Abstract
Abstract
Methylglyoxal (MGO) and glyoxal (GO), α-dicarbonyl compounds found in the Maillard reaction, progressively and irreversibly modify proteins. Beverages are an exogenous source of α-dicarbonyl compounds and may potentially increase MGO and GO levels in vivo. Using GC-FID method, we detected the MGO and GO contents of 86 beverages in Chinese supermarkets. The highest MGO and GO 587.5 µg/100 mL and 716.7 µg/100 mL respectively found in soyamilk and coffee. Herbal beverages, which contained bioactive components, had lower average levels of MGO (48.1 µg/100 mL) and GO (25.9 µg/100 mL). A box-and-whisker plot was used to display variation of the same group drinks, and comparing distributions between six different groups. It was further discovered that fat, protein and flavonoids, in addition to sweeteners, had notable effects on the formation of MGO and GO in soybean milk. The result of LC/MS indicated that quercetin could prevent the formation of MGO by trapping MGO to form the mono-MGO and di-MGO adducts during soybean milk manufacturing.
Graphical Abstract
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2639-z) contains supplementary material, which is available to authorized users.
Keywords: α-Dicarbonyl compounds, Methylglyoxal, Glyoxal, Beverages, Soy milk
Introduction
Nonenzymatic glycation has been shown to play an important pathogenic role in the development of diabetic complications. MGO and GO, products of glycolysis found in biological systems, appear to be major α-dicarbonyl precursors in the glycation process and advanced glycation end-product (AGE) formation in vivo. They react with free amino groups of proteins in a reversible manner and with modified proteins to form AGEs (Lo et al. 1994). α-Dicarbonyl compounds can also easily modify DNA, leading to the formation of AGEs of DNA (Breyer et al. 2008; Frischmann et al. 2005). Moreover, α-dicarbonyl compounds readily react with cellular constituents, modify aminophospholipids to form advanced lipoxidation end-products (ALEs) (Pamplona 2011; Vistoli et al. 2013), and exhibit strong cellular toxicity (Lee et al. 2009; Sheader et al. 2001). Recently, a series of α-dicarbonyl compounds has been found both in various food products and the physiological system via glucose autoxidation, lipid peroxidation and the elimination of phosphate in glycolysis (Frye et al. 1998). Food is the major extrinsic source that could cause the increase in α-dicarbonyl compounds under normal physiological conditions, attracting recent attention. In food, α-dicarbonyl compounds are generated from heat treatments, such as roasting, baking, broiling and frying through caramelization, the Maillard reaction, and lipid oxidation (Degen et al. 2012; Weerawatanakorn 2013). To date, MGO and GO have been detected in soft drinks (Lo et al. 2008), honey (Oelschlaegel et al. 2012), coffee (Daglia et al. 2007), and wine (Revel et al. 2000). Accordingly, increasing attention has been paid to regular consumption of sugar-sweetened drinks by food researchers.
In this study, we monitored for the first time the content of MGO and GO in different groups of beverages in the Chinese market, and a box-and-whisker plot was used to display variation of the same group drinks, and comparing distributions between six different groups. Very popular soy milk (plant protein drink), with highest levels of MGO, was singled out, and then evaluated the effects of sweeteners, protein, fat, and inhibitors on MGO and GO formation, and examined an inhibition strategy against their formation.
Materials and methods
Materials
Methylglyoxal (MGO, 40% in water), glyoxal (GO, 40% in water), 1,2-diaminobenzene (DB), and 2,3-butanedione (dimethylglyoxal, DMGO) were purchased from Sigma-Aldrich (St. Louis, MO, USA). HPLC-grade solvents and other reagents were obtained from Shanghai Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). HPLC-grade water was prepared using a Millipore Milli-Q purification system (Bedford, MA, USA). All beverages were obtained from a local supermarket in Nanjing, China.
Determination of MGO and GO in beverages
Preparation of beverage samples for analysis
Carbonated soft drinks were decarbonated using an ultrasonic bath. Fruit beverages and tea beverages were centrifuged at 10,000 rpm for 15 min. For protein beverages, 2 mL of each solution was mixed with 4 mL of methanol and allowed to precipitate for 2 h at −20 °C. If necessary, stock solutions were diluted with water prior to protein precipitation, after which the mixtures were centrifuged at 10,000 rpm for 15 min, and the supernatant was directly used for 1,2-dicarbonyl compound analysis after additional membrane filtration (0.45 μm, hydrophilic polypropylene). The prepared samples were stored at −20 °C until analysis.
Derivatization of MGO and GO with 1,2-diaminobenzene
A total of 1 mL of 100 mmol/L 1,2-diaminobenzene (derivatization agent) was added to 4 mL of sample and then mixed with 0.5 mL of 2,3-butanedione (internal standard) at 4 mmol/L. The reaction mixture was maintained at 60 °C for 15 min and then cooled in an ice bath, after which 1 mL of 1 mol/L acetaldehyde was added and incubated at 60 °C for 15 min, to react with the rest of the derivatization agent. The mixture was cooled by ice bath and extracted twice with 2 mL of methylene chloride. The organic phase was concentrated to 0.2 mL by a sample concentrator blowing nitrogen, of which 1 μL containing methylquinoxaline and quinoxaline was directly injected into the GC.
GC analysis
The levels of methylquinoxaline and quinoxaline were analyzed with an Agilent Gas Chromatograph (7820 Series, Agilent Technologies, Palo Alto, CA, USA) equipped with a flame ionization detector (FID). The column was an HP-5 MS (5%-Phenyl)-methylpolysiloxane silica capillary (30 m × 0.32 mm id, film thickness 0.25 μm, Agilent, Wilmington, DE, USA). The injector temperature was 250 °C, and the detector temperature was 280 °C, with hydrogen, air, and nitrogen flow rates at 30.0, 300, and 25.0 mL/min, respectively. The injector was in 1:1 split mode. The flow rate was set to a constant 2.0 mL/min of carrier gas (nitrogen). The GC oven temperature was programmed as follows: the initial oven temperature of 40 °C was held for 1 min, increased to 140 °C at a rate of 5 °C/min and held for 1 min, then increased to 250 °C at a rate of 50 °C/min and held for 1 min. The total run time was 25.2 min. The injection volume was 1 μL for each sample solution.
