5 and 20 mg per mL) for samples; (0.05, 0.1, 0.15, 0.2, 0.25 and 0.3 mg per mL) ascorbic acid; (50,100, 200, 300, 400, 500 μmol) Trolox, and (0.05, 0.1, 0.15, 0.2, 0.25, 0.3 mg per mL) BHT. IC50 was expressed as mg per mL for ABTS and DPPH.
Oxygen radical absorbance capacity (ORAC)
Antioxidant activity was measured according to the ORACFL assay described previously (Prior et al. 2003). A DTX 880 Multimode Detector (Beckman Coulter, Brea, CA, USA) was used with excitation and emission wavelengths at 485 and 530 nm, respectively. Sample extracts and Trolox standards (0–400 μM) were diluted with 75 mM phosphate buffer (pH 7.4) prior to transfer into a 96-well microplate. A peroxyl radical was generated using AAPH [2,2′-azobis(2-methylpropionamide)dichloride] (70 mM) during measurement at 37 °C, and fluorescein (87 μM) was used as substrate. Final ORAC values were calculated using a regression equation between the Trolox concentration (0–400 μM) and the net area under the curve and expressed as mmol TE per kg ddb.
Statistical analysis
At least three independent experiments were conducted for all assays with four experimental replicates. Analysis of variance by the general linear models (GLM) procedure, means comparison by Duncan’s test and Pearson correlation were performed according to Statistical Analysis System, SAS 9.1 for Windows.
Results and discussion
Aqueous 80% ethanol produced significantly (P < 0.05) higher yield from RCh than other samples (Table 1). The yields were higher than those previously reported for ethanol extract of two Italian bell pepper cultivars (Loizzo et al. 2013). Furthermore, simulated raw byproducts yields were twice those reported for aqueous methanol (80%) extract of Jalapeño pepper (cv. Ixtapa) grown in Texas, thereby confirming the strong solvent extraction effect (Bae et al. 2012). The scalding process significantly (P < 0.05) reduced extraction yield (13.4%) of simulated raw Chihuahua byproduct (RCh), although that reduction (5%) was insignificant for samples from Sinaloa. Similar reductions in extraction yields (15–36%) have been reported after boiling whole fresh yellow Cayenne and red chili peppers from five Italian cultivars for 10 min (Loizzo et al. 2015).
Table 1.
Hydrophilic yield extraction, total phenols, ascorbic acid and capsaicinoids content (g per kga) of raw and scalded Jalapeño byproduct from Chihuahua and Sinaloa
| Sample | Yield | Total phenolic | Ascorbic acid | Capsaicin | Dihydrocapsaicin | |
|---|---|---|---|---|---|---|
| GAE | CAE | |||||
| RCh | 141.4 | 10.01 ± 0.61b | 12.00 ± 0.41c | 0.0496 ± 0.008 | 0.292 ± 0.304a | 0.1206 ± 0.013b |
| SCh | 123.7 | 13.09 ± 0.38a | 15.57 ± 1.05a | 0.0419 ± 0.003 | 0.165 ± 0.114b | 0.1002 ± 0.004b |
| RSin | 132.5 | 10.70 ± 0.27b | 13.92 ± 0.68b | 0.0394 ± 0.014 | 0.284 ± 0.105a | 0.1622 ± 0.017a |
| SSin | 125.9 | 12.12 ± 0.79a | 14.27 ± 0.76ab | 0.0436 ± 0.015 | 0.142 ± 0.123b | 0.1170 ± 0.006b |
| Raw | 136.9 | 10.35y | 12.96y | 0.0435 | 0.2881x | 0.1414x |
| Scalded | 124.8 | 12.60x | 14.92x | 0.0428 | 0.1534y | 0.1086y |
Mean ± SD (n = 3)
Different letters in the same column indicate significant differences between samples (P < 0.05)
R raw, S scalded, Ch Chihuahua, Sin Sinaloa
a ddb dry defatted byproduct
Total phenolics
Scalding significantly increased total phenolic compared to simulated raw Jalapeño byproduct (22 and 15% when expressed as GAE and CAE, respectively) (Table 1) and were similar to those reported previously (Alvarez-Parrilla et al. 2011). Similar increase in total phenolic content (expressed as g GAE per kg) have been reported during smoking of red Jalapeño pepper (Moreno-Escamilla et al. 2015), and boiling (96 °C, 13.5 min) of Chihuahua Jalapeño peppers (Ornelas-Paz et al. 2010). RCh had significantly lower total phenolic content than those from Sinaloa, indicating environmental differences. Total phenolic content (g GAE per kg) of our simulated raw samples were 60% lower than those reported for the 80% methanol extract of whole Jalapeño pepper (Bae et al. 2012), although phenolic acids are presumed to predominate in the placenta (Materska 2014).
