Abstract
A stevia fraction (ASF) free of steviol glycosides was extracted from Stevia rebaudiana leaves (Stevia UEM-13). ASF essentially constitutes phenolic compounds (52.42%), which were identified by liquid chromatography tandem mass spectrometry (LC–MS/MS) as caffeic acid, quercetin-3-o-glycoside, cyanidin-3-glucoside, kaempferol, quercetin, apigenin, rozmarinic acid, chlorogenic acid and dicaffeoylquinic acid. ASF was used as a multi-functional source of phenolic compounds to fortify the whey protein isolate (WPI) obtained by membrane separation. WPI fortified with 0.2% ASF showed an 80% increase in its antioxidant activity and more pronounced antidiabetic effects than the unfortified WPI, mainly in the glycemic control of diabetic animals induced by streptozotocin. The in vitro and in vivo antioxidant effects of ASF may enhance the effects of WPI. Indeed, this pioneering study revealed that ASF can be used to enrich the antioxidant and antidiabetic properties of WPI.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2638-0) contains supplementary material, which is available to authorized users.
Keywords: Whey protein isolate, Antioxidant fraction of Stevia rebaudiana, Phenolic compounds, Flavonoids, Diabetes mellitus
Introduction
Stevia rebaudiana Bertoni (Bert.) is known worldwide, and its leaves are characterized by the presence of steviol glycosides, which have immense sweetening capacity (Dacome et al. 2005). Among these steviol glycosides, the most prominent compounds are stevioside and rebaudioside A, both of which have been studied in recent decades for their antidiabetic properties (Gregersen et al. 2004; Milani et al. 2016). In addition to steviol glycosides, stevia leaves are a source of phenolic and flavonoid compounds with antioxidant and antidiabetic properties (Ghanta et al. 2007; Shukla et al. 2009; Wölwer-Rieck 2012; Shivanna et al. 2013; Gawel-Beben et al. 2015). Substantial effort has been directed toward isolating and characterizing phenolic compound-rich fractions from stevia leaves and evaluating their potential use as multi-functional sources of phenolic compounds (Ghanta et al. 2007; Shukla et al. 2009; Shivanna et al. 2013; Gawel-Beben et al. 2015). This study describes the isolation of a stevia fraction (ASF) with a high phenolic compound content. The centesimal composition of the fraction was determined, and its main phenolics and flavonoids were identified through liquid chromatography tandem mass spectrometry (LC–MS/MS) analysis. The fraction determined to have potential for fortifying foods was added to a whey protein isolate (WPI), and the developed supplement was tested for its antioxidant potential and antidiabetic effects.
WPI supplements constitute a rich source of proteins, such as β-lactoglobulin and α-lactoalbumin, peptone protease, immunoglobulins, bovine serum albumin, lactoferrin, and lactoperoxidase, and peptides, such as glycomacropeptide, which is an excellent source of branched-chain amino acids. When regularly added to the diet, these concentrated or isolated WPI proteins function as adjuvants for the treatment of diseases such as diabetes mellitus (DM). These compounds may promote insulin secretion and assist in metabolic control, antioxidant capacity and satiety (Sgarbieri 2004; Mortensen et al. 2012; Ebaid 2014). The bioactive compounds in ASF can increase the antioxidant or antidiabetic activities of these proteins, fortify their products and produce even more pronounced effects, especially relating to the metabolic complications of DM.
DM is an endocrine, metabolic disorder that affects thousands of people worldwide and is characterized by deficiency or absence of the production of insulin and its mode of action (Gaudel et al. 2013). This hormone is secreted by β-pancreatic cells and primarily acts in the liver tissue, muscle and adipose tissue to stimulate glycogen, lipid and protein synthesis and inhibit glycogenolysis, lipolysis and proteolysis. In addition to these metabolic effects, insulin plays a fundamental role in capturing glucose from adipose and muscular tissues and releasing it from the liver. Thus, diabetic patients present increased blood glucose levels and decreased antioxidant capacity, which may result in long-term hyperglycemia, further disorders, and even the failure of some organs (Koga et al. 2004).
DM control and management are complex and involve the oral hypoglycemic use of insulin and other strategies, such as lifestyle changes, mainly including the consumption of a balanced diet, to manage the disease. Hypoglycemic and natural antioxidant sources can be important coadjuvants in the treatment of diabetes (Milani et al. 2016; Gaudel et al. 2013).
Therefore, the objectives of this pioneering study were to detect and chemically characterize a phenolic compound-rich fraction of stevia leaf extract and to evaluate the potential use of this fraction to improve the antioxidant and antidiabetic activities of WPI and produce an effective supplement for DM treatment.
Materials and methods
Materials
ASF was obtained from S. rebaudiana leaves (seminal variety: Stevia UEM-13) cultivated at the Universidade Estadual de Maringá (UEM, Maringá, Paraná, Brazil). WPI was obtained from skimmed and pasteurized whey provided by the Flora Milk dairy (Flórida, Paraná, Brazil). The membranes were made of polyethersulfone and polyamide from Koch and Millipore. The reagents used for the extraction, chemical and chromatographic analyses, adipocyte isolation, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, and streptozotocin were purchased from Sigma. The rodents’ feed was obtained from Nuvilab (Colombo, Paraná, Brazil), and the specific kits for the plasma and serum dosages were obtained from Gold Analisa (Belo Horizonte, Minas Gerais, Brazil).
Obtaining the WPI from the milk whey
The WPI was obtained according to Milani et al. (2016) with modifications. The whey was concentrated by ultrafiltration (UF), diafiltration (DF) and nanofiltration (NF). Each concentrated sample was then dried by a spray dryer. The UF and DF processes were performed in a system with polyethersulfone filtering membranes (10-kD cut-off, 50-cm2 area; Koch) in a spiral configuration, and 12 DF cycles were performed. NF was conducted using a reverse osmosis system composed of two polyamide membranes with cut-off molecular masses of 180 Da (Koch) and 500 Da (Millipore), both in a spiral configuration with a 50-cm2 area. The WPI was dried in a spray dryer atomizer (Büchi, B-191) using an input temperature of 170 °C, an output temperature of 105 °C, and a flow of 8 mL/min.