Factors influencing the formation of MGO and GO in soy milk
Influence of sugar and sweeteners on the formation of MGO and GO in soy milk
Soybeans (50.0 g) were thoroughly washed and soaked with tenfold distilled water overnight at room temperature. The soybeans were ground at high speed (21,000 rpm) for 10 min using a soymilk maker (MJ-BL25B1, Guangdong Midea Kitchen Appliances Manufacturing Co., Ltd, Guangzhou, China) and filtered through a 0.15-mm filter. The supernatant was homogenized in a high speed homogenizer (FJ-200, Shanghai Specimen Model Factory, Shanghai, China), with the addition of 6% sucrose, HFCS (F42), fructose, glucose, or xylitol, kept boiling for 2 min, and filled into bottles, which were then capped and autoclaved using a high-pressure steam sterilizer (HVE-50, Hirayama Manufacturing Corporation, Saitama, Japan) at 121 °C for 30 min. These samples were either immediately analyzed by GC or stored at −80 °C.
Influence of fat on the formation of MGO and GO in soy milk
Soybeans (50.0 g) were prepared following the same procedure as 2.2.1, except for the follow processing: the supernatant was centrifuged at 15,000 rpm for 10 min, the upper layer of fat was removed, and then the fat-free soy milk was homogenized in a high speed homogenizer, with the addition of 6% sucrose, in the presence (1.0, 1.5, 2.0, 2.5, or 3.0%) or absence of fat.
Influence of flavonoid on the formation of MGO and GO in soy milk
Soybeans (50.0 g) were prepared following the same procedure as 2.2.1, except for the following processing: the supernatant was homogenized in a high speed homogenizer, with the addition of 6% sucrose, in the presence (0.2, 1.0, or 2.0 mmol/L) or absence of flavonoids (catechin, quercetin, rutin, genistein, or luteolin).
LC/MS analysis
LC/MS analysis was carried out with a Agilent Masshunter System which consisted of an 1290 G4220A BinPump, an 1290 G4226A Wellplate sampler, an G4212A Diode array detector and an 6460 QQQ mass detector (Agilent, Santa Clara, CA, USA) incorporated with electrospray ionization (ESI) interfaces. A 250 × 4.6 mm i.d., 5 µm ZORBAX Eclipse XDB-C18 column (Agilent, Santa Clara, CA, USA) was used for separation at a flow rate of 0.6 mL/min. The column was eluted with 90% solvent B (water with 0.1% formic acid) 5 min, and linear increases in A (acetonitrile with 0.1% formic acid) to 30% from 5 to 30 min, maintaining 30% from 30 to 40 min, and then with 90% B from 40 to 46 min. The LC eluent was introduced into the ESI interface. The negative ion polarity mode was set for ESI ion source with the voltage on the ESI interface maintained at approximately 5 kV. Nitrogen gas was used as the sheath gas at a flow rate of 45 arb units and the auxiliary gas at 5 arb units, respectively. The structural information of quercetin and the major MGO adducts was obtained by tandem mass spectrometry (MS/MS) through collision-induced dissociation (CID) with a relative collision energy setting of 35%. Data acquisition was performed with Qualitative Analysis of Masshunter (Agilent, Santa Clara, CA, USA).
Statistical analysis
Experiments were evaluated using the Wilcoxon test according to the replicates analyzed and are presented as the median (interquartile). Significant differences among treatments were compared using Tukey’s test. Each sample was performed in triplicate and the experiment was done three times with comparable results. Differences among means at p < 0.05 were considered significant.
Results and discussion
GC-FID analysis
A GC method using DB as the derivatization agent was used to analyze MGO and GO. DB reacts with GO, MGO and 2,3-butanedione to form quinoxaline, 2-methylquinoxaline and 2,3-dimethylquinoxaline, respectively. The confirmed peaks were confirmed using standards of quinoxaline, 2-methylquinoxaline and 2,3-dimethylquinoxaline (Supplemental 1). 2,3-Butanedione (2,3-Butanedione was not found in our samples) as the internal standard underwent derivatization along with the endogenous MGO and GO. This approach minimizes the opportunity for systematic operator error. Under the optimized conditions, baseline separation of MGO and GO was obtained, and the total running time was 22.0 min (Supplemental 1). The peaks for quinoxaline and 2-methylquinoxaline appeared at retention times of 15.11 and 17.52 min, respectively. Good linearity was observed in the range of tested concentrations. The calibration curves for MGO and GO were Y = 0.0175X + 0.0031 (r = 0.9998) and Y = 0.0177X + 0.0034 (r = 0.9994), where Y is the ratio of MGO or GO and the 2,3-butanedione (internal standard) peak area, and X is the MGO or GO concentration (μg/mL). Concentration range applied from 0.1 to 50 μg/mL. The limits of detection (LOD) for MGO and GO were 0.02 and 0.03 μg/mL, and the limits of quantitation (LOQ) were 0.06 and 0.08 μg/mL (Supplemental 2–3).
Determination of MGO and GO in beverages
Drinks are potential sources of MGO and GO from sugar, fruit juice or plant protein contents. The quantitative method for α-dicarbonyl determination was performed based on quinoxaline derivative formation. MGO and GO levels of the 86 different beverages were classified into 6 groups, such as Carbonated Soft Drinks (CSD), Fruit and Vegetable Juice (FV), Sports Drinks (SD), Herbal Drinks (HD), Tea Drinks (TD), Protein Beverage (PB) [including of Plant Protein Beverage (PPB), Lactobacillus Acid Beverage (LAB), Coffee Beverages(CB)] (Table 1). The results indicated that almost all samples, even without sugar, contained one or both α-dicarbonyl compounds (MGO and GO). In the majority of these beverages, the average concentration and dispersion of GO was greater than that of MGO (Table 1; Fig. 1). These results revealed that GO was the main product in the beverage. As reported, GO is produced directly from glucose via retro-aldol condensation and is formed indirectly from glucose via a glycoaldehyde intermediate that undergoes autoxidation (Lange et al. 2012). Second, GO can also originate from lipid peroxidation of polyunsaturated fatty acids. As observed here, the level of GO in PPB or CB was about twice the amount of MGO.
Table 1.