Ascorbic acid and capsaicinoids analyses
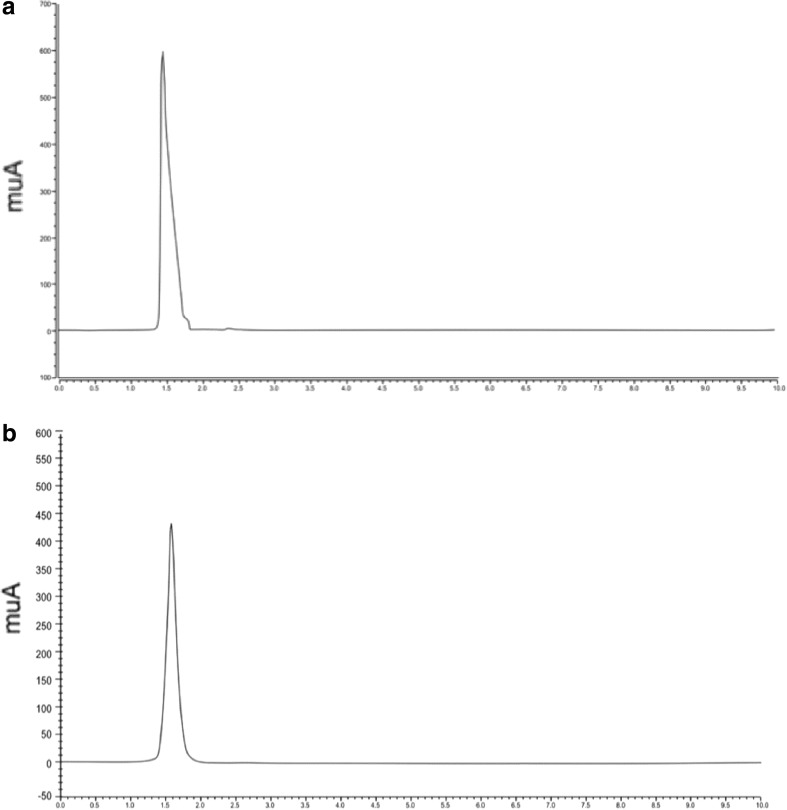
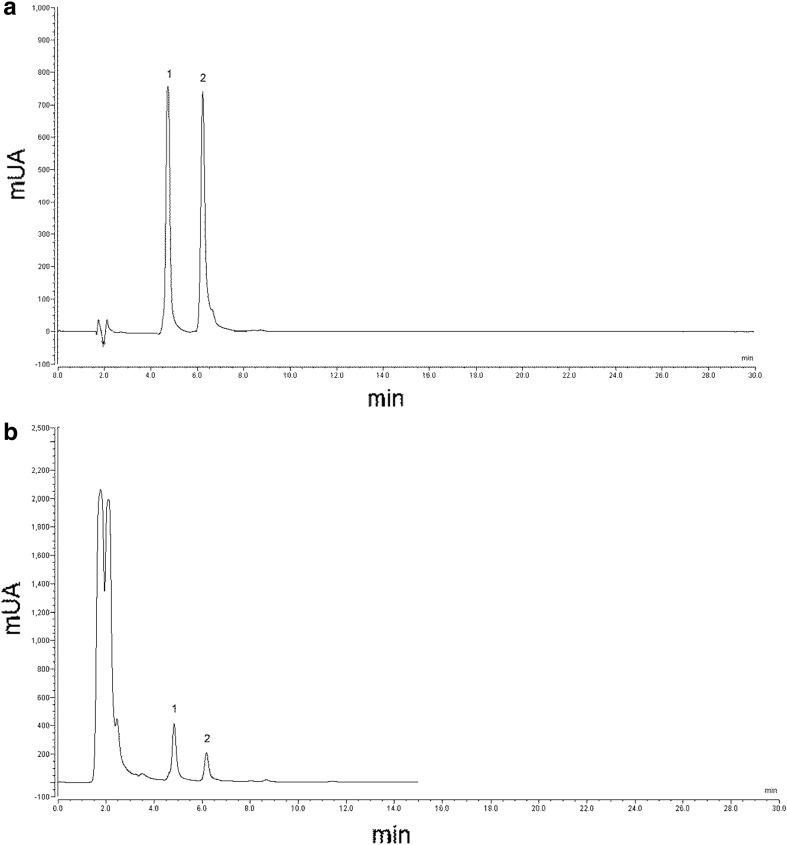
Ascorbic acid content (Table 1; Fig. 1) did not differ significantly among the samples suggesting its retention after the scalding process similar to those observed after blanching and pasteurization of Jalapeño peppers (Howard and Wildman 2006). However, ascorbic acid contents of our samples were lower than those reported earlier (Silva et al. 2013), probably due to the absence of pericarp where ascorbic acid accumulation is presumed to occur (Castro-Concha et al. 2012). Scalding significantly (P < 0.05) reduced capsaicin and dihydrocapsaicin contents of the Jalapeño byproducts (Table 1; Fig. 2) similar to those reported for boiling Jalapeño peppers (Ornelas-Paz et al. 2010), or cooked peppers due to capsaicin oxidation catalyzed by high temperature (Moreno-Escamilla et al. 2015). Furthermore, RSin had significantly (P < 0.05) higher dihydrocapsaicin resulting in its significantly higher retention upon scalding than those from Chihuahua.
Fig. 1.
HPLC-DAD chromatogram a ascorbic acid standard and b scalded Chihuahua extract. UV detection at 245 nm
Fig. 2.