Obtaining the ASF with antioxidant properties
Dry leaves (100 g) that had been previously ground were added to 500 mL of methanol and extracted using a Soxhlet apparatus for 4 h. The extraction was repeated until a colorless methanolic extract was obtained, which then was filtered and evaporated in a rotary evaporator (Buchi) at 50 °C under vacuum. The resulting powder (35.8 g of dry methanolic extract) was hydrated with 400 mL of deionized water and subjected to fractionation with different solvents (hexane, chloroform, ethyl acetate and isobutanol). ASF obtained by fractioning with ethyl acetate contained phenolic compounds and exhibited antioxidant activity; therefore, it was tested for biological activity and used for WPI fortification.
Protein supplement from whey with added ASF (WPI + ASF)
ASF (0.2%) was added to the WPI, and the proportion was determined according to the results obtained by Shivanna et al. (2013), who observed antidiabetic effects in experimental animals fed with feed containing 4% S. rebaudiana leaves.
Physicochemical analysis, bioactive compounds and in vitro antioxidant activity
Whey and WPI
The total protein and lipids, lactose, mineral residues, humidity, pH and soluble solid contents were determined. The total protein and lipid analyses were performed following AOAC (1995) methodology. The lactose concentrations were obtained by high-efficiency LC connected to a refraction index detector [NH2 5-µm column with dimensions of 150 mm × 4.6 mm; acetonitrile and water mobile phase (80:20 v/v)]. The standard and the samples were prepared at a concentration of 0.5 mg/mL. The fixed mineral residue (ash), pH and soluble solid content (°Brix) were quantified according to the Instituto Adolfo Lutz methodology (2005). The WPI antioxidant potential was determined by measuring its 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging ability according to the Blios (1958) methodology. The standard used was gallic acid, and the data were expressed as inhibition percentages. All analyses were conducted in triplicate and were subjected to statistical treatment.
ASF
The proteins, lipids, fixed mineral residues and humidity concentrations were determined following the methodology mentioned in the previous section. The total glycoside concentration was measured using the method described by Dacome et al. (2005). The total phenolic compounds were determined according to Singleton et al. (1999) and the Folin–Ciocalteu method; the data were expressed in gallic acid equivalents, which was used as a standard. The total flavonoid quantity was measured according to Jia et al. (1999), and the results were expressed in standard quercetin equivalents. The antioxidant potential was determined according to the methodology described in the previous section. All analyses were conducted in triplicate and were subjected to statistical treatment.
Identification of phenolic compounds
LC–MS and LC–MS/MS analyses were performed using an LC (Waters 1525µ) and spectrometer (Quattro micro API model) (Beverly, Massachusetts, USA) with a triple quadrupole (QqQ) mass analyzer, electrospray ionization, and a C18 column (250 mm × 4.6 mm; Thermo Scientific). The analyses were performed at 270 nm. The mobile phase consisted of water containing 0.1% of formic acid (solvent A) and acetonitrile containing 0.1% of formic acid (solvent B); the gradient was as follows: 0 min, 50% solvent A and 50% solvent B; 1 min, 30% solvent A and 70% solvent B; 2 min, 15% solvent A and 85% solvent B; 10 min, 5% solvent A and 95% solvent B; 12 min, 50% solvent A and 50% solvent B; and 15 min, 50% solvent A and 50% solvent B. The mass spectra were collected with ESI in positive or negative ion mode. The parameters were as follows: capillary voltage, 2.5 kV; cone voltage, 25 V; source temperature, 150 °C; desolvation temperature, 250 °C; gas caudal cone, 50 Lh-1; and electrovoltage, 30–40 eV. The compounds were identified based on the equipment databases and the following databases and literature: The Metabolomics Innovation Centre (TMIC), METLIN: A Metabolite Mass Spectral Database and Plataform for RIKEN metabolomics and literature data (Shivanna et al. 2013; Gawel-Beben et al. 2015).
Rodents feed
The total protein, total lipids, acidity and gross fiber in the feed provided to the experimental animals were determined in compliance with AOAC (1995) methodology. The ash and humidity were quantified according to the methodology described in “Whey and WPI” section.
MTT assay of the cytotoxicity of ASF
The MTT assay to evaluate the cytotoxicity of ASF was conducted in adipose cells. The adipocytes were isolated according to Rodbell (1964), with some adjustments. Approximately 1 g of retroperitoneal adipose tissue from male Wistar mice was fragmented and added to 4 mL of digestive buffer (Dulbecco’s modified Eagle’s medium [DMEM]/4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid [HEPES], 25 mM; bovine serum albumin [BSA] fraction V, 4%; collagenase II, 1.25 mg/mL; pH 7.4) in a water bath at 37 °C with orbital shaking (90 rpm) for 90 min. Then, the digested tissue was filtered, placed in a conical flask and washed three times with 25 mL of GBT buffer (EARLE/HEPES [20 mM] containing BSA [1%] and sodium pyruvate [1 mM] without glucose; pH 7.4 at 37 °C). The infranatant was aspirated, resulting in a cellular suspension with approximately 3.0 × 106 cells/mL. In each well of a 96-well plate, 200-µL aliquots of GBT buffer (n = 6) containing different ASF concentrations (1, 0.1, 0.01 and 0.001 mg/mL) were added. Then, 60 µL of the cellular suspension (1.8 × 105 cells) was added to each well and incubated for 60 min under 5% CO2 atmosphere at 37 °C. Subsequently, the medium was replaced with 100 µL of MTT solution (0.5 mg/mL) and incubated (37 °C, 5% CO2 atmosphere) for 120 min. Then, the MTT solution was replaced with 100 µL of dimethyl sulfoxide and shaken for 5 min, and the reading was performed at 540 nm. The data were expressed as viability percentages relative to the control.