MGO (GO) content of commercial beverages in the Chinese market
| Sample | GO (μg/100 mL) | MGO (μg/100 mL) | Sugar (%) | Protein (%) | Fat (%) |
|---|---|---|---|---|---|
| Carbonated soft drinks | |||||
| CSD1 | 24.0 ± 5.6 | 28.5 ± 0.3 | 4 | – | – |
| CSD2 | 57.7 ± 2.1 | 44.3 ± 0.2 | 4 | – | – |
| CSD3 | 58.6 ± 2.1 | 59.1 ± 1.2 | 4 | – | – |
| CSD4 | 52.6 ± 3.0 | 46.7 ± 1.1 | 4 | – | – |
| CSD5 | 28.6 ± 1.3 | 59.9 ± 0.9 | 4 | – | – |
| CSD6 | ND | 42.9 ± 0.8 | 4 | – | – |
| CSD7 | 2.8 ± 0.5 | 42.4 ± 0.5 | 4 | – | – |
| CSD8 | 42.1 ± 4.7 | 18.3 ± 0.4 | 3 | – | – |
| CSD9 | ND | 15.7 ± 0.7 | 0 | – | – |
| CSD10 | 19.3 ± 0.2 | 18.5 ± 1.0 | 0 | – | – |
| CSD11 | 118.5 ± 2.9 | 33.4 ± 1.1 | 4 | – | – |
| Fruit and vegetable juice | |||||
| FV1 | 273.8 ± 3.3 | 23.1 ± 0.2 | 2 | – | – |
| FV2 | 98.2 ± 0.6 | 47.4 ± 1.7 | 3 | – | – |
| FV3 | 39.5 ± 1.3 | 43.5 ± 0.6 | 3 | – | – |
| FV4 | 51.0 ± 0.7 | 43.4 ± 0.8 | 3 | – | – |
| FV5 | 53.2 ± 1.0 | 49.9 ± 1.1 | 3 | – | – |
| FV6 | 88.3 ± 0.9 | 50.1 ± 0.6 | 4 | – | – |
| FV7 | 107.2 ± 1.2 | 65.7 ± 1.4 | 2 | – | – |
| FV8 | 27.1 ± 0.4 | 110.6 ± 1.9 | 4 | – | – |
| FV9 | 33.5 ± 0.8 | 80.3 ± 1.6 | 4 | – | – |
| FV10 | ND | 96.7 ± 0.5 | 2 | – | – |
| Sports drinks | |||||
| SD1 | 114.0 ± 1.5 | 33.8 ± 0.4 | 2 | – | – |
| SD2 | 118.1 ± 0.9 | 34.2 ± 0.2 | 4 | – | – |
| SD3 | ND | 23.2 ± 0.3 | 2 | – | – |
| SD4 | 89.3 ± 0.7 | 31.1 ± 0.1 | 2 | – | – |
| SD5 | ND | 33.0 ± 0.7 | 2 | – | – |
| SD6 | 10.9 ± 0.2 | 36.1 ± 0.6 | 4 | – | – |
| SD7 | 83.9 ± 0.4 | 124.2 ± 1.2 | 2 | – | – |
| SD8 | ND | 58.5 ± 2.3 | 8 | – | – |
| SD9 | ND | 45.7 ± 1.0 | 8 | – | – |
| SD10 | ND | 49.5 ± 0.8 | 8 | – | – |
| SD11 | 134.4 ± 1.1 | 40.9 ± 1.0 | 2 | – | – |
| Herbal drinks | |||||
| HD1 | 43.0 ± 0.5 | 58.5 ± 0.5 | 3 | – | – |
| HD2 | 10.7 ± 0.1 | 34.0 ± 0.2 | 2 | – | – |
| HD3 | 21.5 ± 0.2 | 35.6 ± 0.3 | 3 | – | – |
| HD4 | 18.7 ± 0.1 | 33.6 ± 0.6 | 1 | – | – |
| HD5 | 55.7 ± 2.1 | 76.0 ± 0.8 | 3 | – | – |
| HD6 | 35.5 ± 2.1 | 96.5 ± 0.9 | 4 | – | – |
| HD7 | 5.2 ± 0.1 | 14.4 ± 0.3 | 3 | – | – |
| HD8 | ND | 28.5 ± 0.3 | 3 | – | – |
| HD9 | 39.3 ± 0.9 | 43.4 ± 2.1 | 4 | – | – |
| HD10 | 3.3 ± 5.1 | 60.1 ± 0.5 | 2 | – | – |
| Tea drinks | |||||
| TD1 | 16.5 ± 0.1 | 49.0 ± 0.5 | 3 | – | – |
| TD2 | 62.9 ± 0.5 | 41.9 ± 0.6 | 1 | – | – |
| TD3 | ND | 23.5 ± 0.4 | 0 | – | – |
| TD4 | ND | 24.6 ± 0.2 | 0 | – | – |
| TD5 | ND | 45.6 ± 0.3 | 0 | – | – |
| TD6 | ND | 60.7 ± 0.5 | 0 | – | – |
| TD7 | 97.6 ± 1.0 | 37.5 ± 0.1 | 3 | – | – |
| TD8 | 74.8 ± 0.7 | 21.0 ± 0.3 | 3 | – | – |
| TD9 | 90.5 ± 2.0 | 44.7 ± 1.6 | 3 | – | – |
| Plant protein beverage | |||||
| PPB1 | 363.9 ± 3.7 | 174.7 ± 2.0 | 4 | 2 | 4 |
| PPB2 | 282.8 ± 2.6 | 250.1 ± 1.4 | 2 | 2 | 3 |
| PPB3 | 181.6 ± 1.9 | 150.6 ± 0.9 | 2 | 1 | 4 |
| PPB4 | 287.9 ± 0.4 | 53.4 ± 0.7 | 2 | 1 | 4 |
| PPB5 | 500.8 ± 8.1 | 370.8 ± 1.3 | 3 | 1 | 4 |
| PPB6 | 455.5 ± 3.4 | 587.5 ± 7.0 | 4 | 3 | 3 |
| PPB7 | 104.6 ± 0.9 | 164.6 ± 1.5 | 2 | 1 | 3 |
| PPB8 | 499.1 ± 1.2 | 33.3 ± 0.2 | 5 | 1 | 2 |
| PPB9 | 387.3 ± 3.1 | 97.9 ± 0.8 | 3 | 4 | 2 |
| PPB10 | 283.3 ± 2.0 | 109.8 ± 0.6 | 1 | 4 | 2 |
| PPB11 | 226.2 ± 1.3 | 200.1 ± 2.1 | 2 | 1 | 3 |
| PPB12 | 148.1 ± 2.7 | 62.5 ± 0.3 | 2 | 1 | 4 |
| PPB13 | 288.1 ± 0.9 | 87.8 ± 0.8 | 1 | 0 | 2 |
| PPB14 | 215.3 ± 1.2 | 152.8 ± 2.3 | 5 | 5 | 7 |
| Lactobacillus acid beverage | |||||
| LAB1 | 202.3 ± 1.2 | 104.9 ± 0.6 | 5 | 2 | 0 |
| LAB2 | 93.6 ± 0.4 | 169.1 ± 1.4 | 5 | 2 | 0 |
| LAB3 | 107.3 ± 1.4 | 180.9 ± 0.8 | 5 | 1 | 0 |
| LAB4 | 218.3 ± 1.8 | 162.9 ± 2.0 | 5 | 2 | 0 |
| LAB5 | 92.7 ± 0.7 | 143.9 ± 0.7 | 4 | 1 | 0 |
| LAB6 | 274.8 ± 3.1 | 273.2 ± 3.0 | 5 | 2 | 0 |
| LAB7 | 324.8 ± 2.1 | 178.5 ± 2.1 | 5 | 2 | 0 |
| LAB8 | 314.2 ± 0.9 | 178.1 ± 0.3 | 5 | 2 | 0 |
| LAB9 | 156.2 ± 1.1 | 181.4 ± 1.4 | 5 | 2 | 0 |
| LAB10 | 103.7 ± 0.8 | 145.9 ± 0.9 | 5 | 6 | 0 |
| LAB11 | 306.3 ± 5.9 | 209.4 ± 1.6 | 5 | 5 | 5 |
| Coffee beverages | |||||
| CB1 | 650.0 ± 5.1 | 203 ± 2.1 | 2 | 2 | 2 |
| CB2 | 340 ± 3.1 | 144.9 ± 0.6 | 3 | 1 | 0 |
| CB3 | 372.7 ± 1.3 | 151.6 ± 0.8 | 3 | 1 | 2 |
| CB4 | 337.0 ± 2.1 | 110.2 ± 1.0 | 2 | 1 | 0 |
| CB5 | 439.8 ± 0.9 | 184.9 ± 1.2 | 3 | 2 | 2 |