HPLC-DAD chromatogram of a capsaicinoids standards (peaks: 1 capsaicin; 2 dihydrocapsaicin) and b scalded Chihuahua extract. UV detection at 210 nm
HPLC analysis of phenolic compounds
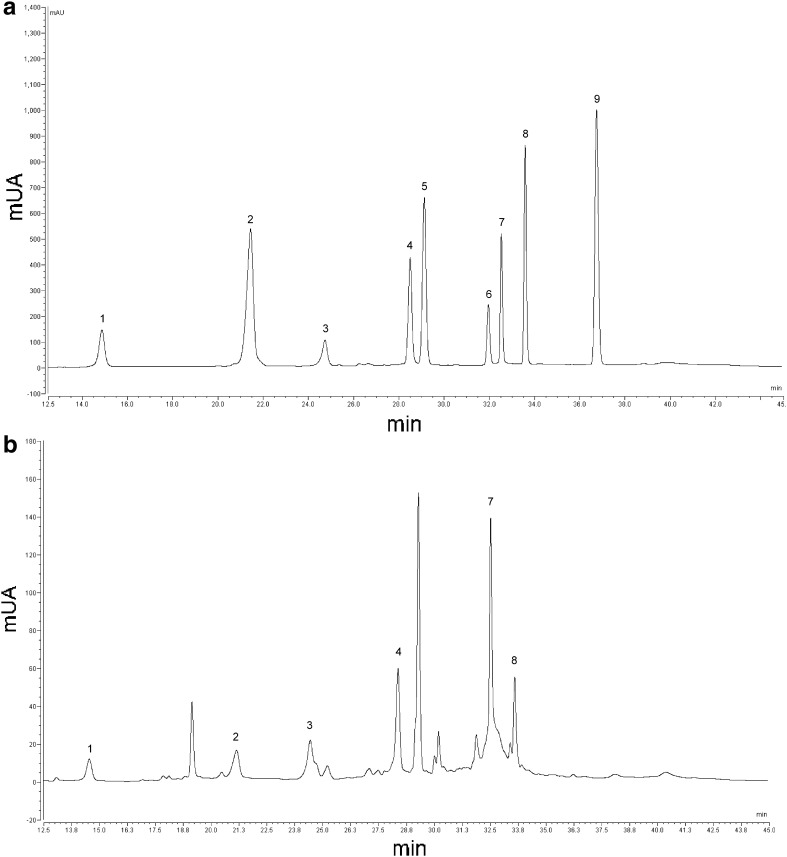
Total phenolic compounds were most abundant in simulated raw Chihuahua than Sinaloa Jalapeño pepper byproducts (19%) and reduced by scalding (22 and 13%, respectively) (Table 2, Fig. 3). The phenolic composition according to the HPLC analysis was not significantly different among the samples, except for caffeic and ferulic acids and rutin (P > 0.05). Thus, RCh and SSin had the highest and the lowest caffeic acid and rutin contents, respectively, whereas ferulic acid content was most and the least abundant in RCh and RSin, respectively. Differences in caffeic acid have been reported with no changes in ferulic acid content of fresh peppers (Troconis-Torres et al. 2012). The major phenolic compounds, rutin, epicatechin and catechin accounted for 90% with rutin comprising 1/3 of the total peak area for all samples. Their high concentration in our samples suggested that these Jalapeño pepper byproducts were excellent sources of flavonoids similar to red pepper pericarp and seed extracts (Sim and Sil 2008). Phenolic compounds in RCh were generally higher than those in RSin, except for catechin. For example, RCh had significantly (P < 0.05) higher caffeic, ferulic and coumaric acids, and lower catechin contents than RSin, indicating thereby differences in Jalapeño origin. Similarly, SCh had significantly (P < 0.05) higher caffeic acid and lower 3,4-dihydroxybenzoic and coumaric acids than SSin. Scalding significantly (P < 0.05) reduced both catechin and rutin and increased coumaric acid contents only in Sinaloa Jalapeño. Coumaric acid content also decreased significantly (P < 0.05) during scalding of Chihuahua Jalapeño. Overall, scalding significantly (P < 0.05) reduced chlorogenic acid when simulated raw Jalapeño was compared to scalded byproducts. Similar reduction in chlorogenic acid (22%) has been reported in Jalapeño pepper due to smoking (Moreno-Escamilla et al. 2015).
Table 2.
Phenolic content (g per kgc) of raw and scalded Jalapeño byproduct from Chihuahua and Sinaloa, determined by HPLC
| Phenolic compounds | RCh | SCh | RSin | SSin |
|---|---|---|---|---|
| Gallic acid | 1.002 ± 0.097a | 0.982 ± 0.090a | 0.971 ± 0.072a | 0.952 ± 0.072a |
| Caffeic acid | 0.449 ± 0.028a | 0.437 ± 0.020a | 0.214 ± 0.015b | 0.187 ± 0.017b |
| Ferulic acid | 1.145 ± 0.081a | 1.037 ± 0.183ab | 0.814 ± 0.027b | 0.934 ± 0.108ab |
| 3,4-Dihydroxybenzoic acid | 0.087 ± 0.028b | 0.094 ± 0.005b | 0.107 ± 0.021b | 0.148 ± 0.015a |
| Chlorogenic acid | 0.682 ± 0.102a | 0.559 ± 0.050a | 0.713 ± 0.119a | 0.569 ± 0.032a |
| o-Coumaric acid | 0.150 ± 0.020a | 0.091 ± 0.026b | 0.071 ± 0.016b | 0.141 ± 0.010a |
| Cathechin | 6.848 ± 0.107ab | 6.357 ± 1.056b | 7.907 ± 0.327a | 7.026 ± 0.192ab |
| Epicathechin | 12.614 ± 3.936a | 10.544 ± 2.036a | 8.849 ± 0.751a | 9.256 ± 2.755a |
| Rutin | 15.611 ± 2.563a | 12.488 ± 2.064ab | 11.441 ± 0.772b | 9.263 ± 0.692b |
| Kaemferol | 0.098 ± 0.034a | 0.101 ± 0.014a | 0.083 ± 0.004a | 0.090 ± 0.008a |
| Total | 38.686 | 32.358 | 31.168 | 28.575 |
Mean ± SD (n = 3)
Different letters in the different columns indicate significant differences between samples (P < 0.05)
R raw, S scalded, Ch Chihuahua, Sin Sinaloa
c ddb dry defatted byproduct
Fig. 3.