Antidiabetic property evaluation
Experimental animals
The experimental protocol was approved by the UEM Ethics Conduct in Using Animals in Experimentation Committee (Protocol n° 8796250415). Male Wistar mice (60 days old) were obtained from the UEM Central Biotherium. The animals were stored in collective cages (46 cm × 24 cm × 20 cm; five animals per cage) or in individual metabolic cages and kept in the sectoral biotherium of the UEM Physiologic Science Department under the following conditions: 23 °C, 12-h light/dark photoperiod, and water and feed (Nuvilab ®, Colombo, PR, Brazil) ad libitum.
Diabetes induction and experimental groups
After overnight fasting for 12 h, the animals were sedated with sodium thiopental (40 mg/kg of p.c, i.p) to induce diabetes (streptozotocin, 40 mg/kg, v.i). On the third day after diabetes induction, the animals that showed fasting glycemia equal to or higher than 200 mg/dL and glycemia in the fed condition equal to or higher than 300 mg/dL were selected. The glycemic values and body weight were used to create four groups of diabetic animals that presented the same degree of diabetes severity before starting the feed supplementation. Five experimental groups were established with n = 10 animals per group: one group of non-diabetic animals (ND = Non-Diabetic) and four groups of diabetic animals (DC = Diabetic Control, DW = Diabetic supplemented with WPI, and DSW = Diabetic supplemented with WPI + ASF).
Feed supplementation
Three types of feed supplements were used: (1) WPI (100 mg/kg), (2) ASF (0.2 mg/kg), and (3) WPI + ASF (0.2%) (100 mg/kg). The oral supplementation was performed daily at 08:00 AM for a 30-day period by gavage (the control diabetic animals group received pure water). The dose and supplementation period were established based on previous studies (Shivanna et al. 2013; Ma and Mu 2016).
Physiological parameters evaluated
Body weight and glycemia
Every week the body weight, fasting glycemia and fed condition glycemia were recorded. The glycemia was determined in blood samples obtained from caudal puncture and analyzed using a glucometer from the MediSence Optium.
Glucose tolerance test (GTT)
At the end of the treatment, after fasting overnight for 12 h, the animals were submitted to oral GTT. After blood sampling (caudal puncture) for glycemia at time zero, the animals received an overload of glucose (1.5 g/kg, gavage). New blood samples were collected 15, 30, 60, 90 and 120 min after the glucose overload, and the glycemia was determined using a glucometer from MediSence Optium.
Twelve-hour glycemic curve
At the end of the treatment, blood sampling (caudal puncture) was performed at 6:00 PM after the animals had been subjected to daytime fasting (12 h). Then, the animals received feed ad libitum, and new blood samples were collected at 20:00, 22:00 and 06:00 on the next day. The glycemia was determined using a glucometer from MediSence Optium.
Water and food intake and excreted urine volume
At the end of the treatment, the animals were put in individual metabolic cages, and their water intake, food intake and excreted urine volume were recorded over a 24-h period.
Animal euthanasia
After fasting overnight for 12 h, the animals were euthanized by anesthetic overload (sodium thiopental, 120 mg/kg, i.p.). After median laparotomy, blood samples were collected through the inferior vena cava, and then, the retroperitoneal and periepididymal fat deposits, gastrocnemius and soleus muscles, testicles, seminal vesicles, kidneys, liver and spleen were removed and weighed.
Biochemical dosage
Blood glucose concentrations, total cholesterol, high-density lipoprotein (HDL), triglycerides, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were determined using colorimetric methods (Gold Analisa®, Belo Horizonte, MG) and spectrophotometry (Bioplus2000®, São Paulo, SP). The 2,2-azinobis-3-ethyl-benzotiazolin-6-sulfonic acid (ABTS) radical was used to analyze the blood total antioxidant capacity (CAT) (Erel 2004).
Statistical analysis
The results were presented as the average ± standard error of the mean (SEM) and were submitted to variance analysis using the Tukey test (p < 0.05). The statistical program SAS (Statistical Analysis System 2006, version 9.1) and GraphPad Prism version 5.0® were used.
Results and discussion
Physicochemical characterization of the obtained WPI
The whey used to produce the WPI had a soluble solid content of 4.4 ºBRIX and pH of 4.8. To obtain whey with a protein content of approximately 90%, 8 L of serum was ultrafiltered at 40 °C and then subjected to 12 cycles of DF and NF. The centesimal compositions of the whey and WPI are shown in Table 1. The procedure used was determined to efficiently concentrate the whey proteins to almost 90%, with only 7% of lactose. According to these results, the product complied with high-quality standards (Patel 2015; Milani et al. 2016). WPI, whey protein concentrate (WPC) and whey protein hydrosylate (WPH) were previously shown to exhibit antioxidant properties (Patel 2015). The WPI obtained in this study showed an antioxidant capacity of 41.7% (concentration: 1 mg/mL), similar to the value reported by Peng et al. (2009).
Table 1.