| CB6 | 439.7 ± 2.3 | 221.6 ± 2.1 | 3 | 1 | 2 |
| CB7 | 481.3 ± 3.0 | 288.3 ± 3.4 | 2 | 2 | 3 |
| CB8 | 494.7 ± 4.2 | 189.5 ± 2.5 | 2 | 3 | 4 |
| CB9 | 396.5 ± 0.7 | 260.3 ± 0.9 | 2 | 0 | 0 |
| CB10 | 716.7 ± 7.8 | 554.6 ± 2.6 | 3 | 1 | 2 |
Concentration unit: μg/100 mL; values are the mean ± standard deviation, n = 3
ND not detectable; sugar, protein, and fat (%): total content on label
Fig. 1.
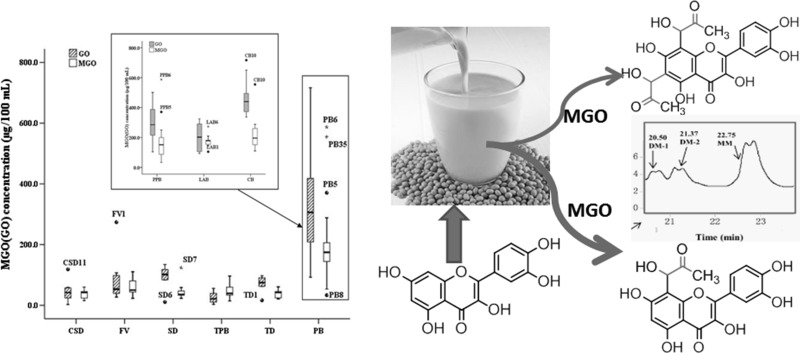
Box-and-whisker plot of minimum to maximum MGO and GO contents in different groups of beverages. CSD carbonated soft drinks, FV fruit and vegetable juice, SD sports drinks, HD herbal drinks, PB plant protein beverage, PPB plant protein beverage, LAB lactobacillus acid, CB coffee beverage
In the carbonated soft drinks (CSD), MGO content was a little lower than that reported earlier (Tan et al. 2008). The reason may be that in China, only a few soft drinks combine high-fructose corn syrup (HFCS, F42) with sucrose for sweetening. However, HFCS, one of the high potential sources of α-dicarbonyl (Lo et al. 2008), is added to nearly all sugared CSDs in the US market (White 2008). MGO was not detected in 11 carbonated soft drinks from the German market (Degen et al. 2012). Because most products are sweetened with only sucrose in Europe, only a few sodas contain small quantities of HFCS. Additionally, our results of MGO and GO contents were similar to findings in the Taiwan market (Lo et al. 2008). Furthermore, without sugar or HFCS sweeteners in CSD9, the levels of MGO (15.7 μg/100 mL) and GO (ND) dropped to the minimum. In addition, a little of MGO and GO, derived from the glucose degradation, was also prevented by the low pH induced by acid in CSD.
For fruit and vegetable juices (FV), higher levels of MGO were observed in juices (FV7–FV10, 65.7–110.6 μg/100 mL) than in soft drinks. One possible explanation is linked to the presence of saccharides in juice, such as glucose, fructose, and sucrose, coming from the fruit material.
In sports drinks (SD), the GO contents displayed a wide dispersion, from no detectable levels (SD3, SD5, SD8–10) to 134.4 μg/100 mL, yet the MGO contents were tightly clustered, ranging between 30 and 50 μg/100 mL, except a single outlier (124.2 μg/100 mL), which was triggered by a peptide contained in the SD. First, GO and MGO might be generated from the sweetener (sugar) through a retro-aldol reaction, where α-dicarbonyl compounds could be derived from condensed juice rich in monosaccharides besides the sweetener. Alternately, minerals in the SD could have promoted sugar autoxidation to produce GO. Finally, ascorbate added to the SD can also spontaneously hydrolyze into GO through an unknown mechanism (Shangari and O’Brien 2004).
In the tea drinks (TD), remarkably lower levels of GO and MGO were observed. Notably, GO was not detected in four drinks (TD3–6). These drinks (TD3–6) had “0” calories, without added sucrose or HFCS. Furthermore, the amount of tea polyphenols in tea drinks must be greater than 150–300 mg/kg on the basis of a national standard (GB/T 21733-2008), and tea polyphenols could effectively reduce MGO and GO generation (Sang et al. 2007).
Among these 6 beverage types, the lowest average and relatively lower overall contents of GO and MGO were found in the herbal drinks (HD). As determined from the beverage ingredient lists, these contained various traditional herbs or their extracts, extracted from medicine-food homologous plants, included in the drink formula for human health. Therefore, lower content of MGO and GO may be due to bioactive compounds, e.g., polyphenols (Lo et al. 2011), chlorogenic acid (Gugliucci et al. 2009) or flavonoids (Li et al. 2014; Shao et al. 2008) from those plants, or possibly even the synergistic effects of various components scavenging the α-dicarbonyl compounds. As for the higher levels of MGO in the HD group, some were due to HFCS in the formulation (Gensberger et al. 2013), (HD 6, 96.5 μg/100 mL and HD 10, 60.1 μg/100 mL), while others are due to higher monosaccharide content (glucose, fructose) originating from raw materials (HD 5, 76.0 μg/100 mL) such as longan, Chinese wolfberry, and jujubae.