HPLC-DAD chromatogram a phenol acids standards (peaks: 1 gallic acid; 2 3,4-dihydroxy benzoic acid; 3 chlorogenic acid; 4 caffeic acid; 5 vanillic acid; 6 syringic acid; 7 ferulic acid; 8 o-coumaric acid; 9 trans-cinnamic acid) and b scalded Sinaloa extract. UV detection at 280 nm
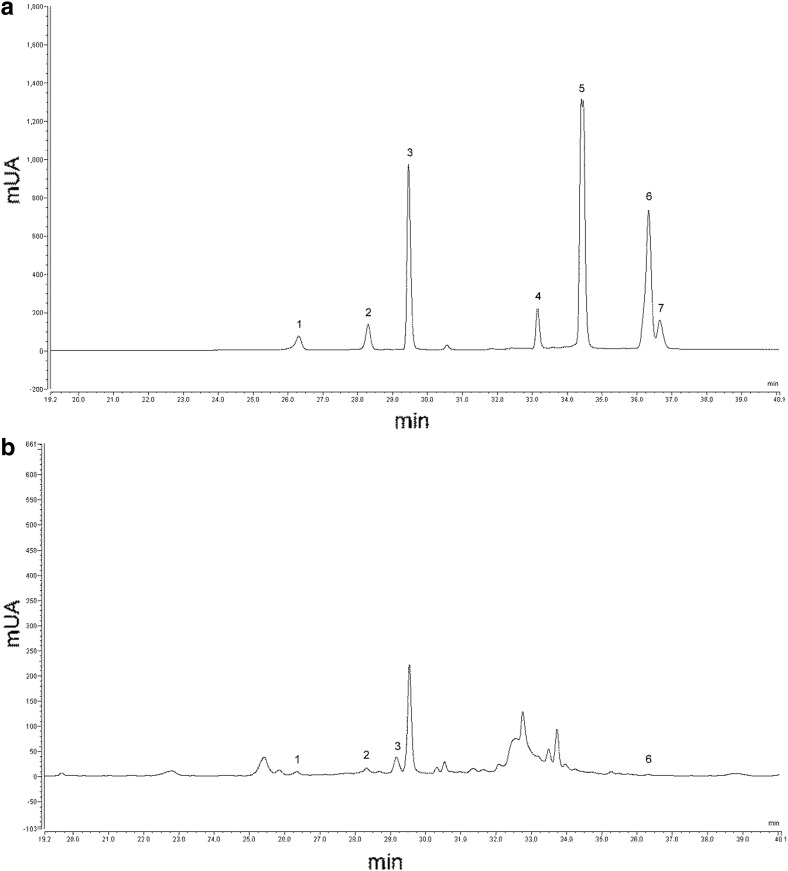
The three major flavonoids, catechin, epicatechin and rutin in our samples have been reported only once before in Jalapeño peppers (Medina-Juárez et al. 2012) (Fig. 4), together with gallic, caffeic and chlorogenic acids. Catechin, rutin, and gallic, caffeic, ferulic and coumaric acids have been reported in Mexican chili peppers (Troconis-Torres et al. 2012). However, the reported values were low since they were expressed in g per kg fresh fruit weight. Rutin content in our samples were 5–9 and 4–7 folds higher than those determined in red pepper and red chili pepper, respectively by the AOAC official methods (Atanassova and Bagdassarian 2012). Chlorogenic and coumaric acids were abundant in the pericarp, placenta and stalk of Jalapeño pepper, although caffeic, ferulic, and 3,4-dihydroxybenzoic acids and kaempferol were also present in the placenta (Chen and Kang 2013). High epicatechin content together with gallic and 3,4-dihydroxybenzoic acids have also been reported in seven Chinese pepper cultivars (Zhuang et al. 2012). The Jalapeño byproducts rich in flavonoids may provide the same coronary disease protection conveyed by red wine flavonoids (Lagiou et al. 2004).
Fig. 4.
HPLC-DAD chromatogram a flavonoids standards (peaks: 1 catechin; 2 epicathechin; 3 rutin; 4 hydroxycoumarin; 5 quercetin; 6 kaempferol; 7 dimethyl hydroxycoumarin) and b flavonoids identified in scalded Sinaloa extract. UV detection at 280 nm
Antioxidant activity
Trolox equivalent antioxidant capacity (TEAC) and DPPH assay
Jalapeño pepper byproducts exhibited antioxidant activity (ABTS and DPPH expressed in mmol Trolox per kg) not significantly different among the samples (Table 3). Byproduct displaying lower antioxidant activity compared to whole fresh Jalapeño fruit has previously been reported; ABTS and DPPH values were between 7–8 and 5–6 times lower, respectively than those reported earlier for whole Jalapeño peppers (Alvarez-Parrilla, et al. 2011; Cervantes-Paz et al. 2012; Moreno-Escamilla et al. 2015). This inferior antioxidant activity of our samples is due to the predominance of seeds, placenta and pericarp known to exhibit low activity compared to whole and/or fresh Jalapeño fruit (Ornelas-Paz et al. 2010). However, the antioxidant activity of the Jalapeño byproducts was 5–10 times higher than those found in tomato byproduct using the same ABTS and DPPH methods (Valdez-Morales et al. 2014). Processing reduced the ABTS and DPPH radical scavenging activities by 7 and 19% after scalding of simulated raw Chihuahua Jalapeño byproduct. Similar reductions in antioxidant activities were reported for South Korean peppers after stir-frying (Hwang et al. 2012). However, this reduction was not observed in processing of simulated raw Jalapeño byproduct from Sinaloa suggesting differential effects due to source/origin of pepper. Furthermore, ABTS values were approximately 37% higher than those of DPPH in accordance with previous reports (Alvarez-Parrilla et al. 2011; Moreno-Escamilla et al. 2015).