Proximate composition (g/100) the whey (W) and whey protein isolate (WPI)
| Analyse | W | WPI |
|---|---|---|
| Total protein | 1.61 ± 0.05 | 89.04 ± 0.02 |
| Total lipids | 0.18 ± 0.03 | 0.07 ± 0.01 |
| Lactose | 4.12 ± 0.02 | 7.01 ± 0.01 |
| Humidity | 93.2 ± 0.01 | 2.03 ± 0.01 |
| Ash | 0.90 ± 0.01 | 1.25 ± 0.01 |
Data were expressed by mean ± SEM
Proximal composition of the obtained ASF
The physicochemical characteristics of the ASF are presented in Table 2. More than 50% of the proximal composition consisted of phenolic compounds, and almost 35% was proteins, with only 0.05% sweetener glycosides. These results suggest that the procedure used was efficient for obtaining the powder fraction with high antioxidant capacity (97.3%) without sweetener function. Wölwer-Rieck (2012), López et al. (2016) and Gawel-Beben et al. (2015) also reported the total phenolic and flavonoid compounds in stevia extracts and indicated that these compounds confer, together with other compounds, important antioxidant activities. Table 2 also shows the main bioactive compounds (caffeic acid, quercetin-3-o-glycoside, cyanidin-3-glucoside, kaempferol, quercetin, apigenin, rozmarinic acid, chologenic acid and dicaffeoylquinic acid) identified by LC–MS (supplementary material). These compounds were also identified by Shivanna et al. (2013) and Gawel-Beben et al. (2015) in aqueous and alcoholic stevia extracts. ASF contained 52.42% phenolic compounds, and when it was added to WPI at a concentration of 0.2% (WPI + ASF), the antioxidant activity increased to 80%. This is the first study in which a fraction extracted from S. rebaudiana leaves was used for WPI fortification. The significant increase in antioxidant activity that resulted from the combination of WPI with ASF may make this material suitable as an antioxidant protein supplement with important applications in clinical nutrition.
Table 2.
Proximate composition (g/100) antioxidante stevia fraction (ASF) and identification by LCMS/MS of phenolics and flavonoids
| Analyse | ASF |
|---|---|
| Total protein | 34.5 ± 0.02a |
| Total lipids | 1.00 ± 0.03 |
| Glicosides | 0.05 ± 0.01 |
| Humidity | 3.04 ± 0.01 |
| Ashes | 2.24 ± 0.01 |
| Flavonoids | 0.038 ± 0.01 |
| Phenolics | 52.42 ± 0.03 |
| Compounds | RT | M/Z |
|---|---|---|
| Caffeic acid | 4.78 | 163 |
| Quercetin-3-o-glicoside | 4.88 | 434 |
| Cyanidin-3-glucoside | 4.51 | 449 |
| Kaempferol | 5.54 | 287 |
| Quercetin | 5.93 | 303 |
| Apigenin | 6.27 | 271 |
| Rosmarinic acid | 6.58 | 361 |
| Chologenic acid | 4.90 | 353 |
| Dicaffeoylquinic acid | 5.33 | 515 |
RT retention time, M/Z mass spectrum
aData were expressed by mean ± SEM
ASF cytotoxicity
The presence of antioxidants in plants and food is related to their beneficial effects for the prevention and treatment of several diseases and metabolic disorders (Krishnaiah et al. 2015). Previous studies have demonstrated that stevia extracts contain significant antioxidant concentrations, especially phenolics and flavonoids (Wölwer-Rieck 2012). However, high levels of antioxidants may increase the toxicity of these extracts, and thus, these extracts must be studied further. The toxicity of various steviol glycoside-rich stevia extracts has been tested in different cellular models (Gawel-Beben et al. 2015). Nevertheless, studies evaluating the toxicity of extracts and fractions rich in other components, such as phenolics and flavonoids, in different cells remain lacking.
No significant differences (data not shown) in the viability percentage of the adipocytes incubated in the absence or presence of different concentrations of ASF (0.001, 0.01, 0.1 and 1 mg/mL) were found using the MTT assay. Concentrations up to a thousand times higher than those added to the WPI were used. Thus, ASF has potential applications in the fortification of food supplements.
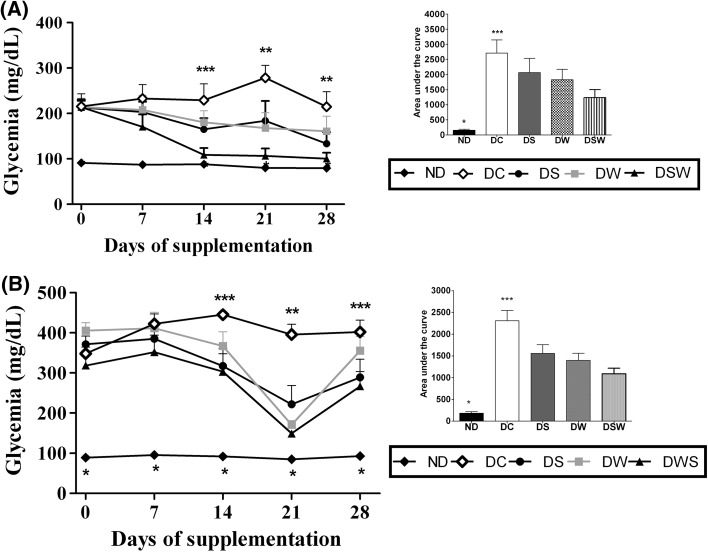
Diabetic condition characterization
The lowest body weight gain (Table 3), fasting hyperglycemia (Fig. 1a) and hyperglycemia in the fed condition (Fig. 1b), excess excreted urine and water intake, and reduction in adipose tissue deposits (Table 3) were observed in the diabetic animal groups compared with those in animals from the non-diabetic group. These results confirmed the induction of diabetes by streptozotocin. This diabetogenic drug causes DNA alkylation and the death of ß-pancreatic cells and, consequently, reduced insulin secretion (Junod et al. 1969; Lenzen 2008).
Table 3.