Conversely, the protein beverages, such as plant protein beverages (PPB) especially soy milk, lactic acid bacteria beverages (LAB) and coffee beverages (CB), possessed notably higher levels of GO (Max. 716.7 μg/100 mL) and MGO (Max. 587.5 μg/100 mL) than all other beverages. These results indicated that ingredients in the formula such as protein or fat were linked to increase in MGO and GO. This was in concordance with previous reports that foods high in protein and fat contained higher amounts of MGO than did carbohydrate-rich foods (Poulsen et al. 2013). The proteins or fat take part in the Maillard reaction. In addition, the fermentation process of lactic acid bacteria was one of the sources of elevating the concentrations of MGO and GO (Yamaguchi et al. 1994). As reported, some α-dicarbonyl compounds were of enzymatic origin in fermented products such as wine (up to 1556 μg/L) (De Revel and Bertrand 1993) and beer (230–1000 μg/L) (Barros et al. 1999). Moreover, a marked increase in MGO and GO levels in coffee beverages was suggested to be induced by thermal reactions occurring during coffee bean roasting (Arribas-Lorenzo and Morales 2010; Zhang et al. 2011).
A box-and-whisker plot depicts the contents of MGO and GO by quartiles from minimum to maximum in different groups of beverages (Fig. 1). The spacings between the different parts of the box indicate the degree of dispersion and skewness in the MGO and GO content. Also shown are outliers notably outside the range of statistical data, such as CSD11, FV1, SD6, TD1, and CB10 for GO content and SD7, PPB5, PPB6, LAB1, LAB6, and CB10 for MGO content. These data scatter groups and the outliers may yield information toward finding the factors that cause MGO and GO to increase or decrease. As shown in Fig. 1, except in the TPB, the dispersion of GO in various beverages was broader than that of MGO, especially in protein beverage groups such as PPB (GOmax, 104.6–500.8 μg/100 mL, median = 287.9 μg/100 mL) and LAB (MGOmin, 143.9–209.4 μg/100 mL, median = 169.1 μg/100 mL). These results indicated that the concentration of GO was largely scattered and highly volatile, revealing GO to be prone to variation from various factors. Therefore, careful attention should be paid to beverage formulation and processing to decrease the generation of GO.
Furthermore, in the same groups of protein beverages, the average level of GO was twice as high as MGO in PPB (GO, 302.8 μg/100 mL; MGO, 128.1 μg/100 mL) and CB (GO, 439.1 μg/100 mL; MGO, 194.9 μg/100 mL) and similar to MGO in LAB (GO, 166.0 μg/100 mL; MGO, 165.9 μg/100 mL) because of the absence of fat. In the different groups, there was approximately half the content of GO in LAB (165.9 μg/100 mL) than in PPB (302.8 μg/100 mL) or CB (439.1 μg/100 mL) because of the presence of fat. Therefore, our observed results may be attributed to the fraction of GO in PPB or CB originating from fat oxidation (Yin and Porter 2005).
Examining MGO, the average content in protein beverages (128.1–194.9 μg/100 mL) was approximately 3–4 times higher than the five beverage groups (38.7–53.7 μg/100 mL) without protein. On the other hand, there were no pronounced differences in the average concentrations in beverages without protein, except in whole juice (53.7 μg/100 mL), which is rich in sugar originating from fruit material. This implies that protein glycation led to higher MGO levels (Poulsen et al. 2013).
In view of the result that soy milk had the highest level of MGO (Max. 587.5 μg/100 mL) among these groups beverage, we further investigated how to control the level of MGO and GO in the soy milk processing.
Influence of sugar and sweeteners on the formation of MGO and GO
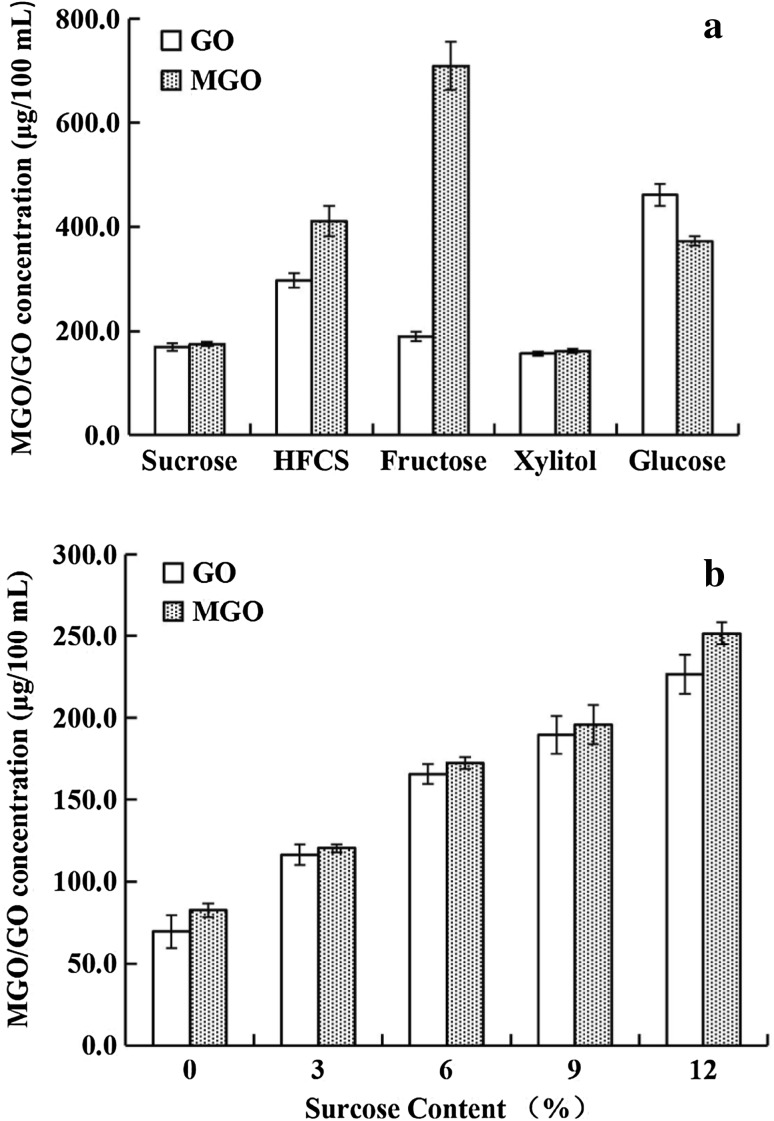
As observed, the different sweeteners had clear impacts on the formation of MGO and GO (Fig. 2a). The concentration of generated MGO successively increased from 161.7 μg/100 mL to 707.3 μg/100 mL using the following sweeteners in soy milk processing: xylitol, sucrose, glucose, HFCS, and fructose. Fructose was the most active, leading to a maximum amount of MGO that was 4.3 times greater than the minimum induced by xylitol. Compared with MGO, the generation of GO (156.5–461.6 μg/100 mL) was not remarkably affected by sweeteners. The sweeteners induced MGO production in the following increasing order: xylitol, sucrose, fructose, HFCS, and glucose.