Table 3.
Antioxidant activity and IC50 of raw and scalded Jalapeño byproduct from Chihuahua and Sinaloa
| Byproduct | ABTS (mmol TE per kgc) | IC50 ABTS (mg per mL) | DPPH (mmol TE per kgc) | IC50 DPPH (mg per mL) | ORAC (mmol TE per kgc) |
|---|---|---|---|---|---|
| RCh | 6.627 ± 0.941a | 0.601 ± 0.013b | 4.383 ± 0.616a | 6.78 ± 0.11c | 16.138 ± 0.941a |
| SCh | 6.190 ± 0.775a | 0.727 ± 0.005a | 3.681 ± 0.635a | 9.39 ± 0.57a | 8.591 ± 0.399b |
| RSin | 6.657 ± 1.042a | 0.621 ± 0.043b | 4.061 ± 0.525a | 6.45 ± 0.06c | 12.181 ± 0.668ab |
| SSin | 6.872 ± 1.273a | 0.717 ± 0.021a | 4.523 ± 0.748a | 7.83 ± 0.20b | 10.978 ± 0.973b |
Mean ± SD (n = 3)
Different letters in the same column indicate significant differences between samples (P < 0.05)
TE trolox equivalents, R raw, S scalded, Ch Chihuahua, Sin Sinaloa
c ddb dry defatted byproduct
ORAC assay
ORAC was the only antioxidant activity methodology that clearly differentiated the samples with the simulated raw and scalded Chihuahua Jalapeño displaying the highest and lowest ORAC values, respectively (Table 3). Both the origin of the Jalapeño pepper and the scalding process affected the ORAC antioxidant activity significantly (P < 0.05) in contrast to previous report (Alvarez-Parrilla et al. 2011), where no significant differences were observed among peppers. Scalding significantly (P < 0.05) reduced ORAC (47% and 10% for SCh and SSin) of their respective simulated raw Jalapeño byproduct values. This concurs with those observed by Loizzo et al. (2015), supporting the claim that peppers differs in their sensitivity to processing.
IC50 values based on ABTS and DPPH radical scavenging activity also showed significant (P < 0.05) differences among samples (Table 3). Furthermore, scalding significantly (P < 0.05) reduced scavenging activity compared to simulated raw Jalapeño byproduct with SCh exhibiting the lowest DPPH scavenging activity. RSin displayed higher scavenging activity (lower IC50 for both ABTS and DPPH) than those from Chihuahua. These IC50 values discriminated samples based both on Jalapeño origin and processing similar to those of ORAC. Antiradical scavenging activity of our samples determined by IC50 values were lower than those reported previously for whole pepper fruit and/or their extracts (Tundis et al. 2011; Loizzo et al. 2013), suggesting that they contained more bioactive compounds than those present in our simulated raw byproducts. Scalding reduced the unit contribution of the total phenolics (g GAE per kg) to antioxidant activity (DPPH) expressed as PAOXI, the antioxidant potential relative to phenolic content (Oomah et al. 2008).
Correlation analysis
The comparison of the byproduct constituents revealed strong correlation with antioxidant activity (Table 4). Total phenolic content (expressed as g GAE or CAE per kg ddb) was inversely associated with capsaicin and ORAC values and positively correlated with IC50DPPH with higher significance for GAE compared to CAE (P < 0.005 vs <0.05). Similar correlation has been reported between IC50DPPH and phenolic contents of eight pepper genotypes (Carvalho et al. 2015). Capsaicin content was positively correlated with dihydrocapsaicin content and ORAC values and inversely associated with IC50 (ABTS and DPPH) values. Similarly, dihydrocapsaicin content and IC50 values were inversely related suggesting strong (P < 0.0001) IC50 ABTS dependence on dihydrocapsaicin. The antioxidant activities determined by ABTS and DPPH were strongly (P < 0.0001) related, but not associated with total phenolic and capsaicinoid contents. Similar relationship between total phenols and ABTS has been reported previously in fresh and processed Jalapeño peppers (Alvarez-Parrilla et al. 2011). The Pearson correlation was low between the two methodologies (DPPH and ORAC) used to determine antioxidant capacity and similar to those reported previously (Kevers et al. 2007). IC50 DPPH was inversely associated with ORAC but correlated positively with IC50 ABTS. Ascorbic acid content showed no association with any analytes or antioxidant activities, although it was moderately correlated (r = 0.646, P < 0.05) with extraction yield (data not shown) contrary to earlier studies (Alvarez-Parrilla et al. 2011). Furthermore, catechin one of the major phenolic compounds, was positively (r = 0.657, P < 0.03) correlated with dihydrocapsaicin and inversely associated (r = −0.665, P < 0.02) with IC50 ABTS. The correlation data suggests that ORAC and IC50 values best represent the association of bioactive compounds with antioxidant activity. Our results contrast with studies where DPPH radical scavenging activity was strongly correlated with total bioactive compounds (capsaicinoids, carotenoids, flavonoids and total phenolics) in pepper cultivars (Bae et al. 2012), and red pepper byproducts (Sim and Sil 2008). The significant correlation between epicatechin and rutin with the free radical ABTS and/or DPPH scavenging activity reported earlier was not substantiated in our study (Medina-Juárez et al. 2012). However, correlation (r = 0.665, P < 0.02) was significant between epicatechin and rutin contents, but not with antioxidant activities in our study.