Body weight gain (∆P), weights of retroperitoneal fat (RF) and periepididymal (PF), water intake (WI), food intake (FI) and urine volume (UV) of non-diabetic and diabetic rats
| ND | DC | DS | DW | DSW | |
|---|---|---|---|---|---|
| ∆P (g) | 91.2 ± 10.6* | 31.8 ± 5.00** | 63.5 ± 13.2 | 50.5 ± 9.70 | 59.8 ± 9.70 |
| RF (g/100 g) | 1.0 ± 0.09* | 0.42 ± 0.03*** | 0.64 ± 0.20 | 0.80 ± 0.09# | 0.54 ± 0.04 |
| PF (g/100 g) | 0.93 ± 0.07* | 0.16 ± 0.02*** | 0.68 ± 0.16 | 0.43 ± 0.17 | 0.50 ± 0.17 |
| WI (mL/dia) | 52.0 ± 2.10* | 149.1 ± 5.40& | 161.2 ± 9.00 | 118.4 ± 8.70 | 101.1 ± 3.40 |
| FI (g/dia) | 39.44 ± 7.40* | 29.06 ± 12.9 | 30.00 ± 12.5 | 33.06 ± 3.30 | 30.50 ± 10.4 |
| UV (mL/dia) | 10.75 ± 1.40* | 70.75 ± 3.60# | 67.00 ± 8.90 | 65.63 ± 7.72 | 49.13 ± 5.50 |
Data were expressed by mean ± SEM
ND non-diabetics, DC diabetic control, DS diabetic supplemented with ASF, DW diabetic supplemented with WPI, DSW diabetic supplemented with WPI + ASF
* differs from other groups (p < 0.05); ** differs from DS and DSW (p < 0.05); *** differs from DS, DW and DSW (p < 0.05); & differs from DW and DSW; # differs from DWS (p < 0.05)
Fig. 1.
Fasting glycemia (a) and in the fed state (b) of non-diabetic mice and diabetic mice. ND non-diabetics, DC diabetic control, DS diabetic supplemented with ASF, DW diabetic supplemented with WPI, DSW diabetic supplemented with WPI + ASF. Data were expressed by mean ± SEM. Detail of area under the curve (AUC). *differs from the other groups (p < 0.05); **differs from the other groups (p < 0.05); ***differs from DSW (p < 0.05)
Feed composition
The functional effects of WPI, ASF and WPI + ASF supplementation were evaluated in diabetic mice fed with commercial mice feed containing 24% protein, 5.4% lipids, 6.5% minerals, 17.3% fiber, 10.4% moinsture and 1.9% acidity.
Evaluating the antidiabetic properties of food supplements
Body weight, adiposity and hydration
The evolution of the body weight during the supplementation period did not differ among the diabetic animal groups. However, animals that received ASF supplementation [the isolate alone (DS group) or added to the whey (DSW group)] showed significantly higher body weight gains (i.e., the difference between the final and initial weights) (p < 0.05) than the control diabetic animals (Table 3). The retroperitoneal and periepididymal fat deposits were significantly larger in the three groups of diabetic animals that received supplementation than in the control (p < 0.05). No significant differences were found in the food intake; however, the excreted urine volume and water intake were significantly smaller in the DSW group (p < 0.05) (Table 3). No significant differences were found in the weights of the liver, kidney, spleen, testicles, seminal vesicles and gastrocnemius and soleus muscles (data not shown). These results showed that the three supplements were efficient in reducing the catabolic effects of streptozotocin-induced diabetes caused by the lack of insulin action and that DSW exhibited the best antidiabetic effect.
Glycemic homeostasis
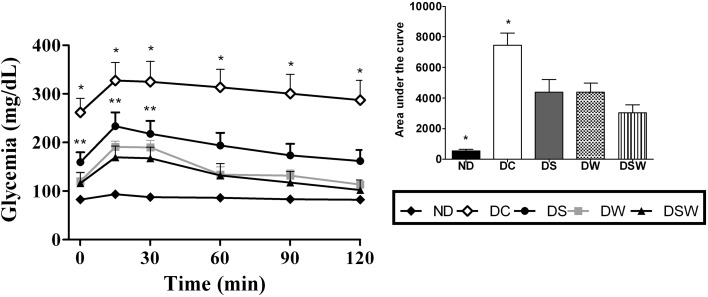
Figure 1a shows the weekly fasting glycemia values (blood sampling at 6:00 PM after 10 h of daytime fasting) during the supplementation period. The DSW group’s AUC (área under the curve) values were significantly lower than those the other groups of diabetic mice (p < 0.05); additionally, lower glycemia values (p < 0.05) were recorded beginning at the 14th day of supplementation. The DW and DS groups also showed reduced fed condition glycemia but only from the 21st day of supplementation onwards. The glycemia in the fed condition (blood sampling at 08:00 AM with food ad libitum overnight) is shown in Fig. 1b. No significant differences were recorded in the ASC values among the groups of diabetic animals, but beginning on the 14th day of supplementation, the DSW group showed values that were significantly lower (p < 0.05) than those of the DC group. Moreover, on the 21st day of the experimental period, all groups of diabetic mice that received food supplementation showed lower fasting glycemia values than the DC group (p < 0.05). Figure 2 presents the glycemia values during the oral GTT. The three groups of diabetic mice that received supplementation showed better glucose tolerance and significantly lower ASC values than the diabetic control group (p < 0.05). The DW and DWS groups exhibited values that were significantly lower than the DC group (p < 0.05) at all of the evaluated time points during the GTT. However, their values did not differ from those of the ND group, indicating that both were effective in restoring glycemic control, even after a glucose overload. The DS group’s levels were significantly lower than those of the DC group at the 15-, 30- and 60-min time points of the GTT, indicating that ASF exerts an important effect on glycemic homeostasis. The 12-h (overnight) glycemic curves revealed that the diabetic animals that received supplementation had lower glycemic values at the four experimental time points (18:00, 20:00, 22:00 and 06:00); however, these differences were not significant relative to those of the DC group. The best glycemic control was observed in the diabetic animals that received supplementation and were in the fasting glycemia group. These results were recorded on the euthanasia day (Table 4) when the animals were subjected to a 12-h (overnight) fast. The values recorded for the supplemented diabetic animal groups were significantly lower than those of the DC group (p < 0.05), and therefore, the three administered food supplements effectively improved the glycemic control in diabetic mice. Notably, the DWS group presented lower glycemia than the DW and DS groups (p < 0.05), suggesting the benefits of fortifying the WPI with ASF and indicating that the fortified supplement (WPI + ASF) had better functional quality and could potentially be used as an adjuvant in DM treatment and to prevent associated diseases. Previous studies have shown that whey protein can be an important functional food for diabetics (Milani et al. 2016; Patel 2015; Ebaid 2014; Gaudel et al. 2013; Tong et al. 2011). Indeed, several studies have confirmed the hypoglycemic and antidiabetic properties of S. rebaudiana products (i.e., extracts and isolate glycosides) (Tadhani et al. 2007; Shivanna et al. 2013; Wölwer-Rieck 2012). The reduction in the glycemia levels determined in fasting and fed conditions in the DS group corroborate the results of these previous studies, demonstrating that stevia polyphenols effectively produce these effects. However, ASF that contains no glycosides cannot be considered to promote hypoglycemic activities (Shivanna et al. 2013; Wölwer-Rieck 2012).