Fig. 2.
Influence of sugar and sweeteners (a) and the concentration of sucrose (b) on the formation of MGO and GO in soy milk
The formation of both MGO and GO in soy milk displays a markedly positive correlation with sucrose concentration (r = 0.9893, p < 0.01 and r = 0.9855, p < 0.01, respectively) (Fig. 2b). This allows us to conclude that the sucrose level in soy milk processing relates equally to the generation of MGO and GO.
Influence of fat on the formation of MGO and GO
As shown in Fig. 3, a highly significant linear correlation (r = 0.90) was observed between the GO concentration and the fat content, while the level of MGO did not obviously vary with increasing fat concentration (1.0–3.0%), although the MGO levels had a large increase from 53.8 μg/100 mL to 176.0 μg/100 mL as the fat content increased from fat-free to 1.0%. Soy milk made from whole soybeans (without defatting) generally contained approximately 1% fat. Thus, the fat content made a greater contribution to increasing the level of GO than MGO in soy milk. This was in concordance with our findings that the GO content doubles in PPB (302.8 μg/100 mL) or CB (439.1 μg/100 mL) from the presence of fat, compared with its levels in LAB (165.9 μg/100 mL), while little difference was observed in the MGO content between LAB (166.0 μg/100 mL) and PPB (128.1) or CB (194.9 μg/100 mL).
Fig. 3.
Influence of fat on the formation of MGO and GO in soy milk
Inhibitory effects of flavonoids on the formation of MGO and GO in soy milk
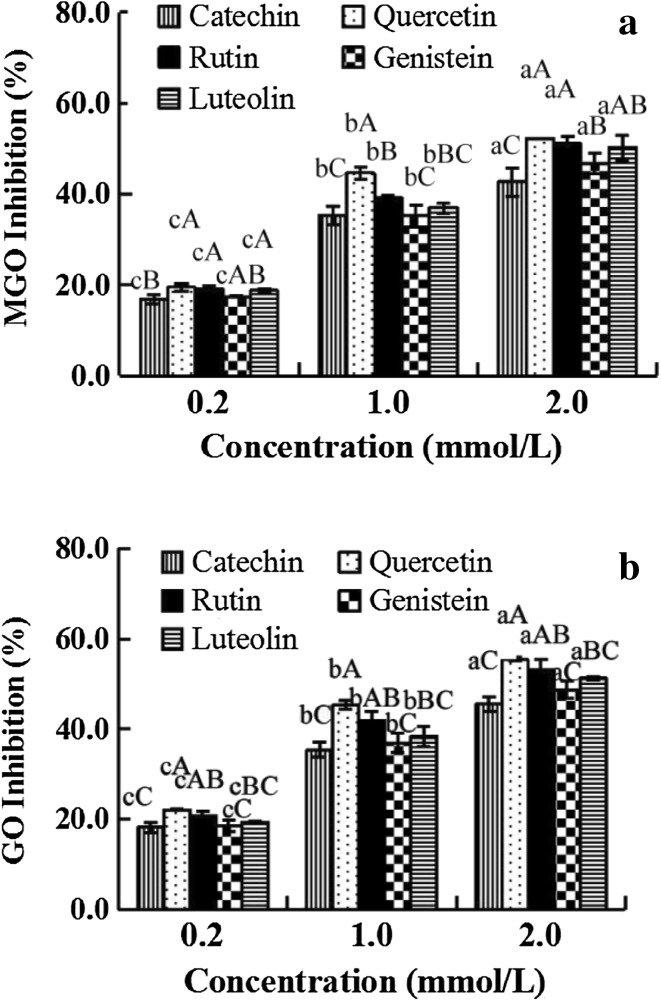
Previous studies have demonstrated that MGO and GO can be effectively trapped by flavonoids in PBS-flavonoid-MGO and GO systems (Lv et al. 2011; Sang et al. 2007; Shao et al. 2014). To verify the efficiency of various flavonoids on the formation of dicarbonyls in soy milk, five dietary flavonoids (catechin, quercetin, rutin, genistein, and luteolin) were added at different concentrations (0.2–2 mmol/L) to soy milk. Our results showed that the five flavonoids could clearly inhibit the formation of MGO and GO, and the flavonoids displayed increasing inhibitory activity in the following order: quercetin, rutin, luteolin, catechin, and genistein at the same concentration (Fig. 4). Furthermore, the inhibition ratio of various flavonoids on MGO and GO responded in a dose-dependent manner (0.2–2 mmol/L). Genistein, derived from soybeans, could simultaneously suppress 45.6% of GO and 42.7% of MGO at a concentration of 2 mmol/L. Thus, our findings also confirmed why the traditional plant beverages and tea beverages possess lower levels of MGO and GO.
Fig. 4.