Table 4.
Correlation coefficients (r) between total phenols, capsaicinoids and antioxidant activity
| TPC GAE | TPC CAT | Capsaicin | Dihydrocapsaicin | ABTS | DPPH | ORAC | IC50ABTS | IC50DPPH | |
|---|---|---|---|---|---|---|---|---|---|
| TPC CAT | 0.775** | ||||||||
| Capsaicin | −0.843** | −0.668*** | |||||||
| Dihydrocapsaicin | −0.567 | −0.216 | 0.606*** | ||||||
| ABTS | −0.012 | 0.111 | 0.038 | 0.107 | |||||
| DPPH | −0.153 | −0.067 | 0.044 | 0.099 | 0.925* | ||||
| ORAC | −0.891** | −0.847** | 0.776** | 0.325 | 0.153 | 0.358 | |||
| IC50ABTS | 0.565 | 0.272 | −0.737*** | −0.913* | −0.052 | 0.036 | −0.321 | ||
| IC50DPPH | 0.857** | 0.662*** | −0.756** | −0.761** | −0.235 | −0.372 | −0.778** | 0.737*** | |
| PAOXI | −0.700** | −0.519 | 0.554 | 0.364 | 0.670*** | 0.802** | 0.802** | −0.275 | −0.741*** |
TPC GAE total phenols expressed as g equivalents of gallic acid per kg, TPC CAT total phenols expressed as g equivalents of (+)-catechin per kg; PAOXI phenol antioxidant index defined as unit contribution of total phenolics to antioxidant activity
Values with single asterisk (*), double asterisk (**) and triple asterisk (***) are significant at P < 0.0001, P < 0.005, P < 0.05, respectively
The abundance of rutin, epicatechin and catechin in Jalapeño byproducts suggests that it can be an excellent source of vegetable flavonoids. These flavonoids are known to exhibit strong antioxidant potency by inhibiting lipid peroxidation (Lagiou et al. 2004). Similar lipid inhibition and protection of LDL cholesterol oxidation have been demonstrated for processed Jalapeño (Alvarez-Parrilla et al. 2012). The control of lipid oxidation was not investigated in our antioxidant activity; however, the similarity of our Jalapeño byproducts with those of processed Jalapeño (Alvarez-Parrilla et al. 2012) suggests that they have strong potential as ingredients for human health. Furthermore, the byproducts and/or their extracts may have other useful applications as previously described (Johnson 2007).
Conclusion
Aqueous 80% ethanol produced a solid byproduct extract with similar content of total phenolics reported in whole fresh and processed pepper fruit. This extract is an excellent source of flavonoids. Although scalding decreased capsaicinoids (up to 42%), HPLC phenolic content (up to 16%) and the antioxidant activity (variable), the Jalapeño pepper byproducts is still an attractive ingredient to develop useful innovative products for potential food/non-food applications simultaneously reducing food loss and waste.
Acknowledgements
The first author is grateful to CONACYT (298133) and BEIFI-IPN scholarships, Instituto Politécnico Nacional Secretaría de Investigación y Posgrado SIP20150798 Project for providing research funds and La Costeña Company, by Jalapeño byproduct provided.
Footnotes
B. Dave Oomah: Formerly with the National Bioproducts and Bioprocesses Program, Pacific Agri-Food Research Centre, Agriculture and Agri-Food Canada, Summerland, BC V0H 1Z0, Canada.
References
- Alós E, Rodrigo MJ, Zacarías L. Transcriptomic analysis of genes involved in the biosynthesis, recycling and degradation of L-ascorbic acid in pepper fruits (Capsicum annuum L.) Plant Sci. 2013;207:2–11. doi: 10.1016/j.plantsci.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Alvarez-Parrilla E, De la Rosa L, Amarowicz R, Shahidi F. Antioxidant activity of fresh and processed Jalapeño and Serrano peppers. J Agric Food Chem. 2011;59:163–173. doi: 10.1021/jf103434u. [DOI] [PubMed] [Google Scholar]
- Alvarez-Parrilla E, De la Rosa L, Amarowicz R, Shahidi F. Protective effect of fresh and processed Jalapeño and Serrano peppers against food lipid and human LDL cholesterol oxidation. Food Chem. 2012;133(3):827–834. doi: 10.1016/j.foodchem.2012.01.100. [DOI] [Google Scholar]
- Atanassova M, Bagdassarian V. Rutin content in plant products. J Chem Technol Metall. 2012;44(2):201–203. [Google Scholar]
- Bae H, Jayaprakasha GK, Jifon J, Patil BS. Variation of antioxidant activity and the levels of bioactive compounds in lipophilic and hydrophilic extracts from hot pepper (Capsicum spp.) cultivars. Food Chem. 2012;134(4):1912–1918. doi: 10.1016/j.foodchem.2012.03.108. [DOI] [PubMed] [Google Scholar]
- Bosland PW, Votava EJ. Peppers: vegetable and spice capsicums. Crop production science in horticulture series. Oxfordshire: CABI; 2012. [Google Scholar]