Fig. 2.
Glycemia (mg/dL) during oral glucose tolerance test of non-diabetic mice and diabetic mice. ND non-diabetics, DC diabetic control, DS diabetic supplemented with ASF, DW diabetic supplemented with WPI, DSW diabetic supplemented with WPI + ASF. Data were expressed by mean ± SEM. Detail of area under the curve (AUC). *differs from the other groups (p < 0.05); **DS differs from the other groups (p < 0.05)
Table 4.
Plasma and serum parameters of non-diabetic and diabetic rats
| ND | DC | DS | DW | DSW | |
|---|---|---|---|---|---|
| GLI (mg/dL) | 96.8 ± 2.5* | 395.6 ± 43.0** | 240.7 ± 24.0 | 213.3 ± 11 | 192.9 ± 9.80# |
| FTA (mmol/L) | 1.00 ± 0.06 | 1.8 ± 0.06** | 1.3 ± 0.10 | 1.12 ± 0.15 | 1.1 ± 0.07 |
| COL (mg/dL) | 75.4 ± 2.70 | 65.7 ± 3.40 | 71.1 ± 4.20 | 73.8 ± 2.90 | 73.6 ± 3.40 |
| HDL (mg/dL) | 39.8 ± 1.50 | 35.3 ± 0.90** | 42.4 ± 2.20 | 45.6 ± 1.30 | 45.5 ± 1.70 |
| TGS (mg/dL) | 43.7 ± 1.50 | 70.1 ± 8.70** | 65.8 ± 7.70*** | 36.3 ± 2.50 | 34.7 ± 4.50 |
| ALT (U/L) | 34.5 ± 0,90* | 67.4 ± 3.20** | 49.4 ± 1.10 | 49.4 ± 1.00 | 45.2 ± 1.00 |
| AST (U/L) | 16.9 ± 0.50* | 75.5 ± 3.20** | 39.0 ± 3.80 | 33.1 ± 5.60 | 37.2 ± 2.90 |
| CAT (µM EQT) | 202.4 ± 11.3** | 227.7 ± 4.10** | 394.1 ± 21.0*** | 324.7 ± 16.80 | 365.2 ± 4.80 |
Data were expressed by mean ± SEM
GLU glucose, FTA fructosamine, COL total cholesterol, HDL HDL cholesterol, TGS triglycerides, ALT alanine aminotransferase, AST aspartate aminotransferase, CAT total antioxidant capacity, ND non-diabetics, DC diabetic control, DS diabetic supplemented with ASF, DW diabetic supplemented with WPI, DSW diabetic supplemented with WPI + ASF
* differs from other groups (p < 0.05); ** differs from DS, DW and DSW (p < 0.05); *** differs from DW and DSW (p < 0.05); # differs from other groups (p < 0.05)
Fructosamine
Another parameter that was related to the best glycemic control in the diabetic animals that received supplementation relative to the control group was the fructosamine plasma concentration. This value represents, in a generic way, the glycated proteins that become stable ketamines, which constitute the parcel most strongly linked to albumin. Thus, the blood fructosamine levels reflect the glycemic control in the past weeks because a direct relationship exists between hyperglycemia and the degree of blood protein glycation. The functional disorders resulting from protein glycation are diverse and are the main determinants of vascular diseases associated with DM (Koga et al. 2004). Significant reductions in the plasma fructosamine levels in the DW, DS and DWS groups were determined in relation to that in the DC group (p < 0.05) (Table 4). Frid et al. (2005) observed reductions in the glycated hemoglobin rates in animals supplemented with whey. Ferri et al. (2006) obtained the same results in animals treated with S. rebaudiana products.
Lipidemia
The diabetic animals from the DW, DS and DWS groups presented the best lipid profiles and higher HDL values (p < 0.05). The total cholesterol concentration was not changed by supplementation, but the triglyceride values in the DW and DWS groups were significantly reduced (p < 0.05) (Table 4). Mortensen et al. (2012) reported decreased triglycerides and total cholesterol levels in diabetic patients who received supplementation with different fractions of whey protein. Milani et al. (2016) found reductions in the triglyceride and total cholesterol concentrations in diabetic animals supplemented with whey protein sweetened with stevia.
Hepatic enzymes
The serum concentrations of AST and ALT decreased (p < 0.05) in all diabetic groups that received supplementation (Table 4). Haraguchi et al. (2009) also observed beneficial effects on these parameters when whey supplements were used.