Flavonoid inhibition of the formation of MGO and GO in soybean milk, a MGO; b GO
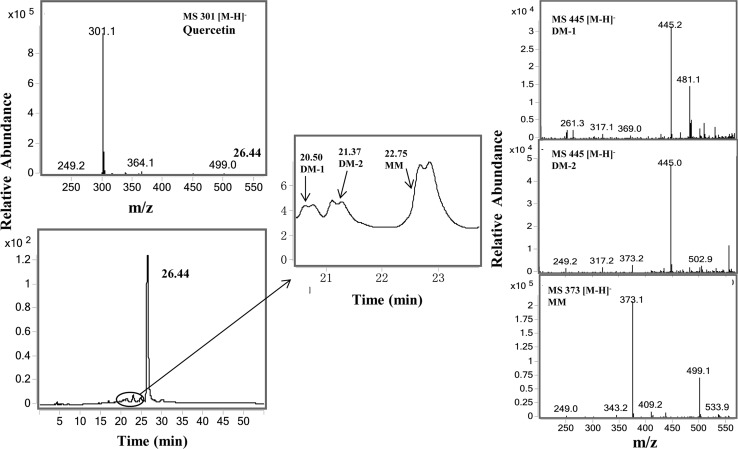
To further determine whether these flavonoids could decrease the levels of MGO and GO via the trapping mechanisms, we used quercetin as an example. The methanol extracts of soybean milk in addition of quercetin were analyzed by LC/MS (Fig. 5). Except the peck of quercetin (tR 26.44 min), there were two new peaks appeared in the LC chromatogram at 20.20 and 21.37 min, both of them had the molecular ion m/z 445 [M–H]− (Fig. 5b), which was 144 mass units heavier than that of quercetin (m/z 301 [M–H]−, indicating that these peaks are the Di-MGO adduct of quercetin (DM-1 and DM-2). Both DM-1 and DM-2 are a mixture of two tautomers. The third new peak observed at 22.75 min, with the molecular ion m/z 373 [M–H]−, which was 72 mass units higher than that of quercetin, suggesting that this peak is the Mono-MGO adduct of quercetin. Mono-MGO is also a mixture of tautomers. This result was consistent with our previous findings that quercetin inhibit the formation of AGEs via trapping MGO to form MGO adducts in the BSA-MGO System under physiological conditions (Li et al. 2014).
Fig. 5.
LC chromatogram of quercetin and mono- and di-MGO adducts of quercetin in soybean milk, and MS spectra of mono-MGO and di-MGO adducts of quercetin
Conclusion
In the present study, a GC-FID method was performed for the detection and quantitative analysis of MGO and GO in beverages. This is the first report of α-dicarbonyl contents of beverages in the Chinese mainland. As we expected, statistical tests of the amounts of MGO and GO in beverages showed strong significant correlation between protein or fat and dicarbonyl compounds, apart from sweeteners. Furthermore, other possible factors may also play an important role in forming MGO and GO, such as manufacturing processes (e.g., fermentation method, heat treatment, temperature and treatment time), alternate sources of α-dicarbonyl compounds, e.g., oil, sugar, HFCS, honey or fruit in beverage formulations. Additionally, herbal or phytochemical components, e.g., flavonoids, added to the beverages might effectively reduce the levels of α-dicarbonyl compounds by trapping MGO to form the momo-MGO or di-MGO adducts in the processing.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Gas chromatogram of the derived MGO and GO detected by FID. Column is an HP-5 MS (5%-Phenyl)-methylpolysiloxane silica capillary (30 m × 0.32 mm id, film thickness 0.25 μm; Peaks: (1) solvent, (2) GO, (3) MGO, and (4) DMGO (TIFF 61 kb)
Acknowledgements
This work was supported by funding from the National Natural Science Foundation of China (Grant No. 31571783) to L. Lv.
Compliance with ethics standards
Conflict of interest
There is no conflict of interest of any author.
Human and animal rights
This article does not contain any studies with human or animal subjects.
Footnotes
Chen Wang and Yongling Lu have contributed equally to this study.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2639-z) contains supplementary material, which is available to authorized users.
References
- Arribas-Lorenzo G, Morales FJ. Analysis, distribution, and dietary exposure of glyoxal and methylglyoxal in cookies and their relationship with other heat-induced contaminants. J Agric Food Chem. 2010;58:2966–2972. doi: 10.1021/jf902815p. [DOI] [PubMed] [Google Scholar]
- Barros A, Rodrigues J, Almeida P, Oliva-Teles M. Determination of glyoxal, methylglyoxal, and diacetyl in selected beer and wine, by HPLC with UV spectrophotometric detection, after derivatization with o-phenylenediamine. J Liq Chromatogr Relat Technol. 1999;22:2061–2069. doi: 10.1081/JLC-100101786. [DOI] [Google Scholar]
- Breyer V, Frischmann M, Bidmon C, Schemm A, Schiebel K, Pischetsrieder M. Analysis and biological relevance of advanced glycation end-products of DNA in eukaryotic cells. FEBS J. 2008;275:914–925. doi: 10.1111/j.1742-4658.2008.06255.x. [DOI] [PubMed] [Google Scholar]
- Daglia M, Papetti A, Aceti C, Sordelli B, Spini V, Gazzani G. Isolation and determination of α-dicarbonyl compounds by RP-HPLC-DAD in green and roasted coffee. J Agric Food Chem. 2007;55:8877–8882. doi: 10.1021/jf071917l. [DOI] [PubMed] [Google Scholar]
- De Revel G, Bertrand A. A method for the detection of carbonyl compounds in wine: glyoxal and methylglyoxal. J Sci Food Agric. 1993;61:267–272. doi: 10.1002/jsfa.2740610221. [DOI] [Google Scholar]
- Degen J, Hellwig M, Henle T. 1,2-Dicarbonyl compounds in commonly consumed foods. J Agric Food Chem. 2012;60:7071–7079. doi: 10.1021/jf301306g. [DOI] [PubMed] [Google Scholar]
- Frischmann M, Bidmon C, Angerer J, Pischetsrieder M. Identification of DNA adducts of methylglyoxal. Chem Res Toxicol. 2005;18:1586–1592. doi: 10.1021/tx0501278. [DOI] [PubMed] [Google Scholar]
- Frye EB, Degenhardt TP, Thorpe SR, Baynes JW. Role of the Maillard reaction in aging of tissue proteins advanced glycation end product-dependent increase in imidazolium cross-links in human lens proteins. J Biol Chem. 1998;273:18714–18719. doi: 10.1074/jbc.273.30.18714. [DOI] [PubMed] [Google Scholar]
- Gensberger S, Glomb MA, Pischetsrieder M. Analysis of sugar degradation products with α-dicarbonyl structure in carbonated soft drinks by UHPLC-DAD-MS/MS. J Agric Food Chem. 2013;61:10238–10245. doi: 10.1021/jf3048466. [DOI] [PubMed] [Google Scholar]