- Cardador-Martínez A, Albores A, Bah M, Calderón-Salinas V, Castaño-Tostado E, Guevara-González R, Shimada-Miyasaka A, Loarca-Piña G. Relationship among antimutagenic, antioxidant and enzymatic activities of methanolic extract from common beans (Phaseolus vulgaris L) Plant Food Hum Nutr. 2006;61:161–168. doi: 10.1007/s11130-006-0026-4. [DOI] [PubMed] [Google Scholar]
- Carvalho AV, De Andrade Mattietto R, De Oliveira Rios A, De Almeida Maciel R, Moresco KS, De Souza Oliveira TC. Bioactive compounds and antioxidant activity of pepper (Capsicum sp.) genotypes. J Food Sci Technol. 2015;52(11):7457–7464. doi: 10.1007/s13197-015-1833-0. [DOI] [Google Scholar]
- Castro-Concha LA, Canche-Chuc I, Miranda-Ham MDL. Determination of antioxidants in fruit tissues from three accessions of habanero pepper (Capsicum chinense Jacq.) J Mex Chem Soc. 2012;56(1):15–18. [Google Scholar]
- Cervantes-Paz B, Yahia EM, Ornelas-Paz JDJ, Gardea-Béjar AA, Ibarra-Junquera V, Pérez-Martínez JD. Effect of heat processing on the profile of pigments and antioxidant capacity of green and red Jalapeño peppers. J Agric Food Chem. 2012;60(43):10822–10833. doi: 10.1021/jf303091u. [DOI] [PubMed] [Google Scholar]
- Chen L, Kang YH. Anti-inflammatory and antioxidant activities of red pepper (Capsicum annuum L.) stalk extracts: comparison of pericarp and placenta extracts. J Funct Foods. 2013;5(4):1724–1731. doi: 10.1016/j.jff.2013.07.018. [DOI] [Google Scholar]
- Espinosa-Alonso LG, Lygin A, Widholm JM, Valverde ME, Paredes-Lopez O. Polyphenols in wild and weedy Mexican common beans (Phaseoulus vulgaris L.) J Agric Food Chem. 2006;54:4436–4444. doi: 10.1021/jf060185e. [DOI] [PubMed] [Google Scholar]
- FAO . Food wastage footprint impacts on natural resources. Rome: Food and Agriculture Organization of the United Nations; 2013. [Google Scholar]
- FAO . Save food: global initiative on food loss and waste reduction. Rome: Food and Agriculture Organization of the United Nations; 2014. [Google Scholar]
- Galicia-Cabrera RM (2005) In: Hui YH (ed) Handbook of food science, technology, and engineering, vol 4. CRC Press, doi:10.1201/b15995-203
- Howard LR, Wildman RE (2006) In: Wildman REC (ed) Handbook of nutraceuticals and functional foods. 2nd edn, CRC Press, pp 165–191. doi:10.1201/9781420006186.ch9
- Hwang IG, Shin YJ, Lee S, Lee J, Yoo SM. Effects of different cooking methods on the antioxidant properties of red pepper (Capsicum annuum L.) Prev Nutr Food Sci. 2012;17(4):286. doi: 10.3746/pnf.2012.17.4.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WJ. Final report on the safety assessment of Capsicum annuum extract, Capsicum annuum fruit extract, Capsicum annuum resin, Capsicum annuum fruit powder, Capsicum frutescens fruit, Capsicum frutescens fruit extract, Capsicum frutescens resin, and capsaicin. Int J Toxicol. 2007;26:3–106. doi: 10.1080/10915810601163939. [DOI] [PubMed] [Google Scholar]
- Kevers C, Falkowski M, Tabart J, Defraigne JO, Dommes J, Pincemail J. Evolution of antioxidant capacity during storage of selected fruits and vegetables. J Agric Food Chem. 2007;55(21):8596–8603. doi: 10.1021/jf071736j. [DOI] [PubMed] [Google Scholar]
- Lagiou P, Samoli E, Lagiou A, Tzonou A, Kalandidi A, Peterson J, Dwyer J, Trichopoulos D. Intake of specific flavonoid classes and coronary heart disease—a case–control study in Greece. Eur J Clin Nutr. 2004;58(12):1643–1648. doi: 10.1038/sj.ejcn.1602022. [DOI] [PubMed] [Google Scholar]
- Loizzo MR, Pugliese A, Bonesi M, De Luca D, O’Brien N, Menichini F, Tundis R. Influence of drying and cooking process on the phytochemical content, antioxidant and hypoglycaemic properties of two bell Capsicum annum L. cultivars. Food Chem Toxicol. 2013;53:392–401. doi: 10.1016/j.fct.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Loizzo MR, Pugliese A, Bonesi M, Menichini F, Tundis R. Evaluation of chemical profile and antioxidant activity of twenty cultivars from Capsicum annuum, Capsicum baccatum, Capsicum chacoense and Capsicum chinense: a comparison between fresh and processed peppers. Food Sci Technol LEB. 2015;64(2):623–631. doi: 10.1016/j.lwt.2015.06.042. [DOI] [Google Scholar]
- Materska M. Bioactive phenolics of fresh and freeze-dried sweet and semi-spicy pepper fruits (Capsicum annuum L.) J Funct Foods. 2014;7:269–277. doi: 10.1016/j.jff.2014.02.002. [DOI] [Google Scholar]
- Medina-Juárez LA, Molina-Quijada DM, Del Toro-Sánchez CL, González-Aguilar GA, Gámez-Meza N. Antioxidant activity of peppers (Capsicum annuum L.) extracts and characterization of their phenolic constituents. Interciencia. 2012;37(8):588–593. [Google Scholar]