Antioxidant capacity
The in vitro antioxidant capacity was evaluated in relation to the DPPH radical scavenging abilities associated with WPI, ASF and WPI + ASF supplementation. The results revealed that WPI presented an antioxidant capacity of approximately 40%, whereas that of ASF was 97% (both were evaluated at the same concentration: 1 mg/mL). WPI + ASF increased the inhibition observed for WPI alone by 80% (at a concentration of 1 mg/mL). Thus, the concentration of the polyphenol-rich fraction tested here increased the WPI antioxidant potential, resulting in superior multi-functional effects in the group of animals that received supplementation (DWS). Ghanta et al. (2007) and Mondaca et al. (2012) also found results indicating that S. rebaudiana may be useful as a potential source of natural antioxidants. The in vivo CAT was also analyzed in plasma samples from all of the animal groups after the supplementation period, and the results are shown in Table 4. The findings corroborated the results of the in vitro test, indicating that ASF produced superior antioxidant effects in the animals from the DS group compared to those from the DW group. Furthermore, the plasma values of the DSW group were better than those of the DW group, proving that ASF has antioxidant potential in addition to antidiabetic properties. This study also suggested that the DS group may have exhibited the best metabolic conditions because of the bioactives present in stevia leaves, which may exhibit higher antioxidant capacities than serum proteins. However, these properties muse be confirmed in future investigations.
Conclusion
WPI from whey through membrane separation was obtained and determined for centesimal composition. The main constituents of ASF obtained from Stevia UEM-13 leaves in a yield of 3.82% were phenolic compounds. The primary identified phenolic acids and flavonoids in ASF were consistent with reports in the literature. When 0.2% ASF was added to WPI, its antioxidant activity increased by 80%. Physiological tests revealed that the three supplements (ASF, WPI and WPI + ASF) contributed to improving the metabolic control of diabetic mice. Additionally, WPI + ASF was identified as food supplement with significant functionality, demonstrating that ASF has potential applications as a fortifier with antioxidant and antidiabetic activities that can be used to enrich foods or supplements.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The researchers would like to thank CAPES and graduate program in Food Science from the State University of Maringa
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2638-0) contains supplementary material, which is available to authorized users.
Contributor Information
Paula Gimenez Milani, Email: paulinhauem@gmail.com, Email: pg52505@uem.br.
Maysa Formigoni, Email: mayformigoni@live.com.
Yago Carvalho Lima, Email: yago7_lima@hotmail.com.
Silvano Piovan, Email: silvanopiovan23@gmail.com.
Giuliana Maria Ledesma Peixoto, Email: giu.peixoto@hotmail.com.
Daiane Montoia Camparsi, Email: daianemontoia@gmail.com.
Willian do Nascimento da Silva Rodrigues, Email: willian_rodrigues7@hotmail.com.
Jordana Quaglia Pereira da Silva, Email: jordanaqps@gmail.com.
Alexandre da Silva Avincola, Email: aleavincola@gmail.com.
Eduardo Jorge Pilau, Email: epilau@gmail.com, Email: ejpilau@uem.br.
Cecília Edna Mareze da Costa, Email: cemcosta@uem.br.
Silvio Cláudio da Costa, Email: sccosta@uem.br, Email: sccosta139@gmail.com.
References
- AOAC International . Official methods of analysis of AOAC International. 16. Arlington: Association of Analytical Communities; 1995. [Google Scholar]
- Blios MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;26:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Dacome AS, Silva CC, Costa CEM, Fontana JD, Adelmann J, Costa SC. Sweet diterpenic glycosides balance of a new cultivar of Stevia rebaudiana (Bert.) Bertoni: isolation and quantitative distribution by chromatographic, spectroscopic, and eletrophoretic methods. Process Biochem. 2005;40:3587–3594. doi: 10.1016/j.procbio.2005.03.035. [DOI] [Google Scholar]
- Ebaid H. Promotion of immune and glycaemic functions in streptozotocin-induced diabetic rats treated with un-denatured camel milk whey proteins. Nutr Metab. 2014;11(31):1–13. doi: 10.1186/1743-7075-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277–285. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Ferri LAF, Alves-Do-Prado W, Yamada SS, Gazola S, Batista MR, Bazotte RB. Investigation of the antihypertensive effect of oral crude stevioside in patients with mild essential hypertension. Phytother Res. 2006;20:732–736. doi: 10.1002/ptr.1944. [DOI] [PubMed] [Google Scholar]
- Frid AH, Nilsson M, Holst JJ, Björck IME. Effect of whey on blood glucose and insulin responses to composite breakfast and lunch meals in type 2 diabetic subjects. Am Soc Clin Nutr. 2005;82:69–75. doi: 10.1093/ajcn.82.1.69. [DOI] [PubMed] [Google Scholar]
- Gaudel C, Nongonierma AB, Maher S, Flynn S, Krause M, Murray BA, Kelly PM, Baird AW, FitzGerald RJ, Newsholme P. a whey protein hydrolysate promotes insulinotropic activity in a clonal pancreatic b-cell line and enhances glycemic function in ob/ob mice. J Nutr. 2013;143(7):1109–1114. doi: 10.3945/jn.113.174912. [DOI] [PubMed] [Google Scholar]