- Gugliucci A, Bastos DHM, Schulze J, Souza MFF. Caffeic and chlorogenic acids in Ilex paraguariensis extracts are the main inhibitors of AGE generation by methylglyoxal in model proteins. Fitoterapia. 2009;80:339–344. doi: 10.1016/j.fitote.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Lange JN, Wood KD, Knight J, Assimos DG, Holmes RP. Glyoxal formation and its role in endogenous oxalate synthesis. Adv Urol. 2012;2012:1–5. doi: 10.1155/2012/819202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Seo I, Suh DJ, Lee HJ, Park HT. A novel mechanism of methylglyoxal cytotoxicity in neuroglial cells. J Neurochem. 2009;108:273–284. doi: 10.1111/j.1471-4159.2008.05764.x. [DOI] [PubMed] [Google Scholar]
- Li X, Zheng T, Sang S, Lv L. Quercetin inhibits advanced glycation end product formation by trapping methylglyoxal and glyoxal. J Agric Food Chem. 2014;62:12152–12158. doi: 10.1021/jf504132x. [DOI] [PubMed] [Google Scholar]
- Lo TWC, Westwood ME, McLellan AC, Selwood T, Thornalley PJ. Binding and modification of proteins by methylglyoxal under physiological conditions. A kinetic and mechanistic study with N alpha-acetylarginine, N alpha-acetylcysteine, and N alpha-acetyllysine, and bovine serum albumin. J Biol Chem. 1994;269:32299–32305. [PubMed] [Google Scholar]
- Lo C-Y, Li S, Wang Y, Tan D, Pan M-H, Sang S, Ho C-T. Reactive dicarbonyl compounds and 5-(hydroxymethyl)-2-furfural in carbonated beverages containing high fructose corn syrup. Food Chem. 2008;107:1099–1105. doi: 10.1016/j.foodchem.2007.09.028. [DOI] [Google Scholar]
- Lo CY, Hsiao WT, Chen XY. Efficiency of trapping methylglyoxal by phenols and phenolic acids. J Food Sci. 2011;76:H90–H96. doi: 10.1111/j.1750-3841.2011.02067.x. [DOI] [PubMed] [Google Scholar]
- Lv L, Shao X, Chen H, Ho C-T, Sang S. Genistein inhibits advanced glycation end product formation by trapping methylglyoxal. Chem Res Toxicol. 2011;24:579–586. doi: 10.1021/tx100457h. [DOI] [PubMed] [Google Scholar]
- Oelschlaegel S, Gruner M, Wang P-N, Boettcher A, Koelling-Speer I, Speer K. Classification and characterization of manuka honeys based on phenolic compounds and methylglyoxal. J Agric Food Chem. 2012;60:7229–7237. doi: 10.1021/jf300888q. [DOI] [PubMed] [Google Scholar]
- Pamplona R. Advanced lipoxidation end-products. Chem Biol Interact. 2011;192:14–20. doi: 10.1016/j.cbi.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Poulsen MW, et al. Advanced glycation endproducts in food and their effects on health. Food Chem Toxicol. 2013;60:10–37. doi: 10.1016/j.fct.2013.06.052. [DOI] [PubMed] [Google Scholar]
- Revel Gd, Pripis-Nicolau L, Barbe JC, Bertrand A. The detection of α-dicarbonyl compounds in wine by formation of quinoxaline derivatives. J Sci Food Agric. 2000;80:102–108. doi: 10.1002/(SICI)1097-0010(20000101)80:1<102::AID-JSFA493>3.0.CO;2-Y. [DOI] [Google Scholar]
- Sang S, Shao X, Bai N, Lo C-Y, Yang CS, Ho C-T. Tea polyphenol (–)-epigallocatechin-3-gallate: a new trapping agent of reactive dicarbonyl species. Chem Res Toxicol. 2007;20:1862–1870. doi: 10.1021/tx700190s. [DOI] [PubMed] [Google Scholar]
- Shangari N, O’Brien PJ. The cytotoxic mechanism of glyoxal involves oxidative stress. Biochem Pharmacol. 2004;68:1433–1442. doi: 10.1016/j.bcp.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Shao X, Bai N, He K, Ho C-T, Yang CS, Sang S. Apple polyphenols, phloretin and phloridzin: new trapping agents of reactive dicarbonyl species. Chem Res Toxicol. 2008;21:2042–2050. doi: 10.1021/tx800227v. [DOI] [PubMed] [Google Scholar]
- Shao X, Chen H, Zhu Y, Sedighi R, Ho C-T, Sang S. Essential structural requirements and additive effects for flavonoids to scavenge methylglyoxal. J Agric Food Chem. 2014;62:3202–3210. doi: 10.1021/jf500204s. [DOI] [PubMed] [Google Scholar]
- Sheader EA, Benson RS, Best L. Cytotoxic action of methylglyoxal on insulin-secreting cells. Biochem Pharmacol. 2001;61:1381–1386. doi: 10.1016/S0006-2952(01)00603-7. [DOI] [PubMed] [Google Scholar]
- Tan D, Wang Y, Lo C-Y, Sang S, Ho C-T. Methylglyoxal: its presence in beverages and potential scavengers. Ann N Y Acad Sci. 2008;1126:72–75. doi: 10.1196/annals.1433.027. [DOI] [PubMed] [Google Scholar]
- Vistoli G, De Maddis D, Cipak A, Zarkovic N, Carini M, Aldini G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): an overview of their mechanisms of formation. Free Radic Res. 2013;47:3–27. doi: 10.3109/10715762.2013.815348. [DOI] [PubMed] [Google Scholar]
- Weerawatanakorn M. Dicarbonyl compounds and sugar contents of Thai commercial beverages. J Sci Technol. 2013;35:631–639. [Google Scholar]
- White JS. Straight talk about high-fructose corn syrup: what it is and what it ain’t. Am J Clin Nutr. 2008;88:1716S–1721S. doi: 10.3945/ajcn.2008.25825B. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Ishida J, Xuan ZX, Nakamura A, Yoshitake T. Determination of glyoxal, methylglyoxal, diacethyl, and 2,3-pentanedione in fermented foods by high-performance liquid chromatography with fluorescence detection. J Liq Chromatogr. 1994;17:203–211. doi: 10.1080/10826079408013445. [DOI] [Google Scholar]
- Yin H, Porter NA. New insights regarding the autoxidation of polyunsaturated fatty acids. Antioxid Redox Signal. 2005;7:170–184. doi: 10.1089/ars.2005.7.170. [DOI] [PubMed] [Google Scholar]
- Zhang G, Huang G, Xiao L, Mitchell AE. Determination of advanced glycation endproducts by LC-MS/MS in raw and roasted almonds (Prunus dulcis) J Agric Food Chem. 2011;59:12037–12046. doi: 10.1021/jf202515k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gas chromatogram of the derived MGO and GO detected by FID. Column is an HP-5 MS (5%-Phenyl)-methylpolysiloxane silica capillary (30 m × 0.32 mm id, film thickness 0.25 μm; Peaks: (1) solvent, (2) GO, (3) MGO, and (4) DMGO (TIFF 61 kb)