- Moreno-Escamilla JO, Laura A, López-Díaz JA, Rodrigo-García J, Núñez-Gastélum JA, Alvarez-Parrilla E. Effect of the smoking process and firewood type in the phytochemical content and antioxidant capacity of red Jalapeño pepper during its transformation to chipotle pepper. Food Res Int. 2015;76:654–660. doi: 10.1016/j.foodres.2015.07.031. [DOI] [PubMed] [Google Scholar]
- Nurmi K, Ossipov V, Haukioja E, Pihlaja K. Variation of total phenolic content and individual low-molecular-weight phenolics in foliage of mountain birch trees (Betulapubescenss sp. tortuosa) J Chem Ecol. 1996;22:2023–2040. doi: 10.1007/BF02040093. [DOI] [PubMed] [Google Scholar]
- Oomah BD, Blanchard C, Balasubramanian P. Phytic acid, phytase, minerals, and antioxidant activity in Canadian dry bean (Phaseolus vulgaris L.) cultivars. J Agric Food Chem. 2008;56:11312–11319. doi: 10.1021/jf801661j. [DOI] [PubMed] [Google Scholar]
- Ornelas-Paz JJ, Martínez-Burrola JM, Ruiz-Cruz S, Santana-Rodríguez V, Ibarra-Junquera V, Olivas GI, Pérez-Martínez JD. Effect of cooking on the capsaicinoids and phenolics contents of Mexican peppers. Food Chem. 2010;119(4):1619–1625. doi: 10.1016/j.foodchem.2009.09.054. [DOI] [Google Scholar]
- Pellegrinini N, Serafini M, Colombi B, Del Rio D, Salvatore S, Bianchi M, Brighenti F. Total antioxidants capacity of plants food, beverages and oils consumed in Italy assessed by three different in vitro assays. J Nutr. 2003;3166:2812–2819. doi: 10.1093/jn/133.9.2812. [DOI] [PubMed] [Google Scholar]
- Prior RL, Gu X, Bacchiocca L, Howard M, Hampsch-Woodill D, Huang B, Ou RJ. Assay for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity ORAC-FL) of plasma and other biological and food samples. J Agric Food Chem. 2003;51:3273–3279. doi: 10.1021/jf0262256. [DOI] [PubMed] [Google Scholar]
- Qin C, Yu C, Shen Y, Fang X, Chen L, Min J, Cheng J, Zhao S, Xu M, Luo Y, Yang Y. Whole-genome sequencing of cultivated and wild peppers provides insights into Capsicum domestication and specialization. Proc Natl Acad Sci USA. 2014;111(14):5135–5140. doi: 10.1073/pnas.1400975111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval-Castro CJ, Valdez-Morales M, Perea-Domínguez XP, Medina-Godoy S, Espinosa-Alonso LG (2014) Antioxidant activity of processed and raw seed byproduct from Jalapeño pepper. J Chem Biol Phys Sci Spec Issue 4(5):26–34. http://www.jcbsc.org/issuespcl.php?conf_nick=biotech&sub_name=food
- SIAP (2015) Servicio de Información Agroalimentaria y Pesquera. Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación. Estados Unidos Mexicanos. http://infosiap.siap.gob.mx/aagricola_siap_gb/icultivo/index.jsp. Accessed 28 Mar 2017
- Silva LR, Azevedo J, Pereira MJ, Valentão P, Andrade PB. Chemical assessment and antioxidant capacity of pepper (Capsicum annuum L.) seeds. Food Chem Toxicol. 2013;53:240–248. doi: 10.1016/j.fct.2012.11.036. [DOI] [PubMed] [Google Scholar]
- Sim KH, Sil HY. Antioxidant activities of red pepper (Capsicum annuum) pericarp and seed extracts. Int J Food Sci Technol. 2008;43(10):1813–1823. doi: 10.1111/j.1365-2621.2008.01715.x. [DOI] [Google Scholar]
- Troconis-Torres IG, Rojas-López M, Hernández-Rodríguez C, Villa-Tanaca L, Maldonado-Mendoza IE, Dorantes-Alvarez L, Tellez-Medina D, Jaramillo-Flores ME. Biochemical and molecular analysis of some commercial samples of chilli peppers from Mexico. J Biomed Biotechnol. 2012 doi: 10.1155/2012/873090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tundis R, Loizzo MR, Menichini F, Bonesi M, Conforti F, Statti G, De Luca D, de Cindio B, Menichini F. Comparative study on the chemical composition, antioxidant properties and hypoglycaemic activities of two Capsicum annuum L. cultivars (Acuminatum small and Cerasiferum) Plant Foods Hum Nutr. 2011;66(3):261–269. doi: 10.1007/s11130-011-0248-y. [DOI] [PubMed] [Google Scholar]
- Valdez-Morales M, Espinosa-Alonso LG, Espinoza-Torres LC, Delgado-Vargas F, Medina-Godoy S. Phenolic content and antioxidant and antimutagenic activities in tomato peel, seeds, and byproducts. J Agric Food Chem. 2014;62:5281–5289. doi: 10.1021/jf5012374. [DOI] [PubMed] [Google Scholar]
- Zhuang Y, Chen L, Sun L, Cao J. Bioactive characteristics and antioxidant activities of nine peppers. J Funct Foods. 2012;4(1):331–338. doi: 10.1016/j.jff.2012.01.001. [DOI] [Google Scholar]