- Gawel-Beben K, Bujak T, Niziol-Lukaszewska Z, Antosiewicz B, Jakubezyk A, Karas M, Rybrzynska K. Stevia rebaudiana Bert. leaf extracts as a multifunctional source of natural antioxidants. Molecules. 2015;20:5468–5486. doi: 10.3390/molecules20045468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanta S, Banerjee A, Poddar A, Chattopadhyay S. Oxidative DNA damage preventive activity and antioxidant potential of Stevia rebaudiana (Bertoni) Bertoni, a natural sweetener. J Agric Food Chem. 2007;55(26):10962–10967. doi: 10.1021/jf071892q. [DOI] [PubMed] [Google Scholar]
- Gregersen S, Jeppesen PB, Holst JJ, Hermansen K. A antihyperglycemic effects of stevioside in type 2 diabetic subjects. Metabolism. 2004;53(1):73–76. doi: 10.1016/j.metabol.2003.07.013. [DOI] [PubMed] [Google Scholar]
- Haraguchi FK, Pedrosa ML, Paula H, Santos RC, Silva ME. Influência das proteínas do soro sobre enzimas hepáticas, perfil lipídico e formação óssea de ratos hipercolesterolêmicos. Revista de Nutrição. 2009;22(4):517–525. doi: 10.1590/S1415-52732009000400007. [DOI] [Google Scholar]
- Instituto Adolfo Lutz (2005) Normas Analíticas do Instituto Adolfo Lutz: métodos químicos e físicos para análises de alimentos, Volume 1, capítulo 4, 4ª edn. IMESP, São Paulo
- Jia Z, Tang M, Wu J. The determination of flavonoid contentes in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
- Junod A, Lambert AE, Stauffacher W, Renold AE. Diabetogenic action of streptozotocin: relationship of dose to metabolic response. J Clin Investig. 1969;48(11):2129–2139. doi: 10.1172/JCI106180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga K, Honda K, Ando S, Harasawa I, Kamiya H, Takano Y. Intrathecal clonidine inhibits mechanical allodynia via activation of the spinal muscarinic M1 receptor in streptozotocin-induced diabetic mice. Eur J Pharmacol. 2004;505(1–3):75–82. doi: 10.1016/j.ejphar.2004.10.033. [DOI] [PubMed] [Google Scholar]
- Krishnaiah D, Bono A, Sarbatly R, Anisuzzaman SM. Antioxidant activity and total phenolic content of an isolated Morinda citrifolia L. methanolic extract from poly-ethersulphone (PES) membrane separator. J King Saud Univ Eng Sci. 2015;27(1):63–67. doi: 10.1016/j.jksus.2014.03.001. [DOI] [Google Scholar]
- Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51(2):216–222. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- López V, Pérez S, Vinuesa A, Zorzettoc C, Abian O. Stevia rebaudiana ethanolic extract exerts better antioxidant properties and antiproliferative effects in tumour cells than its diterpene glycoside stevioside. Food Funct. 2016;7(4):2107–2113. doi: 10.1039/C5FO01586C. [DOI] [PubMed] [Google Scholar]
- Ma M, Mu T. Anti-diabetic effects of soluble and insoluble dietary fibre from deoiled cumin in low-dose streptozotocin and high glucose-fat diet-induced type 2 diabetic rats. J Funct Foods. 2016;25:186–196. doi: 10.1016/j.jff.2016.05.011. [DOI] [Google Scholar]
- Milani PG, Dacome AS, Nalesso CCF, Fiorenti CA, Costa CEM, Costa SC. Functional properties and sensorial testing of whey protein concentrate sweetened with rebaudioside A. Revista de Nutrição. 2016;29(1):125–137. doi: 10.1590/1678-98652016000100012. [DOI] [Google Scholar]
- Mondaca RL, Vega-Gálvez A, Zura-Bravo L, Ah-Hen K. Stevia rebaudiana Bertoni, source of a high-potency natural sweetener: a comprehensive review on the biochemical, nutritional and functional aspects. Food Chem. 2012;132:1121–1132. doi: 10.1016/j.foodchem.2011.11.140. [DOI] [PubMed] [Google Scholar]
- Mortensen LS, Jensen JH, Hartvigsen ML, Jensen VK, Astrup A, Vrese M, Holst JJ, Thomsen C, Hermansen K. Effects of different fractions of whey protein on postprandial lipid and hormone responses in type 2 diabetes. Eur J Clin Nutr. 2012;66:709–805. doi: 10.1038/ejcn.2012.48. [DOI] [PubMed] [Google Scholar]
- Patel S. Emerging trends in nutraceutical applications of whey protein and its derivatives. J Food Sci Technol. 2015;52(11):6847–6858. doi: 10.1007/s13197-015-1894-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Youling L, Xiong BK. Antioxidant activity of peptide fractions from whey protein hydrolysates as measured by electron spin resonance. Food Chem. 2009;113:196–201. doi: 10.1016/j.foodchem.2008.07.068. [DOI] [Google Scholar]
- Rodbell M. Metabolism of isolated fat cells. J Biol Chem. 1964;239(2):375–380. [PubMed] [Google Scholar]
- Sgarbieri VC. Propriedades fisiológicas: funcionais das proteínas do soro do leite. Revista de Nutrição. 2004;17(4):397–409. doi: 10.1590/S1415-52732004000400001. [DOI] [Google Scholar]
- Shivanna N, Naika M, Khanum F, Kaul VK. Antioxidant, anti-diabetic and renal protective properties of Stevia rebaudiana. J Diabetes Complicat. 2013;27(2):103–113. doi: 10.1016/j.jdiacomp.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Shukla S, Mehta A, Bajpai VK, Shukla S. In vitro antioxidante activity and total phenolic content of ethanolic leaf extract of Stevia rebaudiana Bert. Food Chem Toxicol. 2009;47:2338–2343. doi: 10.1016/j.fct.2009.06.024. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation sustrates and antioxidants by means Folin–Ciocalteu reagentes. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Tadhani MB, Patel VH, Subhash R. In vitro antioxidant activities of Stevia rebaudiana leaves and callus. J Food Compos Anal. 2007;20:323–329. doi: 10.1016/j.jfca.2006.08.004. [DOI] [Google Scholar]
- Tong X, Dong J, Wu Z, Li W, Qin L. Whey protein improves insulin resistance via the increase of antioxidant capacity in model rats. Wei Sheng Yan Jiu. 2011;40(5):617–619. [PubMed] [Google Scholar]
- Wölwer-Rieck U. The leaves of Stevia rebaudiana (Bertoni), their constituents and the analyses thereof: a review. J Agric Food Chem. 2012;60:886–895. doi: 10.1021/jf2044907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.